Abstract
In recent years, there has been a great deal of attention toward the field of free radical chemistry. Free radicals reactive oxygen species and reactive nitrogen species are generated by our body by various endogenous systems, exposure to different physiochemical conditions or pathological states. A balance between free radicals and antioxidants is necessary for proper physiological function. If free radicals overwhelm the body's ability to regulate them, a condition known as oxidative stress ensues. Free radicals thus adversely alter lipids, proteins, and DNA and trigger a number of human diseases. Hence application of external source of antioxidants can assist in coping this oxidative stress. Synthetic antioxidants such as butylated hydroxytoluene and butylated hydroxyanisole have recently been reported to be dangerous for human health. Thus, the search for effective, nontoxic natural compounds with antioxidative activity has been intensified in recent years. The present review provides a brief overview on oxidative stress mediated cellular damages and role of dietary antioxidants as functional foods in the management of human diseases.
Keywords: Ageing, antioxidant, free radicals, oxidative stress
INTRODUCTION
The recent growth in the knowledge of free radicals and reactive oxygen species (ROS) in biology is producing a medical revolution that promises a new age of health and disease management.[1] It is ironic that oxygen, an element indispensable for life,[2] under certain situations has deleterious effects on the human body.[3] Most of the potentially harmful effects of oxygen are due to the formation and activity of a number of chemical compounds, known as ROS, which have a tendency to donate oxygen to other substances. Free radicals and antioxidants have become commonly used terms in modern discussions of disease mechanisms.[4]
FREE RADICALS
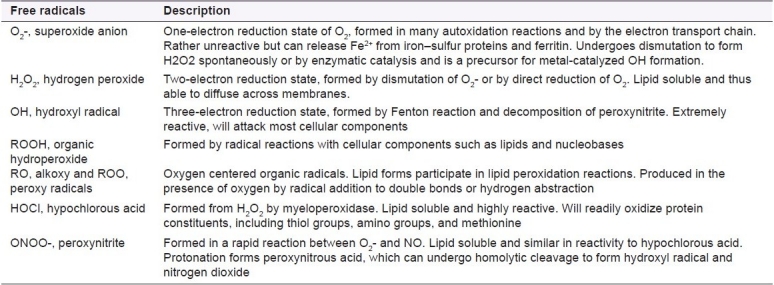
A free radical can be defined as any molecular species capable of independent existence that contains an unpaired electron in an atomic orbital. The presence of an unpaired electron results in certain common properties that are shared by most radicals. Many radicals are unstable and highly reactive. They can either donate an electron to or accept an electron from other molecules, therefore behaving as oxidants or reductants.[5] The most important oxygen-containing free radicals in many disease states are hydroxyl radical, superoxide anion radical, hydrogen peroxide, oxygen singlet, hypochlorite, nitric oxide radical, and peroxynitrite radical. These are highly reactive species, capable in the nucleus, and in the membranes of cells of damaging biologically relevant molecules such as DNA, proteins, carbohydrates, and lipids.[6] Free radicals attack important macromolecules leading to cell damage and homeostatic disruption. Targets of free radicals include all kinds of molecules in the body. Among them, lipids, nucleic acids, and proteins are the major targets.
Production of free radicals in the human body
Free radicals and other ROS are derived either from normal essential metabolic processes in the human body or from external sources such as exposure to X-rays, ozone, cigarette smoking, air pollutants, and industrial chemicals.[3] Free radical formation occurs continuously in the cells as a consequence of both enzymatic and nonenzymatic reactions. Enzymatic reactions, which serve as source of free radicals, include those involved in the respiratory chain, in phagocytosis, in prostaglandin synthesis, and in the cytochrome P-450 system.[7] Free radicals can also be formed in nonenzymatic reactions of oxygen with organic compounds as well as those initiated by ionizing reactions.
Some internally generated sources of free radicals are[8]
Mitochondria
Xanthine oxidase
Peroxisomes
Inflammation
Phagocytosis
Arachidonate pathways
Exercise
Ischemia/reperfusion injury
Some externally generated sources of free radicals are:
Cigarette smoke
Environmental pollutants
Radiation
Certain drugs, pesticides
Industrial solvents
Ozone
Free radicals in biology
Free radical reactions are expected to produce progressive adverse changes that accumulate with age throughout the body [Table 1]. Such “normal” changes with age are relatively common to all. However, superimposed on this common pattern are patterns influenced by genetics and environmental differences that modulate free radical damage. These are manifested as diseases at certain ages determined by genetic and environmental factors. Cancer and atherosclerosis, two major causes of death, are salient “free radical” diseases. Cancer initiation and promotion is associated with chromosomal defects and oncogene activation. It is possible that endogenous free radical reactions, like those initiated by ionizing radiation, may result in tumor formation. The highly significant correlation between consumption of fats and oils and death rates from leukemia and malignant neoplasia of the breast, ovaries, and rectum among persons over 55 years may be a reflection of greater lipid peroxidation.[9] Studies on atherosclerosis reveal the probability that the disease may be due to free radical reactions involving diet-derived lipids in the arterial wall and serum to yield peroxides and other substances. These compounds induce endothelial cell injury and produce changes in the arterial walls.[10]
Table 1.
CONCEPT OF OXIDATIVE STRESS
The term is used to describe the condition of oxidative damage resulting when the critical balance between free radical generation and antioxidant defenses is unfavorable.[14] Oxidative stress, arising as a result of an imbalance between free radical production and antioxidant defenses, is associated with damage to a wide range of molecular species including lipids, proteins, and nucleic acids.[15] Short-term oxidative stress may occur in tissues injured by trauma, infection, heat injury, hypertoxia, toxins, and excessive exercise. These injured tissues produce increased radical generating enzymes (e.g., xanthine oxidase, lipogenase, cyclooxygenase) activation of phagocytes, release of free iron, copper ions, or a disruption of the electron transport chains of oxidative phosphorylation, producing excess ROS. The initiation, promotion, and progression of cancer, as well as the side-effects of radiation and chemotherapy, have been linked to the imbalance between ROS and the antioxidant defense system. ROS have been implicated in the induction and complications of diabetes mellitus, age-related eye disease, and neurodegenerative diseases such as Parkinson's disease.[16]
Oxidative stress and human diseases
A role of oxidative stress has been postulated in many conditions, including anthersclerosis, inflammatory condition, certain cancers, and the process of aging. Oxidative stress is now thought to make a significant contribution to all inflammatory diseases (arthritis, vasculitis, glomerulonephritis, lupus erythematous, adult respiratory diseases syndrome), ischemic diseases (heart diseases, stroke, intestinal ischema), hemochromatosis, acquired immunodeficiency syndrome, emphysema, organ transplantation, gastric ulcers, hypertension and preeclampsia, neurological disorder (Alzheimer's disease, Parkinson's disease, muscular dystrophy), alcoholism, smoking-related diseases, and many others.[17] An excess of oxidative stress can lead to the oxidation of lipids and proteins, which is associated with changes in their structure and functions.
