Abstract
The medicinal plants are widely used by the traditional medicinal practitioners for curing various diseases in their day to day practice. In traditional system of medicine, different parts (leaves, stem, flower, root, seeds and even whole plant) of Ocimum sanctum Linn. have been recommended for the treatment of bronchitis, malaria, diarrhea, dysentery, skin disease, arthritis, eye diseases, insect bites and so on. The O. sanctum L. has also been suggested to possess anti-fertility, anticancer, antidiabetic, antifungal, antimicrobial, cardioprotective, analgesic, antispasmodic and adaptogenic actions. Eugenol (1-hydroxy-2-methoxy-4-allylbenzene), the active constituents present in O. sanctum L. have been found to be largely responsible for the therapeutic potentials. The pharmacological studies reported in the present review confirm the therapeutic value of O. sanctum L. The results of the above studies support the use of this plant for human and animal disease therapy and reinforce the importance of the ethno-botanical approach as a potential source of bioactive substances.
Keywords: Carvacrol, caryophyllene, eugenol, linalool, urosolic acid
INTRODUCTION
Plants are potent biochemists and have been components of phytomedicine since times immemorial; man is able to obtain from them a wondrous assortment of industrial chemicals. A rich heritage of knowledge on preventive and curative medicines was available in ancient scholastic work included in the Atharvaveda (an Indian religious book), Ayurveda (Indian traditional system of medicine) and so on. An estimate suggests that about 13000 plant species worldwide are known to have been used as drugs. Plant-based natural constituents can be derived from any part of the plant like bark, leaves, flowers, roots, fruits, seeds and so on,[1] that is any part of the plant may contain active components. The beneficial medicinal effects of plant materials typically result from the combinations of secondary products present in the plant. The medicinal actions of plants are unique to particular plant species or groups are consistent with this concept as the combination of secondary products in a particular plant is taxonomically distinct.[2] The systematic screening of plant species with the purpose of discovering new bioactive compounds is a routine activity in many laboratories. The research on the medicinal plants should be extended with the identification of the active principles in the plants. Scientific examination of the remedies could lead to standardization and quality control of the products to ensure their safety. It is after such evaluation that they can be approved for use in the primary health care. Such research activities could also lead to the development of new drugs as in the past. Conventional antiasthmatic compounds such as sodium cromolyn and sodium cromoglycate are some of the examples of the lead prepared from the analogs of the naturally occurring furanochromone khelline[3] (Visammin). Exploration of the chemical constituents of the plants and pharmacological screening will thus provide us the basis for developing new life-saving drugs.
Ocimum sanctum L. (also known as Ocimum tenuiflorum, Tulsi) has been used for thousands of years in Ayurveda for its diverse healing properties. Tulsi, the Queen of herbs, the legendary ‘Incomparable one’ of India, is one of the holiest and most cherished of the many healing and healthy giving herbs of the orient. The sacred basil, Tulsi, is renowned[4] for its religious and spiritual sanctity, as well as for its important role in the traditional Ayurvedic and Unani system of holistic health and herbal medicine of the East. It is mentioned by Charaka in the Charaka Samhita; an Ayurvedic text. Tulsi is considered to be an adaptogen, balancing different processes in the body, and helpful for adapting to stress. Marked by its strong aroma and astringent taste, it is regarded in Ayurveda as a kind of ‘elixir of life’ and believed to promote longevity. Tulsi extracts are used in Ayurvedic remedies for common colds, headaches, stomach disorders, inflammation, heart disease, various forms of poisoning and malaria. Traditionally, O. sanctum L. is taken in many forms, as herbal tea, dried power or fresh leaf. For centuries, the dried leaves of Tulsi have been mixed with stored grains to repel insects.[5]
Ocimum sanctum L. (Tulsi) is an erect, much branched sub-shrub 30-60 cm tall, with simple opposite green or purple leaves [Figure 1] that are strongly scented and hairy stems. Leaves have petiole and are ovate, up to 5 cm long, usually somewhat toothed. Flowers are purplish in elongate racemes in close whorls [Figure 2]. Tulsi is native throughout the world tropics and widespread as a cultivated plant and an escaped weed. It is cultivated for religious and medicinal purposes and for its essential oil. Tulsi is an important symbol in many Hindu religious traditions, which link the plant with Goddess figure. The name ‘Tulsi in Sanskrit means ‘the incomparable one’. The presence of a Tulsi plant symbolizes the religious bend of a Hindu family.
Figure 1.

Ocimum sanctum tree (Tulsi)
Figure 2.

Flowers in elongate racemes in close whorls
SCIENTIFIC CLASSIFICATION
Kingdom: Plantae
(unranked) Angiosperms
(unranked) Eudicots
(unranked) Asterids
Order: Lamiales
Family: Lamiaceae
Genus: Ocimum
Species: O. tenuiflorum
Binomial name: Ocimum tenuiflorum or Ocimum sanctum L.
OCIMUM SANCTUM L. (TULSI): A PLANT FROM GENUS OCIMUM
Among the plants known for medicinal value, the plants of genus Ocimum belonging to family Labiate are very important for their therapeutic potentials. Ocimum sanctum L (Tulsi)., O. gratissimum (Ram Tulsi), O. canum (Dulal Tulsi), O. bascilicum (Ban Tulsi), O. kilimandschricum, O. americanum, O. camphora and O. micranthum are examples of known important species of genus Ocimum that grow in different parts of the world and are known to have medicinal properties.[6–8]
NUTRITION VALUE
Contains vitamin C and A, and minerals like[9] calcium, zinc and iron, as well as chlorophyll and many other phytonutrients. Also enhances the efficient digestion, absorption and use of nutrients from food and other herbs. Protein: 30 Kcal, 4.2 g; Fat: 0.5 g; Carbohydrate 2.3 g; Calcium: 25 mg; Phosphorus 287 mg; Iron: 15.1 mg and Edible portion 25 mg vitamin C per 100 g.
PHYTOCHEMICAL CONSTITUENTS
The chemical composition of Tulsi is highly complex, containing many nutrients and other biologically active compounds, the proportions of which may vary considerably between strains and even among plants within the same field. Furthermore, the quantity of many of these constituents is significantly affected by differing growing, harvesting, processing and storage conditions that are not yet well understood.
The nutritional and pharmacological properties of the whole herb in its natural form, as it has been traditionally used, result from synergistic interactions of many different active phytochemicals. Consequently, the overall effects of Tulsi cannot be fully duplicated with isolated compounds or extracts. Because of its inherent botanical and biochemical complexity, Tulsi standardization has, so far, eluded modern science. The leaf volatile oil[10] contains eugenol (1-hydroxy-2-methoxy-4-allylbenzene [Figure 3]), euginal (also called eugenic acid), urosolic acid[11] (2,3,4,5,6,6a,7,8,8a,,10,11,12,13,14b-tetradecahydro-1H-picene-4a-carboxylic acid [Figure 4]), carvacrol (5-isopropyl-2-methylphenol [Figure 5]), linalool (3,7-dimethylocta-1,6-dien-3-ol [Figure 6]), limatrol, caryophyllene (4,11,11-trimethyl-8-methylene-bicyclo[7.2.0]undec-4-ene [Figure 7]), methyl carvicol (also called Estragol: 1-allyl-4-methoxybenzene [Figure 8]) while the seed volatile oil have fatty acids and sitosterol; in addition, the seed mucilage contains some levels of sugars and the anthocyans are present in green leaves. The sugars are composed of xylose and polysaccharides.
Figure 3.
