Abstract
A large number of secondary metabolites like alkaloids, terpenoids, polyphenols and quinones are produced by the plants. These metabolites can be utilized as natural medicines for the reason that they inhibit the activity of DNA topoisomerase which are the clinical targets for anticancer drugs. DNA topoisomerases are the cellular enzymes that change the topological state of DNA through the breaking and rejoining of DNA strands. Synthetic drugs as inhibitors of topoisomerases have been developed and used in the clinical trials but severe side effects are a serious problem for them therefore, there is a need for the development of novel plant-derived natural drugs and their analogs which may serve as appropriate inhibitors with respect to drug designing. The theme for this review is how secondary metabolites or natural products inactivate the action of DNA topoisomerases and open new avenues towards isolation and characterization of compounds for the development of novel drugs with anticancer potential.
Keywords: Anticancer, DNA topoisomerases, secondary metabolities, flavonoids
INTRODUCTION
Secondary metabolites
During the course of evolution an enormous diversity has been developed in nature in the form of different plant species, insects, fungi, algae and prokaryotes. All these species coexist and interact in several ways in the environment sharing a similar biochemistry necessary for a living cell and producing a large amount of metabolites. Bearing in mind the large diversity in nature we focus on plants as a source of metabolites.[1]
Plants form an important part of our everyday diet. Their constituents and nutritional values have been intensively studied for decades. Along with the essential primary metabolites (carbohydrates, amino acids, lipids) they synthesize a wide variety of low molecular weight compounds known as the secondary metabolites. Plant secondary metabolites can be defined as the compounds that play an important role in the interaction of the plant with its environment but have no such role in maintaining the fundamental life processes in plants. Secondary metabolites often have complex and unique structures and are stored in specific cells and or organs that are accumulated in the plant vacuoles. Production of these compounds depends on the physiological and developmental stages of the plant and is enhanced by biotic and abiotic stress.[2] Almost lacs of secondary metabolites have been discovered from the plant kingdom but only half the structures have been fully elucidated.[1] These metabolites are characterized by enormous chemical diversity and every plant species consists its own set of metabolites derived from few building blocks like C2, C9 and C5 units. Depending on the biosynthetic pathways plant secondary metabolites can be structurally divided into five major groups as polyketides, isoprenoids (terpenoids), alkaloids, phenylpropanoids and flavonoids (polyphenols). Biosynthetic pathways are often long, complex, multistep events catalyzed by various enzymes. Polyketides are produced via the acetate-mevalonate pathway, isoprenoids are derived from 5C precursor isopentenyl diphosphate (IPP) or via a classical mevalonate or non-mevalonate pathway, alkaloids are synthesized from various amino acids, phenylpropanoids having a C6-C3 unit are derived from aromatic acids and flavonoids are synthesized by the combination of phenylpropanoids and polyketides.[3]
The presence of various secondary metabolites in the plants implies multiple functions throughout the plants lifecycle. Some of the functions possessed by these metabolites are their role as mediators in the interaction of the plants with its environment (plant-insect, plant-microorganisms, plant-plant interaction), its production as a part of plant defense system (e.g.; production of antifeedants, phytoanticipins and production of phytoalexins), its role in reproduction (insect attractant and male sterility). Secondary metabolites are also known to determine different aspect of food quality (taste, smell, color and flavor). Pigments like anthocyanins and carotenoids are important for the diversity of ornamental plants and flowers. Several secondary metabolites are used in the production of dyes, insecticides, flavors and medicines.[4]
Many of the secondary metabolites show strong biological activities like inhibition of DNA and protein synthesis, inhibition of nervous system, cardiac activity and modulation of microtubule structure.[5] Secondary metabolites thus form an extremely diverse and important class of natural products with industrial and biomedical applications and are interesting targets for drug design.[6] A number of pharmacological agents target the enzyme topoisomerase and consequently research has progressed from DNA enzymology into developmental therapeutics. The study on the basic biochemistry and molecular biology of the enzyme has lead towards its application in clinical pharmacology.
We further focus briefly on DNA topoisomerases, their types, structure and their inhibitors secondary metabolites along with their mode of action.
DNA topoisomerases: An outline of its discovery, classification, structure, and applications.
Research on DNA topoisomerase was inspired by the problem of untwining of the two parental strands of DNA by semi conservative replication. The discovery of circular DNA lead to conclude that a ‘swivel’ must have been introduced into a circular DNA rings to permit strand separation.[7] In 1971, Wang reported that E. coli extracts are capable of relaxing supercoiled DNA.[8] Initially, it was thought that relaxation of the DNA occurred by a cycle of endonucleolytic nicking and resealing of the nick by DNA ligase but subsequent purification of the enzyme however, showed that a single enzyme is capable of relaxing negatively supercoiled DNA. This enzyme was originally designated as the ‘ω’ protein.[9] The enzyme was later renamed as E.coli DNA topoisomerase I which catalyzed relaxation of negatively supercoiled DNA in the absence of any energy cofactor.[10] The discovery of E.coli DNA topoisomerase I led the investigators to isolate many other topoisomerases from both prokaryotes and eukaryotes. In 1972, an enzyme with activity similar to that of E. coli topoisomerase I was isolated from mouse embryo cells.[11] Gellert and his colleagues in 1976 identified an enzyme activity opposing E.coli DNA topoisomerase I by demonstrating that the enzyme (E. coli DNA topoisomerase II, or gyrase) catalyzed conversion of relaxed DNA into negatively supercoiled DNA in a reaction requiring ATP hydrolysis.[12]
In 1979, Liu et al, isolated an enzyme from bacteriophage T4-infected E. coli with three DNA-delay genes encoding the new enzyme (T4 DNA topoisomerase), important for T4 DNA replication. Like the E. coli DNA topoisomerase I or E.coli DNA gyrase, T4 DNA topoisomerase catalyzes relaxation of both positive and negative supercoils in a reaction requiring ATP hydrolysis.[13–14] An archaeal type II topoisomerase activity capable of catalyzing ATP-driven relaxation and decatenation of duplex DNA circles was first discovered in Sulfolobus shibatae by Bergerat et al in 1994.[15] This protein is an A2B2 heterotetramer and based on the structure it is now named as DNA topoisomerase VI.[16] Further research in identification of new enzymes led to the identification of topoisomerase III from E. coli and yeast.[17–19] Topoisomerase IV identified in E. coli (ParC/ParE), shows sequence homology to gyrase and is involved in ‘chromosome partitioning’;[20–21] while topoisomerase V identified in the hyperthermophilic methanogen Methanopyrus kandleri, shows sequence homology to eukaryotic DNA topoisomerase I.[22] From the above investigation it can be assumed that DNA topoisomerases are ubiquitous enzymes found in all living organisms that is from archaebacteria to humans.[23]
DNA topoisomerases are the amazing molecular machines that manage the topological state of DNA in a cell by solving the problems associated with DNA replication, transcription and translation.[24] The enzyme accomplishes this function by transiently breaking a DNA strand and passing another strand through the transient break or by transiently breaking a pair of complementary strands and passing another double-stranded segment. These enzymes also catalyze many interconversions like catenation and decatenation, knotting and unknotting.[25]
All DNA topoisomerases share common characteristic features such as their ability to cleave and reseal the phosphodiester backbone in two successive transesterification reactions. During this transient DNA cleaving a covalent DNA-protein intermediate is formed between a tyrosine hydroxyl group of topoisomerase and DNA phosphate at break site. No energy cofactor is required for DNA breakage and rejoining activity since the bond energy is conserved in the protein-DNA backbone. Another feature is that once an enzyme- cleaved DNA complex is formed the enzyme allows the detached DNA ends to come apart, opening the gate for the passage of the DNA segments.[26]
Classification of topoisomerases
Based on the mode of cleaving DNA, topoisomerases are classified into two classes. Type I class of DNA topoisomerases change the topological state of DNA by transiently breaking one strand of DNA double helix and consequently change the linking number of DNA by one. Type II class of topoisomerases catalyze the strand passing reaction by making transient enzyme bridged double strand breaks and as a result change the linking number of DNA in the multiplies of two.[27] Type I DNA topoisomerases consist of two subfamilies type IA and type IB which are non-homologus and differ in the type of DNA adduct they form. Members of type IA subfamily forms a covalent phoshotyrosine linkage to the 5’ end of DNA during catalysis and thus in earlier times were entitled as type I-5’. The members of type IB are monomeric where the protein is attached to the 3’ end of DNA and thus were previously called as type I-3’. Members of type IA and type IB are included in the following Table 1.
