Abstract
The medicinal properties of hawthorn (Crataegus spp., a genus comprising approximately 300 species) have been utilized by many cultures for a variety of therapeutic purposes for many centuries. In the Western world cardiovascular disease (CVD) has become one of the single most significant causes of premature death. Echoing this situation, more recent research into the therapeutic benefits of hawthorn preparations has focused primarily upon its cardiovascular effects. This review covers research into the various mechanisms of action proposed for Crataegus preparations, clinical trials involving Crataegus preparations, and the herb's safety profile.
Clinical trials reviewed have been inconsistent in terms of criteria used (sample size, preparation, dosage, etc) but have been largely consistent with regard to positive outcomes. An investigation into data available to date regarding hawthorn preparations and herb/drug interactions reveals that theoretical adverse interactions have not been experienced in practice. Further, adverse reactions relating to the use of hawthorn preparations are infrequent and mild, even at higher dosage ranges. A recent retrospective study by Zick et al. has suggested a negative outcome for the long-term use of hawthorn in the prognosis of heart failure. These findings are examined in this paper.
Although further research is needed in certain areas, current research to date suggests that hawthorn may potentially represent a safe, effective, nontoxic agent in the treatment of CVD and ischemic heart disease (IHD).
Keywords: Cardiovascular disease, Crataegus, hawthorn, whitethorn
INTRODUCTION
This paper aims to provide a comprehensive overview of research into the effectiveness of Crataegus preparations in the treatment of cardiovascular disease (CVD) to date. The paper includes information concerning the recent research regarding the identification of perceived active constituents and their mechanisms of action. Current research involving clinical trials utilizing Crataegus preparations is also reviewed. Other areas explored include drug–herb interactions and the safety profile of this remedy.
The World Health Organisation (WHO) lists cardiovascular disease (CVD) as globally the number one cause of death, accounting for 30% of all deaths in 2005.[3] The American Heart Association cites heart disease as the number one killer of American adults,[8] and further commented that for the year 2005, 80,700,000 Americans suffered from some form of CVD.
The use of nonvitamin/nonmineral supplements among the elderly population is increasing.[9] Crataegus supplements have been listed as one of the remedies popular with this age group. There is approximately a one-in-three incidence of complementary and alternative medicine over-the-counter use in patients suffering chronic heart failure (CHF). An increased over-the-counter use of Crataegus preparations, coupled with a high incidence of patients failing to report use of these preparations with concomitant orthodox medications, indicates a need to ensure that Crataegus preparations do not present with any potential complications regarding herb/drug interactions.[10]
The medicinal use of hawthorn in CVD is prevalent in most cultures. Direct comparisons between the phytochemical profiles of these species used worldwide have not been undertaken, although indigenous use would indicate a high degree of confluence.
CONSTITUENTS AND THEIR MECHANISMS OF ACTION
The Irish physician who was first noted to have used the remedy for cardiovascular complaints specifically used berries. The 1983 edition of the British Herbal Pharmacopoeia, regarded by many as the standard text for professional herbalists for many years, cites berries only.[5] This may explain practicing herbalists noted preference for berry over flower, although this trend seems to be changing in recent times, flowers and berries being utilized more interchangeably, and in some cases blended. Historical texts in Western medicine record the use of berries, seeds, and flowers.[11] Leaves are also used.
The identification of constituent groups such as bioflavonoids and proanthocyanidins has shed light on some of the beneficial effects of Crataegus on the cardiovascular system, bioflavonoids now being well established as possessing significant antioxidant activity. Berries, leaves, and flowers of hawthorn are phytochemically similar in composition, differing primarily in the ratio of specific flavonoids and procyanidins present. Berries are rich in hyperoside, while leaves contain higher levels of vitexin-2-rhamnoside. Kingston detected significant levels of vitexin-2-rhamnoside in flowers.[12] Mills states that the flowers contain higher levels of flavonoids, the leaves containing the highest levels of oligomeric procyanidins (OPCs).[6] Higher levels of procyanidins present in leaves was established by Vanhaelen et al.[13] To date a number of different potential actions have been suggested, many of which are theoretical as human studies remain small in number.
Table 1 details research concerning the mechanisms of action of Crataegus. In many studies, whole plant extracts or OPC/flavonoid combinations were utilized rather than specific isolated classes of phytochemicals. This approach, while more realistic in terms of the actions of the whole plant, makes it difficult to attribute mechanisms of action demonstrated to specific active constituents in every case. Also, antioxidant activities appear to be common to both the OPCs and the flavonoid group, conferring a considerable generalized benefit from both of these constituent groups in chronic illness.
Table 1.
Mechanisms of action of hawthorn – Areas of influence on the cardiovascular system
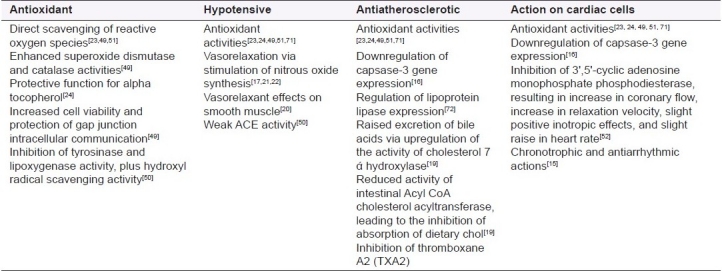
Epidemiological evidence indicates significant links between increased dietary flavonoid intake and reduction in coronary-related mortality.[14] Negative chronotrophic effects and antiarrhythmic effects of crude hawthorn extracts on cultured cardiomyocytes were noted by Long et al.,[15] who further stated that hawthorn's chronotrophic mechanism of action is unlikely to involve β-adrenergic receptor blockade. The study involved cardiomyocytes sourced from mice.