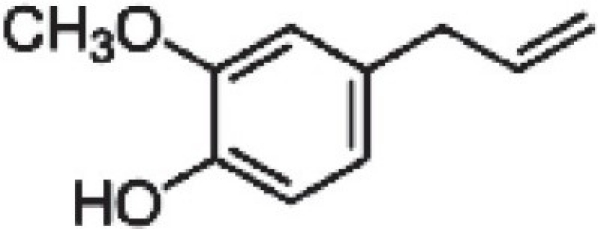
Eugenol (1-hydroxy-2-methoxy-4-allylbenzene)
Figure 4.
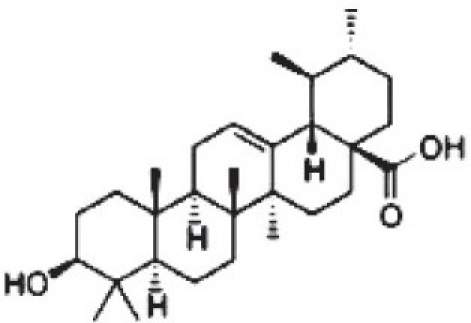
Urosolic acid (2,3,4,5,6,6a,7,8,8a,10,11,12,13,14btetradecahydro-1H-picene-4a-carboxylic acid)
Figure 5.
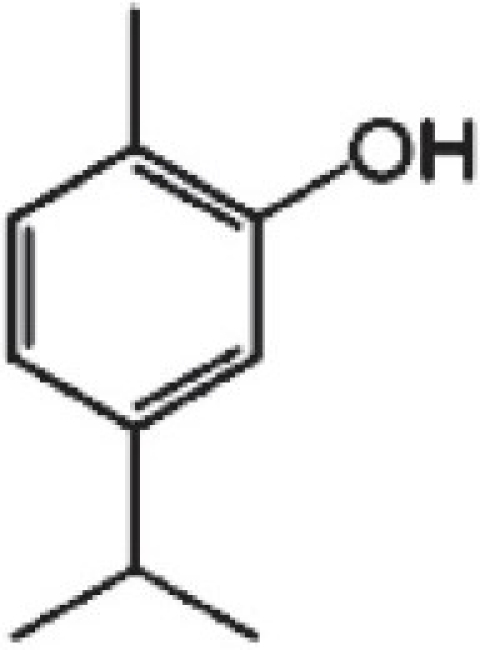
Carvacrol (5-isopropyl-2-methylphenol)
Figure 6.
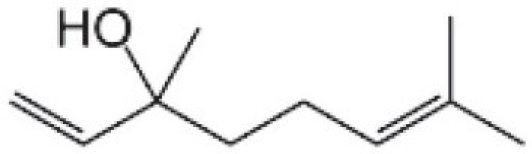
Linalool (3,7-dimethylocta-1,6-dien-3-ol)
Figure 7.
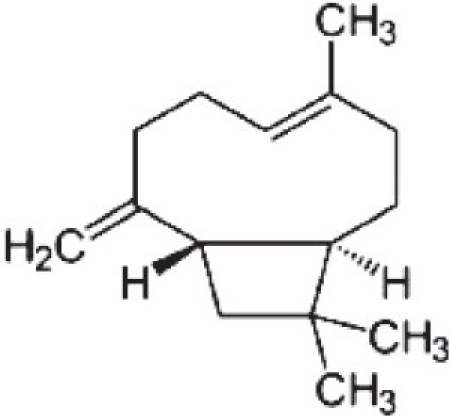
Caryophylline (4,11,11-trimethyl-8-methylene-bicyclo[7.2.0]undec-4-ene)
Figure 8.
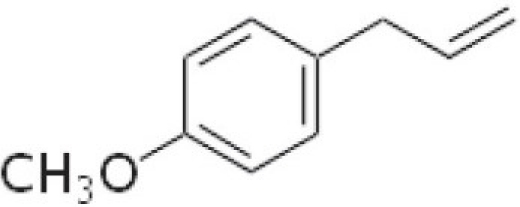
Estragol (1-allyl-4-methoxybenzene)
Although Tulsi is known as a general vitalizer and increases physical endurance, it contains no caffeine or other stimulants. The stem and leaves of holy basil contain a variety of constituents that may have biological activity, including saponins, flavonoids, triterpenoids, and tannins.[12] In addition, the following phenolic actives have been identified, which also exhibit antioxidant and antiinflammatory activities, Rosmarinic acid ((2R)-2-[[(2E)-3-(3,4-Dihydroxyphenyl)-1-oxo-2-propenyl]]oxy]-3-(3,4-dihydroxyphenyl) propanoic acid [Figure 9]), apigenin (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one [Figure 10]), cirsimaritin (5,4’-dihydroxy-6,7-dimethoxyflavone), isothymusin (6,7-dimethoxy-5,8,4’-trihydroxyflavone) and isothymonin. Two water-soluble flavonoids:[13] Orientin (8-C-beta-glucopyranosyl-3’,4’,5,7-tetrahydroxyflav-2-en-3-one) and Vicenin (6-C-beta-D-xylopyranosyl-8-C-beta-D-glucopyranosyl apigenin), have shown to provide protection against radiation-induced chromosomal damage in human blood lymphocytes.
Figure 9.
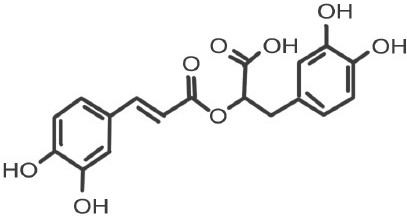
Rosmarinic acid ((2R)-2-[[(2E)-3-(3,4-Dihydroxyphenyl)-1-oxo-2-propenyl]]oxy]-3-(3,4-dihydroxyphenyl)propanoic acid)
Figure 10.
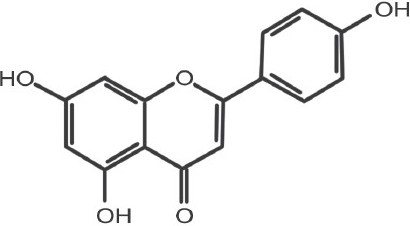
Apigenin (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one)
THERAPEUTIC APPLICATIONS
Pre-Clinical studies (Animal model)
During the last two decades, O. sanctum L. has demonstrated various pre-clinical activities in animal models in vitro testing. Some such notable findings are reported here:
Antidiabetic
Ethanolic extract of O. sanctum L. significantly decreases the blood glucose, glycosylated hemoglobin and urea with a concomitant increase in glycogen, hemoglobin and protein in streptozotocin-induced diabetic rats.[14] This extracts also resulted in an increase in insulin and peptide levels and glucose tolerance.
The constituents of O. sanctum L. leaf extracts have stimulatory effects[15] on physiological pathways of insulin secretion, which may underlie its reported antidiabetic action.
Grovel et al. suggested that treatment with O. sanctum L. extract for 30 days to normal rats fed with fructose for 30 days significantly lowered serum glucose level[16] in comparison with control group. However, O. sanctum L. extract has no significant effect on hyperinsulinemia.
Ghosap et al. unravel the possible mechanism[17] of glucose-lowering activity of O. sanctum L. in male mice. The study suggested that O. sanctum L. decreases the serum concentration of both cortisol and glucose and also exhibited antiperoxidative effect. Therefore O. sanctum L. may potentially regulate corticosteroid- induced diabetic mellitus.
In another study the effect of O. sanctum L. on three important enzymes of carbohydrate metabolism [glucokinase (gk), hexokinase (hk) and phosphofructokinase (PFK) along with glycogen content of insulin-dependent (skeletal muscle and liver) and insulin-independent tissues (kidneys and brain) was studied by Vats et al,[18] in streptozotocin (STZ, 65 mg/kg)-induced model of diabetes for 30 days in rats. Administration of O. sanctum L. extracts 200 mg/kg for 30 days lead to decrease in plasma glucose levels by approximately 9.06 and 24.4% on 15th and 30th day. O. sanctum L. significantly decreased renal but not liver weight (expressed as % of body weight) O. sanctum L. glycogen content in any tissue; also O. sanctum L. partially corrected the activity of glucokinase (gk), hexokinase (hk) and phosphofructokinase (PFK) distributed in the diabetic control.