Table 1.
Type IA and type IB topoisomerases

Type II topoisomerase cleave both the strands of DNA during catalysis. The reaction is ATP dependent and these proteins cleave one DNA duplex, transport a second duplex through the break and then relegate the cleaved duplex. The type II enzymes are dimeric; the enzyme binds to duplex DNA and cleaves the opposing strands with a four base stagger. Cleavage involves covalent attachment of each subunit of the dimer to the 5’ end of the DNA through the phosphotyrosine bond. Cleavage reactions cause a conformational change which pulls the cleaved duplex DNA apart to create an opening called the gate or G-segment DNA. The second region of duplex DNA from either the same molecule or a different molecule is referred as transported or T-segment thus changing the linking number by two. The members of type II are distinguished from each other with respect to the presence of different subunits. Type II enzymes from prokaryotes domains contain two different subunits and are thus heterotetrameric in structure while the members of the eukaryotic enzymes are homodimers. This groups the enzymes as type IIA and type IIB.[28–32]
The enzyme DNA topoisomerase I and II have gained wide importance in clinical research as they have been the chemotherapeutic targets. Various natural drugs have been reported to inhibit the activity of topoisomerases by acting either as topoisomerase suppressors or as poisons. Topoisomerase I suppressors correspond to compounds that inhibit the enzyme but do not stabilize the intermediate DNA- enzyme covalent complex. The interaction of the complex with the free enzyme inhibits binding of topoisomerase I to the DNA cleavage site thus preventing all subsequent steps in the catalytic cycle, while topoisomerase I poisons act after the cleavage of DNA by the enzyme and inhibit the relegation.[33] Further catalytic inhibitors of mammalian topoisomerase have been reported which target the enzyme and inhibit various processes in the cell preventing DNA damage and thus prove to be anticancer agents.[34] The role of mammalian DNA topoisomerases as molecular targets for anticancer drugs have now been recognized and investigators have carried out extensive studies on the mechanism of action of topoisomerase-targeting drugs in the cancer therapy.
Caner is a genetic disease caused due to mutations in genes associated with cell proliferation and cell death that results in DNA damage. The understanding of cancer has revealed large numbers of exciting new targets for the development of effective therapies some of which are in clinical practice. Gene therapies propose promises for future of cancer treatment. This therapy aims in directly attacking the tumor cells but many practical obstacles need to be overcome before the therapy can fulfill its goals in clinical trials. On the other hand conventional therapy that is based on surgery, radiotherapy, chemotherapy or combination of treatments has various side effects because these drugs do not spare normal cells from their devastating actions generating toxic effects in the patients.[35] Furthermore, drug resistance in cancer cells is one of the major problems in cancer chemotherapy. In theory, drug resistance of cancer cells may arise from alterations at any step in the cell-killing pathway of the particular anticancer drug. Predominantly, resistance to various topoisomerase I and II inhibitors has been documented in tissue culture cells with respect to MDR1 over expression; reduced topoisomerase levels, drug resistant mutant topoisomerase, lengthened cell cycle time, and altered DNA repair functions.[36] Hence an altered source for the treatment of cancer is essential.
Natural drugs are thus a novel source towards the treatment of cancer as they have the ability to induce cell cycle arrest and can also repair a range of oxidative radical damages on DNA as well. There is a range of recently discovered compounds which have the ability to inhibit the action of the enzyme topoisomerases and act as promising anticancer agents. These compounds are obtained through bioactivity and mechanism directed isolation and characterization, coupled with rational drug design-based modification and analog synthesis.[37] Further are the examples of few topoisomerase targeting drugs isolated from various source.
Alkaloids as inhibitors of topoisomerase
Plant alkaloids are one of the largest diverse groups of natural products found in more than 20% of plant species. They are defined as the nitrogen containing low molecular weight compounds where the occurrence of the nitrogen ring is in an oxidative state within the heterocyclic ring. This group implies most of the bioactive metabolites which have been reported to have potent pharmacological activities.
Alkaloids are derived from the primary metabolism with amino acids as the precursors but the structural types of alkaloids have independent biosynthetic origins; like the isoquinoline alkaloids (morphine and berberine) are synthesized from tyrosine, indole alkaloids (vinblastine) are produced from tryptophan while the tropane alkaloids (cocaine, scopolamine) are derived from ornithine. These biosynthetic pathways are composed of multiple catalytic steps which form a basic structural nucleus and even alter nascent alkaloids molecules through various carbon ring modifications by multiple reactions such as hydroxylations, methylations, acetylations and glycosylations. Thus because of the above properties, alkaloids have been widely used in pharmaceutical industries in the designing of drugs.[38] Among the array of alkaloids camptothecin has been extensively used as an anticancer drug, also new alkaloids are emerging as topoisomerase inhibitors that can be used in designing of new drugs with anticancer properties.
Camptothecin
Camptothecin (CPT), a potent antitumor drug was isolated for the first time from the bark and stem of the Chinese ornamental tree Camptotheca acuminata (Nyssaceae), also knows as the “tree of joy” [Figure 1].[39] This anticancer drug inhibits DNA topoisomerase I and hence is widely used in clinical trial as an anticancer drug. CPT has also been isolated from Ophiorrhiza pumila and Mapia foetida. It occurs in different plant parts like roots, twigs and leaves. CPT a member of quinolinoalkaloid consists of a pentacyclic ring structure which includes a pyrrole (3, 4 β) quinoline moiety and one asymmetric centre within α-hydroxyl lactone ring with 20S configuration.[40]
Figure 1.