Of the 15 pieces of research considered in Table 1, only four involved the use of human volunteers or human tissue.[16–19] These are of most relevance as they most closely replicate actual human in vivo environments. Vasorelaxant effects on vascular smooth muscles previously artificially contracted by catecholamines were noted by Vierling et al. who postulated that this could have significant clinical importance as raised catecholamine levels are present in the blood stream during heart failure.[20] Endothelial-dependent nitrous oxide induction, triggering vasodilation, has been attributed primarily to oligomeric procyanidins.[17,21,22] Vasorelaxant effects reduce peripheral vascular resistance and increase coronary blood flow. In chronic heart failure, sympathetic nervous system stimulation offers short-term benefits regarding circulatory support but is deleterious in the long term, inducing a hypertensive state.[21] Vasorelaxation would therefore be beneficial.
The antioxidant activity of Crataegus preparations contributes significantly to its therapeutic profile. Gou et al. noted that of 28 fruit pulps tested, the hawthorn pulp (Chinese hawthorn) produced the highest measure of antioxidant activity.[23] A similarly high antioxidant activity in Crataegus aronia, a hawthorn indigenous to Israel, Jordan, and the Palestine, has been found.[24] The IC50 values of specific active constituents have been established in relation to the antioxidant capacity,[25] with values of epicatechin and hyperoside being significantly lower (more effective) than those of established antioxidant drugs (i.e., glutathione and N-acetylcysteine). Oligomeric proanthocyanidins appear to possess a higher antioxidant activity used in isolation than polymeric proanthocyanidins (PPCs) used in isolation.[18] However, the removal of PPCs from a mixture may result in a less actively protective medicine, possibly partially due to their high concentration influencing outcome.[18]
IC50 represents the amount of a substance/drug required to inhibit 50% of a given process.
Significant protective effects on cardiovascular cells were noted by Ling et al. using a whole plant extract of Chinese hawthorn (plant part not stated).[16] It was postulated that this could be mediated by the regulation of the capsase signal pathway, affecting the reduction of endothelial cell apoptosis. The study also highlighted the potential for the four herbs tested to reduce cells’ ability to regenerate and repair a negative finding. The exact nature of the herbal products under study was not given (no plant part stated, no preparation technique elucidated); therefore, this finding is difficult to interpret.
In studies not directly related to cardiovascular effects, immunomodulatory effects were not detected in Crataegus preparations, although the potential for suppression of interleukin 2 has been cited.[26] Flavonoid and procyanidin compounds have demonstrated an antiviral activity in vitro.[27]
Some protective effects in ischemia/reperfusion injury have been reported,[28] primarily relating to ex vivo animal studies. No studies in this area on human tissue appear to have been conducted to date. The pharmacodynamic profile of hawthorn extracts has been examined in animal studies,[29,30] but no studies appear to have been performed on human subjects.
CLINICAL TRIALS AND RELATED RESEARCH
Table 2 provides details of nine trials undertaken between 1990 and 2008. The findings reflect a meta-analysis undertaken by Pittler et al. who studied eight randomized, placebo-controlled trials. This study showed Crataegus extract to demonstrate definite benefits in the treatment of CHF over placebo[31] In common with the data examined by Pittler et al, the parameters utilized in the various study designs detailed below vary significantly.
Table 2.