Cardiovascular diseases
Heart diseases continue to be the biggest killer, responsible for about half of all the deaths. The oxidative events may affect cardiovascular diseases therefore; it has potential to provide enormous benefits to the health and lifespan. Poly unsaturated fatty acids occur as a major part of the low density lipoproteins (LDL) in blood and oxidation of these lipid components in LDL play a vital role in atherosclerosis.[18] The three most important cell types in the vessel wall are endothelial cells; smooth muscle cell and macrophage can release free radical, which affect lipid peroxidation.[19] With continued high level of oxidized lipids, blood vessel damage to the reaction process continues and can lead to generation of foam cells and plaque the symptoms of atherosclerosis. Oxidized LDL is antherogenic and is thought to be important in the formation of anthersclerosis plaques. Furthermore, oxidized LDL is cytotoxic and can directly damage endothelial cells. Antioxidants like B-carotene or vitamin E play a vital role in the prevention of various cardiovascular diseases.
Carcinogenesis
Reactive oxygen and nitrogen species, such as super oxide anion, hydrogen peroxide, hydroxyl radical, and nitric oxide and their biological metabolites also play an important role in carcinogenesis. ROS induce DNA damage, as the reaction of free radicals with DNA includes strand break base modification and DNA protein cross-links. Numerous investigators have proposed participation of free radicals in carcinogenesis, mutation, and transformation; it is clear that their presence in biosystem could lead to mutation, transformation, and ultimately cancer. Induction of mutagenesis, the best known of the biological effect of radiation, occurs mainly through damage of DNA by the HO. Radical and other species are produced by the radiolysis, and also by direct radiation effect on DNA, the reaction effects on DNA. The reaction of HO. Radicals is mainly addition to double bond of pyrimidine bases and abstraction of hydrogen from the sugar moiety resulting in chain reaction of DNA. These effects cause cell mutagenesis and carcinogenesis lipid peroxides are also responsible for the activation of carcinogens.
Antioxidants can decrease oxidative stress induced carcinogenesis by a direct scavenging of ROS and/or by inhibiting cell proliferation secondary to the protein phosphorylation. B-carotene may be protective against cancer through its antioxidant function, because oxidative products can cause genetic damage. Thus, the photo protective properties of B-carotene may protect against ultraviolet light induced carcinogenesis. Immunoenhancement of B-carotene may contribute to cancer protection. B-carotene may also have anticarcinogenic effect by altering the liver metabolism effects of carcinogens.[20] Vitamin C may be helpful in preventing cancer.[21] The possible mechanisms by which vitamin C may affect carcinogenesis include antioxidant effects, blocking of formation of nitrosanimes, enhancement of the immune response, and acceleration of detoxification of liver enzymes. Vitamin E, an important antioxidant, plays a role in immunocompetence by increasing humoral antibody protection, resistance to bacterial infections, cell-mediated immunity, the T-lymphocytes tumor necrosis factor production, inhibition of mutagen formation, repair of membranes in DNA, and blocking micro cell line formation.[22] Hence vitamin E may be useful in cancer prevention and inhibit carcinogenesis by the stimulation of the immune system. The administration of a mixture of the above three antioxidant reveled the highest reduction in risk of developing cardiac cancer.
Free radical and aging
The human body is in constant battle to keep from aging. Research suggests that free radical damage to cells leads to the pathological changes associated with aging.[23] An increasing number of diseases or disorders, as well as aging process itself, demonstrate link either directly or indirectly to these reactive and potentially destructive molecules.[24] The major mechanism of aging attributes to DNA or the accumulation of cellular and functional damage.[25] Reduction of free radicals or decreasing their rate of production may delay aging. Some of the nutritional antioxidants will retard the aging process and prevent disease. Based on these studies, it appears that increased oxidative stress commonly occurs during the aging process, and antioxidant status may significantly influence the effects of oxidative damage associated with advancing age. Research suggests that free radicals have a significant influence on aging, that free radical damage can be controlled with adequate antioxidant defense, and that optimal intake of antioxidant nutrient may contribute to enhanced quality of life. Recent research indicates that antioxidant may even positively influence life span.
Oxidative damage to protein and DNA
Oxidative damage to protein
Proteins can be oxidatively modified in three ways: oxidative modification of specific amino acid, free radical mediated peptide cleavage, and formation of protein cross-linkage due to reaction with lipid peroxidation products. Protein containing amino acids such as methionine, cystein, arginine, and histidine seem to be the most vulnerable to oxidation.[26] Free radical mediated protein modification increases susceptibility to enzyme proteolysis. Oxidative damage to protein products may affect the activity of enzymes, receptors, and membrane transport. Oxidatively damaged protein products may contain very reactive groups that may contribute to damage to membrane and many cellular functions. Peroxyl radical is usually considered to be free radical species for the oxidation of proteins. ROS can damage proteins and produce carbonyls and other amino acids modification including formation of methionine sulfoxide and protein carbonyls and other amino acids modification including formation of methionine sulfoxide and protein peroxide. Protein oxidation affects the alteration of signal transduction mechanism, enzyme activity, heat stability, and proteolysis susceptibility, which leads to aging.
