Abstract
Crocus sativus L. belonging to the family Iridaceae (syn - kesar) comprises the dried red stigma and is widely cultivated in Iran and other countries such as India and Greece. Saffron contains more than 150 volatile and aroma-yielding compounds mainly terpenes, terpene alcohol, and their esters. The bitter taste and an iodoform or hay-like fragrance are caused by chemicals picrocrocin and safranal. C. sativus possesses a number of medicinally important activities such as antihypertensive, anticonvulsant, antitussive, antigenototoxic and cytotoxic effects, anxiolytic aphrodisiac, antioxidant, antidepressant, antinociceptive , anti-inflammatory, and relaxant activity. It also improves memory and learning skills, and increases blood flow in retina and choroid. The present review explores the historical background, chemical constituents, pharmacological actions, uses, substitutes and adulterants, and toxicity. It also deals with its evaluation, formulations, and chemical tests in detail.
Keywords: Anticonvulsant, antinociceptive, aphrodisiac, crocin, Crocus sativus, safranal
INTRODUCTION
Crocus sativus L. (Iridaceae), commonly known as saffron, is a perennial stemless herb that is widely cultivated in Iran and other countries such as India and Greece. Commercial saffron comprises the dried red stigma with a small portion of the yellowish style attached.[1] The earliest apparent reference to its cultivation goes back to about 2300 BC; Sargon, the founder of the Aceadian empire, was born at the otherwise unknown village on the Euphrates called Azupirano - the name meaning perhaps “Saffron town.”[2] A definite identification of saffron crocuses dates from about 1700-1600 BC, in the form of a fresco painting in the Palace of Minos at Knossos in Crete. The wild precursor of domesticated saffron crocus was Crocus cartwrightianus. Experts believe saffron was first documented in a 7th centaury BC Assyrian botanical reference compiled under Ashurbanipal. Since then, documentation of saffron's use over the span of 4000 years in the treatment of some 90 illnesses has been uncovered. It is in leaf from October to May, and in flower in October. The flowers are hermaphrodite (have both male and female organs) and are pollinated by bees and butterflies. The plant prefers light (sandy) and medium (loamy) soils, requires well-drained soil, and can grow in nutritionally poor soil. The flower has three stigmas, which are the distal ends of the plant's carpels. Together with the style, the stalk connecting the stigmas to the rest of the plant are often dried and used in cooking as a seasoning and coloring agent. Saffron blooms only once a year and should be collected within a very short duration. It is picked during 3-4 weeks in October-November. The method for the cultivation of saffron contributes greatly to its high price. According to some reports, this species is a sterile triploid and so does not produce fertile seeds. Germination can take 1-6 months at 18°C.[15] It takes 3 years for plants to flower from seed.[3–6] Saffron is characterized by a bitter taste and an iodoform or hay-like fragrance, which are caused by chemicals picrocrocin and safranal.[7] The value of saffron (stigmas of C. sativus L.) is determined by the existence of three main secondary metabolites: crocin, picrocrocin, and safranal.[8,9] Saffron is used for depression in Persian traditional medicine.[10–13] Pistils of saffron are generally used in traditional Indian medicine as analgesics and cardio-protective agents, as well as in the treatment of various kinds of mental illnesses. A crude extract of pistils of saffron improves recovery in ischemia/reperfusion injury and learning and memory in rats. In traditional medicines, saffron is recommended as an aphrodisiac agent.[14]
SCIENTIFIC CLASSIFICATION
Kingdom : Plantae
Division : Magnoliophyta
Class : Liliopsida
Order : Asparagales
Family : Iridaceae
Genus : Crocus
Species : C. sativus
SYNONYMS
Hindi - kesar, zaffran; Sanskrit - avarakta, saurab, mangalya, agnishikha, kumkuma, mangal, kusrunam; English- saffron; Arab and Persian - zafrah, zipharana; Ben - jafran; Bom - safran, kessar; Mah - kecara; Guj - keshar; Tel - kunkuma-purva, kunkumma-purru; Tam. and Mal. - kunkumappu; Can. and Kon. - kunkuma-kesara; Fr.and Ger. - safran.[15]
MACROSCOPY
Color - stigma dark red to reddish brown. Style is yellowish brown to yellowish orange.
Odor - strong, characteristic, and aromatic. Taste - characteristic and bitter. Size- stigmas are 25-mm long, and styles are about 10-mm long. Shape - stigma trifid and styles cylindrical.[16]
MICROSCOPY
If the soaked drug is examined under a lens or microscope, the stigmas will be found either separate or united in three to the apex of yellowish styles. Each stigma is about 25-mm long and has the shape of a slender funnel, the rim of which is dentate or fimbricate.[3]
CHEMICAL CONSTITUENTS