C. acuminata (tree of joy), structure of the anticancer drug Camptothecin
CPT is a potent cytotoxic drug and inhibits the DNA topoisomerase I by causing many single stranded DNA breaks while the prolonged incubation does not lead to more cleavage. CPT interferes with the breakage reunion reaction of the enzyme by trapping the reaction intermediate, the “cleavable complex” and prevents relegation.[41] This cleavable complex is stabilized and becomes non-productive in the relaxation reaction in the presence of CPT. CPT is also a highly phase specific cytotoxic drug. It is selectively cytotoxic to S-phase cells, arrests cells in the G-2 phase and induces fragmentation of chromosomal DNA by inhibiting DNA synthesis through strand scission, thus causing cell death during the S-phase of the cycle.
Earlier reports suggested that the complete pentacyclic ring structure is essential for the antitumor activity but later on it was reported that the D ring pyrridone is required for its activity also the presence of E ring lactone form with 20S configuration gives better activity. CPT because of its severe toxicity cannot be used as a drug and hence several semi synthetic derivatives have been developed by modifying its ring structure. The most successful CPT analogues widely used in the clinical trial are topotecan and irinotecan (water-soluble) which are the obtained by modifying the A and B rings of CPT [Figure 2].
Figure 2.

Camptothecin and its analogues topotecan and irinotecan
The modification at the C and D rings of CPT led to complete loss of cytotoxicity which may be because the CPT molecule loses its planarity that is supposed to be essential for enzyme-DNA-CPT ternary complex stabilization.
Campothecin and its analogues exhibit a broad spectrum of antitumor activity and represent a very promising class of agents. CPT and its analogues shows anticancer activity against solid tumors mainly by inhibiting the action of topoisomerase I and is used in the treatment of colon and pancreatic cancer cells while its analogues are used in the treatment of breast, liver and prostrate cancer.[42–45] Alkaloids from various plants possessing topoisomerase inhibitory activity are listed in Table 2.
Table 2.
List of plants and their active principles (alkaloids) acting as DNA topoisomerase inhibitors

Terpenoids as inhibitors of topoisomerase
Terpenoids are the most structurally diverse class of plant natural products. The name terpenoid, or terpene, was derived from the compounds isolated from turpentine (“terpentin” in German). All terpenoids are derived by repetitive fusion of branched five-carbon unit based on isopentane skeleton. The isoprene units of terpenoids on thermal decomposition yield the alkene gas isoprene as a product. Appropriate chemical conditions can induce isoprene to polymerize in multiples of five carbons, generating numerous terpenoid skeletons. Thus terpenoids are often called isoprenoids.[59]
Taxol a complex polyoxygenated diterpenoid was isolated from the pacific yew, Taxus brevifolia, [Figure 3] by Dr. Wall and Dr.Wani.[60] Taxol as a drug has been developed by the National Cancer Institute, USA and is used in the treatment of ovarian cancer, metastatic breast and lung cancer and Kaposi's sarcoma. It has a basic pentadecane, tetracyclic ring system and a N-benzoyl-b-phenylisoserine side chain attached at the C-13 hydroxyl as an ester linkage. This side chain is essential for the anticancer activity.[61] Taxol has several side effects like numbness, nausea, tingling in toes and also reduction in the W.B.C's as it affects the bone marrow and hence cannot be used directly. Taxotere one of the semisynthetic derivative of taxol is a potent anticancer drug as it has improved water solubility and acts at the microtubules enhancing polymerization of tubulin into stable microtubule bundles leading to apoptosis. It is used for the treatment of breast cancer and non small cell lung cancer.[62,63] Terpenoids from various plants possessing topoisomerase inhibitory activity are listed in Table 3.
Figure 3.

Taxus brevifolia and the structure of the anticancer drug taxol and taxotere
Table 3.
List of plants and their active principles (terpenoids) acting as DNA topoisomerase inhibitors

Flavonoids as inhibitors of topoisomerases
Flavonoids are low molecular weight polyphenolic compounds, ubiquitous in the plant kingdom. These polyphenolic compounds have variable chemical structures and are found in vegetables, fruits, grains, tree barks, roots, stems, flowers and also in wine. This diverse group of secondary metabolites has a vast array of biological functions. Flavonoids are classified into several subclasses such as isoflavonoids, chalcones, flavanones, flavones, dihydroflavonols, flavonols, anthocyanidins and catechins.[69–72] The basic structural feature of flavonoids compound consist of the flavone nucleus composed of two benzene rings A and B linked through a heterocyclic pyrane C ring. Based on the basis of the position of the benzenoid ring B the flavonoids class is divided into flavonoids (2-position) and isoflavonoids (three -position).[73] Flavonoids have wide range of biological activities like antiviral, antiinflammatory, antitumor, antimicrobial, estrogenic, antiestrogenic and antioxidant, mutagenic and antimutagenic because of which they are emerging as nutraceuticals in pharmaceutical industries.[74]
Austin et al, studied the inhibitory activities of plant derived flavonoids baicalein, quercetin, quercetagetin and myricetin and two catechins (-) epicatechin gallate and (-) epigallocatechin gallate isolated from Camellia sinensis on mammalian DNA topoisomerase II. They reported that the flavonoids quercetin, quercetagetin, myricetin and baicalein altered the linking number of DNA as compared to the control while catechin derivatives did not show any effect on the DNA topology. The decatenation assay of topoisomerase II showed that quercetin inhibited the activity of topoisomerase II while catechins showed less inhibitory activity.[75]
Constantinour and co-workers studied the inhibitory activity of topoisomerase I and II by performing topoisomerase I relaxation assay, topoisomerase II unknotting assay and plasmid linearization assay. The activity of 20 flavonoid compounds was assessed and it was reported that myricetin, quercetin, fisetin, and morin inhibited both enzymes, while phloretin, kaempferol, and 4’, 6, 7-trihydroxyisoflavone inhibited topo II without inhibiting topo I. Topoisomerase II inhibiting flavonoids can function as topo II poisons, antagonists, or both depending on the position of hydroxyl groups in the A and B rings of the molecule. So, flavonoid antagonists may bind with a spatial orientation that neither interferes with the DNA cleavage/relegation equilibrium, nor opposes the DNA strand-passage step of the reaction but rather they inhibit enzymatic turnover through a mechanism requiring ATP hydrolysis. Flavonoid poisons, on the other hand, because of a different spatial arrangement, may stabilize the (normally transient) DNA-enzyme complex and favor the DNA cleavage component of the reaction.[76]
Boege et al, reported that quercetin and related natural flavone derivatives, such as acacetin, apigenin, kaempferol, and morin, stabilize the covalent DNA topoisomerase I-DNA post-cleavage complex by inhibiting the relegation process.[77]
Bernard et al, reported that the glycosylated flavones present in the cotton seed flour were selective poisons of E.coli topoisomerase IV. Among the flavones rutin was the most potent in stimulating topoisomerase IV- dependent DNA cleavage and it also blocked the catalytic activity of topoisomerase IV.[78] Flavonoids from various plants possessing topoisomerase inhibitory activity are listed in Table 4.