Lipid peroxidation
Oxidative stress and oxidative modification of biomolecules are involved in a number of physiological and pathophysiological processes such as aging, artheroscleosis, inflammation and carcinogenesis, and drug toxicity. Lipid peroxidation is a free radical process involving a source of secondary free radical, which further can act as second messenger or can directly react with other biomolecule, enhancing biochemical lesions. Lipid peroxidation occurs on polysaturated fatty acid located on the cell membranes and it further proceeds with radical chain reaction. Hydroxyl radical is thought to initiate ROS and remove hydrogen atom, thus producing lipid radical and further converted into diene conjugate. Further, by addition of oxygen it forms peroxyl radical; this highly reactive radical attacks another fatty acid forming lipid hydroperoxide (LOOH) and a new radical. Thus lipid peroxidation is propagated. Due to lipid peroxidation, a number of compounds are formed, for example, alkanes, malanoaldehyde, and isoprotanes. These compounds are used as markers in lipid peroxidation assay and have been verified in many diseases such as neurogenerative diseases, ischemic reperfusion injury, and diabetes.[27]
Oxidative damage to DNA
Many experiments clearly provide evidences that DNA and RNA are susceptible to oxidative damage. It has been reported that especially in aging and cancer, DNA is considered as a major target.[28] Oxidative nucleotide as glycol, dTG, and 8-hydroxy-2-deoxyguanosine is found to be increased during oxidative damage to DNA under UV radiation or free radical damage. It has been reported that mitochondrial DNA are more susceptible to oxidative damage that have role in many diseases including cancer. It has been suggested that 8-hydroxy-2-deoxyguanosine can be used as biological marker for oxidative stress.[29]
ANTIOXIDANTS
An antioxidant is a molecule stable enough to donate an electron to a rampaging free radical and neutralize it, thus reducing its capacity to damage. These antioxidants delay or inhibit cellular damage mainly through their free radical scavenging property.[30] These low-molecular-weight antioxidants can safely interact with free radicals and terminate the chain reaction before vital molecules are damaged. Some of such antioxidants, including glutathione, ubiquinol, and uric acid, are produced during normal metabolism in the body.[31] Other lighter antioxidants are found in the diet. Although there are several enzymes system within the body that scavenge free radicals, the principle micronutrient (vitamins) antioxidants are vitamin E (α-tocopherol), vitamin C (ascorbic acid), and B-carotene.[32] The body cannot manufacture these micronutrients, so they must be supplied in the diet.
History
The term antioxidant originally was used to refer specifically to a chemical that prevented the consumption of oxygen. In the late 19th and early 20th century, extensive study was devoted to the uses of antioxidants in important industrial processes, such as the prevention of metal corrosion, the vulcanization of rubber, and the polymerization of fuels in the fouling of internal combustion engines.[33]
Early research on the role of antioxidants in biology focused on their use in preventing the oxidation of unsaturated fats, which is the cause of rancidity.[34] Antioxidant activity could be measured simply by placing the fat in a closed container with oxygen and measuring the rate of oxygen consumption. However, it was the identification of vitamins A, C, and E as antioxidants that revolutionized the field and led to the realization of the importance of antioxidants in the biochemistry of living organisms.[35,36] The possible mechanisms of action of antioxidants were first explored when it was recognized that a substance with antioxidative activity is likely to be one that is itself readily oxidized.[37] Research into how vitamin E prevents the process of lipid peroxidation led to the identification of antioxidants as reducing agents that prevent oxidative reactions, often by scavenging ROS before they can damage cells.[38]
Antioxidant defense system
Antioxidants act as radical scavenger, hydrogen donor, electron donor, peroxide decomposer, singlet oxygen quencher, enzyme inhibitor, synergist, and metal-chelating agents. Both enzymatic and nonenzymatic antioxidants exist in the intracellular and extracellular environment to detoxify ROS.[39]
Mechanism of action of antioxidants
Two principle mechanisms of action have been proposed for antioxidants.[40] The first is a chain- breaking mechanism by which the primary antioxidant donates an electron to the free radical present in the systems. The second mechanism involves removal of ROS/reactive nitrogen species initiators (secondary antioxidants) by quenching chain-initiating catalyst. Antioxidants may exert their effect on biological systems by different mechanisms including electron donation, metal ion chelation, co-antioxidants, or by gene expression regulation.[41]
Levels of antioxidant action
The antioxidants acting in the defense systems act at different levels such as preventive, radical scavenging, repair and de novo, and the fourth line of defense, i.e., the adaptation.
The first line of defense is the preventive antioxidants, which suppress the formation of free radicals. Although the precise mechanism and site of radical formation in vivo are not well elucidated yet, the metal-induced decompositions of hydroperoxides and hydrogen peroxide must be one of the important sources. To suppress such reactions, some antioxidants reduce hydroperoxides and hydrogen peroxide beforehand to alcohols and water, respectively, without generation of free radicals and some proteins sequester metal ions.
Glutathione peroxidase, glutathione-s-transferase, phospholipid hydroperoxide glutathione peroxidase (PHGPX), and peroxidase are known to decompose lipid hydroperoxides to corresponding alcohols. PHGPX is unique in that it can reduce hydroperoxides of phospholipids integrated into biomembranes. Glutathione peroxidase and catalase reduce hydrogen peroxide to water.
The second line of defense is the antioxidants that scavenge the active radicals to suppress chain initiation and/or break the chain propagation reactions. Various endogenous radical-scavenging antioxidants are known: some are hydrophilic and others are lipophilic. Vitamin C, uric acid, bilirubin, albumin, and thiols are hydrophilic, radical-scavenging antioxidants, while vitamin E and ubiquinol are lipophilic radical-scavenging antioxidants. Vitamin E is accepted as the most potent radical-scavenging lipophilic antioxidant.
The third line of defense is the repair and de novo antioxidants. The proteolytic enzymes, proteinases, proteases, and peptidases, present in the cytosol and in the mitochondria of mammalian cells, recognize, degrade, and remove oxidatively modified proteins and prevent the accumulation of oxidized proteins.
The DNA repair systems also play an important role in the total defense system against oxidative damage. Various kinds of enzymes such as glycosylases and nucleases, which repair the damaged DNA, are known.