In view of its wide range of medical uses, the saffron has undergone extensive phytochemical and biochemical studies and variety of biologically active ingredients has been isolated [Tables 1, 2]. Characteristic components of saffron are crocin -(responsible for the color), picrocrocin- (responsible for the bitter taste), and safranal- (responsible for odor and aroma) [Figure 1].[3] Saffron contains more than 150 volatile and aroma-yielding compounds. It also has many non-volatile active components, many of which are carotenoids including zeaxanthin, lycopene, and various α- and β-carotenes.[17] The volatiles with a very strong odor are consistent of more than 34 components that are mainly terpenes, terpene alcohols, and their esters. Non-volatiles include crocins 14 that are responsible for the red or reddish brown color of stigmas together with carotenes, crocetin, picrocrocin (a glycosidic precursor of safranal), the bitter substance and safranal the major organoleptic principle of stigmas.[4] However saffron's golden yellow-orange color is primarily due to α-crocin. This crocin is trans-crocetin di-(β-D-gentiobiosyl) ester. Systematic (IUPAC) name: 8, 8-diapo-8, 8-carotenoic acid. This means that the crocin underlying saffron's aroma is a digentiobiose ester of the carotenoid crocetin. Crocins themselves are a series of hydrophilic carotenoids that are either monoglycosyl or di-glycosyl polyene esters of crocetin.[17] Meanwhile crocetin is a conjugated polyene dicarboxylic acid that is hydrophobic and thus oil soluble. When crocetin is esterified with two water-soluble gentiobioses (which are sugars), a product results that is itself water soluble. The resultant α-crocin is a carotenoid pigment that may comprise more than 10% of dry saffron's mass. The two esterified gentiobioses make α-crocin ideal for coloring waterbased (nonfatty) foods such as rice dishes.[4] A hypothetical protocrocin of the fresh plant is decomposed on drying into one molecule of crocin and two molecules of picrocrocin. Crocin on hydrolysis yields gentiobiose and crocetin, while picrocrocin yields glucose and safranal.[3] The bitter glucoside picrocrocin is responsible for saffron's flavor. Picrocrocin (chemical formula: C16H26O7, systematic name: 4-(β-d-glucopyranosyloxy)-2, 6.6-trimethylcyclohex-1-ene-1-carboxaldehyde) is a union of an aldehyde subelement known as safranal (systematic name: 2, 6, 6-trimethylcyclohexa-1,3-dien-1-carboxaldehyde) and a carbohydrate. It has insecticidal and pesticidal properties and may comprise upto 4% of dry saffron. Safranal is less bitter than picrocrocin and may comprise up to 70% of dry saffron's volatile fraction in some samples. A second element underlying saffron's aroma is 2-hydroxy-4, 4 ,6-trimethyl-2,5-cyclohexa-dien-1-one, the scent which has been described as “saffron, dried hay-like.”[18] Callus cultures at ρH: 7.0-7.6 with added uridine-diphosphoglucose are able to transform all trans-crocetin into its related glycosides. An antioxidant 3,8-dihydroxy-1-methylanthroquinone-2-carboxylic, claimed to be superior to vitamin E in its inhibition of oxidation of linoleic acid, has been isolated from callus stem tissue of saffron.[3] Dry saffron is highly sensitive to fluctuating pH levels and rapidly breaks down chemically in the presence of light and oxidizing agent. It must, therefore, be stored in air-tight containers in order to minimize contact with atmospheric oxygen. C. sativus has been shown to have anti-depressant effects, two active ingredients are crocin and safranal.[19] As preliminary phytochemical results indicated, it could be suggested that the antinociceptive and anti-inflammatory effects of the petal extracts may be due to their content of flavonoids, tannins, and anthocyanins. Other studies have demonstrated that various flavonoids such as rutin, quercetin, luteolin, hesperidin, and bioflavonoids are present.[20–23]
Table 1.
Chemical composition of saffron

Table 2.
Proximate analysis of saffron
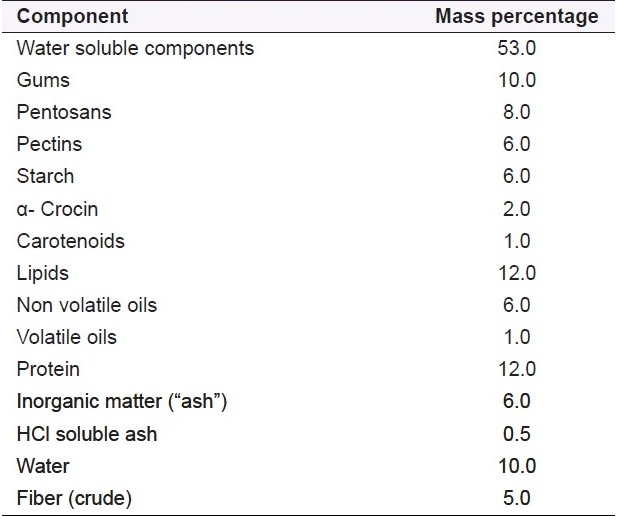
Figure 1.
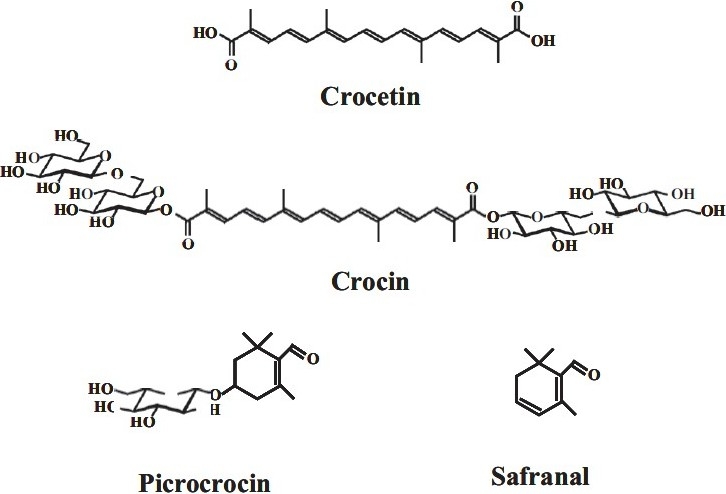
Structures of the chemical constituents
PHARMACOLOGICAL ACTIONS
Antihypertensive activity
Fatehi and others investigated the effects of C. sativus petals’ extract on blood pressure in anesthetized rats and also on responses of the isolated rat vas deferens and guinea -pig ileum induced by electrical field stimulation (EFS). Aqueous and ethanol extracts of C. sativus petals’ reduced the blood pressure in a dose-dependent manner. Administration of 50 mg/g of aqueous extract changed the blood pressure from 133.5 ± 3.9 to 117 ± 2.1 (mmHg). This reduction could either be due to the effect of the C. sativus petals’ extracts on the heart itself/total peripheral resistance, or both. The effect of extracts on peripheral resistance seems to be more important.[23] In the rat isolated vas deferens, contractile responses to EFS were decreased by the petals’ extracts. Contractions of the vas deferens to EFS are mediated by a combination of noradrenaline and ATP released as cotransmitters from sympathetic nerves.[24] The ethanol extract induced greater changes in EFS in the rat isolated vas deferens and guinea- pig ileum than the aqueous extract.[23]
Anticonvulsant activity
The anticonvulsant activities of C. sativus stigma constituents, safranal and crocin, were evaluated in mice using pentylenetetrazole (PTZ)-induced convulsions in mice. Safranal (0.15 and 0.35 ml/kg body weight, i.p.) reduced the seizure duration, delayed the onset of tonic convulsions, and protected mice from death. Crocin (22 mg/kg, i.p.) did not show anticonvulsant activity.[25]
Antitussive activity
The antitussive activity of C. sativus stigma and petal extracts and its components, safranal and crocin, was evaluated using the nebolized solution of citric acid 20% in guinea pigs. The ethanolic extract of C. sativus (100-800 mg/kg) and safranal (0.25-0.75 ml/kg) reduced the number of cough. The ethanolic and aqueous extracts of petal and crocin did not show antitussive activity.[26]
Antigenototoxic and cytotoxic effects of saffron
