Abstract
Background & objectives:
The mechanisms that protect female upper genital tract from ascending infection by microbes present in vagina are only partially understood. It is expected that epithelial cells in mucosal surfaces and their secretions directly interfere with microbial colonization and invasion. This study was aimed to demonstrate the expression of 2 kDa antimicrobial peptide which was identified and purified from female genital tract tissues using chromatographic techniques.
Methods:
Low molecular weight proteins were isolated from human female reproductive tract tissues obtained from premenopausal women. Antimicrobial activity of these LMW proteins was assessed against different reproductive tract pathogens viz., Neisseria gonorrhoeae, Group B streptococcus, Gardnerella vaginalis, Escherechia coli and Candida albicans. The expression of these peptides were also documented in reproductive tract tissues with the help of hyperimmune sera raised against the rabbits. The purified peptide was characterized by N-terminal sequencing.
Results:
Immunohistochemical and immunofluorescence studies demonstrated that 2 kDa peptide was expressed in the stratified squamous epithelial cells of the ectocervix while it was absent in columnar epithelial cells of upper genital tract. Upregulation of the expression of this peptide was observed in patients of chronic non-specific cervicitis and acute on chronic cervicitis. This purified antimicrobial peptide also showed broad spectrum antimicrobial activity against different reproductive tract pathogens.
Interpretation & conclusions:
Considering the emerging bacterial resistance against conventional antibiotics, isolation and understanding of the expression of antimicrobial peptides from female reproductive tissue extracts may provide some leads towards the development of strategies for the treatment of reproductive tract infections.
Keywords: Antimicrobials, antimicrobial peptides, female reproductive tract, innate immunity, sexually transmitted infection
Living organisms of all types produce a large repertoire of antimicrobial peptides (AMPs) that play an important role in innate immunity to microbial invasion. In higher organisms these are mainly produced on epithelial surfaces and phagocytic cells that play a crucial role in innate as well as adaptive defence systems1. AMPs, the main oxygen-independent bactericidal mechanism of phagocytic cells are stored in intracellular granules and reach high concentration within the phagolysosomes leading to rapid killing of microorganisms. Hundreds of AMPs have been isolated so far and irrespective of their origin, spectrum of activity and structure, most of the peptides share several common properties. The interaction of AMPs with membranes alters the organization of bilayer and makes it permeable, causing membrane depolarization2.
The widespread increase of bacterial resistance towards many conventional antibiotics has resulted in an intensive search for alternative antimicrobial agents. In this respect, it is encouraging to believe that AMPs which are being developed for use either as antibiotics for topical use in healthcare or as preservative in the food industry, are on the brink of breakthrough3–5. With the discovery of broad spectrum AMPs within the bovine tracheal epithelium, murine small intestine and the paneth cells of small intestine, the mucosal epithelium can no longer be considered the inert barrier to microbial invasion it was once perceived to be6–8. These AMPs are all expressed by cells which are directly exposed to microbial milieu. This observation suggests that similar AMPs may be expressed in the mucosal surfaces of the human female genital tract.
Although the human female genital tract is colonized by a variety of indigenous microorganisms and constantly exposed to a broad range of potential pathogens, the actual incidence of infection remains relatively low. The mechanisms that protect upper genital tract from ascending infection by microbes present in the vagina are partially understood. The stratified squamous epithelia of vagina and ectocervix provide a substantive cellular barrier to reproductive tract infections (RTIs) and this is supported by an indigenous microflora, an acidic microenvironment (pH 3.5 to 5.0), physical barrier of mucus derived from endocervical epithelial cells9, secretory IgA and antibacterial molecules such as lactoferrin, lysozyme and zinc10,11. The fallopian tubes and uterus are classically described as sterile sites, but microorganisms can ascend from the lower genital tract and cause pelvic inflammatory disease (PID). Several antimicrobial agents that have been described previously confer protection against infections to female reproductive tissues. These include lysozyme in amniotic fluid, cervical mucus, placenta, amnion, chorion and deciduas, β-lysin, spermines, leukins, peroxidases, transferrins and various low molecular weight peptides in amniotic fluid10–14. As the organisms causing RTIs show resistance to currently most commonly used drugs, there is an urgent need of a new antimicrobial agent for the treatment of diseases caused by such pathogens, which if remain untreated can lead to serious complications. Recently it has been observed that AMPs, e.g. human intestinal defensin-5 may be expressed in the mucosal surfaces of the human female genital tract and may provide an innate host defence against these microorganisms15–17.
Most of the reproductive tract bacteria like Chlamydia trachomatis and N. gonorrhoeae are known to infect the columnar epithelial cells of upper reproductive tract without infecting lower genital tract including ectocervix and vagina which are lined by stratified squamous epithelial cells. However, the exact mechanism which protects the cells of ectocervix and vagina from RTIs are not clearly understood. Many AMPs display selective or broad-spectrum activity against Gram-negative and Gram-positive bacteria and fungi, the molecular basis of which is not completely understood18. Women with PID secondary to N. gonorrhoeae, Trichomanas vaginalis or C. trachomatis had higher median levels of neutrophil defensins in vagina than the uninfected women and the mean levels of these AMPs were strongly associated with the presence of endometritis19. Human β-defensin-1 was shown to be expressed constitutively in healthy renal tissue, whereas induction of β-defensin-1 gene and protein expression were demonstrated only in tubulus epithelia with chronic pyelonephritis20. Although some investigators have worked on mRNA levels of some AMPs, e.g. HβD-117, defensin-515, HD-516, there are perhaps no reports on isolation, and purification of AMPs directly from human female reproductive tissues. Therefore, in the present study the AMPs from human female reproductive tract were isolated, purified and characterized. Their antimicrobial activity against different reproductive tract pathogens in vitro was noted and their expression was seen in healthy lower female genital tract and different genital tract infections.