There is another important function called adaptation where the signal for the production and reactions of free radicals induces formation and transport of the appropriate antioxidant to the right site.[42]
ENZYMATIC
Types of antioxidants
Cells are protected against oxidative stress by an interacting network of antioxidant enzymes.[43] Here, the superoxide released by processes such as oxidative phosphorylation is first converted to hydrogen peroxide and then further reduced to give water. This detoxification pathway is the result of multiple enzymes, with superoxide dismutases catalyzing the first step and then catalases and various peroxidases removing hydrogen peroxide.[44]
Superoxide dismutase
Superoxide dismutases (SODs) are a class of closely related enzymes that catalyze the breakdown of the superoxide anion into oxygen and hydrogen peroxide.[45,46] SOD enzymes are present in almost all aerobic cells and in extracellular fluids.[47] There are three major families of superoxide dismutase, depending on the metal cofactor: Cu/Zn (which binds both copper and zinc), Fe and Mn types (which bind either iron or manganese), and finally the Ni type which binds nickel.[48] In higher plants, SOD isozymes have been localized in different cell compartments. Mn-SOD is present in mitochondria and peroxisomes. Fe-SOD has been found mainly in chloroplasts but has also been detected in peroxisomes, and CuZn-SOD has been localized in cytosol, chloroplasts, peroxisomes, and apoplast.[48–50]
In humans (as in all other mammals and most chordates), three forms of superoxide dismutase are present. SOD1 is located in the cytoplasm, SOD2 in the mitochondria, and SOD3 is extracellular. The first is a dimer (consists of two units), while the others are tetramers (four subunits). SOD1 and SOD3 contain copper and zinc, while SOD2 has manganese in its reactive center.[51]
Catalase
Catalase is a common enzyme found in nearly all living organisms, which are exposed to oxygen, where it functions to catalyze the decomposition of hydrogen peroxide to water and oxygen.[52] Hydrogen peroxide is a harmful by-product of many normal metabolic processes: to prevent damage, it must be quickly converted into other, less dangerous substances. To this end, catalase is frequently used by cells to rapidly catalyze the decomposition of hydrogen peroxide into less reactive gaseous oxygen and water molecules.[53] All known animals use catalase in every organ, with particularly high concentrations occurring in the liver.[54]
Glutathione systems
The glutathione system includes glutathione, glutathione reductase, glutathione peroxidases, and glutathione S-transferases. This system is found in animals, plants, and microorganisms.[55] Glutathione peroxidase is an enzyme containing four selenium-cofactors that catalyze the breakdown of hydrogen peroxide and organic hydroperoxides. There are at least four different glutathione peroxidase isozymes in animals.[56] Glutathione peroxidase 1 is the most abundant and is a very efficient scavenger of hydrogen peroxide, while glutathione peroxidase 4 is most active with lipid hydroperoxides. The glutathione S-transferases show high activity with lipid peroxides. These enzymes are at particularly high levels in the liver and also serve in detoxification metabolism.[57]
NONENZYMATIC
Ascorbic acid
Ascorbic acid or “vitamin C” is a monosaccharide antioxidantfound in both animals and plants. As it cannot be synthesized in humans and must be obtained from the diet, it is a vitamin.[58] Most other animals are able to produce this compound in their bodies and do not require it in their diets. In cells, it is maintained in its reduced form by reaction with glutathione, which can be catalyzed by protein disulfide isomerase and glutaredoxins.[59] Ascorbic acid is a reducing agent and can reduce and thereby neutralize ROS such as hydrogen peroxide.[60] In addition to its direct antioxidant effects, ascorbic acid is also a substrate for the antioxidant enzyme ascorbate peroxidase, a function that is particularly important in stress resistance in plants.[61]
Glutathione
Glutathione is a cysteine-containing peptide found in mostforms of aerobic life.[62] It is not required in the diet and is instead synthesized in cells from its constituent amino acids. Glutathione has antioxidant properties since the thiol group in its cysteine moiety is a reducing agent and can be reversibly oxidized and reduced. In cells, glutathione is maintained in the reduced form by the enzyme glutathione reductase and in turn reduces other metabolites and enzyme systems as well as reacting directly with oxidants.[63] Due to its high concentration and central role in maintaining the cell's redox state, glutathione is one of the most important cellular antioxidants.[33] In some organisms, glutathione is replaced by other thiols, such as by mycothiol in the actinomycetes, or by trypanothione in the kinetoplastids.[64]
Melatonin
Melatonin, also known chemically as N-acetyl-5-methoxytryptamine,[65] is a naturally occurring hormone found in animals and in some other living organisms, including algae.[66] Melatonin is a powerful antioxidant that can easily cross cell membranes and the blood–brain barrier.[67] Unlike other antioxidants, melatonin does not undergo redox cycling, which is the ability of a molecule to undergo repeated reduction and oxidation. Melatonin, once oxidized, cannot be reduced to its former state because it forms several stable end-products upon reacting with free radicals. Therefore, it has been referred to as a terminal (or suicidal) antioxidant.[68]
Tocopherols and tocotrienols (Vitamin E)
Vitamin E is the collective name for a set of eight related tocopherols and tocotrienols, which are fat-soluble vitamins with antioxidant properties.[69] Of these, α-tocopherol has been most studied as it has the highest bioavailability, with the body preferentially absorbing and metabolizing this form.[70] It has been claimed that the α-tocopherol form is the most important lipid-soluble antioxidant, and that it protects membranes from oxidation by reacting with lipid radicals produced in the lipid peroxidation chain reaction.[71] This removes the free radical intermediates and prevents the propagation reaction from continuing. This reaction produces oxidized α-tocopheroxyl radicals that can be recycled back to the active reduced form through reduction by other antioxidants, such as ascorbate, retinol, or ubiquinol.[72]
Uric acid
Uric acid accounts for roughly half the antioxidant ability of plasma. In fact, uric acid may have substituted for ascorbate in human evolution.[73] However, like ascorbate, uric acid can also mediate the production of active oxygen species.