The antimutagenic, comutagenic, and cytotoxic effects were assessed using the Ames/Salmonella test system, two well-known mutagen (BP, 2AA), the in vitro colony-forming assay, and four different cultured human normal (CCD-18LU) and malignant (Hela,a-204 and Hepg2) cells. When only using the TA98 strain in the Ames/Salmonella test system, saffron showed nonmutagenic, as well as non-antimutagenic activity against BP-induced mutagenicity and demonstrated a dose-dependent co-mutagenic effect on 2-AA-induced antimutagenicity. The saffron component responsible for this unusual co-mutagenic effect was safranal. In the in vitro colony-forming test system, saffron displayed a dose-dependent inhibitory effect only against human malignant cells. All isolated carotenoid ingredients of saffron demonstrated cytotoxic activity against in vitro tumor cells. Saffron crocin derivatives possessed a stronger inhibitory effect on tumor cell colony formation. Overall, these results suggest that saffron itself, as well as its carotenoid components, might be used as potential cancer chemopreventive agents.[27]
Effect on sexual behavior
The aphrodisiac activities of C. sativus stigma aqueous extract and its constituents, safranal and crocin, were evaluated in male rats. The aqueous extract (80, 160, and 320 mg/kg body wt.), crocin (100, 200, and 400 mg/kg body wt.), safranal (0.1, 0.2, and 0.4 ml/kg), sildenafil (60 mg/kg body wt., as a positive control), and saline were administered intraperitoneally to male rats. Mounting frequency (MF), mount latency (ML), intromission latency (IL), and ejaculation latency (EL) were the factors evaluated during the sexual behavior study. Crocin, at all doses, and the extract, especially at doses 160 and 320 mg/kg body wt., increased MF, IF, and EF behaviors and reduced EL, IL, and ML parameters. Safranal did not show aphrodisiac effects. This study exhibited an aphrodisiac activity of saffron aqueous extract and its constituent crocin.[28]
Anxiolytic activity
This study was designed to investigate in rodents whether or not crocins possess anxiolytic properties. For this aim, the light\dark test was selected. Either crocins, at a dose which did not influence animals’ motor activity (50 mg/kg), or diazepam (1.5 mg/kg), increased the latency to enter the dark compartment and prolonged the time spent in the lit chamber in the rats. Conversely, lower doses of crocins (15-30 mg/kg) did not substantially modify animals’ behavior. The present results indicate that treatment with these active constituents of C. sativus L. induces anxiolytic-like effects in the rat.[29]
Relaxant property
To study the mechanism(s) of the relaxant effects of C. sativus (Iridaceae), the stimulatory effect of aqueous-ethanolic extracts of this plant and one of its constituent, safranal, was examined on β-adrenoreceptors in tracheal chains of guinea pigs. The β2-adrenergic stimulatory was tested by performing the cumulative concentration-response curves of isoprenaline-induced relaxation of pre-contracted isolated guinea pig tracheal chains. The studied solutions included two concentrations of aqueous ethanolic extracts from C. sativus (0.1 and 0.2 g%), safranal (1.25 and 2.5 μg), 10 nM propranalol, and saline. The study was done in two different conditions including non-incubated (group 1, n = 9) and incubated tissues with 1 μM chlorpheniramine (group 2, n = 6). The results showed clear leftward shifts in isoprenaline curves obtained in the presence of only higher concentration of the extract in group 1 and its both concentrations in group 2 compared with that of saline. The EC50 (the effective concentration of isoprenaline, causing 50% of maximum response) obtained in the presence of both concentrations of the extract (0.17 ± 0.06 and 0.12 ± 0.02) and safranal (0.22 ± 0.05 and 0.22 ± 0.05) in group 1 and only in the presence of two concentrations of the extract (1.16 ± 0.31 and 0.68 ± 0.21) in group two was significantly lower compared to saline. The maximum responses obtained in the presence of both concentrations of the extract and safranal in group 1 were significantly lower than that of saline. The results indicated a relatively potent stimulatory effect of the extract from C. sativus on β2-adrenoreceptors, which is partially due to its constituent, safranal. A possible inhibitory effect of the plant on histamine (H1) receptors was also suggested.[30]
Effect on depression
The efficacy of petal of C. sativus was assessed in the treatment of mild-to-moderate depression in a 6-week double-blind, placebo-controlled and randomized trial. Forty adult outpatients who met the Diagnostic and Statistical Manual (DSM) of Mental Disorders, fourth edition for major depression based on the structural clinical interview for DSM IV, participated in the trial. In this double-blind, placebo-controlled and randomized trial, patients were randomly assigned to receive capsule of petal of C. sativus 30 mg/day (b.d.) (Group 1) and capsule of placebo (b.d.) (Group 2) for a 6-week study. At 6 weeks, petal of C. sativus produced a significantly better outcome on Hamilton Depression Rating Scale than placebo (d.f. = 1, F = 16.87, P<0.001). There were no significant differences in the two groups in terms of observed side-effects. The results of this study indicate the efficacy of petal of C. sativus in the treatment of mild-to-moderate depression.[31] In further preliminary work, saffron was compared to the drug fluoxetine; it was found that the saffron performed as well as the drug in the treatment of depression.[13] In addition, in a recent pre-clinical study, it has been reported that petal of C. sativus, the part of this herb that is very cheap compared to stigma of C. sativus (saffron), has antidepressant effect.[11]