Table 4.
List of plants and their active principles (fl avonoids) acting as DNA topoisomerase inhibitors

Cantero et al, conducted a comparative study of luteolin and quercetin to check its effect on topoisomerase II activity from Chinese hamster ovary AA8 cells and found that both the compounds inhibited topoisomerase II catalytic activity resulting in extraordinarily high yields of metaphases showing diplochromosomes. The studies on luteolin and quercetin showed that the compound acts as a lead for cancer treatment.[83]
Lopez-Lazaro reported that the dietary flavonoids genistein and luteolin as topoisomerase I and II poisons in the cell based assay. They also reported that the flavonoids functioned as the catalytic inhibitors in K562 leukemia cells.[84]
Vega et al, reported the activity of the flavonoids and flavonoid fraction from Annona dioica. The methanolic extracts of the leaves of Annona dioca showed the presence of four flavonoids kaempferol (1), 3-O-[3”, 6”-di-O-p-hydroxycinnamoyl]-β-galactopyranosyl-kaempferol(2),6”-O-phydroxycinnamoyl-β-galactopyranosyl-kaempferol(3) and 3-O-β-galactopyranosyl-kaempferol (4). They observed that all the tested flavonoids except the flavonoid fraction showed inhibitory relaxation effects as compared to quercetin which was used as the positive control. The inhibitory effects on DNA-topoisomerase II-α was also evaluated, by the relaxation assays using supercoiled pBR322 plasmid DNA in the presence of ATP. They observed that all assayed flavonoids, (except 4) including the FF fraction showed significant inhibitory effect as compared to etoposide.[85]
Stilbenes as inhibitors of topoisomerase
Stilbenes are the naturally occurring, low molecular weight compounds found in a wide range of plant sources. These compounds are synthesized via the phenylpropanoid pathway. Upon attack by pathogens, the plant host activates the phenylpropanoid pathway producing stilbenes. Stilbenes act as natural protective mediators to defend the plants against the viral and microbial attack, exposure to ultraviolet radiation and diseases. These compounds have some structural similarities with estrogen. Stilbenes exists in the stereo isomeric forms like the E and Z forms, depending on the position of where the functional groups are attached in relation to one another on either side of the double bond. Combretastatins, piceatannol, pinosylvin, rhapontigenin, pterostilbene and resveratrol are some of the naturally occurring stilbenes. Of these combretastatins and resveratrol has been extensively studied as it has been used in anticancer, antioxidant and anti-inflammatory activities.[86]
In 1982 Pettit et al, isolated combretastatins from the bark of the South African tree Combretum caffrum. Combretastatin are the first members of a series of biologically active bibenzyls, stilbenes and phenantherenes.[87–89] Combretastatins A-1 and A-4 are stilbene derivatives having two phenyl rings separated by a C-C double bond [Figure 4]. Ring-A has three methoxy groups in 3,4,5-positions while in ring B one hydroxyl group is at the C-3 position and one methoxy group at the C-4 position. Combretastatin A-4 [20,cis-1-(3, 4, 5-trimethoxyphenyl)-2-(30-hydroxy-40-methoxy phenyl) ethene], a simple stilbene has been found to be a potent cytotoxic agent that strongly inhibits the polymerization of brain tubulin by binding to the colchicine site.[90] The compound is active against colon, lung and leukemia cancers.
Figure 4.

Combretum caffrum and its stilbene Combretastatin A-4
In vitro studies have shown that CA-4 competes with colchicine for binding sites on tubulin. Hence, it is a member of the colchicine-like inhibitors of microtubulin.[91] Further, by making modifications in the structure of combretastatin and by developing its derivatives new drugs with activities similar to or better than combretastatin can be obtained. Thus combretastatin or its analogues may come up as anticancer drugs of choice in near future. Stilbenes from various plants possessing topoisomerase inhibitory activity are listed in Table 5.
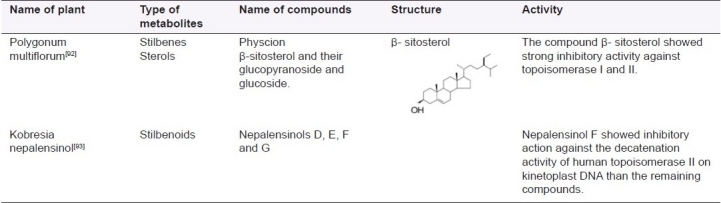
Table 5.
List of plants and their active principles (stilbenes) acting as DNA topoisomerase inhibitors
Chalcones as inhibitors of DNA topoisomerase
Chalcones are substances widely present in the plant and are the intermediates in the biosynthesis of flavonoids. They have a wide range of biological activities like antibacterial, antifungal, antitumor and antiinflammatory. Structurally they are aromatic ketones which act as a central core for a variety of biological activities. Further are few chalcones and its derivatives with anticancer properties.
Yoon et al, isolated retrochalcones (compounds that are structurally distinguished from normal chalcones by their lack of oxygen molecule at the C-2’ and C-6’ positions) from the roots of Glycyrrhiza inflata (Leguminosae) [Figure 5]. The activity of DNA topoisomerase I was determined by measuring the relaxation of supercoiled DNA pBR322. The licochalcones A and E inhibited the activity of topoisomerase I in a dose dependent manner. The compounds showed potent inhibition of topoisomerase I and thus can serve as lead structures for the development of anticancer drugs.[94]
Figure 5.

Glycyrrhiza inflata and the structure of the licochalcones A and E
Lignans as topoisomerase inhibitors
Another important addition to the anticancer drug armamentarium is the class of lignans. Lignans are a family of natural products originated as secondary metabolites through the shikimic acid pathway. They are formed by the combination of two phenylpropane units and constitute a complex family of skeletons and characteristic chemical functions, which can be subdivided into four groups: Lignans, neolignans, oxyneolignans and trimers, higher analogues and mixed lignanoids Among the lignans, cyclolignans present a carbocycle between both phenylpropane units, created by two single carbon-carbon bonds through the side chains, one of them between the β-β’ positions. The aryltetralin structure of podophyllotoxin belongs to cyclolignans.[95]
Podophyllotoxin (PDT), a bioactive lignan, was first isolated by Podwyssotzki in 1880 from the North American plant Podophyllum peltatum [Figure 6]. This compound chemically an aryltetralin lignan has a lactone ring.[96] These PDT lignans block the catalytic activity of DNA topoisomerase II by stabilizing a cleavage enzyme-DNA complex in which the DNA is cleaved and covalently linked to the enzyme.[97–98] Podophyllotoxin shows strong cytotoxic activity against various cancer cell lines. It is effective in the treatment of Wilms tumors, various genital tumors and in non- Hodgkin's and other lymphomas and lung cancer.[99–101] The drug when used in the treatment of human neoplasia showed severe side effects and hence could not be used as such in further treatment. Therefore various modifications were performed to obtain a more potent and less toxic drug. This lead to the synthesis of two new analoges etoposide and teniposide which are widely used for the treatment of various cancers.[102] Lignans from various plants possessing topoisomerase inhibitory activity are listed in Table 6.