PLANTS AS SOURCE OF ANTIOXIDANTS
Synthetic and natural food antioxidants are used routinely in foods and medicine especially those containing oils and fats to protect the food against oxidation. There are a number of synthetic phenolic antioxidants, butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) being prominent examples. These compounds have been widely uses as antioxidants in food industry, cosmetics, and therapeutic industry. However, some physical properties of BHT and BHA such as their high volatility and instability at elevated temperature, strict legislation on the use of synthetic food additives, carcinogenic nature of some synthetic antioxidants, and consumer preferences have shifted the attention of manufacturers from synthetic to natural antioxidants.[74] In view of increasing risk factors of human to various deadly diseases, there has been a global trend toward the use of natural substance present in medicinal plants and dietary plats as therapeutic antioxidants. It has been reported that there is an inverse relationship between the dietary intake of antioxidant-rich food and medicinal plants and incidence of human diseases. The use of natural antioxidants in food, cosmetic, and therapeutic industry would be promising alternative for synthetic antioxidants in respect of low cost, highly compatible with dietary intake and no harmful effects inside the human body. Many antioxidant compounds, naturally occurring in plant sources have been identified as free radical or active oxygen scavengers.[75] Attempts have been made to study the antioxidant potential of a wide variety of vegetables like potato, spinach, tomatoes, and legumes.[76] There are several reports showing antioxidant potential of fruits.[77] Strong antioxidants activities have been found in berries, cherries, citrus, prunes, and olives. Green and black teas have been extensively studied in the recent past for antioxidant properties since they contain up to 30% of the dry weight as phenolic compounds.[78]
Apart from the dietary sources, Indian medicinal plants also provide antioxidants and these include (with common/ayurvedic names in brackets) Acacia catechu (kair), Aegle marmelos (Bengal quince, Bel), Allium cepa (Onion), A. sativum (Garlic, Lahasuna), Aleo vera (Indain aloe, Ghritkumari), Amomum subulatum (Greater cardamom, Bari elachi), Andrographis paniculata (Kiryat), Asparagus recemosus (Shatavari), Azadirachta indica (Neem, Nimba), Bacopa monniera (Brahmi), Butea monosperma (Palas, Dhak), Camellia sinensis (Green tea), Cinnamomum verum (Cinnamon), Cinnamomum tamala (Tejpat), Curcma longa (Turmeric, Haridra), Emblica officinalis (Inhian gooseberry, Amlaki), Glycyrrhiza glapra (Yashtimudhu), Hemidesmus indicus (Indian Sarasparilla, Anantamul), Indigofera tinctoria, Mangifera indica (Mango, Amra), Momordica charantia (Bitter gourd), Murraya koenigii (Curry leaf), Nigella sativa (Black cumin), Ocimum sanctum (Holy basil, Tusil), Onosma echioides (Ratanjyot), Picrorrhiza kurroa (Katuka), Piper beetle, Plumbago zeylancia (Chitrak), Sesamum indicum, Sida cordifolia,Spirulina fusiformis (Alga), Swertia decursata, Syzigium cumini (Jamun), Terminalia ariuna (Arjun), Terminalia bellarica (Beheda), Tinospora cordifolia (Heart leaved moonseed, Guduchi), Trigonella foenum-graecium (Fenugreek), Withania somifera (Winter cherry, Ashwangandha), and Zingiber officinalis (Ginger).[79]
ANTIOXIDANT POTENTIAL OF INDIAN FUNCTIONAL FOODS
Concepts of functional foods and nutraceuticals
In the last decade, preventive medicine has undergone a great advance, especially in developed countries. Research has demonstrated that nutrition plays a crucial role in the prevention of chronic diseases, as most of them can be related to diet. Functional food enters the concept of considering food not only necessary for living but also as a source of mental and physical well-being, contributing to the prevention and reduction of risk factors for several diseases or enhancing certain physiological functions.[80] A food can be regarded as functional if it is satisfactorily demonstrated to affect beneficially one or more target functions in the body, beyond adequate nutritional effects, in a way which is relevant to either the state of well being and health or reduction of the risk of a disease. The beneficial effects could be either maintenance or promotion of a state of well being or health and/or a reduction of risk of a pathologic process or a disease.[81] Whole foods represent the simplest example of functional food. Broccoli, carrots, and tomatoes are considered functional foods because of their high contents of physiologically active components (sulforaphen, B-carotene, and lycopene, respectively). Green vegetables and spices like mustard and turmeric, used extensively in Indian cuisine, also can fall under this category.[82] “Nutraceutical” is a term coined in 1979 by Stephen DeFelice.[83] It is defined “as a food or parts of food that provide medical or health benefits, including the prevention and treatment of disease.” Nutraceuticals may range from isolated nutrients, dietary supplements, and diets to genetically engineered “designer” food, herbal products, and processed products such as cereals, soups, and beverages. A nutraceutical is any nontoxic food extract supplement that has scientifically proven health benefits for both the treatment and prevention of disease.[84] The increasing interest in nutraceuticals reflects the fact that consumers hear about epidemiological studies indicating that a specific diet or component of the diet is associated with a lower risk for a certain disease. The major active nutraceutical ingredients in plants are flavonoids. As is typical for phenolic compounds, they can act as potent antioxidants and metal chelators. They also have long been recognized to possess anti-inflammatory, antiallergic, hepatoprotective, antithrombotic, antiviral, and anticarcinogenic activities.[85]
Indian dietary and medicinal plants as functional foods
Ingredients that make food functional are dietary fibers, vitamins, minerals, antioxidants, oligosaccharides, essential fatty acids (omega-3), lactic acid bacteria cultures, and lignins. Many of these are present in medicinal plants. Indian systems of medicine believe that complex diseases can be treated with complex combination of botanicals unlike in west, with single drugs. Whole foods are hence used in India as functional foods rather than supplements. Some medicinal plants and dietary constituents having functional attributes are spices such as onion, garlic, mustard, red chilies, turmeric, clove, cinnamon, saffron, curry leaf, fenugreek, and ginger. Some herbs as Bixa orellana and vegetables like amla, wheat grass, soyabean, and Gracinia cambogia have antitumor effects. Other medicinal plants with functional properties include A.marmelos, A. cepa, Aloe vera, A. paniculata, Azadirachta india, and Brassica juncea.[86]
CONCLUSION
Free radicals damage contributes to the etiology of many chronic health problems such as cardiovascular and inflammatory disease, cataract, and cancer. Antioxidants prevent free radical induced tissue damage by preventing the formation of radicals, scavenging them, or by promoting their decomposition. Synthetic antioxidants are recently reported to be dangerous to human health. Thus the search for effective, nontoxic natural compounds with antioxidative activity has been intensified in recent years. In addition to endogenous antioxidant defense systems, consumption of dietary and plant-derived antioxidants appears to be a suitable alternative. Dietary and other components of plants form a major source of antioxidants. The traditional Indian diet, spices, and medicinal plants are rich sources of natural antioxidants; higher intake of foods with functional attributes including high level of antioxidants in antioxidants in functional foods is one strategy that is gaining importance.