Effect on learning behavior and long-term potentiation
The saffron extract and two of its main ingredients, crocin and crocetin, improved memory and learning skills in ethanol-induced learning behavior impairments in mice and rats. Oral administration of saffron may be useful in the treatment of neurodegenerative disorders and related memory impairment.[8,32]
Effects on ocular blood flow and retinal function
Crocin analogs isolated from saffron significantly increased the blood flow in the retina and choroid as well as facilitated retinal function recovery and it could be used to treat ischemic retinopathy and/or age-related macular degeneration.[33]
Effect on coronary artery disease
Fifty milligrams of saffron dissolved in 100 ml of milk was administered twice a day to human subjects, and the significant decrease in lipoprotein oxidation susceptibility in patients with coronary artery disease (CAD) indicates the potential of saffron as an antioxidant.[34]
Antinociceptive and anti-inflammatory effects
Saffron stigma and petal extracts exhibited antinociceptive effects in chemically induced pain test as well as acute and/or chronic anti-inflammatory activity, and these effects might be due to the presence of flavonoids, tannins, anthocyanins, alkaloids, and saponins.[10]
EVALUATION
Tablet assay
C. sativus stigma tablets were evaluated for short-term safety and tolerability in healthy adult volunteers. The study was a double-blind, placebo-controlled design consisting of a 1-week treatment of saffron tablets. Volunteers were divided into three groups of 10 each (five males and five females). Group 1 received placebo; groups 2 and 3 received 200 and 400 mg saffron tablets, respectively, for 7 days. General measures of health were recorded during the study such as hematological, biochemical, and electrocardiographic parameters done in pre- and post-treatment periods. Clinical examination showed no gross changes in all volunteers after invention. Saffron with higher dose (400 mg) decreased standing systolic blood pressure and mean arterial pressures significantly. Saffron decreased slightly some hematological parameters such as red blood cells, hemoglobin, hematocrit, and platelets. Saffron increased sodium, blood urea nitrogen, and creatinine. This study showed that saffron tablets may change some hematological and biochemical parameters. However these alternations were in normal ranges and they were not important clinically.[35]
Quantitative structure-retention relationship
Quantitative structure-retention relationship (QSRR) studies were performed for predicting the retention times of 43 constituents of saffron aroma, which were analyzed by solid-phase micro-extraction gas chromatography-mass spectrometry (SPME-GC-MS). The chemical descriptors were calculated from the molecular structures of the constituents of saffron aroma alone, and the linear and non-linear QSRR modes were constructed using the best-multi-linear regression (BMLR) and pursuit regression (PPR) methods. The predicted results of the two approaches were in agreement with the experimental data. The coefficients of determination (R2) of the linear BMLR model were 0.9434 and 0.8725 for the training and test sets, respectively. The other non-linear PPR model gave a more accurate prediction with R2 values of 0.9806 (training set) and 0.9456 (test set). The proposed models could also identify and provide some insights into the structural features that may play a role on the retention behaviors of the constituents of saffron aroma in the SPME-GC-MS system. This study affords a simple but efficient approach for studying the retention behaviors of other similar plants and herbs.[36]
Chromatography and spectral analysis
A variety of analytical methods, for example, TLC, HPLC, HPTLC, have been developed for analytical separation and qualitative studies of crocins; quantitative methods for crocins have recently been reported. Methods reported for preparative chromatography of crocins include aluminum oxide column chromatography,[37] TLC, gel column chromatography, and multi-layer coil counter current chromatography, with silica gel, aluminum oxide, Sephadex LH-20 as stationary phases. By use of these methods, the yellow pigments have been separated into seven fractions at most. A preparative HPLC method for saffron has also been repoted; [Figures 2–5][38] high purity crocins have not, however, been obtained. Eleven certified saffron samples (C. sativus L.), one each from Azerbaijan, China, Greece, France, India, Iran, Italy, New Zealand, Spain, Turkey, and the Sigma Chemical Company, were analyzed by using an HPLC photodiode array detection method. This analysis quantified the 10 major saffron compounds in each sample and their concentration was analyzed at three different wavelengths. Result indicated that the Greek Indian, New Zealand, and Spanish saffron extracts possessed the highest concentrations of water-soluble glycosidic carotenoids (≥8.0%) suggesting that they could be a good source of this type of metabolite for further biological evaluation.[39]
Figure 2.
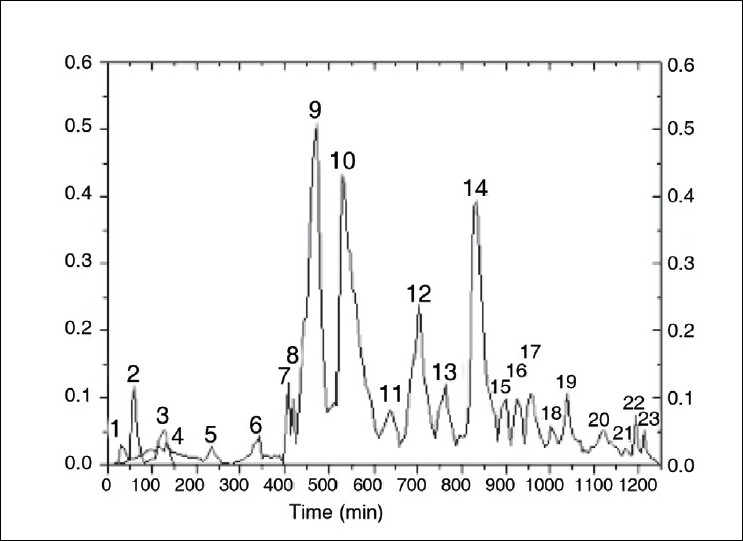
Preparative chromatogram obtained from the saffron extract. For fractions 1 and 2, the detection wavelength was 560 nm. For fraction 3, the detection wavelength was 620 nm. For fractions 4-23, the detection wavelength was 440 nm.
Figure 5.
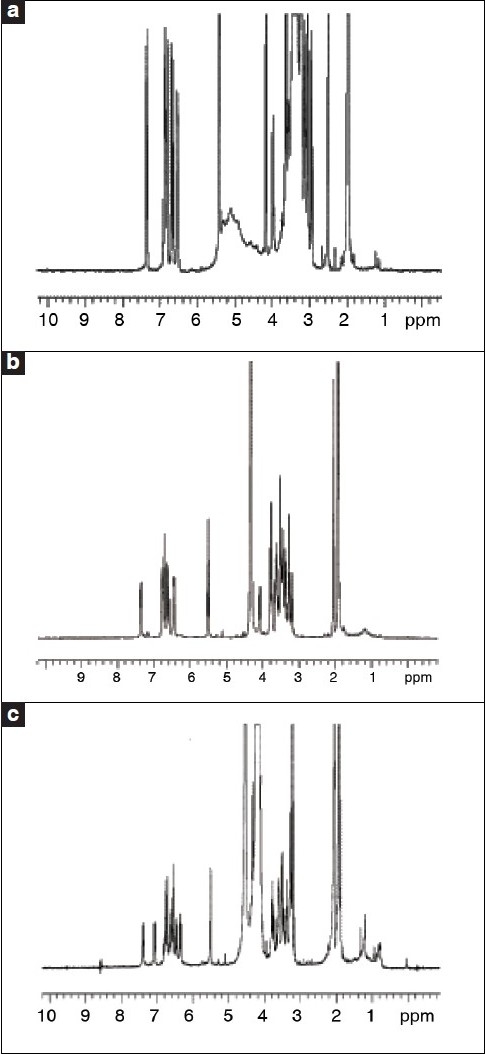
1H-NMR spectra of crocin 1 (a), crocin 2 (b), crocin 3 (c).