Figure 6.

Podophyllum peltatum and the lignan podophyllotoxin and its analogue etoposide
Table 6.
List of plants and their active principles (lignans) acting as DNA topoisomerase inhibitors

CONCLUSIONS
Secondary metabolites isolated from the plants have been a principal source of most effectual conventional drugs for the treatment of various forms of diseases especially different forms of cancers. Many times the legitimate compounds isolated from the plants may not serve as the novel drug because of the side effects (e.g.: Taxol, CPT) and hence it leads to the development of potential novel analogue with better activity.
The enzyme, essential for the topological changes in DNA that is DNA topoisomerase also called the double-edged sword are clinical targets in cancer therapy. This review summarizes the clinical implications of various inhibitors of DNA topoisomerase because of their strong antitumour activities. Natural drugs and their analogues have been extensively characterized with respect to their mode of action, application to the functional analysis of the enzyme to various genetic processes in the cell and especially their allegation in cancer chemotherapy. Supplementary research in the isolation, characterization of plant based natural drug and their mode of action may lead to the development and designing of various drugs with anticancer potential. Thus research in metaboliomics may open new avenues in development on drugs targeting the enzyme and preventing the genetic illness.[104]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Verpoorte R. Exploration of nature's chemodiversity: The role of secondary metabolites as leads in drug development. DDT. 1998:3. [Google Scholar]
- 2.Oksman-Caldentey KM, Inzé D. Plant cell factories in the post-genomic era: New ways to produce designer secondary metabolites. Trends Plant Sci. 2004;9:433–40. doi: 10.1016/j.tplants.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Verpoorte R. Secondary metabolism. In: Verpoorte R, Alfermann AW, editors. Metabolic Engineering of Plant Secondary Metabolism. Kluwer Academic Publishers; 2000. pp. 1–29. [Google Scholar]
- 4.Verpoorte R, Memelink J. Engineering secondary metabolite production in plants. Curr Opin Biotechnol. 2002;13:181–7. doi: 10.1016/s0958-1669(02)00308-7. [DOI] [PubMed] [Google Scholar]
- 5.Yazaki K. ABC transporters involved in the transport of plant secondary metabolites. FEBS Lett. 2006;580:1183–91. doi: 10.1016/j.febslet.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Mijts BN, Schmidt-Dannert C. Engineering of secondary metabolite pathways. Curr Opin Biotechnol. 2003;14:597–602. doi: 10.1016/j.copbio.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Cairns J. The chromosome of Escherichia coli.Cold Spring Harbor. Symp Quant Biol. 1963;28:43–6. doi: 10.1101/sqb.1974.038.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Wang JC. Interaction between DNA and an Escherichia coli protein omega. J Mol Biol. 1971;55:523–33. doi: 10.1016/0022-2836(71)90334-2. [DOI] [PubMed] [Google Scholar]
- 9.Depew RE, Liu LF, Wang JC. Interaction between DNA and Escherichia coli protein omega: Formation of a complex between single-stranded DNA and omega protein. J Biol Chem. 1978;253:511–8. [PubMed] [Google Scholar]
- 10.Wang JC, Liu LF. New York: Academic Press, Inc; 1979. DNA topoisomerases: Enzymes that catalyze the concerted breaking and rejoining of DNA backbone bonds J Molecular genetics, part III; pp. 65–88. [Google Scholar]
- 11.Champoux JJ, Dulbecco R. An activity from mammalian cells that untwists superhelical DNA: A possible swivel for DNA replication (polyoma-ethidium bromide-mouse-embryo cells-dye binding assay) Proc Natl Acad Sci U S A. 1972;69:143–6. doi: 10.1073/pnas.69.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gellert M, Mizuuchi K, O’Dea MH, Nash HA. DNA gyrase: An enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976;73:3872–6. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu LF, Liu CC, Alberts BM. T4 DNA topoisomerase.A new ATP-dependent enzyme essential for the initiation of T4 bacteriophage DNA replication. Nature. 1979;281:456–61. doi: 10.1038/281456a0. [DOI] [PubMed] [Google Scholar]
- 14.Stetler GL, King GJ, Huang WM. T4 DNA-delay proteins, required for specific DNA replications, form a complex that has ATP-dependent DNA topoisomerase activity. Proc Natl Acad Sci U S A. 1979;76:3737–41. doi: 10.1073/pnas.76.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergerat A, Gadelle D, Forterre P. Purification of a DNA topoisomerase II from the hyperthermophilic archaeon Sulfolobus shibatae: A thermostable enzyme with both bacterial and eucaryal features. J Biol Chem. 1994;269:27663–9. [PubMed] [Google Scholar]
- 16.Bergerat A, de Massy B, Gadelle D, Varoutas PC, Nicolas A, Forterre P. An atypical topoisomerase II from Archaea with implications for meiotic recombination. Nature. 1997;386:414–7. doi: 10.1038/386414a0. [DOI] [PubMed] [Google Scholar]
- 17.Srivenugopal KS, Lockshon D, Morris DR. Escherichia coil DNA topoisomerase III: Purification and characterization of a new type I enzyme. Biochemistry. 1984;23:1899–906. doi: 10.1021/bi00304a002. [DOI] [PubMed] [Google Scholar]
- 18.Kim RA, Wang JC. Identification of the yeast TOP3 gene product as a single strand-specific DNA topoisomerase. J Biol Chem. 1992;267:17178–85. [PubMed] [Google Scholar]
- 19.Wallis JW, Chrebet G, Brodsky G, Rolfe M, Rothstein R. A hyper-recombination mutation in S.cerevisiae identifies a novel eukaryotic topoisomerase. Cell. 1989;58:409–19. doi: 10.1016/0092-8674(89)90855-6. [DOI] [PubMed] [Google Scholar]
- 20.Kato J, Nishimura Y, Imamura R, Niki H, Hiraga S, Suzuki H. New topoisomerase essential for chromosome segregation in E.coli. Cell. 1990;63:393–404. doi: 10.1016/0092-8674(90)90172-b. [DOI] [PubMed] [Google Scholar]