Newer approaches utilizing collaborative research and modern technology in combination with established traditional health principles will yield dividends in near future in improving health, especially among people who do not have access to the use of costlier western systems of medicine.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Aruoma OI. Methodological consideration for characterization for potential antioxidant actions of bioactive components in plants foods. Mutat Res. 2003;532:9–20. doi: 10.1016/s0027-5107(02)00317-2. [DOI] [PubMed] [Google Scholar]
- 2.Mohammed AA, Ibrahim AA. Pathological roles of reactive oxygen species and their defence mechanism. Saudi Pharm J. 2004;12:1–18. [Google Scholar]
- 3.Bagchi K, Puri S. Free radicals and antioxidants in health and disease. East Mediterranean Health Jr. 1998;4:350–60. [Google Scholar]
- 4.Aruoma OI. Nutrition and health aspects of free radicals and antioxidants. Food Chem Toxicol. 1994;32:671–83. doi: 10.1016/0278-6915(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 5.Cheeseman KH, Slater TF. An introduction to free radicals chemistry. Br Med Bull. 1993;49:481–93. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- 6.Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol. 2001;54:176–86. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu T, Stern A, Roberts LJ. The isoprostanes: Novel prostanglandin-like products of the free radical catalyzed peroxidation of arachidonic acid. J Biomed Sci. 1999;6:226–35. doi: 10.1007/BF02253564. [DOI] [PubMed] [Google Scholar]
- 8.Ebadi M. Antioxidants and free radicals in health and disease: An introduction to reactive oxygen species, oxidative injury, neuronal cell death and therapy in neurodegenerative diseases. Arizona: Prominent Press; 2001. [Google Scholar]
- 9.Lea AJ. Dietary factors associated with death rates from certain neoplasms in man. Lancet. 1966;2:332–3. doi: 10.1016/s0140-6736(66)92615-8. [DOI] [PubMed] [Google Scholar]
- 10.Harman D. Role of free radicals in aging and disease. Ann N Y Acad Sci. 1992;673:126–41. doi: 10.1111/j.1749-6632.1992.tb27444.x. [DOI] [PubMed] [Google Scholar]
- 11.Sies H. Oxidative stress: Introductory remarks. In: Sies H, editor. Oxidative Stress. San Diego: Academic Press; 1985. pp. 1–7. [Google Scholar]
- 12.Docampo R. Antioxidant mechanisms. In: Marr J, Müller M, editors. Biochemistry and Molecular Biology of Parasites. London: Academic Press; 1995. pp. 147–60. [Google Scholar]
- 13.Rice-Evans CA, Gopinathan V. Oxygen toxicity, free radicals and antioxidants in human disease: Biochemical implications in atherosclerosis and the problems of premature neonates. Essays Biochem. 1995;29:39–63. [PubMed] [Google Scholar]
- 14.Rock CL, Jacob RA, Bowen PE. Update o biological characteristics of the antioxidant micronutrients- Vitamin C, Vitamin E and the carotenoids. J Am Diet Assoc. 1996;96:693–702. doi: 10.1016/S0002-8223(96)00190-3. [DOI] [PubMed] [Google Scholar]
- 15.Mc Cord JM. The evolution of free radicals and oxidative stress. Am J Med. 2000;108:652–9. doi: 10.1016/s0002-9343(00)00412-5. [DOI] [PubMed] [Google Scholar]
- 16.Rao AL, Bharani M, Pallavi V. Role of antioxidants and free radicals in health and disease. Adv Pharmacol Toxicol. 2006;7:29–38. [Google Scholar]
- 17.Stefanis L, Burke RE, Greene LA. Apoptosis in neurodegenerative disorders. Curr Opin Neurol. 1997;10:299–305. doi: 10.1097/00019052-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Esterbauer H, Pubi H, Dieber-Rothender M. Effect of antioxidants on oxidative modification of LDL. Ann Med. 1991;23:573–81. doi: 10.3109/07853899109150520. [DOI] [PubMed] [Google Scholar]
- 19.Neuzil J, Thomas SR, Stocker R. Requirement for promotion, or inhibition of α- tocopherol of radical induced initiation of plasma lipoprotein lipid peroxidation. Free Radic Biol Med. 1997;22:57–71. doi: 10.1016/s0891-5849(96)00224-9. [DOI] [PubMed] [Google Scholar]
- 20.Poppel GV, Golddbohm RA. Epidemiologic evidence for β – carotene and cancer prevention. Am J Clin Nutr. 1995;62:1393–5. doi: 10.1093/ajcn/62.6.1393S. [DOI] [PubMed] [Google Scholar]
- 21.Glatthaar BE, Horing DH, Moser U. The role of ascorbic acid in carcinogenesis. Adv Exp Med Biol. 1986;206:357–77. doi: 10.1007/978-1-4613-1835-4_27. [DOI] [PubMed] [Google Scholar]
- 22.Sokol RJ. Vitamin E deficiency and neurologic diseses. Annu Rev Nutr. 1988;8:351–73. doi: 10.1146/annurev.nu.08.070188.002031. [DOI] [PubMed] [Google Scholar]
- 23.Ashok BT, Ali R. The aging paradox: Free radical theory of aging. Exp Gerontol. 1999;34:293–303. doi: 10.1016/s0531-5565(99)00005-4. [DOI] [PubMed] [Google Scholar]
- 24.Sastre J, Pellardo FV, Vina J. Glutathione, oxidative stress and aging. Age. 1996;19:129–39. [Google Scholar]
- 25.Cantuti-Castelvetri I, Shukitt-Hale B, Joseph JA. Neurobehavioral aspects of antioxidants in aging. Int J Dev Neurosci. 2000;18:367–81. doi: 10.1016/s0736-5748(00)00008-3. [DOI] [PubMed] [Google Scholar]
- 26.Freeman BA, Crapo JD. Biology of disease: Free radicals and tissue injury. Lab Invest. 1982;47:412–26. [PubMed] [Google Scholar]