Clinical trials and related studies conducted to date
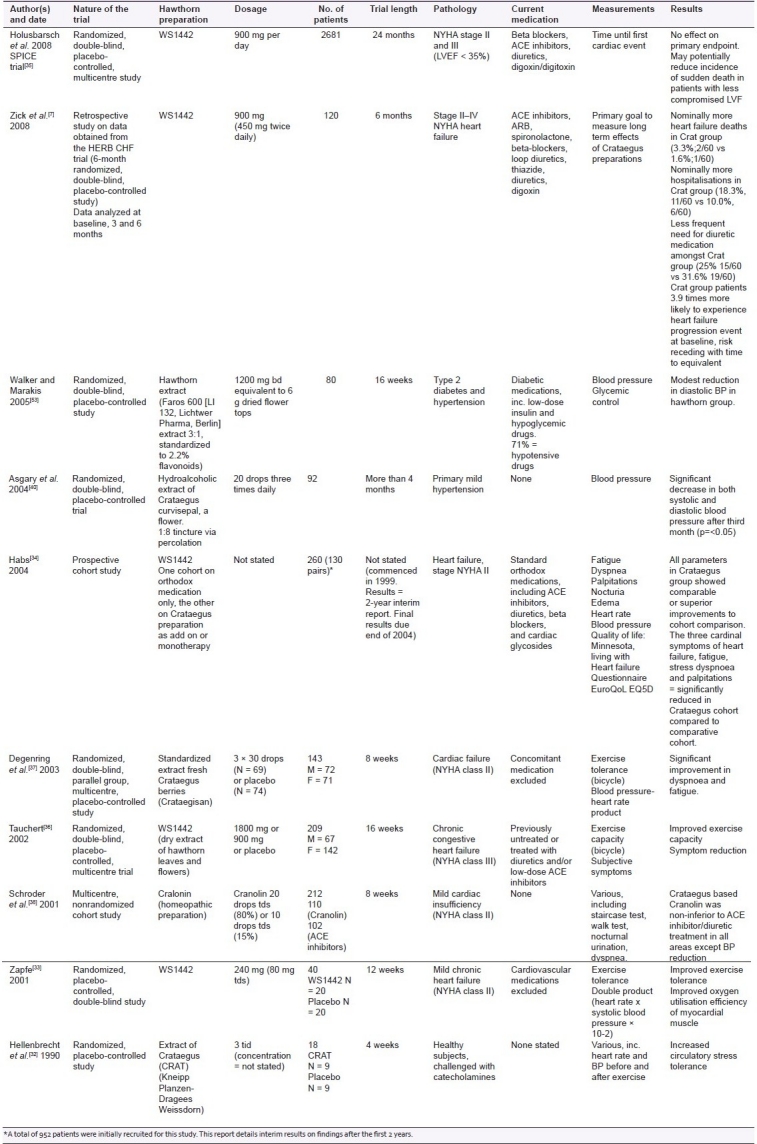
A heterogeneous array of Crataegus-based medicines is represented in the clinical trials reviewed here, with WS1442 being the most common. Numbers of patients involved in the trials varied, those undertaken by Hellenbrecht et al.[32] and Zapfe et al.[33] being so low (18 and 40, respectively) as to question the significance of the results. The use of the cohort study technique by Habs may result in significant dissimilarities existing in the two study cohorts, as the physician's criteria for determining who receives the Crataegus extract and who does not may introduce bias. Thus there is a danger that the study is not comparing like with like. The study's strengths lie in the large number of participants, the extended time scale (>2 years), and the matched-pairs technique employed to minimize dissimilarities between the two groups. Long-term results from this study do not appear to be available. Where consistency was present concerning the remedy used (i.e., WS1442), dosage administered to participants varied from 240 mg per day to 1800 mg per day. Four studies involved patients also taking concurrent orthodox cardiovascular medications,[7,34–36] while four did not (one involved patients on orthodox diabetic medications). Three of the studies were of short duration, ranging from 8 to 12 weeks.[32,37,38] Two of these studies investigated exercise tolerance/circulatory stress tolerance and found improvements within this timeframe. The third study (8 weeks) recorded improvements in all parameters except blood pressure.[38] Furey and Tassell[39] however note that the findings of Asgary et al. demonstrated that hypotensive effects were specifically detected after 3 months of treatment.[40] Thus the 8 weeks of the trial might not have been the sufficient study length for the hypotensive effects to become apparent.
The study by Holusbarsch et al.[35] failed to establish significant differences in rates of cardiac death, nonfatal myocardial infarction, and hospitalization between the two treatment groups, but highlighted potential benefits for the remedy in the treatment of patients with heart failure at risk of sudden cardiac death. The ambivalent findings recorded by Holubarsch et al. regarding the primary endpoint may be in part due to insufficient dosage levels being utilized among this patient group, a significant number of whom have NYHA III heart failure.[39] Tauchert's findings of dose-dependent improvements in clinical signs and symptoms, coupled with improved exercise tolerance among patients with NYHA III heart failure, would indicate higher dosage levels as desirable. Specifically, this study noted significant improvements at the higher dosage level of 1800 mg per day, as opposed to the data used by Zick et al.[7] and Holubarsch et al.[41] who used 900 mg per day. Future trials to determine the optimal therapeutic dosage for this patient group have been suggested.
Although the study by Holubarsh did not detect the clinical improvements sought, neither did it detect significant adverse findings, and many of the parameters measured yielded positive, although not statistically significant, results. Significantly fewer patients in the Crataegus cohort were noted by Habs et al. to require orthodox medications such as ACE inhibitors and cardiac glycosides than in the comparative cohort.[34] Diuretics and beta blockers were also less frequently prescribed. This finding was also noted by Zick et al.[7] with regard to reduced need for diuretic medications in the Crataegus cohort.
The varied nature of parameters used in the above trials, although potentially confusing, is of interest as the outcomes are largely the same. No matter what parameters change the outcome is positive. This is significant in its own way, although the concept of publication bias in clinical research should also be acknowledged.[42] The only obvious exception to this finding is the change in the patient profile in terms of the increased severity of illness (NYHA stage III and above) which may require higher dosage levels to elicit therapeutic benefit. The Expanded Commission E monographs specifically approved use of the hawthorn leaf with the flower in the treatment of the decreasing cardiac output as described in functional stage II of NYHA.[43] Findings by Holsbarsch et al.[35] suggest that patients with milder forms of heart failure (left ventricular ejection fraction 25–35%) would benefit most from WS1442 in terms of the overall reduction of sudden cardiac death (at the dosage levels used).[44]
The phenomenon of the increased likelihood of patients in the Crataegus Special Extract WS1442 group in the recent study by Zick et al.[7] to experience a heart failure progression event at baseline (3.9 times more likely), and the subsequent decrease of this risk over time have no rational explanation and have not been observed by other studies, indicating anomalous findings. Holubarsch et al. reported a statistically nonsignificant increase in hospitalization due to progression of heart failure, but this was countered by a significant decrease in the incidence of sudden cardiac death, and overall number of patients experiencing a cardiac event was lower (statistically nonsignificant) in the WS1442 cohort.