Figure 3.
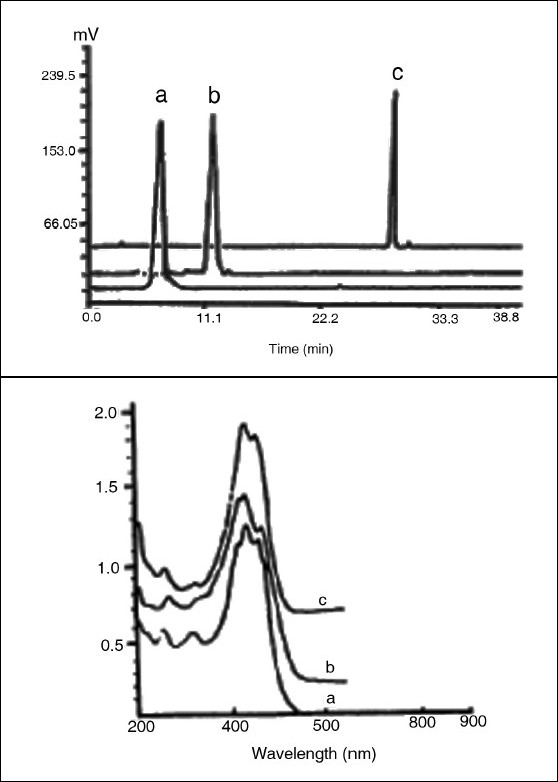
Chromatogram and UV-visible spectra of the crocins: a = crocin 1; b = crocin 2; c = crocin 3.
Mass spectrometric analysis of the crocins was performed with a MALDI-TDFMS instrument with an α-cyanocinnamic acid as matrix. 1H NMR spectra were recorded at 25°C (crocin 1 in DMSO σ-6, crocin 2 and crocin 3 in CD3OD-CD3COCD3-D2O, 1:1:1) by means of a Varian Inova-400 spectrometer (400 MHz) equipped with a 5-mm1H/13C/19F/31P probe for 1H spectra.[38]
Figure 4.
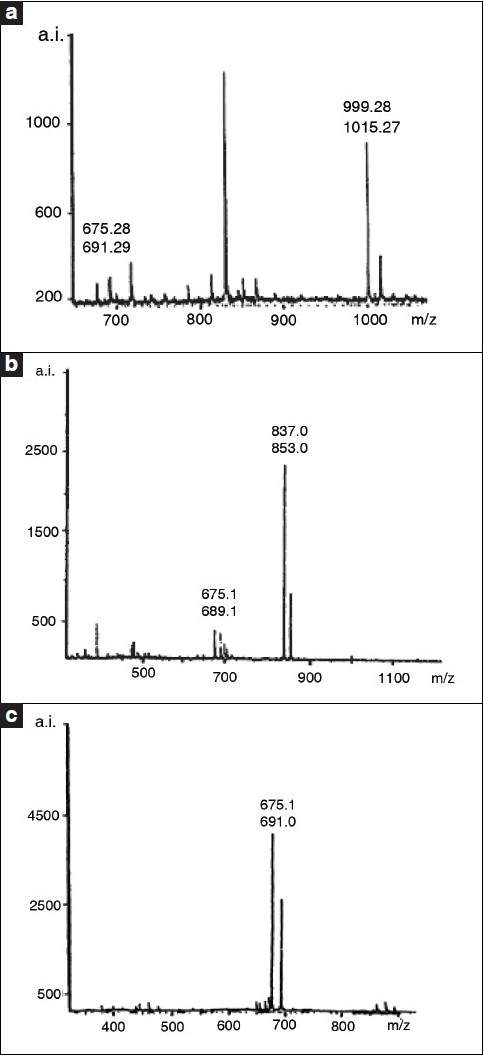
Mass spectra of the crocins: a = crocin 1; b = crocin 2; c = crocin.
Thermal desorption-gas chromatography was applied to samples of saffron to quantify the safranal content that is responsible for the distinctive aroma. Safranal constituted 72% of the volatile fraction. Using β-cyclocitral as internal standard and submitting the sample to a temperature of 70°C for 8 min, 83% of the total was determined with the first desorptions, and 100% with three consecutive desorptions.[40]
TOXICITY STUDIES
Saffron is nontoxic in animal studies (LD50 - 20.7 g/kg), noncytotoxic in in- vitro studies (LD50 -200 m kg/mL). The corms are toxic to young animals. In respect to LD50 values and maximum non-fatal doses, the stigma extracts were more toxic than the petals’ extract and have been reported as 1.6 g/kg, i.p. and 3.38 g/kg, i.p. in mice. According to a toxicity classification, stigma and petals extracts are “relatively toxic” and “low toxic,” respectively. Treatment with the C. sativus extract also significantly prolonged the life span of cisplatin-treated mice almost threefold.[41] Toxicity studies showed that the hematological and the biochemical parameters were within normal range with saffron extract.[6]
SUBSTITUTES AND ADULTERANTS
Because of the cost, saffron is frequently adulterated with cheaper substitutes such as marigold flowers and safflower. The flower styles are used as a tea substitute.
The substances used may be grouped in three categories, viz.;(a) substitution of other materials that have some external resemblance to saffron; (b) exhausted saffron recovered by dyes; and (c) substances added to saffron in order to increase its weight.
Materials used as substitutes may be mixed with saffron or supplied in place of saffron, the following have been used: styles of crocus, which are yellowish, slender, and unbranched; stamens and strips of the corolla of saffron crocus; ligulate carollas of florets of the marigold, Calendula officinalis, which are often colored with methyl orange and sometimes known as feminell or Chinese safflower; ligulate florets of safflower, Carthmus tinctorious. Linn often found in the cake saffron of commerce; slender stems and roots of some monocotyledons (e.g., Carex), colored artificially and stigmas of Zea mays Linn, known as corn silk.[4]
FORMULATIONS
-
(1)
Itch cream, a topical proprietary polyherbal formulation, is marketed for its antipruritic and emollient properties in xerotic and pruritic skin disorders.