- 27.Lovell MA, Ehmann WD, Buffer BM, Markesberry WR. Elevated thiobarbituric acid reactive substances and antioxidant enzyme activity in the brain in Alzemers disease. Neurology. 1995;45:1594–601. doi: 10.1212/wnl.45.8.1594. [DOI] [PubMed] [Google Scholar]
- 28.Woo RA, Melure KG, Lee PW. DNA dependent protein kinase acts upstream of p53 in response to DNA damage. Nature. 1998;394:700–4. doi: 10.1038/29343. [DOI] [PubMed] [Google Scholar]
- 29.Hattori Y, Nishigori C, Tanaka T, Ushida K, Nikaido O, Osawa T. 8 Hydroxy-2-deoxyguanosine is increased in epidermal cells of hairless mice after chronic ultraviolet B exposure. J Invest Dermatol. 1997;89:10405–9. doi: 10.1111/1523-1747.ep12365625. [DOI] [PubMed] [Google Scholar]
- 30.Halliwell B. How to characterize an antioxidant- An update. Biochem Soc Symp. 1995;61:73–101. doi: 10.1042/bss0610073. [DOI] [PubMed] [Google Scholar]
- 31.Shi HL, Noguchi N, Niki N. Comparative study on dynamics of antioxidative action of α- tocopheryl hydroquinone, ubiquinol and α- tocopherol, against lipid peroxidation. Free Radic Biol Med. 1999;27:334–46. doi: 10.1016/s0891-5849(99)00053-2. [DOI] [PubMed] [Google Scholar]
- 32.Levine M, Ramsey SC, Daruwara R. Criteria and recommendation for Vitamin C intake. JAMA. 1991;281:1415–23. doi: 10.1001/jama.281.15.1415. [DOI] [PubMed] [Google Scholar]
- 33.Matill HA. Antioxidants. Annu Rev Biochem. 1947;16:177–92. doi: 10.1146/annurev.bi.16.070147.001141. [DOI] [PubMed] [Google Scholar]
- 34.German J. Food processing and lipid oxidation. Adv Exp Med Biol. 1999;459:23–50. doi: 10.1007/978-1-4615-4853-9_3. [DOI] [PubMed] [Google Scholar]
- 35.Jacob R. Three eras of vitamin C discovery. Subcell Biochem. 1996;25:1–16. [PubMed] [Google Scholar]
- 36.Knight J. Free radicals: Their history and current status in aging and disease. Ann Clin Lab Sci. 1998;28:331–46. [PubMed] [Google Scholar]
- 37.Moreau, Dufraisse Comptes Rendus des Séances et Mémoires de la Société de Biologie. 1922;86:321. [Google Scholar]
- 38.Wolf G. The discovery of the antioxidant function of vitamin E: The contribution of Henry A. Mattill. J Nutr. 2005;135:363–6. doi: 10.1093/jn/135.3.363. [DOI] [PubMed] [Google Scholar]
- 39.Frie B, Stocker R, Ames BN. Antioxidant defences and lipid peroxidation in human blood plasma. Proc Natl Acad Sci. 1988;37:569–71. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rice-Evans CA, Diplock AT. Current status of antioxidant therapy. Free Radic Biol Med. 1993;15:77–96. doi: 10.1016/0891-5849(93)90127-g. [DOI] [PubMed] [Google Scholar]
- 41.Krinsky NI. Mechanism of action of biological antioxidants. Proc Soc Exp Biol Med. 1992;200:248–54. doi: 10.3181/00379727-200-43429. [DOI] [PubMed] [Google Scholar]
- 42.Niki E. Antioxidant defenses in eukaryotic cells. In: Poli G, Albano E, Dianzani MU, editors. Free radicals: From basic science to medicine. Basel, Switzerland: Birkhauser Verlag; 1993. pp. 365–73. [Google Scholar]
- 43.Sies H. Oxidative stress: Oxidants and antioxidants. Exp Physiol. 1997;82:291–5. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 44.Magnenat JL, Garganoam M, Cao J. The nature of antioxidant defense mechanisms: A lesson from transgenic studies. Environ Health Perspect. 1998;106:1219–28. doi: 10.1289/ehp.98106s51219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zelko I, Mariani T, Folz R. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–49. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 46.Banniste J, Bannister W, Rotilio G. Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit Rev Biochem. 1987;22:111–80. doi: 10.3109/10409238709083738. [DOI] [PubMed] [Google Scholar]
- 47.Johnson F, Giulivi C. Superoxide dismutases and their impact upon human health. Mol Aspects Med. 2005;26:340–52. doi: 10.1016/j.mam.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Wuerges J, Lee JW, Yim YI, Yim HS, Kang SO, Djinovic Carugo K. Crystal structure of nickel-containing superoxide dismutase reveals another type of active site. Proc Natl Acad Sci. 2004;101:8569–74. doi: 10.1073/pnas.0308514101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corpas FJ, Barroso JB, del Río LA. Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci. 2001;6:145–50. doi: 10.1016/s1360-1385(01)01898-2. [DOI] [PubMed] [Google Scholar]
- 50.Corpas FJ, Fernández-Ocaña A, Carreras A, Valderrama R, Luque F, Esteban FJ, et al. The expression of different superoxide dismutase forms is cell-type dependent in olive (Olea europaea L.) leaves. Plant Cell Physiol. 2006;47:984–94. doi: 10.1093/pcp/pcj071. [DOI] [PubMed] [Google Scholar]
- 51.Cao X, Antonyuk SV, Seetharaman SV, Whitson LJ, Taylor AB, Holloway SP, et al. Structures of the G85R variant of SOD1 in familial amyotrophic lateral sclerosis. J Biol Chem. 2008;283:16169–77. doi: 10.1074/jbc.M801522200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci. 2004;61:192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaetani G, Ferraris A, Rolfo M, Mangerini R, Arena S, Kirkman H. Predominant role of catalase in the disposal of hydrogen peroxide within human erythrocytes. Blood. 1996;87:1595–9. [PubMed] [Google Scholar]