Material & Methods
Samples, microbial strains and animals: Reproductive tract tissues (fallopian tubes, endometrium, endocervix and ectocervix) were collected in operation theatre at the time of hysterectomies performed for various benign indications in Department of Obstetrics and Gynaecology, Postgraduate Institute of Medical Education & Research (PGIMER), Chandigarh, from July, 2001 to December, 2005. All women were premenopausal and were not taking any hormonal or antibiotic therapy prior to two weeks of surgery. Different reproductive tract pathogens, N. gonorrhoeae (standard WHO F strain, procured from department of Neisseria, Statens Serum Institute, Copenhagen, Denmark), Group B Streptococcus (GBS, a clinical strain, obtained from department of Medical Microbiology, section Bacteriology, PGIMER), Gardnerella vaginalis (a clinical strain, obtained from department of Medical Microbiology section STD, PGIMER), Escherichia coli (NCTC-10418, Hi-Media, Mumbai, India) and Candida albicans (ATCC-24433, Hi-Media India) were used for antimicrobial susceptibility testing. Young healthy male rabbits (approximately 2 kg body weight, obtained from central animal house, PGIMER, Chandigarh) were used for raising the hyperimmune serum. The study was approved by Institute Ethical Committee.
Isolation of protein from female reproductive tract: Low molecular weight proteins were isolated from human female reproductive tract tissues as per the standard method8. In brief, tissues were flushed with 0.9 per cent NaCl containing 1mM chilled EDTA and weighed. These were then minced, powdered in liquid nitrogen and homogenized in chilled phosphate buffer saline containing 0.5 mM dithiothrietol, 1.5 mM EDTA and protease inhibitors (Roche Diagnostics, USA). Homogenate was extracted overnight with 5 per cent acetic acid under continuous stirring at 4°C and centrifuged at 27,000×g for 15 min at 4°C. Supernatant (supernatant 1) was collected and insoluble residue was sonicated (3 cycles, 12 sec each). The sonicated residue was re-extracted with minimum volume of 5 per cent acetic acid for 4 h with continuous stirring at 4°C, followed by centrifugation (27000×g) for 15 min at 4°C to collect supernatant II. Supernatants I and II were mixed and kept in benzyolated dialysis bags with a cut-off value of 1.2 kDa (SIGMA, USA) and dialyzed against 5 per cent acetic acid (dialysis buffer) for 48 h at 4°C with continuous stirring and every 6 hourly change of dialysis buffer. The protein was concentrated to powder form using lyophilizer (Thermo Savant, USA). Protein estimation at various steps during study was done by the modified Lowry's method21. Absorbance was read at 750 nm. Reagent blank and standard protein (BSA 1 mg/ml, Roche Diagnostics, USA?) were run along with the test protein samples. Acid Urea - Polyacryamide Gel Electrophoresis (AU-PAGE) was performed to reveal the protein profile of the acid extracted proteins by the standard method22. The photographs of the gels were taken using Gel Documentation System (Alpha Imager, 2000, USA) and analyzed with Gel Pro Analyzer software (Media Cybernetics, USA). Acid extracted crude protein was analysed for antimicrobial susceptibility testing by radial diffusion assay (RDA). Later on acid extracted crude extract was used for separation by the centrifugation filters, centripepes (Millipore, India) having cut-off value of 10 kDa, by centrifugation for 4 h (3000×g at 4°C). After separation the retentate (> 10 kDa) and filtrate (< 10 kDa) were kept in different tubes and the profile of the protein was checked by AU-PAGE. The filtrate and retentate were then lyophilized to store at -20°C for further use.
Purification of peptide: Lyophilized protein was dissolved in 1 per cent acetic acid by continuous stirring for 2 h at 4°C, incubated overnight at 4°C, clarified by centrifugation (21000×g) at 4°C for 15 min. The supernatant was subjected to gel filtration chromatography using Bio gel P-10 gel filtration media (Bio-Rad, Laboratories, USA). Approximately 140 fractions (3.0 ml each) were collected at the flow rate of 25 ml/h with 1 per cent acetic acid as eluting buffer and absorbance (OD) was measured at 280 nm. Fractions were divided in to ten different groups after plotting their OD values.
Antimicrobial susceptibility testing: Both acid extracted crude protein and proteins (retentate and filtrate) separated by centripepes were subjected to antimicrobial susceptibility testing against E. coli by radial diffusion assay22. Briefly, E.coli (Standard inoculum) were incorporated in to 1 mm thick agarose gels. Four wells (3 mm in diameter) were punched in the gel. Antibacterial activity was tested by placing 5 μl of protein samples (suspended in 0.01% acetic acid) in each well. The plates were incubated for 3 h to permit the antimicrobial peptides to diffuse in to agarose, and pouring a thin nutrient rich overlay that allowed the surviving bacteria to grow and form colonies. After overnight incubation the zone of inhibition due to antimicrobial activity of protein was calculated as: Zone of inhibition (Clear zone) = Diameter of total clear zone – Diameter of well (i.e. 3 mm).