It contains the following ingredients (v/w basis):
Extracts of turmeric 16% (Curcuma longa), saffron 0.025% (C. sativus), sandalwood 8% (Santalum album), vetiver 0.5% (Vetiveriazizanoides), lata kasturi 0.1% (Abelmoschus moschatus), mehendi 3% (Lawsonia inermis), tulsi 3% (Ocimum sanctum), yashtimadhu 0.5% (Glycyrrhiza glabra), turmeric oil 6.1%, surasar 0.5%, and swarna bhasma 0.00032% in a non-greasy cream base q.s.[42]
-
(2)
Scar remover skin cream
Each 100 g contains wheat germ oil (3.50 ml), Curcuma longa Linn. (20.0 g), Azadirachta indica (2.00 ml), Santalum album Linn. (1.00 ml), Citrus sinensis rind (2.00 g), Rosemary oil (5.00 ml), Aloe vera gel (2.00 ml), C. sativus (1.00g), cream base q.s. to make (100.00 g).[43]
-
(3)
Tincture dose- 5-20 minims
-
(4)
Infusion (saffron tea- 1 in 80)
Dose- 1 to 4 ounce[15]
USES
Saffron in therapeutics
The Ebers papyrus (ca 1550 B.C.) mentions saffron as an ingredient in a cure for kidney problems.[44] It was recommended as an addition to each meal as “a cheering cardiac medicament,” but with a warning that excessive quantities acted as an appetite depressant,[45] although a reasonable amount would stimulate appetite and ease headaches and hangovers. In the recent times, it is being used as a remedy for catarrhal infections, for melancholia, to treat liver enlargement, as a nerve sedative,[46] as a carminative, diaphoretic, and emmenagogue.[47] Its extensive use as an abortifacient decreased following reports of fatalities; death has occurred after ingestion of 1.5 g. Saffron has been discovered to be easily the richest known source of riboflavin, with about 100 γ/g.[48] Saffron would be likely to offset the decreased diffusivity of oxygen caused by elevated plasma protein and cholesterol level, reduced the severity of atherosclerosis. In addition, serum cholesterol levels were reduced by half.[49] The addition of crocetin to an appropriate nutrient fermentation broth was found to increase the yield of antibiotics and other products.[50] Modern pharmacological studies have demonstrated that saffron extract or its constituents have antidepressant,[51] anti-inflammatory,[10] anti-tumor effects, radical-scavenging, learning and memory improving properties.[52–54] Saffron extract also has chemoprotective properties and protects from genotoxin-induced oxidative stress in mice.[55–58] Anticonvulsant effects have been reported in both PTZ and maximal electroshock (MES) models in mice.[59]
Saffron as dye
Dyes and colored garments (principal pigment of saffron is α-crocin, a water-soluble carotenoid). Saffron has been used as a histological stain, i.e., as a dye for connective tissue.[60]
Saffron as perfume
A pleasantly odoriferous compound, safranal,[61] develops during the drying process,[62] probably by enzymatic[61] or thermal dissociation[63] of the bitter compound, picrocrocin.
Saffron in food
It performs the functions of a spice, adding its faint, delicate aroma, pleasing flavor, and magnificent yellow color to enhance palatability.[64,65]
CHEMICAL TESTS
The U.S.P. provides the following tests for saffron: saffron should not include the yellow styles. When pressed between filtering paper, it should not leave an oily stain. When chewed, it tinges the saliva deep orange-yellow. When soaked in water, it should not deposit any pulverulent, mineral matter, nor show the presence of organic substances differing in shape from that described. On agitating 1 part of saffron with 100,000 parts of water, the liquid should acquire a distinct yellow color. No color is imparted to benzine agitated with saffron (absence of picric acid and some other coal-tar colors). On drying saffron at 100°C (212°F), it should not lose more than 14% of its weight (absence of added water). When thus dried, and ignited with the free access of air, 100 parts of the dry saffron should not leave more than 7.5% ash (absence of foreign inorganic substances).[66]
CONTRAINDICATION
Contraindicated in pregnancy.[67]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Zargari A. Medicinal Plant. Tehran: Tehran University Press; 1990. p. 574. [Google Scholar]
- 2.Gadd CJ. In: The dynasty of Agade and the Guitan invasion. Edwards I.E.S, Gadd C.J, Hammand N.G.L, editors. Cambridge: Cambridge University Press; 1971. pp. 417–63. [Google Scholar]
- 3.Evans WC. Trease and Evans-Pharmacognosy. China: Saunders© Elsevier Limited; 1996. p. 438. [Google Scholar]
- 4.Wallis TE. Textbook of Pharmacognosy. New Delhi: CBS Publishers and Distributors; 2005. pp. 163–5. [Google Scholar]
- 5.Kalia AN. Textbook of Industrial Pharmacognosy. New Delhi: CBS Publishers and Distributors; pp. 235–6. [Google Scholar]
- 6.Nair SC, Pannikar B, Pannikar KR. Antitumour activity of saffron. Cancer letters. 1991;57:109–14. doi: 10.1016/0304-3835(91)90203-t. [DOI] [PubMed] [Google Scholar]
- 7.Katzer G. Saffron (Crocus sativus L.) Gernot Katzer's Spice Pages. [last accessed on 2006 Jan 10]. Available from: http://www.unigraz.at/~katzer/engl/croc_sat.html .
- 8.Abe K, Saito H. Effects of saffon and its constituent crocin on learning behavior and long-term potentiation. Phytother Res. 2000;14:149–52. doi: 10.1002/(sici)1099-1573(200005)14:3<149::aid-ptr665>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Abdullaev FI. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.) Exp Biol Med. 2002;227:20–5. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 10.Hosseinzadeh H, Younesi H. Petal and stigma extracts of Crocus sativus L. have antinoceceptive and anti-inflammatory effects in mice. BMC Pharmacol. 2002;2:7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karimi G, Hosseinzadeh H, Khaleghpanah P. Study of antidepresant effect of aqueous and ethanolic extract of Crocus sativus in mice. Iran J Basic Med Sci. 2001;4:11–5. [Google Scholar]
- 12.Akhondzadeh S, Fallah Pour H, Afkham K, Jamshidi AH, Khalighi-Cigarodi F. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: a pilot double-blind randomized trial. BMC Complement Altern Med. 2004;4:12. doi: 10.1186/1472-6882-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noorbala AA, Akhondzadeh S, Tamacebi-Pour N, Jamshedi AH. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind randomized pilot trial. J Ethnopharmacol. 2005;97:281–4. doi: 10.1016/j.jep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Madan CL, Kapur BM, Gupta US. Saffron. Econ Bot. 1996;20:377. [Google Scholar]
- 15.Nadkarni KM. Indian Materia Medica. Vol. 1. Bombay: Popular Prakashan; 2000. pp. 390–1. [Google Scholar]
- 16.Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy. Pune: Nirali Prakashan; 2006. p. 390. [Google Scholar]
- 17.Liakopulou-Kyriakides M, Kyriakides DA. Crocus sativus-Biological active Constituents. Stud Nat Prod Chem. 2002;26:293–312. [Google Scholar]
- 18.Antidepressant effect of Crocus sativus L. stigma extracts and their constituents,crocin and safranal, in mice. [last accessed on 2008 Mar 2]. Available from: http://www.cababstractplus.org .