- 54.Eisner T, Aneshansley DJ. Spray aiming in the bombardier beetle: Photographic evidence. Proc Natl Acad Sci USA. 1999;96:9705–9. doi: 10.1073/pnas.96.17.9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meister A, Anderson M. Glutathione. Annu Rev Biochem. 1983;52:711–60. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 56.Brigelius-Flohe R. Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med. 1999;27:951–65. doi: 10.1016/s0891-5849(99)00173-2. [DOI] [PubMed] [Google Scholar]
- 57.Hayes J, Flanagan J, Jowsey I. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 58.Smirnoff N. L-ascorbicacid biosynthesis. Vitam Horm. 2001;61:241–66. doi: 10.1016/s0083-6729(01)61008-2. [DOI] [PubMed] [Google Scholar]
- 59.Meister A. Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem. 1994;269:9397–400. [PubMed] [Google Scholar]
- 60.Padayatty S, Katz A, Wang Y, Eck P, Kwon O, Lee J, et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22:18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 61.Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, et al. Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot. 2002;53:1305–19. [PubMed] [Google Scholar]
- 62.Meister A, Anderson A. Glutathione. Annu Rev Biochem. 1983;52:711–60. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 63.Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–8. [PubMed] [Google Scholar]
- 64.Fairlamb AH, Cerami A. Metabolism and functions of trypanothione in the Kinetoplastida. Annu Rev Microbiol. 1992;46:695–729. doi: 10.1146/annurev.mi.46.100192.003403. [DOI] [PubMed] [Google Scholar]
- 65.Nassar E, Mulligan C, Taylor L, Kerksick C, Galbreath M, Greenwood M, et al. Effects of a single dose of N-Acetyl-5-methoxytryptamine (Melatonin) and resistance exercise on the growth hormone/IGF-1 axis in young males and females. J Int Soc Sports Nutr. 2007;4:14. doi: 10.1186/1550-2783-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caniato R, Filippini R, Piovan A, Puricelli L, Borsarini A, Cappelletti E. Melatonin in plants. Adv Exp Med Biol. 2003;527:593–7. doi: 10.1007/978-1-4615-0135-0_68. [DOI] [PubMed] [Google Scholar]
- 67.Reiter RJ, Carneiro RC, Oh CS. Melatonin in relation to cellular antioxidative defense mechanisms. Horm Metab Res. 1997;29:363–72. doi: 10.1055/s-2007-979057. [DOI] [PubMed] [Google Scholar]
- 68.Tan DX, Manchester LC, Reiter RJ, Qi WB, Karbownik M, Calvo JR. Significance of melatonin in antioxidative defense system: Reactions and products. Biol Signals Recept. 2000;9:137–59. doi: 10.1159/000014635. [DOI] [PubMed] [Google Scholar]
- 69.Herrera E, Barbas C. Vitamin E: Action, metabolism and perspectives. J Physiol Biochem. 2001;57:43–56. [PubMed] [Google Scholar]
- 70.Brigelius-Flohe R, Traber M. Vitamin E: Function and metabolism. FASEB J. 1999;13:1145–55. [PubMed] [Google Scholar]
- 71.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang X, Quinn P. Vitamin E and its function in membranes. Prog Lipid Res. 1999;38:309–36. doi: 10.1016/s0163-7827(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 73.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–76. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 74.Papas AM. Diet and antioxidant status. Food Chem Toxicol. 1999;37:999–1007. doi: 10.1016/s0278-6915(99)00088-5. [DOI] [PubMed] [Google Scholar]
- 75.Brown JE, Rice-Evan CA. Luteolin-rich Artichoke extract protects low density lipoprotein from oxidation in vitro. Free Radic Res. 1998;29:247–255. doi: 10.1080/10715769800300281. [DOI] [PubMed] [Google Scholar]
- 76.Furuta S, Nishiba Y, Suda I. Fluorometric assay for screening antioxidative activities of vegetables. J Food Sci. 1997;62:526–8. [Google Scholar]
- 77.Wang H, Cao G, Prior RL. Total antioxidant capacity of fruits. J Agric Food Chem. 1996;44:701–5. [Google Scholar]
- 78.Lin JK, Lin CH, Ling YC, Lin-Shian SY, Juan IM. Survey of catechins, gallic acid and methylxantines in green, oolong, puerh and black teas. J Agric Food Chem. 1998;46:3635–42. [Google Scholar]
- 79.Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free radicals and antioxidants in Human Health: Current status and future prospects. J Assoc Physicians India. 2004;52:794–803. [PubMed] [Google Scholar]
- 80.López-Varela S, González-Gross M, Marcos A. Functional foods and the immune system: A review. Eur J Clin Nutr. 2002;56:S29–33. doi: 10.1038/sj.ejcn.1601481. [DOI] [PubMed] [Google Scholar]
- 81.Roberfroid MB. What is beneficial for health? The concept of functional food. Food Chem Toxicol. 1999;37:1034–41. doi: 10.1016/s0278-6915(99)00080-0. [DOI] [PubMed] [Google Scholar]
- 82.Krishnaswamy K. Indian functional food: Role in prevention of cancer. Nutr Rev. 1996;54:127–31. doi: 10.1111/j.1753-4887.1996.tb03832.x. [DOI] [PubMed] [Google Scholar]
- 83.DeFelice SL. Nutraceuticals: Opportunities in an Emerging Market. Scrip Mag. 1992;9:14–5. [Google Scholar]
- 84.Dillard CJ, German JB. Phytochemicals: Nutraceuticals and human health. J Sci Food Agric. 2000;80:1744–56. [Google Scholar]
- 85.Tapas AR, Sakarkar DM, Kakde RB. Review article flavonoids as nutraceuticals: A review. Trop J Pharm Res. 2008;7:1089–99. [Google Scholar]
- 86.Vidya AD, Devasagayam TP. Current status of Herbal drug in India: An overview. J Clin Biochem Nutr. 2007;41:1–11. doi: 10.3164/jcbn.2007001. [DOI] [PMC free article] [PubMed] [Google Scholar]