Purified protein fractions obtained after gel filtration chromatography were also tested for their antimicrobial activity against N. gonorrhoeae, GBS, E, coli, G. vaginalis, and C. albicans. The fractions showing antimicrobial activity were then taken for further studies.
Raising of hyperimmune serum in rabbit: The purified peptide was conjugated to bovine serum albumin (BSA) (peptide: BSA:: 4:1) to increase the immunogenicity23. Hyperimmune serum against the purified peptide was raised in adult New Zealand white rabbits (2 kg weight). Pre-immunization serum was collected and stored at -20°C. The conjugated peptide (125 μg) was emulsified in 1 : 1 ratio with Complete Freund's Adjuvant (Bangalore Genei, India) and injected subcutaneously as the primary dose. Three booster doses containing 50 μg of conjugated peptide emulsified in 1: 1 ratio with Incomplete Freund's Adjuvant (Bangalore Genei, India) were injected intramuscular at intervals of 10 days. Antibody titre of the serum was estimated by standard protocol of enzyme linked immunosorbant assay (ELISA) at various steps of immunization protocol. The specificity of raised antiserum was tested by Western blot assay.
Western blotting enzyme immunoassay: Western blotting was done by the standard method of Valore et al17. After separation on AU- PAGE, the crude protein sample and purified 2 kDa peptide sample were transferred to PVDF membrane [0.2 μm pore size (Bio-Rad Laboratories, California, USA)] for 40 min with 0.7 per cent acetic acid and 10 per cent methanol at 0.18 amperes using a transblot apparatus (Bio-Rad Laboratories). Blots (strip A containing crude protein sample and strips B, C & D containing purified 2 kDa peptide) were then fixed for 2 h in 0.05 per cent glutaraldehyde in tris buffered saline (TBS), blocked overnight at 4°C with 5 per cent skim milk in TBS, and then washed with TTBS (3-4 times, 15 min each washing). Later on blots (strips A, C & D ) were incubated for 2 h at 37°C in 1: 200 diluted rabbit serum (primary antibody) in TTBS on a rocker and strip B was incubated with pre-immune serum of rabbit for the same duration. After washing with TTBS (3-4 times), blots were incubated for 1 h at 37°C on rocker with 1: 2000 dilution of Swine anti-rabbit horse raddish peroxidase (HRPO) conjugated antibody (secondary antibody) in TTBS. Blots were then washed with TTBS (4- 5 times) and developed with 6 mg of 3-3’- diaminebenzidine (DAB) in 20 ml of TBS and 30μl of H2O2. The reaction was stopped by washing with DW.
Expression studies: Peptide expression studies were performed in normal tissues of the human female reproductive tract and tissues from women with various diseases (chronic non-specific cervicitis, acute on chronic cervicitis, chronic cervicitis, chronic salpingitis, acute on chronic salpingo-oopheritis and acute on chronic endometritis). Approximately 3-5 tissue samples (non-inflamed and inflamed) were obtained from different patients with different reproductive tract conditions. First of all paraffin blocks were prepared using the tissue samples following by tissue sectioning. Processed tissue slides were stained with haematoxylin and eosine stain and immunofluorescence studies were done by using hyperimmune serum raised against the 2 kDa purified peptide.
For haematoxylin and eosine staining of tissue samples, sections (5 μm) were cut from the paraffin blocks, fixed for 10-15 min at 65°C in hot air oven and dewaxed in xylene three times (for 3-4 min each). Then tissue sections were hydrated by passing through absolute, 90 and 70 per cent alcohol (for 2 min each), washed in water and kept in nuclear stain haematoxylin for 20-30 min. Slides containing tissue sections were washed in water and differentiated by giving two dips in 1 per cent acid alcohol, kept in scotts tap water for 3-5 min, washed in water, and dehydrated by passing through 70, 90 per cent and absolute alcohol (for 2 min each). Slides containing tissue sections were dried and cleared in xylene (2-3 dips), mounted in DPX and viewed under light microscope.
Immunofluorescent staining of tissue sections: Sections (5 μm) were cut from the paraffin blocks prepared from human female reproductive tract tissues (normal and diseased tissue). Slides containing tissue sections were kept at 65 °C for 5 min, dewaxed in xylene two times (for 10 min each) and then hydrated by passing through absolute, 90, 70 and 50 per cent alcohol (for 10 min each). After hydration, sections were washed for 1.5 h in phosphate buffer saline (PBS, pH 7.2) and dried. Sections were then incubated with 1 : 200 dilution of rabbit serum (primary antibody) at 37° C for 45 min in a moist chamber, washed three times with PBS (10 min each washing). After washing slides were dried and sections on slides were incubated with 1: 20 dilution of FITC – labelled goat anti-rabbit IgG (secondary antibody), at 37°C for 30 min in a moist chamber. Sections were dried and on to the sections one drop of Evans blue (0.1% solution) and one drop of fixative (9 : 1 v/v glycerol : normal saline) was placed. After keeping the coverslip the sections were observed under the fluorescent microscope (Nikon, Japan).