Zick et al. commented that their results may be due to chance as the sample size was small, rendering it more vulnerable to a significant impact from chance outcomes which would produce anomalies in the findings. Also the trial in question was not specifically designed to examine these areas; therefore, other factors might have influenced results and important influences might have been overlooked. The issue of dosage, as discussed above, may be of considerable significance here also, particularly as the study group contained patients suffering from stage IV NYHA heart failure, a group who until now had not been included in clinical trials and who may well be too ill for this treatment to be appropriate. The aldosterone antagonist spironolactone used by some patients in the HRBC CHF trial displays potential for a range of serious side effects, including electrolyte imbalances and hepatotoxicity.[45] The expected incidence of adverse HF events in a patient group of this nature has not been discussed. These data, if available, would be crucial.
The SPICE trial[46] specifically looked at morbidity and mortality as endpoints. The study concluded that the primary endpoints, reductions in cardiac death, nonfatal MI, and hospitalization due to progressive heart failure, were not achieved. The study did find however that deaths of a sudden cardiac cause, deaths due to progressive heart failure, and fatal myocardial infarctions (MI) were all lower in the WS1442 group, although these figures did not reach statistical significance. It was suggested that WS1442 may reduce sudden cardiac deaths in patients with LVEF between 25% and 35%.
Camphor–Crataegus combinations have also been used in the treatment of CVD, notably in cases of orthostatic hypotension. Belz et al. reported findings of dose-response-related efficacy of a camphor–Crataegus product (Korodin) in randomized, placebo-controlled studies.[47] A further study in 2003 suggested that the d-camphor component of this product is responsible for the rapid initial effect, whereas Crataegus berries add a longer lasting pressoric effect.[48] This would confirm the findings of Asgary et al. concerning the 3-month time span for pressor effects of Crataregus to become measurable.[40]
Both the HERB CHF study and the SPICE trial involved patients who were significantly more ill than those in many of the previous studies. This may well have influenced the outcome. Crataegus-based remedies at the dosage utilized may be primarily suited to mild or moderate cases of heart failure only. The slightly higher number of NYHA stage III patients in the Crataegus group (N = 30, 50%) in the HERB CHF study compared to those in the placebo group (N = 27, 45%) might have a detrimental impact on the results where participant numbers are small.
Mechanisms of action of Crataegus postulated to date reveal a remedy with potentially broad-based influence on the cardiovascular system. These effects include a hypotensive activity[17,22] via vasorelaxation resulting from nitrous oxide stimulation, significant antioxidant activity,[49–51] and a tonic action on cardiac myocytes.[15,52]
SAFETY PROFILE OF CRATAEGUS PREPARATIONS
Side effects
Crataegus preparations have been consistently proven to be well tolerated by patients with low/negligible levels of side effects.[28,33,36,37,53–55] Daniele et al. looked at data from 24 clinical trials and a total of 5577 patients.[56] They concluded that hawthorn preparations are generally well tolerated and noted that adverse effects were significantly lower in treatment groups using WS1442. It was noted that Crataegus appears to prevent dizziness rather than causing it. Further examination of spontaneous reporting schemes highlighted 18 case reports following Crataegus treatment, but stated that in many cases insufficient data were supplied to prove any association between Crataegus and specific adverse effects.[56] There appears to be no substantial body of evidence to suggest that Crataegus causes anything other than infrequent, mild adverse effects. There are also no known contraindications to its use during pregnancy,[6] although expert advice should be sought in this circumstance. Crataegus demonstrates low toxicity, with an LD50 of 25 mg/kg[57] and a high therapeutic index.[58] The clinical trial conducted by Tauchert et al. utilized a high dose of WS1442 (1800 mg) with no reported side effects.[36] Animal studies on Crataegus toxicity, using doses of WS1442 up to 100 times normal dose, showed no evidence of toxicity. Studies on human models of this nature have not been undertaken. The inotropic properties of Crataegus may theoretically cause concern, as the use of inotropes in the treatment of heart failure has been strongly linked to increased mortality rates.[59] The one orthodox inotrope not associated with this phenomenon is digoxin, which demonstrates weak inotropic effects, coupled with therapeutic benefits via other mechanisms (i.e., neurohormonal).[59] In that, it is not simply an inotroph but possesses a broad spectrum of other actions; Crataegus shares its therapeutic profile with digoxin and is thus unlikely to present with long-term adverse effects. An increase in the mortality rate resulting from the positive inotrophic effect of Crataegus was not detected in most recent clinical trials.[60] Kernan et al. highlighted concern regarding toxic side effects associated with digitalis medications. Specifically, they examined the potential for acute, coincident illness to engender digitalis toxicity due to decreased drug clearance. This potential risk appears to be largely avoided in Crataegus treatments due to its wide therapeutic index, coupled with the minimal incidence of serious side effects.[61] The need for good quality clinical studies examining mortality as an endpoint has been highlighted.[55,58,62] This issue has been addressed by Holusbarsh et al.[60] in a study lasting 24 months, which showed no significant statistical differences between placebo or treatment groups in terms of mortality.