- 21.Kato J, Suzuki H, Ikeda H. Purification and characterization of DNA topoisomerase IV in Escherichia coli. J Biol Chem. 1992;267:25676–84. [PubMed] [Google Scholar]
- 22.Slesarev AI, Stetter KO, Lake JA, Gellert M, Krah R, Kozyavkin SA. DNA topoisomerase V is a relative of eukaryotic topoisomerase I from a hyperthermophilic prokaryote. Nature. 1993;364:735–7. doi: 10.1038/364735a0. [DOI] [PubMed] [Google Scholar]
- 23.Pommier Y. Diversity of DNA topoisomerases I and inhibitors. Biochimie. 1998;80:255–70. doi: 10.1016/s0300-9084(98)80008-4. [DOI] [PubMed] [Google Scholar]
- 24.Champoux JJ. DNA topoisomerases: Structure, function, and mechanism. Annu Rev Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- 25.Wang JC. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–97. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- 26.Roca J. The mechanisms of DNA topoisomerases. Trends Biochem Sci. 1995;20:156–60. doi: 10.1016/s0968-0004(00)88993-8. [DOI] [PubMed] [Google Scholar]
- 27.Liu LF. DNA topoisomerase poisons as antitumour drugs. Annu Rev Biochem. 1989;58:351–75. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- 28.Watt PM, Hickson ID. Structure and function of type II DNA topoisomerases. Biochem J. 1994;303:681–95. doi: 10.1042/bj3030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nitiss JL. Investigating the biological functions of DNA topoisomerases in eukaryotic cells. Biochim Biophys Acta. 1998;1400:63–81. doi: 10.1016/s0167-4781(98)00128-6. [DOI] [PubMed] [Google Scholar]
- 30.Li W, Wang JC. Mammalian DNA topoisomerase IIIalpha is essential in early embryogenesis. Proc Natl Acad Sci U S A. 1998;95:1010–3. doi: 10.1073/pnas.95.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger JM. Structure of DNA topoisomerases. Biochim Biophys Acta. 1998;1400:3–18. doi: 10.1016/s0167-4781(98)00124-9. [DOI] [PubMed] [Google Scholar]
- 32.Corbett KD, Berger JM. Structure, molecular mechanisms, and evolutionary relationships in DNA topoisomerases. Annu Rev Biophys Biomol Struct. 2004;33:95–118. doi: 10.1146/annurev.biophys.33.110502.140357. [DOI] [PubMed] [Google Scholar]
- 33.Bailly C. Topoisomerase I poisons and suppressors as anticancer drugs. Curr Med Chem. 2000;7:39–58. doi: 10.2174/0929867003375489. [DOI] [PubMed] [Google Scholar]
- 34.Andoh T, Ishida R. Catalytic inhibitors of DNA topoisomerase II. Biochim Biophys Acta. 1998;1400:155–71. doi: 10.1016/s0167-4781(98)00133-x. [DOI] [PubMed] [Google Scholar]
- 35.Bertram JS. The molecular biology of cancer. Mol Aspects Med. 2000;21:167–223. doi: 10.1016/s0098-2997(00)00007-8. [DOI] [PubMed] [Google Scholar]
- 36.Chen AY, Liu LF. DNA topoisomerase: Essential enzymes and lethal targets. Annu Rev Pharmacol Toxicol. 1994;34:191–218. doi: 10.1146/annurev.pa.34.040194.001203. [DOI] [PubMed] [Google Scholar]
- 37.Dholwani KK, Saluja AK, Gupta AR, Shah DR. A review on plant-derived natural products and their analogs with anti-tumor activity. Indian J Pharmacol. 2008;40:49–58. doi: 10.4103/0253-7613.41038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Facchini PJ, Bird DA, St-Pierre B. Can Arabidopsis make complex alkaloids? Trends Plant Sci. 2004;9:116–22. doi: 10.1016/j.tplants.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Wall ME, Wani MC, Cooke CE, Palmer KH, Mcphail AT. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J Am Chem Soc. 1966;88:3888–90. [Google Scholar]
- 40.Srivastava V, Negi AS, Kumar JK, Gupta MM, Khanuja SP. Plant-based anticancer molecules: A chemical and biological profile of some important leads. Bioorg Med Chem. 2005;13:5892–908. doi: 10.1016/j.bmc.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 41.Hsiang YH, Hertzberg R, Hecht S, Liu LF. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985;260:14873–8. [PubMed] [Google Scholar]
- 42.Pommier Y, Pourquier P, Fan Y, Strumberg D. Mechanism of action of eukaryotic DNA topoisomerase I and drugs targeted to the enzyme. Biochim Biophys Acta. 1998;1400:83–105. doi: 10.1016/s0167-4781(98)00129-8. [DOI] [PubMed] [Google Scholar]
- 43.Gupta M, Fujimori A, Pommier Y. Eukaryotic DNA topoisomerases I. Biochim Biophys Acta. 1995;1262:1–14. doi: 10.1016/0167-4781(95)00029-g. [DOI] [PubMed] [Google Scholar]
- 44.Thomas CJ, Rahier NJ, Hecht SM. Camptothecin: Current perspectives. Bioorg Med Chem. 2004;12:1585–604. doi: 10.1016/j.bmc.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 45.Ewesuedo RB, Ratain MJ. Topoisomerase I inhibitors. Oncologist. 1997;2:359–64. [PubMed] [Google Scholar]
- 46.Fang SD, Wang LK, Hecht SM. Inhibitors of DNA topoisomerase I isolated from roots of Zanthoxylum nitidum. J Organic Chem. 1993;19:5025–7. [Google Scholar]
- 47.Wang LK, Johnson RK, Hecht SM. Inhibition of topoisomerase I function by nitidine and fagaronine. Chem Res Toxicol. 1993;6:813–8. doi: 10.1021/tx00036a010. [DOI] [PubMed] [Google Scholar]
- 48.Hazra B, Sur P, Roy DK, Sur B, Banerjee A. Biological activity of diospyrin towards Ehrlich ascites carcinoma in Swiss A mice. Planta Med. 1984;50:295–7. doi: 10.1055/s-2007-969713. [DOI] [PubMed] [Google Scholar]
- 49.Ray S, Hazra B, Mittra B, Das A, Majumder HK. Diospyrin, a bisnaphthoquinone: A novel inhibitor of type I DNA topoisomerase of Leishmania donovani. Mol Pharmacol. 1998;54:994–9. doi: 10.1124/mol.54.6.994. [DOI] [PubMed] [Google Scholar]
- 50.Pecere T, Gazzola MV, Mucignat C, Parolin C, Vecchia FD, Cavaggioni A, et al. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res. 2000;60:2800–4. [PubMed] [Google Scholar]
- 51.Hara H, Maruyama N, Yamashita S, Hayashi Y, Lee KH, Bastow KF, et al. Elecanacin, a novel new naphthoquinone from the bulb of Eleutherine americana. Chem Pharm Bull (Tokyo) 1997;45:1714–6. [Google Scholar]