Biochemical characterization of purified peptide: For biochemical characterization of this peptide the amino-acid sequence was determined, for which the purified peptide was subjected to N-terminal sequencing for 10 cycles. Peptide was purified by gel filtration chromatography using Bio gel P-10 gel filtration media (Bio-Rad, Laboratories). The purified peptide was vacuum concentrated and run on AU-PAGE. After electrophoresis the band of purified protein was blotted on to PVDF membrane (0.2μm pore size, Bio-Rad Laboratories, California, USA) in CAPS (3-cyclohexylamino-1-propanesulphonic acid) buffer as the transfer buffer for blotting using a transblot apparatus (Bio-Rad Laboratories). After transfer the band was stained with the reversible stain i.e. Ponceau S. The PVDF membrane containing the purified peptide was cut with a sterile blade, destained in 5 per cent acetic acid, dried properly and sent to National Facility for Protein Sequencing, Indian Institute of Technology, Powai, Mumbai.
Results
Antimicrobial peptide purification: The protein profile of acid extracted crude protein from the human female reproductive tract tissue was analysed by AU-PAGE (Fig. 1a). The gel stained with Coomassie brilliant blue R-250 was analysed by using Gel Pro analyzer software (Media Cybernetics, USA) by comparing intensity and mobilities of bands of molecular weight marker. Thirteen protein bands corresponding to molecular weight ranging between approx. < 3 to ~ 50 kDa were identified. The graph plotted by the software (Fig. 1b) showed peaks representing relative intensity and relative front values of individual bands. The low molecular weight bands of peptides below 3 kDa which were not clearly visible in the gel during AU-PAGE due to less peptide concentration were easily visualized by peaks in graph below 3 kDa of molecular weight marker. Fig. 1c shows the band patterns of centriprep (centrifugation filters, cut-off 10 kDa) purified protein sample. It was observed that the molecular weight of the bands in centriprep purified protein samples ranged from approx. < 3kDa to 10 kDa (Lane 2) as compared to crude protein where the molecular weight of the protein bands was observed to be in the range of approx. < 3 to 50 kDa (lane 4).
Fig. 1.
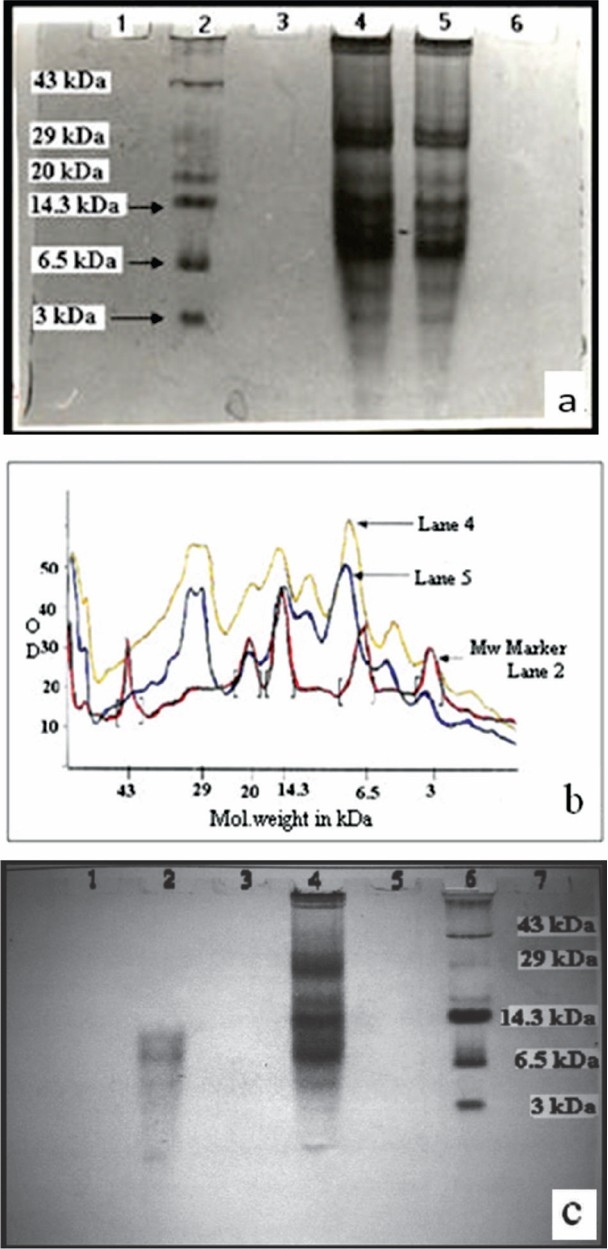
(a): AU-PAGE gel stained with Coomassie brilliant blue R-250. Lane 2 - low molecular weight marker (3-43 kDa), lanes 4 & 5 - acid extracted crude protein sample (250 μg & 200 μg/well respectively), lane 1, 3 & 6 were empty, (b): Protein profile analysis with Gel Pro analyzer software (Media Cybernetics, USA). Lane 2 (red)-low molecular weight marker (3-43 kDa), lanes 4 (yellow) and 5 (blue) - acid extracted crude protein samples (250 μg & 200 μg/well respectively), (c): AU-PAGE gel stained with Coomassie brilliant blue R-250. Lane 6 - low molecular weight marker, lane 2 - filtrate (< 10 kDa) and lane 4 - unfiltered protein sample. Lanes 1, 3, 5 and 7 were empty.