- 19.Bittar M, deSouza MM, Yunus RA, Lento R, Monache FD, Cechinel VF. Antinociceptive Activity of I-3,II8-binarigenin,a biflavonoid present in plants of the guttiferae. Planta Med. 2000;66:84–6. doi: 10.1055/s-0029-1243118. [DOI] [PubMed] [Google Scholar]
- 20.Calixto JB, Beirith A, Ferreira J, Santos AR, Filho Cechinel V, Yunus RA. Naturally occurring antinociceptive substances from plants. Phytother Res. 2000;14:401–18. doi: 10.1002/1099-1573(200009)14:6<401::aid-ptr762>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 21.Galati EM, Monforte MT, Kirjavainen S, Forestieri AM, Torento A, Tripodo MM. Biological Effects Of hesperidin, a citrus flavonoid (Note-I):anti-inflammatory and analgesic activity. Farmaco. 1994;40:709–12. [PubMed] [Google Scholar]
- 22.Ramesh M, Rao YN, Rao AV, Prabhakar MC, Rao CS, Muuralidhar N, et al. Antinociceptive and anti-inflammatory activity of a flavonoid isolated from Carraluma attenuata. J Ethnopharmacol. 1998;62:63–6. doi: 10.1016/s0378-8741(98)00048-8. [DOI] [PubMed] [Google Scholar]
- 23.Fatehi M, Rashidabady T, Fatehi-Hassanabad Z. Effects of Crocus sativus petals’ extract on rat blood pressure and on response induced by electrical field stimulation in the rat isolated vas deferens and guinea-pig ileum. J Ethnopharmacology. 2003;84:199–203. doi: 10.1016/s0378-8741(02)00299-4. [DOI] [PubMed] [Google Scholar]
- 24.Hoyle CH, Burnstock G. ATP receptors and their physiological roles. In: Stone TW, editor. Adenosine in the Nervous system. London: Academic Press; 1991. pp. 43–76. [Google Scholar]
- 25.Hosseinzadeh H, Talebzadeh F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia. 2005;76:722–4. doi: 10.1016/j.fitote.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Hosseinzadeh H, Ghenaati J. Evaluation of the antitussive effect of stigma and petals of saffron(Crocus sativus)and its components, safranal and crocin in guinea pigs. Fitoterapia. 2006;77:446–8. doi: 10.1016/j.fitote.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Abdullaev FI, Riverón-Negrete L, Caballero-Ortega H, Manuel Hernández J, Pérez-López I, Pereda-Miranda R, et al. Use of in vitro assays to assess the potential antigenototoxic and cytotoxic effect of saffron (Crocus sativus) Toxicol In Vitro. 2003;17:731–6. doi: 10.1016/s0887-2333(03)00098-5. [DOI] [PubMed] [Google Scholar]
- 28.Hosseinzadeh H, Ziaee T, Sadeghi A. The effect of saffron, Crocus sativus stigma extract and its constituents, safranal and crocin on sexual behaviors in normal male rats. Phytomedicine. 2008;15:491–5. doi: 10.1016/j.phymed.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 29.Pitsikas N, Boultadakis A, Gergiadou G, Tarantilis PA, Sakellaridis N. Effects of the active constituents of Crocus sativus L. in an animal model of anxiety. Phytomedicine. 2008;15:1135–9. doi: 10.1016/j.phymed.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Nemati H, Boskabady MH, Ahmadzadef Vortakolaei H. Stimulatory effects of Crocus sativus (Saffron) on ß2 adrenoreceptors of guinea pig tracheal chains. Phytomedicine. 2008;15:1038–45. doi: 10.1016/j.phymed.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Moshiri E, Basti AA, Noorbala AA, Jamshidi AM, Abbasi SH, Akhondzadeh S. Crocus sativus L.(petal) in the treatment of mild-to-moderate depression: A double-blind, randomised and placebo-controlled trial. Phytomedicine. 2006;13:607–11. doi: 10.1016/j.phymed.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Sigiura M, Saito H, Abe K. Ethanol extract of Crocus sativus L.antagonizes the inhibitory action of ethanol on hippocampal long-term potentiation in vivo. Phytother Res. 1995;9:100–4. [Google Scholar]
- 33.Xuan B. Effects of crocin analogs on ocular flow and retinal function. J Ocul Pharmacol Ther. 1999;15:143–52. doi: 10.1089/jop.1999.15.143. [DOI] [PubMed] [Google Scholar]
- 34.Verma SK, Bordia A. Antioxidant property of saffron in man. Indian J Med Sci. 1998;52:205–7. [PubMed] [Google Scholar]
- 35.Modaghegh MH, Shahabian M, Esmaeili HA, Rajbai O, Hosseinzadeh H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine. 2008;15:1032–7. doi: 10.1016/j.phymed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Du H, Wang J, Hu Z, Yao X. Quantitative Structure-Retention Relationship study of the constituents of saffron aroma in SPME-GC-MS based on the Projection Pursuit Regression method. Talanta. 2008;77:360–5. doi: 10.1016/j.talanta.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 37.Escribano J, Alonso GL, Coca-Prados M, Fernandez JA. Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Lett. 1996;100:23–30. doi: 10.1016/0304-3835(95)04067-6. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Zeng Y, Yan F, Chen F, Zhang X, Liu M, et al. Semi-Preparative Isolation of Crocins from Saffron (Crocus sativus L.) Chromatographia. 2004;59:691–6. [Google Scholar]
- 39.Caballero-Ortega H, Pereda-Miranda R, Abdullaev FI. HPLC quantification of major active components from 11 different saffron (Crocus sativus L.) Sources. 2007;100:1126–31. [Google Scholar]