Tulsi (O. sanctum L.) leaf powder[19] was fed at the 1% level in normal and diabetic rats for a period of one month and the result indicated a significant reduction in fasting blood sugar urogenic acid, total amino acids level. This observation indicates the hypoglycemic effect of O. sanctum L. in diabetic rats.
Chattopadyay also reported that oral administration of alcoholic extract of leaves of O. sanctum L. led to marked lowering of blood sugar[20] level in normal, glucose-fed hyperglycemic and streptozotocin-induced diabetic rats. Furthermore, the extract potentiates the action of exogenous insulin in normal rats. The activity of the extract was 91.55 and 70.43% of that of Tolbutamide in normal and diabetic rats, respectively.
Cardiac activity
Oral feeding of hydroalcoholic extract of O. sanctum L. (100 mg/kg) to male Wister rats subjected to chronic-resistant stress (6 h/day for 21 days) significantly prevented the chronic-resistant stress/induced rise in plasma cAMP level, myocardial superoxide dismutase and catalase activities[21] as well as the light microscopic changes in the myocardium.
Wister rats fed with fresh leaf homogenate of O. sanctum L. (50 and 100 mg/kg body weight) daily 30 days inhibit isoproterenol-induced changes[22] in myocardial superoxide dismutase, glutathione peroxidase and reduced glutathione.
In another study effect of pre- and co-treatment of hydroalcoholic extract of O. sanctum L. at different doses (25, 50, 75, 100, 200 and 400 mg/kg) was investigated against isoproterenol (ISO, 20 mg/kg, Sc) myocardial infarction[23] in rats. O. sanctum L. at the dose of 25, 50, 75 and 100 mg/kg significantly reduced glutathione (GSH), superoxide dismutase and LDH levels. In this study, it was observed that O. sanctum L. at the dose of 50 mg/kg was found to demonstrate maximum cardioprotective effect.
The generation of drug-induced oxygen radicals in heart cells led to cardiac lipid[24] membrane peroxidation. Urosolic acid(UA) isolated from O. sanctum L. have been identified as a protector against Adriamycin (ADR)-induced lipid peroxidation. Protection with UA was 13 and 17% in liver and heart microsomes, respectively. On combination with oleanolic acid (OA) isolated from Eugenia jumbolata , it increased to 69%.
Wound healing activity
Shetty et al[25] evaluated the wound healing effect of aqueous extract of O. sanctum L. in rats. Wound-breaking strength in incision wound model, epithelization period and percent wound concentration in excision wound model were studied owing to increased per cent wound contraction. Ocimum sanctum L. may be useful in the management of abnormal healing such as keloids and hypertropic scars.
Ethanolic extract of leaves of O. sanctum L. was investigated for normal wound healing and dexamethasone-depressed healing.[26] The extract significantly increased the wound breaking strength, wound epithelializes fast and wound contraction was significantly increased along with increase in wet and dry granulation tissue weight and granulation tissue breaking strength. The extract also significantly decreases the anti-healing activities of dexamethasone in all wound healing models.
Radio-protective effect
Radio-protective effect[27] of aqueous extract of O. sanctum L. (40 mg/kg, for 15 days) in mice exposed to high doses (3.7 MBq) of oral 131 iodine was investigated by studying the organ weights, lipid peroxidation and antioxidant defense enzyme in various target organs like liver, kidney, salivary glands and stomach at 24 h after exposure. Pretreatment with O. sanctum L. in radioiodine-exposed group showed significant reduction in lipid peroxidation in both kidney and salivary glands. In liver, reduced glutathione (GSH) levels showed significant reduction after radiation exposure while pretreatment with O. sanctum L. exhibited less depletion in GSH level even after 131 iodine exposure. However, no such changes were observed in the stomach. The results indicate the possibility of using aqueous extract of O. sanctum L. for ameliorating 131 iodine induced damage to the salivary glands.
Two polysaccharides isolated from O. sanctum L. could prevent oxidative damage[28] to liposomal lipids and plasmid DNA induced by various oxidants such as iron, AAPH and gamma radiation.
Vrinda et al. reported that two water-soluble flavonoids, Orientin (Ot) and Vicenin (Vc), isolated from the leaves of O. sanctum L. provide significant protection against radiation,[13,29] lethality and chromosomal aberration in vivo. In order to select the most effective drug concentration, fresh whole blood was exposed to 4 Gy of cobalt-60 gamma radiation with O. sanctum L. without a 30 min pretreatment with 6.25, 12.5, 15, 17.5 and 20 micron of Ot/Vc in micronucleus test. Radiation significantly increased the micronucleus (MN) frequently. Pretreatment with either Ot or Vc at all concentration-dependent manner, with optimum effect at 17.5 μm.
The effect of aqueous extract (OE) of leaves of O. sanctum L. against radiation lethality[30] and chromosome damage was studied by radiation-induced lipid peroxidation in liver. Adult Swiss mice were injected with 10 mg/kg of gamma radiation 30 min after last injection. Glutathione (GSH) and the antioxidant enzymes glutathione transferase (GST), reductase (GSRx), peroxidase (GSPx) and superoxide dismutase (SOD) as well as lipid peroxide (LPx) activity were estimated in the liver at 15 min, 30 min, 1, 2, 4 and 8 h post-treatment. Aqueous extract itself increased the GSH and enzymes significantly above normal level, whereas radiation significantly reduced all the values and significantly increased the lipid peroxidation rate, reaching a maximum value at 2 h after exposure (3.5 times of control). Aqueous extract significantly reduced the lipid peroxidation and accelerated recovery to normal levels.
In a comparative study[31] of radioprotection by ocimum flavonoids and synthetic aminothiol protectors in mouse showed Ocimum flavonoids as promising human radiation protectant. In this study, adult Swiss mice were injected intraperitoneally with 50 μg/kg body weight of Orientin (OT) or vicenin (Vc) 20 mg/kg body weight of 2-ercaptopropionyl glycine (MPG) 150 mg/kg body weight of WR2721 and exposed to whole body irradiation of 2 Gy gamma radiation 30 min later. After 24 hours, chromosomal aberrations were studied in the bone marrow of the femur by routine metaphase preparation after colchicines treatment. Pretreatment with all the protective compounds resulted in a significant reduction in the percentage of aberrant metaphases. Vicenin produced the maximum reduction in per cent aberrant cells while MPG was the least effective; OT and WR-2721 showed an almost similar effect.
Ganasoundari et al.[32] investigated the radio-protective effect of the leaf extract of O. sanctum L. (OE) in combination with WR-2721 (WR) on mouse bone marrow. Adult Swiss mice were injected intraperitoneally with OE (10 mg/kg for five consecutive days) alone or 100-400 mg/kg WR (Single dose) O. sanctum L. combination of the two and whole body was exposed to 4.5Gy gamma irradiation (RT). Metaphase plates were prepared from femur bone marrow on days 1, 2, 7 and 14 post-treatment and chromosomal aberrations were scored. Pretreatment with OE or WR individually resulted in a significant decrease in aberrant cells as well as different types of aberrations. The combination of the two further enhanced this effect; resulting in a two-fold increase in the protection factors (PF = 6.68) compared to 400 mg/kg WR alone.
Genotoxicity
In vivo cytogenetic assay[33] in Allium cepa root tip cells has been carried out to detect the modifying effect of O. sanctum L. aqueous leaf extract against chromium (Cr) and mercury (Hg)-induced genotoxicity. It was observed that the roots post-treated with the leaf extract showed highly significant recovery in mitotic index (MI) and chromosomal aberrations. When compared to pre-treated (Cr/Hg) samples, the lower doses of the leaf extract were found to be more effective than the higher doses.