Antimicrobial susceptibility testing of Centriprep purified protein against E. coli (NCTC-10418) showed that the protein fraction with molecular weight of above 10 kDa did not show any antimicrobial activity (well 1, Fig. 1d) whereas significant antimicrobial activity was exhibited by fraction containing peptides less than 10 kDa (wells 2, 3 and 4; Fig. 1d).
Susceptibility testing of purified peptide against E.coli shows clear antimicrobial activity (Fig. 2a) of a low molecular weight peptide (~2 kDa) eluted in the fractions 9 and 10 during gel filtration chromatography (Fig. 2 b & c).
Fig. 2.
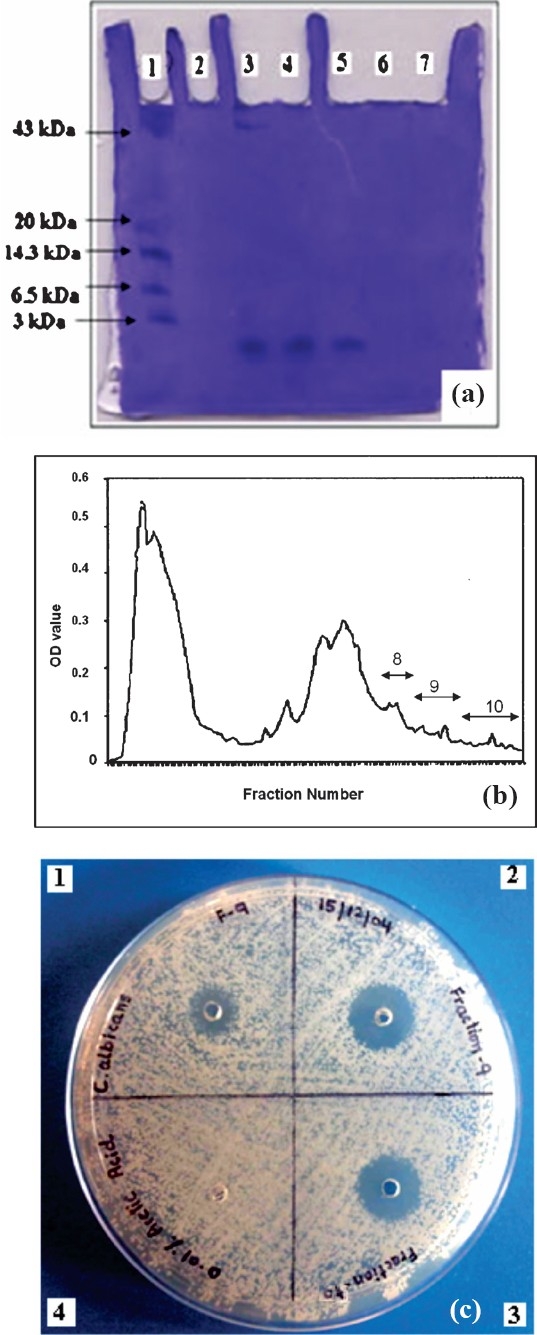
(a): AU-PAGE gel stained with Coomassie brilliant blue R-250, lane 1 - low molecular weight marker (range 3-43 kDa), lanes 3, 4, and 5 - fractions 8, 9 and 10 of gel filtration column chromatography respectively, lanes 2, 6 and 7 were empty, (b): Protein purification by gel filtration chromatography. Acid extracted protein was chromatographed in 1 % acetic acid on a Biogel P-10 column. Fractions 9 and 10 containing purified peptide were pooled (Graph plotted by taking UV absorbance of fractions at 280 nm), (c): Radial diffusion assay showing antifungal activity against Candida albicans. Wells 1 & 2 contained fraction 9 i.e.5 μg and 10 μg respectively, well 3 contained fraction 10, 10 μg. Well 4 contained 0.01% acetic acid (negative control).
Antimicrobial susceptibility testing: During the antimicrobial susceptibility testing against reproductive tract pathogens of human female reproductive tract it was observed that N. gonorrhoeae and Group B Streptococcus were susceptible to 2 kDa peptide in the same concentration (5 μg/well). In E. coli and G. vaginalis it has shown better antimicrobial activity as zone of inhibition was bigger than in case of N. gonorrhoeae and Group B Streptococcus. However, the results of antimicrobial susceptibility testing showed that C. albicans appears to be less sensitive to these peptides, since double amount (10 μg/well) of peptide identified in this study, was found to produce zone of clearance equivalent to in case of other organisms where the concentration of peptide used was 5 μg/well (Fig. 3).
Fig. 3.