Drug/Herb interactions
Many different theoretical interactions between Crataegus and orthodox medications have been postulated. None have been substantiated. An interaction study between digoxin and Crataegus preparation WS1442 concluded that both of these remedies may be co-administered safely.[63] Three randomized clinical trials and one observational study reviewed by Daniele et al. involved concomitant use of cardioactive glycoside medications.[56] None of these studies raised any issues regarding herb/drug interactions. Vasodilatory effects of hawthorn have been cited as theoretically causing complications when used with other vasodilatory agents (i.e., caffeine, theophylline).[54] No reports of adverse effects relating to this issue have been cited to date. Inotropic actions of hawthorn have been cited as potentially affecting the hypotensive effects of beta blockers.[64] This also remains unsubstantiated. The proposed theoretical herb/drug interaction between Crataegus preparations and orthodox medications remains theoretical, possibly due to the complex effects of whole plant medicines upon the system.
The impact of the phytochemical synergistic interactions occurring within whole plant remedies is vital to a more intelligent and rational understanding of the nature of their mechanisms of action. Pharmacodynamic and pharmacokinetic interactions have already been researched[65] in whole plant medicines and the concept that identified constituents, singly or in groups, do not act in isolation but exert their effects in a more interactive and synergistic manner has been increasingly postulated by researchers.[15,25,66–69] This issue may well have relevance in the question of drug–herb interactions, where the broad-based nature of whole plant remedies poses a significantly reduced risk. This whole area requires further research if optimal therapeutic benefits are to be derived.
CONCLUSIONS
Results recorded from clinical trials, experiences of professionally qualified medical herbalists, and the low/negligible incidence of side effects experienced by patients would indicate that Crataegus preparations hold significant potential as a useful remedy in the treatment of CVD.
Clinical trials have until recently been largely confined to patients presenting with NYHA stage I or II heart failure. More recently, the inclusion of patients with more advanced CVD in clinical trials might have affected outcomes, particularly where dosages were not adjusted to reflect the severity of illness.
Studies by Holusbarsch et al.[35] would indicate efficacy of Crataegus preparations in the treatment of mild to moderate heart failure (NYHA I–II). The more seriously ill patient may need higher dosages (1800 mg) as used by Tauchert[36] for significant improvements to be obtained. Ultimately, an examination of the data to date is encouraging but would point to the need for a more targeted approach in terms of dosage related to severity of illness. It is possible that this remedy might have limited the benefit for more seriously ill patients but, used in the early stages of disease progression, may significantly enhance prognosis.
The excellent safety profile of this remedy, coupled with the lack of herb–drug interactions detected to date in clinical trials would further support its inclusion in treatment strategies surrounding CVD, especially in the early stages of disease progression.
A robust and succinct response to a letter to the editor of the European Journal of Heart Failure, criticizing the inclusion of a clinical trial involving the use of a homeopathic Crataegus preparation in the treatment of heart failure[70] called for more open-mindedness within the scientific community and a celebration “of the success of bringing different medical cultures together to focus on patients unmet needs.” Whole plant hawthorn remedies represent an excellent opportunity for this commendable concept to be taken forward.
ACKNOWLEDGMENTS
We gratefully acknowledge funding from Technology Sector Research: Strand 1; Post-Graduate RandD Skills Programme in Institutes of Technology – 2005 for M. C. Tassell and the Council of Directors, Technological Sector Research – Strand III 2006 Grant Scheme, awarded to Dr. A. Furey.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Albarouki E, Peterson A. Molecular and morphological characterization of Crataegus L. species (Rosaceae) in southern Syria. Botanical Journal of the Linnean Society. 2007 [Google Scholar]
- 2.British HF. British Heart Foundation. Statistics. 2006. Nov 3rd, [cited in 2007]. Available from: http://www.heartstats.org/datapage.asp?id=713 .
- 3.World HO. World Health Organisation Website. [cited in 2007]. Available from: http://www.who.int/topics/cardiovascular_diseasees/en/.
- 4.National S. Heart disease leading cause of death in England and Wales. Health Statistics Quarterly. 2006 [Google Scholar]
- 5.Bournemouth: British Herbal Medicines Association; 1984. Herbal Medicines Association, B., British Herbal Pharmacopoeia. [Google Scholar]
- 6.Mills S, Bone K. London: Churchill Livingstone; 2000. Principles and Practice of Phytotherapy. [Google Scholar]
- 7.Zick SM, Gillespie B, Aaronson K. The Effect of Crataegus oxyacantha special extract WS 1442 on clinical progression in patients with mild to moderate symptoms of heart failure. European Journal of Heart Failure. 2008:387–93. doi: 10.1016/j.ejheart.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American and H. Association. Coronary Risk Profi le. [cited in 2008]. Available from: http://www.americanheart.org/presenter.jhtml?identifi er=4528 .