Immu-21, a poly-herbal formulation containing O. sanctum L. and other herbal extracts when given at 100 mg per kg daily over 7 days and 300 mg/kg daily over 14 days inhibited both cyclophosphamide (40 mg/kg i.p.)-induced classical and non-classical chromosomal aberration[34] (40–60% of control). This also reduces the increase in micronuclei in the bone marrow erythrocytes of mice treated with cyclophosphamide.
Antioxidant
The antioxidant capacity of[35] essential oils obtained by steam hydrodistillation from O. sanctum L. was evaluated using a high-performance liquid chromatography (HPLC) based hypoxanthine xanthine oxidase and OPPH assays. In hypoxanthine xanthine oxidase assay, strong antioxidant capacity was evident from O. sanctum L. (IC50 = 0.46 μL/ml).
In another study the aqueous extract of O. sanctum L. significantly increases the activity of anti-oxidant[36] enzymes such as superoxide dismutase, catalase level in extract-treated group compared to control.
Aqueous extract of O. sanctum L. inhibit the hypercholesterolemia-induced[37] erythrocyte lipid peroxidation activity in a dose-dependent manner in male albino rabbits. Oral feeding also provides significant leaver and aortic tissue protection from hypercholestrolemia-induced peroxidative damage.
The effect of methanolic extract of O. sanctum L. leaves in cerebral reperfusion injury[38] as well as long-term hypoperfusion was studied by Yanpallewar et al. Osimum sanctum L. pretreatment (200 mg/kg/day for 7 days) prevented reperfusion-induced rise in lipid peroxidation and superoxide dismutase. Osimum sanctum L. pretreatment also stabilized the levels of tissue total sulfhydryl group during reperfusion.
Hypolipidemic
Administration of O. sanctum L. seed oil (0.8 gm/kg body weight/day) for four weeks, in cholesterol-fed (100 mg/kg body weight/day) rabbits significantly decreases serum cholesterol, triacylglycerol and LDL + VLDL cholesterol as compared to untreated cholesterol-fed group suggesting the hypo-cholesterolemic[35] activity of O. sanctum L.
Fresh leaves of O. sanctum L. mixed OS 1 and 2 g in 100 gm of diet given for four weeks brought about significant changes in the lipid[39] of normal albino rabbits. This resulted in significant lowering in serum total cholesterol, triglyceride, phospholipids and LDL-cholesterol level and significant increase in the HDL-cholesterol and total fecal sterol contents.
Antimicrobial
Singh et al[40] in his study suggested that higher content of linoleic acid in O. sanctum L. fixed oil could contribute towards its antibacterial activity. The oil show good antibacterial activity against Staphylococcus aureus, Bacillus pumius and Pseudomonas aeruginosa, where S. aureus was the most sensitive organism.
Geeta et al[41] studied that the aqueous extract of O. sanctum L. (60 mg/kg) show wide zones of inhibition compared to alcoholic extract against Klebsiella, E. coli, Proteus, S. aureus and Candida albicans when studied by agar diffusion method. Alcoholic extract showed wider zone for Vibrio cholerae.
Effect on gene transcription
The genes that have direct role in artherogenesis include LDRL, LxRalpha, PPARs, CD-36 because these genes control lipid metabolism, cytotoxin production and cellular activity within the arterial wall. To know whether or not the polyphenols extracted from O. sanctum L. have any effect on the transcription[42] of these genes, Kaul et al. cultured human mononuclear cells in the presence of polyphenols extracted from O. sanctum L. Transcriptional expression of these genes was measured by using RT-PCR and SCION IMAGE analysis software. These polyphenolic extracts were found to have the inherent capacity to inhibit the transcriptional expression of these genes.
Gastroprotective
The standardized methanolic extract of leaves of O. sanctum L. (OSE) given in doses of 50–200 mg/kg orally twice daily for five days showed dose-dependent ulcer protective effect against cold-restraint stress-induced gastric ulcers. Optimal effective dose (100 mg/kg) of OSE showed significant ulcer protection[43] against ethanol and pyloric ligation induced gastric ulcer but was ineffective against aspirin-induced ulcer. OSE (100 mg/kg) also inhibits the offensive acid pepsin secretion and lipid peroxidation and increases the gastric defensive factors like mucin secretion, cellular mucus and lifespan of mucosal cells.
Dharmani et al. evaluated the anti-ulcerogenic activity[44] in cold-restraint (CRU), aspirin (ASP), alcohol (Al), pyloric ligation (PL) induced gastric ulcer models in rats, histamine-induced (HST) duodenal ulcer in guinea pigs and ulcer healing activity in acetic acid induced (AC) chronic ulcer model. Osimum sanctum L. at a dose of 100 mg/kg was found to be effective in CRU (65.07%), ASP (63.49%), Al (53.87%), PL (62.06%) and HST (61.76%) induced ulcer models and significantly reduced free, total acidity and peptic activity by 72.58, 58.63 and 57.6%, respectively, and increased mucin secretion by 34.61% conclusively Osimum sanctum L. could act as a potent therapeutic agent against peptic ulcer disease.
The antiulcerogenic[45] property of O. sanctum L. was studied in pyloric-ligated and aspirin-treated rats. The extract of reduced ulcer index, free and total acidity on acute and chronic administration seven days pretreatment increased the mucus secretion also. So it may be concluded that O. sanctum L. extract has anti-ulcerogenic property against experimental ulcers and it is due to its ability to reduce acid secretion and increase mucus secretion.
Immunomodulatory effect
Immunotherapeutic potential[46] of aqueous extract of O. sanctum L. leaf in bovine sub-clinical mastitis (SCM) was investigated after intramammary infusion of aqueous extract. The results revealed that the aqueous extract of O. sanctum L. treatment reduced the total bacterial count and increased neutrophil and lymphocyte counts with enhanced phagocytic activity and phagocytic index.
In another study, the immunomodulatory effect of O. sanctum L. seed oil (OSSO) was evaluated in both non-stressed and stressed animals.[47] Osimum sanctum L. seed oil (3 ml/kg, Ip ) produced a significant increase in anti-sheep red blood cells (SRBC) antibody titer and a decrease in percentage histamine release from peritoneal mast cell of sensitized rats (humoral immune responses) and decrease in food pad thickness and percentage leucocyte migration inhibition (cell-mediated immune responses). Co-administration of diazepam (1 mg/kg, Sc), a benzodiazepine (BZD) with OSSO (1 mg/kg, IP) enhanced the effect of OSSO on resistant stress induced changes in both humoral and cell-mediated immune responses. Further, flumazenil (5 mg/kg, IP) a central BZD receptor antagonist inhibited the immunomodulatory action of OSSO on resistant stress induced immune responsiveness. Thus, OSSO apparatus to modulate both humoral and cell-mediated immune responsiveness and these immunomodulatory effects may be mediated by GABAnergic pathway.
Godhwani et al.[48] investigated the immunoregulatory profile of methanolic extract and an aqueous suspension of O. sanctum L. leaves to antigenic challenge of Salmonella typhosa and sheep erythrocytes by quantifying agglutinating antibodies employing the Widal agglutination and sheep erythrocyte agglutination tests and E-rosette formation in albino rats. The data of the study indicate an immunostimulation of humoral immunogenic response as represented by an increase in antibody titer in both the Widal and sheep erythrocyte agglutination tests as well as by cellular immunologic response represented by E-rosette formation and lymphocytosis.
Sexually transmitted disease
Extract of O. sanctum L. caused inhibition of Neisseria gonorrhoeae clinical isolates[49] and WHO organization strains. The activity is comparable to penicillin and ciprofloxacin.