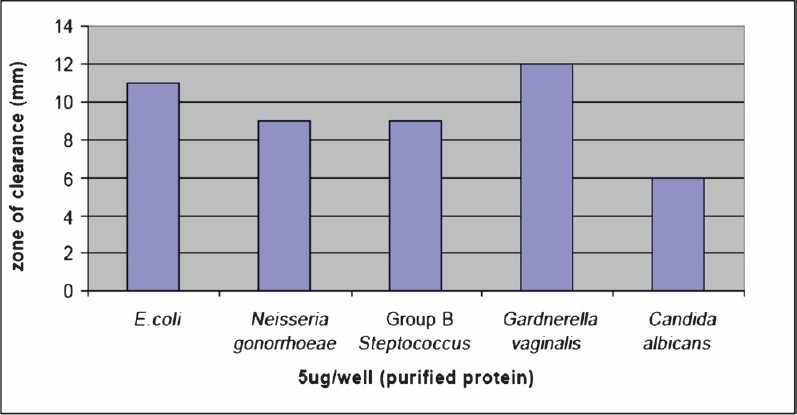
Activities of purified peptide against E.coli, N. gonorrhoeae, Group B Steptococcus, G. vaginalis and C. albicans. Radial diffusion assays were performed and the clear zone diameter were measured.
Expression of 2 kDa peptide identified in the study was seen by immunofluorescence staining of tissue sections using antiserum raised against purified peptides. Sections examined finally and included in the study were selected after haematoxyline and eosine (H & E) staining (Fig. 4).
Fig. 4.
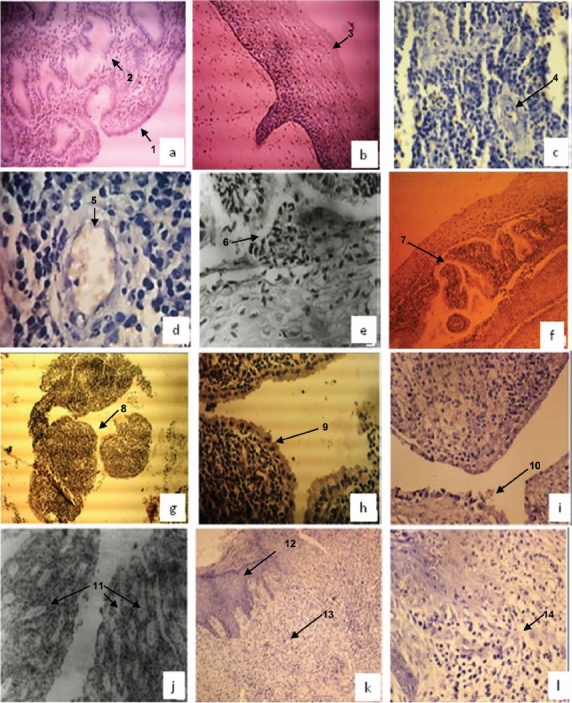
Hematoxyline & Eosine stained tissue sections of normal endometrium; [a (10X), 1: columnar epithelial cells of endometrial lining, 2: endometrial glands], normal ectocervix [b (10X) 3: stratified squamous epithelial cells of ectocervix], and patients with chronic endometritis [c & d (20X & 40X), 4 & 5: Endometrial curettings showed mainly acute inflammatory exudate consisting of polymorphs and endometrial glands infiltrated by lymphocytes], chronic non-specific cervicitis [e & f (40X & 10X), 6 & 7: focal squamous metaplasia], chronic cervicitis [g & h, (10X & 40X), 8 & 9: endocervical curettings with fragmented endocervical tissue lined by endocervical lining epithelium of columnar cells] chronic salpingitis [i, (10X), 10: tissue sections from fallopian tube showed flattened tubal lining epithelium with loss of plica and some sections showed plical adhesions], chronic salpingo-oopheritis [j (40X), 11: fallopian tubes showed inflammatory infiltrate comprising plasma cells, lymphocytes and polymorphs}, acute on chronic cervicitis] [k (10X), 12 & 13: cervix showed keratinised lymphoplastic squamous epithelial lining and stroma with mild chronic inflammation, l (40X), 14: infammed stroma].
Expression of peptide in normal female reproductive tract tissues: On immunofluorescence staining this peptide was cytosolic in nature and well expressed by the stratified squamous epithelial cells of ectocervix of human female reproductive tract (Fig. 5b to d). Clear apple green fluorescence was observed in these cells which indicated the location of the purified 2 kDa peptide. When immunofluorescence staining of normal cervix (stratified squamous epithelial cells) with rabbit pre-immune serum was done, no fluorescence was observed (Fig 5a).
Fig. 5.
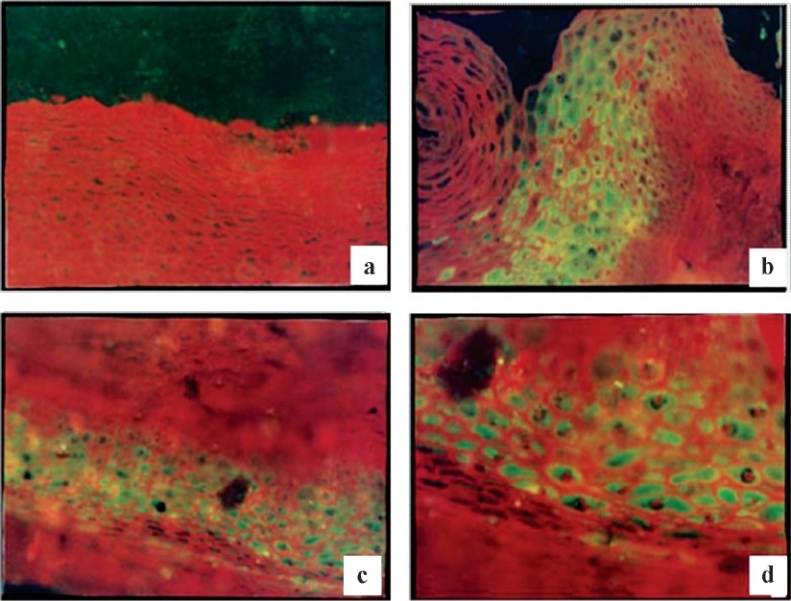
Immunofluorescent staining of normal ectocervix; a (10X), stratified squamous epithelial cells stained with rabbit pre immune serum as negative control; b, c (20X) & d (30X) stratified squamous epithelial cells stained with hyperimmune serum raised against 2 kDa peptide.