- 40.Alonso GL, Salinas MR, Esteban-Infantes FJ, Sanchez-Fernandez MA. Determination of Safranal from Saffron (Crocus sativus L.) by Thermal Desorption- Gas Chromatography. [last cited on 2010 Mar 2];J Agric Food Chem. 1996 44:185–8. Available from: http://www.pubs.acs.org/doi/citedby/10.1021/jf940665i . [Google Scholar]
- 41.Nair SC, Salomi MJ, Panikkar B, Panikkar KR. Modulatory Effects Of Crocus sativus and Nigella sativa extracts on Cisplatin induced toxicity in mice. J Ethnopharmacol. 1991;31:75–84. doi: 10.1016/0378-8741(91)90146-5. [DOI] [PubMed] [Google Scholar]
- 42.Chatterjee S, Datta RN, Bhattacharyya D, Bandopadhyay SK. Emollient and anti-pruritic effect of itch cream in dermatological disorders: A randomized controlled trial. Indian J Pharmacol. 2005;37:253–4. [Google Scholar]
- 43.Kalia AN. Textbook of Industrial Pharmacognosy. New Delhi: CBS Publishers and Distributors; 2005. p. 270. [Google Scholar]
- 44.Baumann BB. The botanical aspects of ancient Egyptian embalming and burial. Econ Bot. 1960;14:84–104. [Google Scholar]
- 45.Maimonides M. In: On the causes of symptoms (12th century) Leibowitz JO, Marcus S, editors. Berkeley, CA: Univ California Press; 1974. [Google Scholar]
- 46.Encyclopaedia Britannica, Saffron . Vol. 9. Chicago, IL: Encyclopaedia Britannica Inc., Encyclopaedia Britannica; 1974. p. 891. [Google Scholar]
- 47.Grisolia S. Hypoxia, saffron, and cardiovascular disease. Lancet. 1974;2:41–2. doi: 10.1016/s0140-6736(74)91367-1. [DOI] [PubMed] [Google Scholar]
- 48.Bhat JV, Broker R. Riboflavin and thiamine content of saffron.Crocus sativus Linn. Nature. 1953;172:544. doi: 10.1038/172544a0. [DOI] [PubMed] [Google Scholar]
- 49.Gainer JW, Chisolm GM. Oxygen diffusion and atherosclerosis. Athersclerosis. 1974;19:135–8. doi: 10.1016/0021-9150(74)90049-5. [DOI] [PubMed] [Google Scholar]
- 50.Gainer JL. Increasing fermentation yields. U.S. Pat. 4038144. 1977 [Google Scholar]
- 51.Hosseinzadeh H, Karimi Gh, Niapoor M. Antidepressant effects of Crocus sativus stigma extracts and its constituents, crocin and safranal, in mice. Acta Hort. 2004;650:435–45. [Google Scholar]
- 52.Abdullaev FJ. Biological effects of saffron. Biofactors. 1993;4:83–6. [PubMed] [Google Scholar]
- 53.Zhang YX, Sugiura M, Saito H, Shoyama Y. Acute effects of Crocus sativus L. on passive avoidance performance in mice. Biol Pharmacol Bull. 1994;17:217–21. doi: 10.1248/bpb.17.217. [DOI] [PubMed] [Google Scholar]
- 54.Abe K, Sugiura M, Ymaguchi S, Shoyama Y, Saito H. Saffron extract prevents acetaldehyde-induced inhibition of long-term potentiation in the rat dentate gyrus in vivo. Brain Res. 1999;851:287–9. doi: 10.1016/s0006-8993(99)02174-5. [DOI] [PubMed] [Google Scholar]
- 55.Abdullaev Jafarova F, Caballero-Ortega H, Riverón-Negrete L, Pereda-Miranda R, Rivera-Luna R, Manuel Hernández J, et al. In vitro evaluation of chemoprotective potential of saffron. Rev Inves Clin. 2002;54:430–6. [PubMed] [Google Scholar]
- 56.Nair SC, Kurumboor SK, Hasegawa JH. Saffron chemoprotective in biology and medicine: a review. Cancer Biother. 1995;10:257–64. doi: 10.1089/cbr.1995.10.257. [DOI] [PubMed] [Google Scholar]
- 57.Premkumar K, Abraham SK, Santhiya ST, Gopinath PM, Ramesh A. Inhibition of genotoxicity by saffron (Crocus sativus L.) in mice. Drug Chem Toxicol. 2001;24:421–8. doi: 10.1081/dct-100106266. [DOI] [PubMed] [Google Scholar]
- 58.Premkumar K, Abraham SK, Santhiya ST, Ramesh A. Protective effects of saffron (Crocin sativus L.) on genotoxins-induced oxidative stress in swiss albino mice. Phytother Res. 2003;17:614–7. doi: 10.1002/ptr.1209. [DOI] [PubMed] [Google Scholar]
- 59.Hosseinzadeh H, Khosravan V. Anticonvulsant effects of aqueous and ethanolic extracts of Crocus sativus L. stigma in mice. Arch Irn Med. 2002;5:44–7. [Google Scholar]
- 60.Lewis FT. The introduction of biological stains: employment of saffron by Vieussens and Leeuwenhoek. Anat Rec. 1942;83:229–53. [Google Scholar]
- 61.Guenther E. The Essential Oils. New York: Van Nostrand; 1952. p. 348. [Google Scholar]
- 62.Zarghami N.S. Ph.D. Thesis. CA: Univ. California, Davis; 1970. The volatile constituents of saffron (Crocus sativus L.) [Google Scholar]
- 63.Stahl E, Wagner C. TAS-method for the microanalysis of important constituents of saffron. J Chromatogr. 1969;40:308. doi: 10.1016/s0021-9673(01)96666-x. [DOI] [PubMed] [Google Scholar]
- 64.Sastry LV, Srinivasan M, Subrahmanyam V. Saffron (Crocus sativus Linn.) J Sci Industr Res (India) 1955;14A:178–84. [Google Scholar]
- 65.Basker D, Negbi M. Uses of Saffron. Economic Botany. 1983;37:228–36. [Google Scholar]
- 66.Crocus (U.S.P.) Saffron. [last accessed on 2009 Mar 16]. Available from: http://www.henriettesherbal.com/crocus sativus.html .
- 67.Khare CP. Encyclopedia of Indian Medicinal Plants. Germany: Springer; 2004. p. 166. [Google Scholar]