Effect on central nervous system (CNS)
Different extracts of stem, leaf and stem callus (induced on slightly modified Murashige and Skoog's medium and supplemented with 2,4-dichlorophenonyacetic acid and kinetin) were tested for anticonvulsant activity[50] by maximal electroshock model using Phenytoin as standard. It was observed that ethanol and chloroform extractives of stem, leaf and stem calli were effective in preventing tonic convulsions induced by transcorneal electroshock.
Ethanolic extract of leaves of O. sanctum L. prolonged the time of lost reflex in mice due to pentobarbital,[51] decreased the recovery time and severity of electroshock and pentylenetetrazole-induced convulsions and decreased apomorphine-induced fighting time and ambulation in ‘open field’ studies. In the forced swimming behavioral despair model, the extract lowered immobility in a manner comparable to Imipramine. This action was blocked by haloperidol and sulpiride, indicating a possible action involving dopaminergic neurons. In similar studies, there was a synergistic action when the extract was combined with bromocriptine, a potent D2-receptor agonist.
Nootropic agents are a new class of drugs used in situations where there is organic disorder in learning abilities. Joshi and Parle[52] assessed the potential of O. sanctum L. extract as a nootropic and anti-amensic agent in mice. Aqueous extract of derived whole plant of O. sanctum L. ameliorated the amensic effect of scopolamine (0.04 mg/kg), diazepam (1 mg/kg) and aging-induced memory deficits in mice. Elevated plus maze and passive avoidance paradigm served as the exteroceptive behavioral models. O. sanctum L. extract decreased transfer latency and increased step-down latency, when compared to control (piracetam-treated), scopolamine and aged groups of mice significantly. So O. sanctum L. preparation could be beneficial in the treatment of cognitive disorders such as dementia and Alzheimer's disease.
Methanolic extract of O. sanctum L. root extract[53] at a dose of 400 mg/kg (ip increases the swimming time of mouse in a despair swim test model, suggesting a central nervous system stimulant and/or anti-stress activity of O. sanctum L.
Antinociceptive (Analgesic)
The analgesic[54] activity of alcoholic leaf extract of O. sanctum L. (50, 100 mg/kg, ip; 50, 100, 200 mg/kg, po) was tested in mice using glacial acetic acid induced writhing test. O. sanctum L. reduced the number of writhes. Osimum sanctum L. (50, 100 mg/kg ip) also increased the tail withdrawal latency in mice.
Anti-fertility
Benzene extract of O. sanctum L. leaves have a reversible anti-fertility[55] effect, as O. sanctum L. extract (250 mg/kg body weight) for 48 days decreases the total sperm count, sperm motility and forward velocity. The percentage of abnormal sperm increased in caudal epididymal fluid and the fructose content decreased in the caudal plasma of the epididymis and the seminal vesicles. All these parameters returned to normal two week after the withdrawal of the treatment.
Anthelmintic activity
The anthelmintic activity[56] of the essential oil from O. sanctum L. was evaluated by Caenorhabditis elegance model. Eugenol exhibited an ED50 of 62.1 μg/ml and being the predominant component of the essential oil, it was suggested as the putative anthelmintic principle.
Antiinflammatory
Compounds isolated from O. sanctum L. extract, Civsilineol, Civsimavatine , Isothymonin, Apigenin, Rosavinic acid and Eugenol were observed for their anti-inflammatory activity[57] or cyclooxygenase inhibitory activity. Eugenol demonstrated 97% cyclooxygenase-1 inhibitory activity when assayed at 1000 μM concentration (pn). Civsilineol, Civsimavitin, Isothymonin, Apigenin and Rosavinic acid displayed 37, 50, 37, 65 and 58% cyclooxygenase-1 inhibitory activity, respectively, when assayed at 1000 μM concentrations. The activities of these compounds were comparable to Ibuprofen, Naproxen and aspirin at 10, 10 and 1000 μM concentrations.
Singh in his study[58] reported that linoleic acid present in different amount in the fixed oil of different species of O. sanctum L. has the capacity to block both the cyclooxygenase and lipoxygenase pathways of arachidonate metabolism and could be responsible for the anti-inflammatory activity.
A methanolic extract[59] and an aqueous suspension of O. sanctum L. (500 mg/kg) inhibited acute as well as chronic inflammation in rats as tested by carrageenin-induced pedal edema and cratonoil -induced granuloma and exudates, respectively, and the response was comparable to the response observed with 300 mg/kg of sodium salicylate. Both the extract and suspension showed analgesic activity in mouse hot plate procedure, and the methanol extract caused an increase in tail withdrawal reaction time of a sub-analgesic dose of morphine. Both preparations reduced typhoid–paratyphoid A–B vaccine-induced pyrexia. The antipyretic action of methanol extract and aqueous suspension was weak and of shorter duration than that of 300 mg/kg sodium salicylate.
Anticancer
Fresh leaf paste (topically) aqueous and ethanolic extract (orally) for their chemopreventive activity against 7,12-dimethylbenzaanthracene (DMBA) induced (0.5%) hamster buccal pouch[60] carcinogenesis. Incidence of papillomas and squamous cell carcinomas were significantly reduced and increased the survival rate in the topically applied leaf paste and orally administered extracts to animals. Histopathological observation made on the mucosa confirmed the profound effect of the orally administered aqueous extract than other.
Prasbar et al. in their study reported that O. sanctum L. leaf extract blocks or suppresses the events associated with chemical carcinogenesis[61] by inhibiting metabolic activation of the carcinogen. In this study, primary cultures of rat hepatocytes were treated with 0–500 μg of O. sanctum L. extract for 24 h and then with 7,12-dimethaylbenz[a] anthracene (DMBA, 10 or 50 μg) for 18 h. Cells were then harvested and their DNA was isolated and analyzed by 32p post-labeling. A significant reduction in the levels of DMBA/DNA adducts was observed in all cultures pretreated with O. sanctum L. extract. Hepatocytes that were treated with the highest dose of extract (500 μg) showed a maximum reduction of 93% in the mean values of DMBA/DNA adducts. This suggests the inhibition of metabolic activation of carcinogen.
The chemopreventive activity[62] of seed oil of O. sanctum L. was evaluated against subsequently injected 20-methyl cholanthrene-induced fibrosarcoma tumors in the thigh region of Swiss albino mice. Supplementation of maximal-tolerated dose (100 μl/kg body wt.) of the oil significantly reduced 20-methaylcholathrene-induced tumor incidence and tumor volume. The enhanced survival rate and delay in tumor incidence was observed in seed oil supplemented mice. Liver enzymatic, non-enzymatic antioxidants and lipid peroxidation end product, malondialdehyde level were significantly modulated with oil treatment as compared to untreated 20-methylcholathrene injected mice. The chemopreventive efficacy of 100 μl/kg seed oil was comparable to that of 80 mg/kg vita-E.
Thyroid activity
The extract of O. sanctum L. leaf extract (OSE) on the changes in the concentrations of serum Triiodothyronine (T3), Thyronine (T4) and serum cholesterol were investigated.[63] OSE at the dose of 0.5 g/kg body weight for 15 days significantly decreased serum T4 concentration; however, no marked changes were observed in serum T3 level, T3/T4 ratio and in the concentration of serum cholesterol. It appears that OSE is antithyroidic in nature.
Miscellaneous activity
Graded dose[64] of 100, 150, 200 and 400 mg/kg of O. sanctum L. leaves extract significantly decreases the sexual behavioral score in adult male Wistar rats. Broadband white noise exposure[65] (100 dB) in Wistar strain male albino rats significantly increased the level of dopamine (DA), serotonin (5-HT) and 5-HT turnover in many of the discrete brain regions during sub-chronic noise exposure (4 h daily, 15 days). In acute (4 h for 1 day) and chronic noise exposures (4 h daily for 30 days) the levels were significantly altered in certain region. The intraperitoneal administration of 70% ethanolic extract of O. sanctum L. at dosage of 100 mg/kg body weight to animals subjected to noise exposure has prevented the noise-induced increase in neurotransmitter levels without affecting the normal levels. This suggests that O. sanctum L. can be a probable herbal remedy for noise-induced biogenic amine alterations. Gupta studied the anticataract effect[66] of O. sanctum L. extract on selenite-induced cataract (25 micromole/kg body weight) in 9 day old rat pups. Osimum sanctum L. (5 and 10 mg/kg body wt.) injected intraperitoneally 4 h prior to selenite challenge reduces the incidence of selenite cataract by 20 and 60%, respectively, and prevented protein insolubilization as well.