Expression of peptide in infected female reproductive tract tissue: On immunofluorescence staining the sections from the patients with chronic non-specific cervicitis and chronic cervicitis showed apple green fluorescence indicating the expression of peptides (Fig. 6 a–d). This expression was cytosolic in nature. The expression of peptide was absent in all other cases.
Fig. 6.
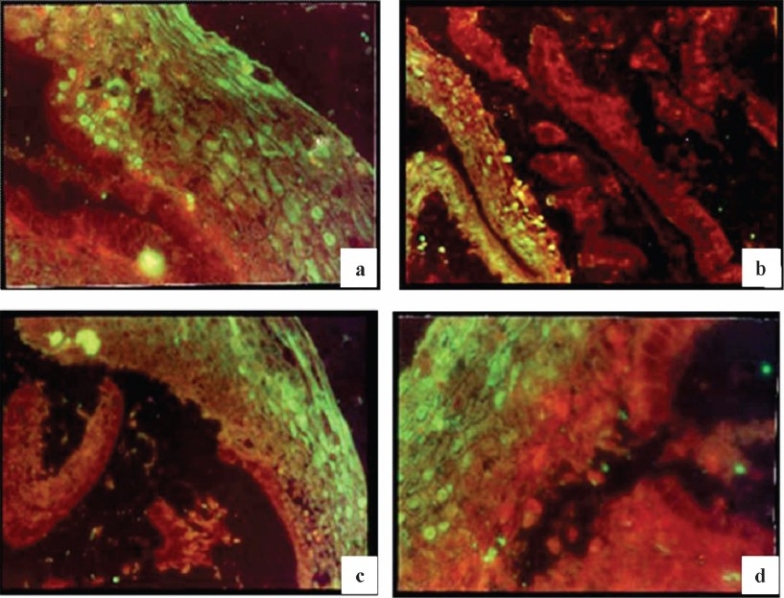
Immunofluorescent staining of inflamed cervix (stratified squamous epithelial cells) from patient with chronic non-specific cervicitis using hyperimmune serum raised against 2 kDa peptide; a & d (30X), b & c (30X).
Biochemical characterization of purified peptide: Protein sequencing results obtained from National Facility for Protein Sequencing showed that peptide constitutes of 10 amino acids viz., isoleucine, valine, glutamine, asparagine, phenylalanine, alanine, glutamic acid, phenylalanine, aspartic acid, praline.
Discussion
During the study a 2 kDa peptide was isolated and purified and it had broad spectrum antimicrobial activity against various reproductive pathogens in vitro. Expression of the peptide was studied by immunofluorescence studies which showed that this protein was a cytosolic protein localized in the cervix of the female reproductive tract. Hence, it is hypothesized that this particular peptide might be exerting antimicrobial effects in human female reproductive tract in natural conditions, particularly to the cells of ectocervix and vagina. Expression of the peptide by the stratified squamous epithelial cells of ectocervix and vagina of healthy females and upregulation of the same in cases of chronic non-specific cervicitis and acute on chronic cervicitis also support the hypothesis. Upregulation of the peptide expression in these tissues was probably induced by infections and inflammation.
The widespread increase of bacterial resistance against many conventional antimicrobial agents has resulted in an intensive search for alternative antimicrobial agents. In this context, AMPs are of major significance. The use of topical microbicides has advanced as a new health initiative to prevent the spread of sexually transmitted diseases. Topical administrations of gels containing cationic AMPs had also been shown to decrease T. vaginalis colonization of vaginal epithelium in mice24. Thus, the identification of a new peptide with broad spectrum antimicrobial activity could be the primary step towards the development of a new antimicrobial agent to treat such diseases. Detailed studies are required to understand the mechanisms of their action.