- 52.Krishnan P, Bastow KF. Novel mechanisms of DNA topoisomerase II inhibition by pyranonaphthoquinone derivatives-eleutherin, alpha lapachone, and bet lapachone. Biochem Pharmacol. 2000;60:1367–79. doi: 10.1016/s0006-2952(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 53.Sobhani AM, Ebrahimi SA, Mahmoudian M. An in vitro evaluation of human DNA topoisomerase I inhibition by Peganum harmala L.seeds extract and its β-carboline alkaloids. J Pharm Pharm Sci. 2002;5:19–23. [PubMed] [Google Scholar]
- 54.Ting CY, Hsu CT, Hsu HT, Su JS, Chen TY, Tarn WY, et al. Isodiospyrin as a novel human DNA topoisomerase I inhibitor. Biochem Pharmacol. 2003;66:1981–91. doi: 10.1016/j.bcp.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Prescott TA, Sadler IH, Kiapranis R, Maciver SK. Lunacridine from Lunasia amara is a DNA intercalating topoisomerase II inhibitor. J Ethnopharmacol. 2007;109:289–94. doi: 10.1016/j.jep.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 56.Son JK, Jung SJ, Jung JH, Fang Z, Lee CS, Seo CS, et al. Anticancer constituents from the roots of Rubia cordifolia L. Chem Pharm Bull (Tokyo) 2008;56:213–6. doi: 10.1248/cpb.56.213. [DOI] [PubMed] [Google Scholar]
- 57.Fujii N, Yamashita Y, Arima Y, Nagashima M, Nakano H. Induction of topoisomerase II-mediated DNA cleavage by the plant naphthoquinones plumbagin and shikonin. Antimicrob Agents Chemother. 1992;36:2589–94. doi: 10.1128/aac.36.12.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thind TS, Agrawal SK, Saxena AK, Arora S. Studies on cytotoxic, hydroxyl radical scavenging and topoisomerase inhibitory activities of extracts of Tabernaemontana divaricata (L.) R.Br. ex Roem. and Schult. Food Chem Toxicol. 2008;46:2922–7. doi: 10.1016/j.fct.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 59.Croteau R, Kutchan TM, Lewis NG. Natural Products (Secondary Metabolites) Biochem Mole Biol Plants. 2000;24:1250–18. [Google Scholar]
- 60.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–7. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 61.Kingston DG. The chemistry of taxol. Pharmacol Ther. 1991;52:1–34. doi: 10.1016/0163-7258(91)90085-z. [DOI] [PubMed] [Google Scholar]
- 62.Geney R, Chen J, Ojima I. Recent advances in the new generation taxane anticancer agents. Med Chem. 2005;1:125–39. doi: 10.2174/1573406053175292. [DOI] [PubMed] [Google Scholar]
- 63.Itokawa H, Morris-Natschke SL, Akiyama T, Lee KH. Plant-derived natural product research aimed at new drug discovery. J Nat Med. 2008;62:263–80. doi: 10.1007/s11418-008-0246-z. [DOI] [PubMed] [Google Scholar]
- 64.Miyata S, Wang LY, Yoshida C, Kitanaka S. Inhibition of cellular proliferation by diterpenes, topoisomerase II inhibitor. Bioorg Med Chem. 2006;14:2048–51. doi: 10.1016/j.bmc.2005.10.059. [DOI] [PubMed] [Google Scholar]
- 65.Li CH, Chen PY, Chang UM, Kan LS, Fang WH, Tsai KS, et al. Ganoderic acid X, a lanostanoid triterpene, inhibits topoisomerases and induces apoptosis of cancer cells. Life Sci. 2005;77:252–65. doi: 10.1016/j.lfs.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 66.Meng LH, Zhang JS, Ding J. Salvicine, a novel DNA topoisomerase II inhibitor, exerting its effects by trapping enzyme-DNA cleavage complexes. Biochem Pharmacol. 2001;62:733–41. doi: 10.1016/s0006-2952(01)00732-8. [DOI] [PubMed] [Google Scholar]
- 67.Tanaka N, Kitamura A, Mizushina Y, Sugawara F, Sakaguchi K. Fomitellic acids, triterpenoid inhibitors of eukaryotic DNA polymerases from a basidiomycete, Fomitella fraxinea. J Nat Prod. 1998;61:193–7. doi: 10.1021/np970127a. [DOI] [PubMed] [Google Scholar]
- 68.Mizushina Y, Iida A, Ohta K, Sugawara F, Sakaguchi K. Novel triterpenoids inhibit both DNA polymerase and DNA topoisomerase. Biochem J. 2000;350:757–63. [PMC free article] [PubMed] [Google Scholar]
- 69.Dixon RA, Steele CL. Flavonoids and isoflavonoids: A gold mine for metabolic engineering. Trends Plant Sci. 1999;4:394–400. doi: 10.1016/s1360-1385(99)01471-5. [DOI] [PubMed] [Google Scholar]
- 70.Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: A review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–25. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 71.Akoi T, Akashi T, Ayabe S. Flavonoids of leguminous plants: Structure, biological activity and biosynthesis. J Plant Res. 2000;113:475–88. [Google Scholar]
- 72.López-Lázaro M. Flavonoids as anticancer agents: Structure-activity relationship study. Curr Med Chem Anticancer Agents. 2002;2:691–714. doi: 10.2174/1568011023353714. [DOI] [PubMed] [Google Scholar]
- 73.Jedinak A, Farago J, Psenakova I, Maliar T. Approaches to flavonoid production in plant tissue cultures. Biologia Bratislava. 2004;59:697–10. [Google Scholar]
- 74.Messina MJ. Legumes and soybeans: Overview of their nutritional profiles and health effects. Am J Clin Nutr. 1999;70:439S–50S. doi: 10.1093/ajcn/70.3.439s. [DOI] [PubMed] [Google Scholar]
- 75.Austin CA, Patel S, Ono K, Nakane H, Fisher LM. Site-specific DNA cleavage by mammalian DNA topoisomerase II induced by novel flavone and catechin derivatives. Biochem J. 1992;282:883–9. doi: 10.1042/bj2820883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Constantinou A, Mehta R, Runyan C, Rao K, Vaughan A, Moon R. Flavonoids as DNA topoisomerase antagonists and poisons: Structure-activity relationships. J Nat Prod. 1995;58:217–25. doi: 10.1021/np50116a009. [DOI] [PubMed] [Google Scholar]
- 77.Boege F, Straub T, Kehr A, Boesenberg C, Christiansen K, Andersen A, et al. Selected novel flavones inhibit the DNA binding or the DNA relegation step of eukaryotic topoisomerase I. J Biol Chem. 1996;271:2262–70. doi: 10.1074/jbc.271.4.2262. [DOI] [PubMed] [Google Scholar]