Halder et al[67] found aqueous extract of O. sanctum L. as the most effective aldose reductase (AR) inhibitor with a significant inhibition of 38.05% considering the AR activity of normal rat lenses as 100%. The IC50 value was found to be 20 μg/ml.
Ocimum sanctum L. extract (10 mg/kg body wt., PO) before and after mercury (HgCl 2 ) intoxication (5 mg/kg body wt.) showed a significant decrease in lipid peroxidation.[68] Serum glutamate pyruvate transaminase (SGPT) activities compared to HgCl2-induced values suggests that O. sanctum L. extract provides protection against HgCl2-induced toxicity in mice.
Ocimum sanctum L. fixed oil increases blood clotting[69] time and percentage increase was comparable to aspirin and could be due to inhibition of platelet aggregation. The oil also increased pentobarbitone-induced sleeping time in rats indicating probable inhibitory effect of oil towards cytochromic enzyme responsible for hepatic metabolism of pentobarbitone.
Noise stress causes[70] leucopenia, increased corticosterone level and enhances the neutrophil functions as indicated by increase in the candida phagocytosis and nitro blue tetrazolium reduction. Pre-treatment with the O. sanctum L. extract brought back the stress altered values to normal levels indicating the stress alleviating effect of O. sanctum L.
ARE THERE ANY SIDE EFFECTS OR INTERACTIONS?
Two animal studies suggested that large amounts of holy basil might negatively affect fertility[71,72] but no adverse reactions have been reported in human clinical trials. Safety during pregnancy and lactation has not been investigated; until more is known, holy basil should probably be avoided at those times.[73]
CONCLUSION
In recent years there has been a resurgence of interest in investing the traditional health promoting uses of Tulsi. The nutritional and pharmacological properties of the whole herb in its natural form, as it has been traditionally used, may results from synergistic interactions of many different active phytochemicals. Consequently, the overall effects of Tulsi cannot be fully duplicated with isolated compounds or extracts. Because of its inherent botanical and biochemical complexity, Tulsi standardization has, so far, eluded modern science. Although, Tulsi is known as a general vitalizer and increase physical endurance, it contains no caffeine or other stimulant.
In spite of the many impressive accomplishment of western medical science, the typically fragmented approach of modern allopathic medicine has not been able to cope with the growing array of chronic degenerative environmental, lifestyle and personal stress-related disorders that plague modern society. A significant complementary role is emerging for traditional herbal medicines and holistic approaches to health in the prevention and treatment of the passive illness of modern civilization. Recognizing the importance of broadening western medical perspective, the World Health Organization has recommended that traditional health and folk medicine systems be integrated with modern medical therapies to more effectively address health problems worldwide. Substantial evidence has accumulated that, in addition to Tulsi's many specific therapeutic applications, the herb's powerful general adaptogenic properties offer significant preventive and curative potential with respect to the stress-related degenerative diseases endemic to industrialized societies. Ongoing clinical investigation of Tulsi's health promoting qualities is sure to bear rich fruit.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Gordon MC, David JN. Naturan product drug discovery in the next millennium. Pharm Boil. 2001;39:8–17. doi: 10.1076/phbi.39.s1.8.0009. [DOI] [PubMed] [Google Scholar]
- 2.Wink M. Introduction Biochemistry, role and biotechnology of secondary products. In: Wink M, editor. Biochemistry of Secondary product Metabolism. Florida: CRC press, Boca Raton; 2000. pp. 1–16. [Google Scholar]
- 3.Cor JS, Beach JF, Blair A, Clark AJ, King J, Lee TB, et al. Disodium chromoglycate. Adv Drug Res. 1970;5:190–6. [PubMed] [Google Scholar]
- 4.Warrier PK. In: Indian Medicinal Plants. Longman O, editor. New Delhi: CBS publication; 1995. p. 168. [Google Scholar]
- 5.Biswas NP, Biswas AK. Evaluation of some leaf dusts as grain protectant against rice weevil Sitophilus oryzae (Linn.) Environ Ecol. 2005;23:485–8. [Google Scholar]
- 6.Sen P. Therapeutic potential of Tulsi: From experience to facts. Drug News Views. 1993;1:15–21. [Google Scholar]
- 7.Gupta SK, Prakash J, Srivastav S. Validation of traditional claim of Tulsi, Ocimum sanctum Linn. as a medicinal plant. Indian J Exp Biol. 2002;40:765–73. [PubMed] [Google Scholar]
- 8.Chopra RN, Nayer SI, Chopra IC. New Delhi: CSIR; 1956. Glossary of Indian Medicinal plants. [Google Scholar]
- 9.Anbarasu K, Vijayalakshmi G. Improved shelf life of protein-rich tofu using Ocimum sanctum (tulsi) extracts to benefit Indian rural population. J Food Sci. 2007;72:M300–05. doi: 10.1111/j.1750-3841.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- 10.Kelm MA, Nair MG, Strasburg GM, DeWitt DL. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine. 2000;7:7–13. doi: 10.1016/S0944-7113(00)80015-X. [DOI] [PubMed] [Google Scholar]
- 11.Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. Urosolic acidinhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: Correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003;63:4375–83. [PubMed] [Google Scholar]
- 12.Jaggi RK, Madaan R, Singh B. Anticonvulsant potential of holy basil, Ocimum sanctum Linn., and its cultures. Indian J Exp Biol. 2003;41:1329–33. [PubMed] [Google Scholar]
- 13.Uma Devi P, Ganasoundari A, Vrinda B, Srinivasan KK, Unnikrishnan MK. Radiation protection by the Ocimum flavonoids orientin and vicenin: Mechanisms of action. Radiat Res. 2000;154:455–60. doi: 10.1667/0033-7587(2000)154[0455:rpbtof]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Narendhirakannan RT, Subramanian S, Kandaswamy M. Biochemical evaluation of antidiabetogenic properties of some commonly used Indian plants on streptozotocin-induced diabetes in experimental rats. Clin Exp Pharmacol Physiol. 2006;33:1150–7. doi: 10.1111/j.1440-1681.2006.04507.x. [DOI] [PubMed] [Google Scholar]
- 15.Hannan JM, Marenah L, Ali L, Rokeya B, Flatt PR, Abdel-Wahab YH. Ocimum sanctum leaf extracts stimulate insulin secretion from perfusd pancreas, isolated islets and clonal pancreatic beta-cells. J Endocrinol. 2006;189:127–36. doi: 10.1677/joe.1.06615. [DOI] [PubMed] [Google Scholar]