References
- 1.Hancock RE, Scott MG. The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci USA. 2000;97:8856–61. doi: 10.1073/pnas.97.16.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsuzaki K, Yoneyama S, Fujii N, Miyajima K, Yamada K, Kirino Y, et al. Membrane permeabilization mechanisms of a cyclic antimicrobial peptide, tachyplesin I, and its linear analog. Biochemistry. 1997;36:9799–806. doi: 10.1021/bi970588v. [DOI] [PubMed] [Google Scholar]
- 3.Nes IF, Holo H. Class II antimicrobial peptides from lactic acid bacteria. Biopolymers. 2000;55:50–61. doi: 10.1002/1097-0282(2000)55:1<50::AID-BIP50>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Zasloff M. Reconstructing one of nature's designs. Trends Pharmacol Sci. 2000;21:236–8. doi: 10.1016/s0165-6147(00)01490-5. [DOI] [PubMed] [Google Scholar]
- 5.Cleveland J, Montville TJ, Nes IF, Chikindas ML. Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol. 2001;71:1–20. doi: 10.1016/s0168-1605(01)00560-8. [DOI] [PubMed] [Google Scholar]
- 6.Diamond G, Zasloff M, Eck H, Brasseur M, Maloy WL, Bevins CL. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci USA. 1991;88:3952–6. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones DE, Bevins CL. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 1993;315:187–92. doi: 10.1016/0014-5793(93)81160-2. [DOI] [PubMed] [Google Scholar]
- 8.Eisenhauer PB, Harwig SS, Lehrer RI. Cryptdins: antimicrobial defensins of the murine small intestine. Infect Immun. 1992;60:3556–65. doi: 10.1128/iai.60.9.3556-3565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schumacher GF. Biochemistry of cervical mucus. Fertil Steril. 1970;21:697–705. [PubMed] [Google Scholar]
- 10.Chimura T, Hirayama T, Takase M. Lysozyme in cervical mucus of patients with chorioamnionitis. Jpn J Antibiot. 1993;46:726–9. [PubMed] [Google Scholar]
- 11.Cohen MS, Britigan BE, French M, Bean K. Preliminary observations on lactoferrin secretion in human vaginal mucus: variation during the menstrual cycle, evidence of hormonal regulation, and implications for infection with Neisseria gonorrhoeae. Am J Obstet Gynecol. 1987;157:1122–5. doi: 10.1016/s0002-9378(87)80274-0. [DOI] [PubMed] [Google Scholar]
- 12.Rozansky R, Persky S, Bercovice B. Antibacterial action of human cervical mucus. Proc Soc Exp Biol Med. 1962;110:876–81. doi: 10.3181/00379727-110-27678. [DOI] [PubMed] [Google Scholar]
- 13.Barling PM, John MJ, Walsh JR, Niall HD. The isolation and characterization of lysozyme from human foetal membranes: a comparison with the enzyme from other sources. Comp Biochem Physiol B. 1985;81:509–13. doi: 10.1016/0305-0491(85)90352-9. [DOI] [PubMed] [Google Scholar]
- 14.Scane TM, Hawkins DF. Antibacterial activity in human amniotic fluid: relationship to zinc and phosphate. Br J Obstet Gynaecol. 1984;91:342–8. doi: 10.1111/j.1471-0528.1984.tb05920.x. [DOI] [PubMed] [Google Scholar]
- 15.Svinarich DM, Wolf NA, Gomez R, Gonik B, Romero R. Detection of human defensin 5 in reproductive tissues. Am J Obstet Gynecol. 1997;176:470–5. doi: 10.1016/s0002-9378(97)70517-9. [DOI] [PubMed] [Google Scholar]
- 16.Quayle AJ, Porter EM, Nussbaum AA, Wang YM, Brabec C, Yip KP, et al. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol. 1998;152:1247–58. [PMC free article] [PubMed] [Google Scholar]
- 17.Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB, Jr, Ganz T. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101:1633–42. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyasaki KT, Lehrer RI. Beta-sheet antibiotic peptides as potential dental therapeutics. Int J Antimicrob Agents. 1998;9:269–80. doi: 10.1016/s0924-8579(98)00006-5. [DOI] [PubMed] [Google Scholar]
- 19.Wiesenfeld HC, Heine RP, Krohn MA, Hillier SL, Amortegui AA, Nicolazzo M, et al. Association between elevated neutrophil defensin levels and endometritis. J Infect Dis. 2002;186:792–7. doi: 10.1086/342417. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann J, Retz M, Harder J, Krams M, Kellner U, Hartmann J, et al. Expression of human beta-defensins 1 and 2 in kidneys with chronic bacterial infection. BMC Infect Dis. 2002;2:20. doi: 10.1186/1471-2334-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shakir FK, Audilet D, Drake AJ, 3rd, Shakir KM. A rapid protein determination by modification of the Lowry procedure. Anal Biochem. 1994;216:232–3. doi: 10.1006/abio.1994.1031. [DOI] [PubMed] [Google Scholar]
- 22.Lehrer RI, Rosenman M, Harwig SS, Jackson R, Eisenhauer P. Ultrasensitive assays for endogenous antimicrobial polypeptides. J Immunol Methods. 1991;137:167–73. doi: 10.1016/0022-1759(91)90021-7. [DOI] [PubMed] [Google Scholar]
- 23.Adrian TE. Production of Antisera using Peptide Conjugates. In: Walker JM, editor. The protein protocols handbook. Fredericksburg USA: Humana Press; 2002. pp. 953–62. [Google Scholar]
- 24.Lushbaugh WB, Blossom AC, Shah PH, Banga AK, Jaynes JM, Cleary JD, et al. Use of intravaginal microbicides to prevent acquisition of Trichomonas vaginalis infection in Lactobacillus-pretreated, estrogenized young mice. Am J Trop Med Hyg. 2000;63:284–9. [PubMed] [Google Scholar]