- 9.Wold RS, Lopez ST, Yau CL, Butler LM, Pareo-Tubbeh SL, Waters DL, et al. Increasing trends in elderly persons’ use of nonvitamin, nonmineral dietary supplements and concurrent use of medications. J Am Diet Assoc. 2005;105:54–63. doi: 10.1016/j.jada.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Miller LG. Selected Clinical Considerations Focussing on Known or Potential Drug-Herb Interactions. Archives of Internal Medicine. 1998;158:2200–11. doi: 10.1001/archinte.158.20.2200. [DOI] [PubMed] [Google Scholar]
- 11.Culpepper N. Halifax: Nicholson and Co; 1820. The British Herbal and Family Physician. [Google Scholar]
- 12.Kingston R. A phyotchemical analysis of selected constituents of Crataegus flos and fruct. to determine whether ethanol, whiskey and brandy solvents affect the chemical constituent profi le of the herbal preparations 2007, Scottish School of Herbal Medicine [Google Scholar]
- 13.Vanhaelen M, Vanhaelen-Fastre R. TLC-densitometric determination of 2,3-cis-procyanidin monomer and oligomers from hawthorn (Crataegus laevigata and C.monogyna) J Pharm Biomed Anal. 1989;7:1871–5. doi: 10.1016/0731-7085(89)80206-7. [DOI] [PubMed] [Google Scholar]
- 14.Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, et al. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113:71–88. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 15.Long SR, Carey RA, Crofoot KM, Proteau PJ, Filtz TM. Effect of hawthorn (Crataegus oxycantha) crude extract and chromatographic fractions on multiple activities in a cultured cardiomyocyte assay. Phytomedicine. 2006;13:643–50. doi: 10.1016/j.phymed.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Ling S. Effects of four medicinal herbs on human vascular endothelial cells in culture. Int Jrnl cardio. 2007 doi: 10.1016/j.ijcard.2007.05.111. [DOI] [PubMed] [Google Scholar]
- 17.Brixius K, Willms S, Napp A, Tossios P, Ladage D, Bloch W, et al. Crataegus special extract WS 1442 induces an endothelium-dependent, NO-mediated vasorelaxation via eNOS-phosphorylation at serine 1177. Cardiovasc Drugs Ther. 2006;20:177–84. doi: 10.1007/s10557-006-8723-7. [DOI] [PubMed] [Google Scholar]
- 18.Quettier-Deleu C, Voiselle G, Fruchart JC, Duriez P, Teissier E, Bailleul F, et al. Hawthorn extracts inhibit LDL oxidation. Pharmazie. 2003;58:577–81. [PubMed] [Google Scholar]
- 19.Zhang Z. Hypercholesterolaemic activity of Hawthorn fruit is mediated by regulation of cholesterol 7 ά Hydroxylase and acyl CoA: cholesterol acyltransferase. Food Res Int. 2002;35:885–91. [Google Scholar]
- 20.Vierling W, Brand N, Gaedcke F, Sensch KH, Schneider E, Scholz M. Investigation of the pharmaceutical and pharmacological equivalence of different Hawthorn extracts. Phytomedicine. 2003;10:8–16. doi: 10.1078/094471103321648601. [DOI] [PubMed] [Google Scholar]
- 21.Tsuyaki RT. β-Blockers for congestive heart failure.What is the current consensus? Drugs Aging. 2000;16:1–7. doi: 10.2165/00002512-200016010-00001. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Kang KW, Kim KW, Kim ND. Procyanidins in crataegus extract evoke endothelium-dependent vasorelaxation in rat aorta. Life Sci. 2000;67:121–31. doi: 10.1016/s0024-3205(00)00608-1. [DOI] [PubMed] [Google Scholar]
- 23.Guo C. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nut Res. 2003;23:1719–26. [Google Scholar]
- 24.Zhang Z. Characterisation of antioxidants present in Hawthorn fruits. J Nutr Biol. 2000;12:144–52. doi: 10.1016/s0955-2863(00)00137-6. [DOI] [PubMed] [Google Scholar]
- 25.Bahorun T, Gressier B, Trotin F, Brunet C, Dine T, Luyckx M, et al. Oxygen species scavenging activity of phenolic extracts from hawthorn fresh plant organs and pharmaceutical preparations. Arzneimittelforschung. 1996;46:1086–9. [PubMed] [Google Scholar]
- 26.Bleske BE. Evaluation of Hawthorn extract on immodulatory biomarkers in a pressure overload model of heart failure. Med Sci Mon. 2007;13:255–8. [PubMed] [Google Scholar]
- 27.Orhan I. HPLC Quantification of vitexine-2”-O-rhamnoside and hyperoside in three crataegus species and their antimicrobial and antiviral activities. Chromato Suppl. 2007;66:153–7. [Google Scholar]
- 28.Chang WT, Dao J, Shao ZH. Hawthorn: Potential Roles in Cardiovascular Disease. Amer Jrnl Chin Med. 2005;33:1–10. doi: 10.1142/S0192415X05002606. [DOI] [PubMed] [Google Scholar]
- 29.Chang Q, Zuo Z, Ho WK, Chow MS. Comparison of the pharmacokinetics of hawthorn phenolics in extract versus individual pure compound. J Clin Pharmacol. 2005;45:106–12. doi: 10.1177/0091270004270500. [DOI] [PubMed] [Google Scholar]