- 16.Grover JK, Vats V, Yadav SS. Pterocarpus marsupium extract (Vijayasar) prevented the alteration in metabolic patterns induced in the normal rat by feeding an adequate diet containing fructose as sole carbohydrate. Diabetes Obes Metab. 2005;7:414–20. doi: 10.1111/j.1463-1326.2005.00414.x. [DOI] [PubMed] [Google Scholar]
- 17.Gholap S, Kar A. Hypoglycemic effects of some plant extracts are possibly mediated through inhibition in corticosteroid concentration. Pharmazie. 2004;59:876–8. [PubMed] [Google Scholar]
- 18.Vats V, Yadav SP, Grover JK. Ethanolic extract of Ocimum sanctum leaves partially attenuates sterptozotocin-induced alterations in glycogen content and carbohydrate metabolism in rats. J Ethnopharmacol. 2004;90:155–60. doi: 10.1016/j.jep.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 19.Rai V, Iyer U, Mani UV. Effect of Tulasi (Ocimum sanctum) leaf power supplementation on blood sugar levels, serum lipids and tissue lipids in diabetic rats. Plant Foods Hum Nutr. 1997;50:9–16. doi: 10.1007/BF02436038. [DOI] [PubMed] [Google Scholar]
- 20.Chattopadhyay RR. Hypoglycemic effect of Ocimum sanctum leaf extract in normal and streptozotocin diabetic rats. Indian J Exp Biol. 1993;31:891–3. [PubMed] [Google Scholar]
- 21.Sood S, Narang D, Thomas MK, Gupta YK, Maulik SK. Effect of Ocimum sanctum Linn.on cardiac changes in rats subjected to chronic restraint stress. J Ethnopharmacol. 2006;108:423–7. doi: 10.1016/j.jep.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Sood S, Narang D, Dinda AK, Maulik SK. Chronic oral administration of Ocimum sanctum Linn.augments cardiac endogenous antioxidants and prevents isoproterenol-induced myocardial necrosis in rats. J Pharm Pharmacol. 2005;57:127–33. doi: 10.1211/0022357055146. [DOI] [PubMed] [Google Scholar]
- 23.Sharm M, Kishore K, Gupta SK, Joshi S, Arya DS. Cardiaprotective potential of Ocimum sanctum Linn in isoproterenol induced myocardial infraction in rats. Mol Cell Biochem. 2001;498:39–46. doi: 10.1023/a:1012220908636. [DOI] [PubMed] [Google Scholar]
- 24.Balanehru S, Nagarajan B. Intervention of adriamycin induced free radical damage. Biochem Int. 1992;28:735–44. [PubMed] [Google Scholar]
- 25.Shetty S, Udupa S, Udupa L, Somayaji N. Wound healing activity of Ocimum sanctum Linn with supportive role of antioxidant enzymes. Indian J Physiol Pharmacol. 2006;50:163–8. [PubMed] [Google Scholar]
- 26.Udupa SL, Shetty S, Udupa AL, Somayaji SN. Effect of Ocimum sanctum Linn.on normal and dexamethasone suppressed wound healing. Indian J Exp Biol. 2006;44:49–54. [PubMed] [Google Scholar]
- 27.Bhartiya US, Raut YS, Joseph LJ. Protective effect of Ocimum sanctum L after high-dose 131iodine exposure in mice: An in vivo study. Indian J Exp Biol. 2006;44:647–52. [PubMed] [Google Scholar]
- 28.Subramanian M, Chintalwar GJ, Chattopadhyay S. Antioxidant and radioprotective properties of an Ocimum sanctum polysaccharide. Redox Rep. 2005;10:257–64. doi: 10.1179/135100005X70206. [DOI] [PubMed] [Google Scholar]
- 29.Vrinda B, Uma Devi P. Radiation protection of human lymphocyte chromosomes in vitro by orientin and vicenin. Mutat Res. 2001;498:39–46. doi: 10.1016/s1383-5718(01)00263-7. [DOI] [PubMed] [Google Scholar]
- 30.Devi PU, Ganasoundari A. Modulation of glutathione and antioxidant enzymes by Ocimum sanctum and its role in protection against radiation injury. Indian J Exp Biol. 1999;37:262–8. [PubMed] [Google Scholar]
- 31.Devi PU, Bisht KS, Vinitha M. A comparative study of radioprotection by Ocimum flavonoids and synthetic aminothiol protectors in the mouse. Br J Radiol. 1998;71:782–4. doi: 10.1259/bjr.71.847.9771390. [DOI] [PubMed] [Google Scholar]
- 32.Ganasoundari A, Devi PU, Rao BS. Enhancement of bone marrow radioprotection and reduction of WR-2721 toxicity by Ocimum sanctum. Mutat Res. 1998;397:303–12. doi: 10.1016/s0027-5107(97)00230-3. [DOI] [PubMed] [Google Scholar]
- 33.Babu K, Uma Maheswari KC. In vivo studies on the effect of Ocimum sanctum L. leaf extract in mordifying the genotoxicity induced by chromium and mercury in Allium root meristems. J Environ Biol. 2006;27:93–5. [PubMed] [Google Scholar]
- 34.Jena GB, Nemmani KV, Kaul CL, Ramarao P. Protective effect of a polyherbal formulation (Immu-21) against cyclophosphamide-induced mutagenicity in mice. Phytother Res. 2003;17:306–10. doi: 10.1002/ptr.1125. [DOI] [PubMed] [Google Scholar]
- 35.Trevisan MT, Vasconcelos Silva MG, Pfundstein B, Spiegelhalder B, Owen RW. Characterization of the volatile pattern and antioxidant capacity of essential oils from different species of the genus Ocimum. J Agric Food Chem. 2006;54:4378–82. doi: 10.1021/jf060181+. [DOI] [PubMed] [Google Scholar]
- 36.Gupta S, Mediratta PK, Singh S, Sharma KK, Shukla R. Antidiabetic, antihypercholesterolaemic and antioxidant effect of Ocimum sanctum (Linn) seed oil. Indian J Exp Biol. 2006;44:300–4. [PubMed] [Google Scholar]
- 37.Geetha RK, Vasudevan DM. Inhibition of lipid peroxidation by botanical extracts of Ocimum sanctum: In vivo and in vitro studies. Life Sci. 2004;76:21–8. doi: 10.1016/j.lfs.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 38.Yanpallewar SU, Rai S, Kumar M, Acharya SB. Evaluation of antioxidant and neuroprotective effect of Ocimum sanctum on transient cerebral ischemia and long-term cerebral hypoperfusion. Pharmacol Biochem Behav. 2004;79:155–64. doi: 10.1016/j.pbb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Sarkar A, Lavania SC, Pandey DN, Pant C. Changes in the blood lipid profile after administration of Ocimum sanctum (Tulsi) leaves in the normal albino rabbits. Indian J Physiol Pharmacol. 1994;38:311–2. [PubMed] [Google Scholar]
- 40.Singh S, Malhotra M, Majumdar DK. Antibacterial activity of Ocimum sanctum L.fixed oil. Indian J Exp Biol. 2005;43:835–7. [PubMed] [Google Scholar]
- 41.Geeta, Vasudevan DM, Kedlaya R, Deepa S, Ballal M. Activity of Ocimum sanctum (the traditional Indian medicinal plant) against the enteric pathogens. (472).Indian J Med Sci. 2001;55:434–8. [PubMed] [Google Scholar]
- 42.Kaul D, Sukla AR, Sikand K, Dhawan V. Effect of herbal polyphenols on artherogenic transcriptome. Mol Cell Biochem. 2005;278:177–84. doi: 10.1007/s11010-005-7497-8. [DOI] [PubMed] [Google Scholar]
- 43.Goel RK, Sairam K, Dorababu M, Prabha T, Rao ChV. Effect of standardized extract of Ocimum sanctum Linn.on gastric mucosal offensive and defensive factors. Indian J Exp Biol. 2005;43:715–21. [PubMed] [Google Scholar]