- 78.Bernard FX, Sablé S, Cameron B, Provost J, Desnottes JF, Crouzet J, et al. Glycosylated flavones as selective inhibitors of topoisomerase IV. Antimicrob Agents Chemother. 1997;41:992–8. doi: 10.1128/aac.41.5.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zahir A, Jossang A, Bodo B, Provost J, Cosson JP, Sévenet T. DNA topoisomerase I inhibitors: Cytotoxic flavones from Lethedon tannaensis. J Nat Prod. 1996;59:701–3. doi: 10.1021/np960336f. [DOI] [PubMed] [Google Scholar]
- 80.Sun NJ, Woo SH, Cassady JM, Snapka RM. DNA polymerase and topoisomerase II inhibitors from Psoralea corylifolia. J Nat Prod. 1998;61:362–6. doi: 10.1021/np970488q. [DOI] [PubMed] [Google Scholar]
- 81.Dutta PK, Chowdhury US, Chakraborty AK, Achari B, Pakrashi SC. Studies on Indian medicinal plants: Nishindaside: a novel iridoid glycoside from Vitex negundo. Tetrahedron. 1983;39:3067–72. [Google Scholar]
- 82.Chowdhury AR, Sharma S, Mandal S, Goswami A, Mukhopadhyay S, Majumder HK. Luteolin, an emerging anti-cancer flavonoid, poisons eukaryotic DNA topoisomerase I. Biochem J. 2002;366:653–61. doi: 10.1042/BJ20020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cantero G, Campanella C, Mateos S, Cortés F. Topoisomerase II inhibition and high yield of endoreduplication induced by the flavonoids luteolin and quercetin. Mutagenesis. 2006;21:321–5. doi: 10.1093/mutage/gel033. [DOI] [PubMed] [Google Scholar]
- 84.López-Lazaro M, Willmore E, Austin CA. Cells lacking DNA topoisomerase II beta are resistant to genistein. J Nat Prod. 2007;70:763–7. doi: 10.1021/np060609z. [DOI] [PubMed] [Google Scholar]
- 85.Vega MR, Esteves-Souza A, Vieira IJ, Mathias L, Braz-Filhob R, Echevarria A. Flavonoids from Annona dioica leaves and their effects in Ehrlich carcinoma cells, DNA-topoisomerase I and II. J Braz Chem Soc. 2007;18:1554–9. [Google Scholar]
- 86.Roupe KA, Remsberg CM, Yáñez JA, Davies NM. Pharmacometrics of stilbenes: Seguing towards the clinic. Curr Clin Pharmacol. 2006;1:81–101. doi: 10.2174/157488406775268246. [DOI] [PubMed] [Google Scholar]
- 87.Pettit GR, Cragg GM, Herald DL, Schmidt JM, Lohavanijaya P. Isolation and structure of combretastatin. Can J Chem. 1982;60:1374. [Google Scholar]
- 88.Pettit GR, Singh SB, Cragg GM. Synthesis of natural (-)-Combretastatin. J Org Chem. 1985;50:3404–6. [Google Scholar]
- 89.Pettit GR, Cragg GM, Singh SB. Antineoplastic agents, 122: Constituents of Combretum caffrum. J Nat Prod. 1987;50:386–91. doi: 10.1021/np50051a008. [DOI] [PubMed] [Google Scholar]
- 90.Hamel E, Lin CM. Interactions of combretastatin: a new plantderived antimitotic agent, with tubulin. Biochem Pharmacol. 1983;32:3864–7. doi: 10.1016/0006-2952(83)90163-6. [DOI] [PubMed] [Google Scholar]
- 91.Lin CM, Ho HH, Pettit GR, Hamel E. Antimitotic natural products combretastatin A-4 and combretastatin A-2: Studies on the mechanism of their inhibition of the binding of colchicine to tubulin. Biochemistry. 1989;28:6984–91. doi: 10.1021/bi00443a031. [DOI] [PubMed] [Google Scholar]
- 92.Xu ML, Zheng MS, Lee YK, Moon DC, Lee CS, Woo MH, et al. A new stilbene glucoside from the roots of Polygonum multiflorum Thunb. Arch Pharm Res. 2006;29:946–51. doi: 10.1007/BF02969276. [DOI] [PubMed] [Google Scholar]
- 93.Yamada M, Hayashi K, Hayashi H, Tsuji R, Kakumoto K, Ikeda S, et al. Nepalensinols D-G, new resveratrol oligomers from Kobresia nepalensis (Cyperaceae) as potent inhibitors of DNA topoisomerase II. Chem Pharm Bull (Tokyo) 2006;54:354–8. doi: 10.1248/cpb.54.354. [DOI] [PubMed] [Google Scholar]
- 94.Yoon G, Kang BY, Cheon SH. Topoisomerase I inhibition and cytotoxicity of licochalcones A and E from Glycyrrhiza inflata. Arch Pharm Res. 2007;30:313–6. doi: 10.1007/BF02977611. [DOI] [PubMed] [Google Scholar]
- 95.Moss GP. Nomenclature of lignans and neolignans. Pure Appl Chem. 2000;72:1493–23. [Google Scholar]
- 96.Imbert TF. Discovery of podophyllotoxins. Biochimie. 1998;80:207–22. doi: 10.1016/s0300-9084(98)80004-7. [DOI] [PubMed] [Google Scholar]
- 97.Gordaliza M, Castro MA, del Corral JM, Feliciano AS. Antitumor properties of podophyllotoxin and related compounds. Curr Pharm Des. 2000;6:1811–39. doi: 10.2174/1381612003398582. [DOI] [PubMed] [Google Scholar]
- 98.Gordaliza M, García PA, del Corral JM, Castro MA, Gómez-Zurita MA. Podophyllotoxin: Distribution, sources, applications and new cytotoxic derivatives. Toxicon. 2004;44:441–59. doi: 10.1016/j.toxicon.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 99.Ayres DC, Loike Lignans JD. Cambridge: Cambridge University Press; 1990. Chemical, biological and clinical properties. [Google Scholar]
- 100.Utsugi T, Shibata J, Sugimoto Y, Aoyagi K, Wierzba K, Kobunai T, et al. Antitumor activity of a novel podophyllotoxin derivative (TOP-53) against lung cancer and lung metastatic cancer. Cancer Res. 1996;56:2809–14. [PubMed] [Google Scholar]
- 101.Subrahmanyam D, Renuka B, Rao CV, Sagar PS, Deevi DS, Babu JM, et al. Novel D-ring analogues of podophyllotoxin as potent anti-cancer agents. Bioorg Med Chem Lett. 1998;8:1391–6. doi: 10.1016/s0960-894x(98)00232-7. [DOI] [PubMed] [Google Scholar]
- 102.Stähelin HF, von Wartburg A. The chemical and biological route from podophyllotoxin glucoside to etoposide: Ninth Cain memorial Award lecture. Cancer Res. 1991;51:5–15. [PubMed] [Google Scholar]
- 103.Li G, Lee CS, Woo MH, Lee SH, Chang HW, Son JK. Lignans from the bark of Machilus thunbergii and their DNA topoisomerases I and II inhibition and cytotoxicity. Biol Pharm Bull. 2004;27:1147–50. doi: 10.1248/bpb.27.1147. [DOI] [PubMed] [Google Scholar]
- 104.Kashiwada Y, Bastow KF, Lee K. Novel lignan derivatives as selective inhibitors of DNA topoisomerase II. Bioorg Med Chem Lett. 1995;5:905–8. [Google Scholar]