- 30.Liang M, Xu W, Zhang W, Zhang C, Liu R, Shen Y, et al. Quantitative LC/MS/MS method and in vivo pharmacokinetic studies of vitexin rhamnoside, a bioactive constituent on cardiovascular system from hawthorn. Biomed Chromatogr. 2007;21:422–9. doi: 10.1002/bmc.777. [DOI] [PubMed] [Google Scholar]
- 31.Pittler MH, Schmidt K, Ernst E. Hawthorn extract for treating chronic heart failure: meta-analysis of randomized trials. Am J Med. 2003;114:665–74. doi: 10.1016/s0002-9343(03)00131-1. [DOI] [PubMed] [Google Scholar]
- 32.Hellenbrecht D. Randomised, placebo controlled study with crataegus on exercise tests and challenge by catecholamines. Eur J Pharmacol. 1990;183:525–6. [Google Scholar]
- 33.Zapfe jun G. Clinical efficacy of crataegus extract WS 1442 in congestive heart failure NYHA class II. Phytomedicine. 2001;8:262–6. doi: 10.1078/0944-7113-00041. [DOI] [PubMed] [Google Scholar]
- 34.Habs M. Prospective, comparative Cohort Studies and Their Contribution to the benefit Assessments of Therapeutic Options: Heart Failure Treatment with and without Hawthorn Special Extract WS 1442. Forsch Komplementarmed Klass Naturheilkd. 2004;11:36–9. doi: 10.1159/000080574. [DOI] [PubMed] [Google Scholar]
- 35.Holubarsch CJ, Colucci WS, Meinertz T, Gaus W, Tendera M. The effi cacy and safety of Crataegus extract WS 1442 in patients with heart failure: the SPICE trial. Eur J Heart Fail. 2008;10:1255–63. [Google Scholar]
- 36.Tauchert M. Efficacy and safety of crataegus extract WS 1442 in comparison with placebo in patients with chronic stable New York Heart Association class-III heart failure. Am Heart J. 2002;143:910–5. doi: 10.1067/mhj.2002.121463. [DOI] [PubMed] [Google Scholar]
- 37.Degenring FH, Suter A, Weber M, Saller R. A randomised double blind placebo controlled clinical trial of a standardised extract of fresh Crataegus berries (Crataegisan) in the treatment of patients with congestive heart failure NYHA II. Phytomedicine. 2003;10:363–9. doi: 10.1078/0944-7113-00312. [DOI] [PubMed] [Google Scholar]
- 38.Schroder D, Weiser M, Klein P. Efficacy of a homeopathic Crataegus preparation compared with usual therapy for mild (NYHA II) cardiac insufficiency: results of an observational cohort study. Eur J Heart Fail. 2002;5:319–26. doi: 10.1016/s1388-9842(02)00237-4. [DOI] [PubMed] [Google Scholar]
- 39.Furey A, Tassell M. Towards a systematic scientific approach in the assessment of efficacy of an herbal preparation: Hawthorn (Crataegus spp.) Eur J Heart Fail. 2008;10:1153–7. doi: 10.1016/j.ejheart.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Asgary S, Naderi GH, Sadeghi M, Kelishadi R, Amiri M. Antihypertensive effect of Iranian Crataegus curvisepala Lind: a randomized, double-blind study. Drugs Exp Clin Res. 2004;30:221–5. [PubMed] [Google Scholar]
- 41.Holubarsch CJ, Colucci WS, Meinertz T, Gaus W, Tendera M. Survival and prognosis: investigation of Crataegus extract WS 1442 in congestive heart failure (SPICE)--rationale, study design and study protocol. Eur J Heart Fail. 2000;2:431–7. doi: 10.1016/s1388-9842(00)00109-4. [DOI] [PubMed] [Google Scholar]
- 42.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–72. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 43.ESCOP, Expanded Commision E monographs. 1994 [Google Scholar]
- 44.Brown D. High Dose Hawthorn Extract for Advanced Congestive Heart Failure. (28).Herbalgram. 2003;57:24–25. [Google Scholar]
- 45.Association BM. British National Formulary. In: Mehta DK, editor. 2005. [Google Scholar]
- 46.Holubarsch CJ, Colucci WS, Meinertz T, Gaus W, Tendera M. The effi cacy and safety of Crataegus extract WS 1442 in patients with heart failure: the SPICE trial. Eur J Heart Fail. 2008;10:1255–63. [Google Scholar]
- 47.Belz GG, Butzer R, Gaus W, Loew D. Camphor-Crataegus berry extract combination dose-dependently reduces tilt induced fall in blood pressure in orthostatic hypotension. Phytomedicine. 2002;9:581–588. doi: 10.1078/094471102321616382. [DOI] [PubMed] [Google Scholar]
- 48.Belz GG, Loew D. Dose-response related efficacy in orthostatic hypotension of a fixed combination of D-camphor and an extract from fresh crataegus berries and the contribution of the single components. Phytomedicine. 2003;10:61–7. doi: 10.1078/1433-187x-00303. [DOI] [PubMed] [Google Scholar]
- 49.Yoo KM. Relative antioxidant and cytoprotective activities of common herbs. Food Chem. 2007;106:929–36. [Google Scholar]