- 44.Dharmani P, Kuchibhotla VK, Maurya R, Srivastava S, Sharma S, Patil G. Evaluation of anti-ulcerogenic and ulcer-healing properties of Ocimum sanctum Linn. J Ethnophamacol. 2004;93:197–206. doi: 10.1016/j.jep.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 45.Mandal S, Das DN, De K, Ray K, Roy G, Chaudhuri SB, et al. Ocimum sanctum Linn: A study on gastric ulceration and gastric secretion in rats. Indian J Physiol Pharmacol. 1993;37:91–2. [PubMed] [Google Scholar]
- 46.Mukherjee R, Dash PK, Ram GC. Immunotherapeutic potential of Ocimum sanctum (L) in bovine subclinical mastitis. Res Vet Sci. 2005;79:37–43. doi: 10.1016/j.rvsc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Mediratta PK, Sharma KK, Singh S. Evaluation of immunomodulatory potential of Ocimum sanctum seed oil and its possible mechanism of action. J Ethnopharmacol. 2002;80:15–20. doi: 10.1016/s0378-8741(01)00373-7. [DOI] [PubMed] [Google Scholar]
- 48.Godhwani S, Godhwani JL, Vyas DS. Ocimum sanctum: A prelimnary study evaluating its immunoregulatory profile in albino rats. J Ethnopharmacol. 1988;24:193–8. doi: 10.1016/0378-8741(88)90151-1. [DOI] [PubMed] [Google Scholar]
- 49.Shokeen P, Ray K, Bala M, Tondon V. Prelimnary studies on activity of Ocimum sanctum, Drynaria quercifolia, and Annona squamosa against Neisseria gonorrohoeae. Sex Transm Dis. 2005;32:106–11. doi: 10.1097/01.olq.0000152821.23777.90. [DOI] [PubMed] [Google Scholar]
- 50.Jaggi RK, Madaan R, Singh B. Anticonvulsant potential of holy basil, Ocimum sanctum Linn., and its cultures. Indian J Exp Biol. 2003;41:1329–33. [PubMed] [Google Scholar]
- 51.Sakina MR, Dandiya PC, Hamdard ME, Hameed A. Prelimnary psychopharmacological evaluation of Ocimum sanctum leaf extract. J Ethnopharmacol. 1990;28:143–50. doi: 10.1016/0378-8741(90)90023-m. [DOI] [PubMed] [Google Scholar]
- 52.Joshi H, Parle M. Evaluation of nootropic potential of Ocimum sanctum Linn.in mice. Indian J Exp Biol. 2006;44:133–6. [PubMed] [Google Scholar]
- 53.Maity TK, Mandal SC, Saha BP, Pal M. Effect of Ocimum sanctum roots extract on swimming performance in mice. Phytother Res. 2000;14:120–1. doi: 10.1002/(sici)1099-1573(200003)14:2<120::aid-ptr557>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 54.Khanna N, Bhatia J. Antinociceptive action of Ocimum sanctum (Tulsi) in mice: Possible mechanisms involved. J Ethnopharmacol. 2003;88:293–6. doi: 10.1016/s0378-8741(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 55.Ahmed M, Ahamed RN, Aladakatti RH, Ghosesawar MG. Reversible anti-fertility effect of benzene extract of Ocimum sanctum leaves on sperm parameters and fructose content in rats. J Basic Clin Physiol Pharmacol. 2002;13:51–9. doi: 10.1515/jbcpp.2002.13.1.51. [DOI] [PubMed] [Google Scholar]
- 56.Asha MK, Prashanth D, Murali B, Padmaja R, Amit A. Anthelmintic activity of essential oil of Ocimum sanctum and eugenol. Fitoterapia. 2001;72:669–70. doi: 10.1016/s0367-326x(01)00270-2. [DOI] [PubMed] [Google Scholar]
- 57.Kelm MA, Nair MG, Stasburg GM, DeWitt DL. Antioxidant and cyclooxygenase inhibitiory phenolic compounds from Ocimum sanctum Linn. Phytomedicine. 2000;7:7–13. doi: 10.1016/S0944-7113(00)80015-X. [DOI] [PubMed] [Google Scholar]
- 58.Singh S. Comparative evalution of antiinflammatory potential of fixed oil of different species of Ocimum and its possible mechanism of action. Indian J Exp Biol. 1998;36:1028–31. [PubMed] [Google Scholar]
- 59.Godhwani S, Godhwani JL, Vyas DS. Ocimum sanctum: An experimental study evaluating its anti-inflammatory, analgesic and antipyretic activity in animals. J Ethnopharmacol. 1987;21:153–63. doi: 10.1016/0378-8741(87)90125-5. [DOI] [PubMed] [Google Scholar]
- 60.Karthikeyan K, Ravichandran P, Govindasamy S. Chemopreventive effect of Ocimum sanctum on DMBA-induced hamster buccal pouch carcinogenesis. Oral Oncol. 1999;35:112–9. doi: 10.1016/s1368-8375(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 61.Prashar R, Kumar A, Hewer A, Cole KJ, Davis W, Phillips DH. Inhibition by an extract of Ocimum sanctum of DNA-binding activity of 7,12-dimethylbenz[a]anthracene in rat hepatocytes in vitro. Cancer Lett. 1998;128:155–60. doi: 10.1016/s0304-3835(98)00068-8. [DOI] [PubMed] [Google Scholar]
- 62.Prakash J, Gupta SK. Chemopreventive activity of Ocimum sanctum seed oil. J Ethnopharmacol. 2000;72:29–34. doi: 10.1016/s0378-8741(00)00194-x. [DOI] [PubMed] [Google Scholar]
- 63.Panda S, Kar A. Ocimum sanctum leaf extract in the regulation of thyroid function in the male mouse. Pharmacol Res. 1998;38:107–10. doi: 10.1006/phrs.1998.0338. [DOI] [PubMed] [Google Scholar]
- 64.Kantak NM, Gogate MG. Effect of short term administration of Tulsi (Ocimum sanctum Linn.) on reproductive behavior of adult male rats. Indian J Physiol Pharmacol. 1992;36:109–11. [PubMed] [Google Scholar]
- 65.Samson J, Sheela Devi R, Ravindran R, Senthilvelan M. Biogenic amine changes in brain regions and attenuating action of Ocimum sanctumin noise exposure. Pharmacol Biochem Behav. 2006;83:67–75. doi: 10.1016/j.pbb.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 66.Gupta SK, Srivastava S, Trivedi D, Joshi S, Halder N. Ocimum sanctum modulates selenite-induced cataractogenic changes and prevents rat lens opaciification. Curr Eye Res. 2005;30:583–91. doi: 10.1080/02713680590968132. [DOI] [PubMed] [Google Scholar]
- 67.Halder N, Joshi S, Gupta SK. Lens aldose reductase inhibiting potential of some indigenous plants. J Ethnopharmacol. 2003;86:113–6. doi: 10.1016/s0378-8741(03)00052-7. [DOI] [PubMed] [Google Scholar]
- 68.Sharma MK, Kumar M, Kumar A. Ocimum sanctum aqueous leaf extract provides protection against mercury induced toxicity in Swiss albino mice. Indian J Exp Biol. 2002;40:1079–82. [PubMed] [Google Scholar]
- 69.Singh S, Rehan HM, Majumdar DK. Effect of Ocimum sanctum fixed oil on blood pressure, blood clotting time and pentobarbitone-induced sleeping time. J Ethnopharmacol. 2001;78:139–43. doi: 10.1016/s0378-8741(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 70.Archana R, Namasivayam A. Effect of Ocimum sanctum on noise induced changes in neutrophil functions. J Ethnopharmacol. 2000;73:81–5. doi: 10.1016/s0378-8741(00)00281-6. [DOI] [PubMed] [Google Scholar]
- 71.Seth SD, Johri N, Sundaram KR. Antispermatogenic effect of Ocimum sanctum. Indian J Exp Biol. 1981;19:975–6. [PubMed] [Google Scholar]
- 72.Kasinathan S, Ramakrishnan S, Basu SL. Antifertility effect of Ocimum sanctum L. Indian J Exp Biol. 1972;10:23–5. [PubMed] [Google Scholar]
- 73.Brinker F. NewYork: Eclectic Medical Publications; 1998. Herb Contraindications and Drug Interactions; p. 33. [Google Scholar]