- 50.Cui T, Nakamura K, Tian S, Kayahara H, Tian YL. Polyphenolic content and physiological activities of Chinese hawthorn extracts. Biosci Biotechnol Biochem. 2006;70:2948–56. doi: 10.1271/bbb.60361. [DOI] [PubMed] [Google Scholar]
- 51.Ljubuncic P, Portnaya I, Cogan U, Azaizeh H, Bomzon A. Antioxidant activity of Crataegus aronia aqueous extract used in traditional Arab medicine in Israel. J Ethnopharmacol. 2005;101:153–61. doi: 10.1016/j.jep.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 52.Schüssler M, Hölzl J, Fricke U. Myocardial effects of flavonoids from Crataegus species. Arzneimittelforschung. 1995;45:842–5. [PubMed] [Google Scholar]
- 53.Walker AF, Marakis G, Simpson E, Hope JL, Robinson PA, Hassanein M, et al. Hypotensive effects of hawthorn for patients with diabetes taking prescription drugs: a randomised controlled trial. Br J Gen Pract. 2005;56:437–43. [PMC free article] [PubMed] [Google Scholar]
- 54.Rigelsky JM, Sweet BV. Hawthorn: pharmacology and therapeutic uses. Am J Health Syst Pharm. 2002;59:417–22. doi: 10.1093/ajhp/59.5.417. [DOI] [PubMed] [Google Scholar]
- 55.Fong HH, Bauman JL. Hawthorn. J Cardiovasc Nurs. 2002;16:1–8. doi: 10.1097/00005082-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Daniele C, Mazzanti G, Pittler MH, Ernst E. Adverse-event profile of Crataegus spp.: a systematic review. Drug Saf. 2006;29:523–35. doi: 10.2165/00002018-200629060-00005. [DOI] [PubMed] [Google Scholar]
- 57.Thorne R. Crataegus oxyacantha. Alt Med Rev. 1998;3:138–9. [Google Scholar]
- 58.Loew D. Phytotherapy in Heart Failure. Phytomedicine. 1997;4:267–71. doi: 10.1016/S0944-7113(97)80080-3. [DOI] [PubMed] [Google Scholar]
- 59.Felker GM, O’Connor CM. Inotropic therapy for heart failure:An evidence based approach. American Heart J. 2001;42:393–401. doi: 10.1067/mhj.2001.117606. [DOI] [PubMed] [Google Scholar]
- 60.Holubarsch CJ, Colucci WS, Meinertz T, Gaus W, Tendera M. Survival and prognosis: investigation of Crataegus extract WS 1442 in congestive heart failure (SPICE)--rationale, study design and study protocol. Eur J Heart Fail. 2000;2:431–7. doi: 10.1016/s1388-9842(00)00109-4. [DOI] [PubMed] [Google Scholar]
- 61.Kernan WN, Castellsague J, Perlman GD, Ostfeld A. Incidence of hospitalization for digitalis toxicity among elderly Americans. Am J Med. 1994;96:426–31. doi: 10.1016/0002-9343(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 62.Baughman KL, Bradley DJ. Hawthorn extract: is it time to turn over a new leaf? Am J Med. 2003;114:700–1. doi: 10.1016/s0002-9343(03)00160-8. [DOI] [PubMed] [Google Scholar]
- 63.Tankanow R, Tamer HR, Streetman DS, Smith SG, Welton JL, Annesley T, et al. Interaction study between digoxin and a preparation of hawthorn (Crataegus oxyacantha) J Clin Pharmacol. 2003;43:637–42. [PubMed] [Google Scholar]
- 64.Kendler BS. Recent nutritional approaches to the prevention and therapy of cardiovascular disease. Prog Cardiovasc Nurs. 1997;12:3–23. [PubMed] [Google Scholar]
- 65.Spinella M. The importance of pharmacological synergy in psychoactive herbal medicines - Herbal Synergy Review. Alternative Medicine Review. 2002:130–7. [PubMed] [Google Scholar]
- 66.Skegert M. Phenols, procyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89:191–8. [Google Scholar]
- 67.Cirico TL, Omaye ST. Additive or synergetic effects of phenolic compounds on human low density lipoprotein oxidation. Food Chem Toxicol. 2005;44:510–6. doi: 10.1016/j.fct.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 68.Sokol-Letowska A, Oszmianski J, Wojdylo A. Antioxidant activity of the phenolic compounds of hawthorn, pine and skullcap. Food Chem. 2006;103:853–9. [Google Scholar]
- 69.Zapatero JM. Selections from current literature: effects of hawthorn on the cardiovascular system. Fam Pract. 1999;16:534–8. doi: 10.1093/fampra/16.5.534. [DOI] [PubMed] [Google Scholar]
- 70.Cleland JGF. Response from editor to letter to the editor: Alternative approaches to the management of heart failure:Editors response. Eur J Heart Fail. 2004;6:517–8. [Google Scholar]
- 71.Wojdyto A, Oszmianski J. Influence of polyphenols isoalted from Scutellaria baiacalensis Georgi and Crataegus oxyacantha on the oxidative stability of cholesterol in butter stored in various conditions. Euro Food Res Techno. 2006;224:635–42. [Google Scholar]
- 72.Fan C, Yan J, Qian Y, Wo X, Gao L. Regulation of lipoprotein lipase expression by effect of hawthorn flavonoids on peroxisome proliferator response element pathway. J Pharmacol Sci. 2005;100:51–8. doi: 10.1254/jphs.fp0050748. [DOI] [PubMed] [Google Scholar]


