Abstract
Background & objectives:
Mycobacterium w (M.w) is a saprophytic cultivable mycobacterium and shares several antigens with M. tuberculosis. It has shown good immunomodulation in leprosy patients. Hence in the present study, the efficacy of M.w immunotherapy, alone or in combination with multi drug chemotherapeutic regimens was investigated against drug sensitive M. tuberculosis H37Rv and three clinical isolates with variable degree of drug resistance in mice.
Methods:
BALB/c mice were infected with M. tuberculosis H37Rv (susceptible to all first and second line drugs) and three clinical isolates taken from the epository of the Institute. The dose of 200 bacilli was used for infection via respiratory route in an aerosol chamber. Chemotherapy (5 days/wk) was given one month after infection and the vaccinated group was given a dose of 1×107 bacilli by subcutaneous route. Bacterial load was measured at 4 and 6 wk after initiation of chemotherapy.
Results:
M.w when given along with chemotherapy (4 and 6 wk) led to a greater reduction in the bacterial load in lungs and other organs of TB infected animals compared to. However, the reduction was significantly (P<0.05) more in terms of colony forming units (cfu) in both organs (lungs and spleen).
Conclusion:
M.w (as immunomodulator) has beneficial therapeutic effect as an adjunct to chemotherapy.
Keywords: Chemotherapy, immunotherapy, Mycobacterium tuberculosis, Mycobacterium w
Tuberculosis (TB) is an air borne communicable disease caused by Mycobacterium tuberculosis. Despite five decade of control programmes and the availability of efficacious drugs, TB still kills about two million people annually1. TB control is further complicated by its unfortunate synergism with human immunodeficiency virus (HIV)2 and is the most common cause of morbidity and mortality in HIV positive patients. There is an alarming rise of incidence linked to the devastating impact of HIV epidemics, population movement and deficiencies of current tuberculosis control programme. The estimates of the global burden of disease caused by TB in 2009 are: 9.4 million incident cases (range, 8.9-9.9 million), 14 million prevalent cases (range, 12-16 million), 1.3 million deaths among HIV-negative people (range, 1.2-1.5 million) and 0.38 million deaths among HIV-positive people (range, 0.32-0.45 million)3. In India, about 2.0 million people develop tuberculosis every year and the TB mortality in the country has reduced from over 42/100,000 population in 1990 to 23/100,000 population in 20104.
Variable protective efficacies of vaccination with M. bovis BCG have been reported from different parts of the world5. During the last decade, extensive work has been done on the development of potential tuberculosis vaccine candidates using the mice and guinea pig models, and more than 200 candidate vaccines have been tested. Some of the promising candidates have been identified and at least nine vaccines have entered in clinical evaluation process. However, there is a need to continue the search for development of much better animal models of chemotherapy and latency, additional vaccine candidates or vaccination strategies and testing them in human subjects6,7. Immunotherapy need to be investigated especially in multi drug resistant disease as well as for improving the cure rates by eradication of persisters, but all these aspects can be considered only after adequate experimentation8. Hence novel vaccination strategies are warranted.
Mycobacterium w (M.w) is a saprophytic soil cultivable mycobacterium, which shares several antigens with M. tuberculosis and M. leprae. Mycobacteriu w (M.w) named as Mycobacterium indicus prani9, was isolated from sputum of a patient and Talwar and his team demonstrated that vaccination of mice with killed Mycobacterium w protected the animals against subsequent tuberculosis10. Further, it has been observed that the addition of immunotherapy (both BCG and M.w) to chemotherapy specially in highly bacillated cases of leprosy helped in achieving faster bacteriological and histological response11,12. Immunization with Mycobacterium w followed by M. tuberculosis infection showed protection from TB in BCG non responder strains of mice13. Promising effect of addition of immunotherapy with M.w has also been reported in pulmonary tuberculosis cases14. Studies in animals (different animal models like mice, guinea pigs, etc.) need to be done to evaluate the beneficial therapeutic effects of M.w vaccine in drug resistant tuberculosis.
The present study was thus undertaken to evaluate the efficacy of M.w vaccine alone and in combination with different anti-tuberculosis agents in murine model of TB against different drug resistant isolates of M. tuberculosis.
Material & Methods
Drugs & isolates:
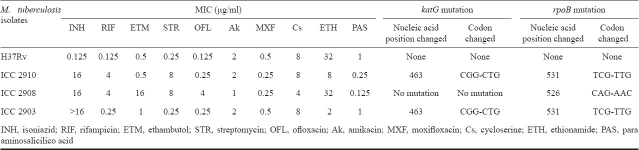
Isoniazid (INH), rifampicin (RIF), ethambutol (ETM), pyrazinamide (PZA), cyclosyrine (Cs), para amino salisalic acid (PAS), amikacin (Ak), and ethionamide (ETH) were purchased from Sigma Chemical Co., USA. Moxifloxacin (MXF) was provided by Bayer, Milan, Itlay. RIF and ETH were dissolved in dimethyl sulphoxide and was subsequently diluted in distilled water. INH, PZA, Cs, Ak, PAS were dissolved in distilled water, MXF was dissolved in 0.1M NaOH and was subsequently diluted in distilled water. M. w was purchased from Cadila Pharmaceuticals Ltd. (Le Sante) Ahmedabad, India. M. tuberculosis H37Rv (TMC 102, which is susceptible to all first-line as well as second line drugs) and three clinical isolates (ICC 2910,ICC 2908,ICC 2903) of M. tuberculosis (which were isolated from the patients) were taken from repository of National JALMA Institute for Leprosy and Other Mycobacterial Diseases, Agra. The identity of the clinical isolates was confirmed by PCR-RFLP detection based on primers rpoB15 and hsp 65 kD16 and 1.8 kd fragment of 16-23S rRNA region17. The MICs of isolates against INH, RIF, ETM, PZA, Cs, PAS, Ak, Eth and MXF. Were determined by Resazurin microtitre assay (REMA) method18,19.
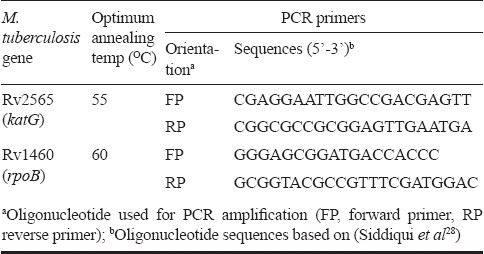
Sequencing of katG and rpoB from M. tuberculosis isolates included in the study - Oligonucleotides used in PCRs are given in Table I. PCR was performed in the reaction volume of 20 μl, which contained 1X buffer [200 mM Tris-Cl (pH 8.0), 500 mM KCl and 25 mM MgCl2], 0.2 mmol of each deoxynucleotide (dATP, dGTP, dCTP, dTTP), 10 pmol of each primer (10 pmol/μl), 1.5U of Taq DNA polymerase and target sample. The PCR assay was carried out in a DNA thermal cycler (ABL, USA) using the following amplification conditions; 5 min at 94°C, followed by 35 cycles, each of 1 min at 94°C, 1 min at 60°C and1 min at 72°C, with a final extension of 10 min at 72°C for rpoB and 5 min at 94°C, followed by 35 cycles, each of 1 min at 94°C, 1 min at 55°C and 1 min at 72°C for katG, with a final extension of 10 min at 72°C. PCR products were electrophoresed in 1.5 per cent agarose gel and visualized under UV light and photographed. Each amplification yielded one band of the expected molecular size. The resulting PCR-amplified products of 414 and 350 bp for katG and rpoB, respectively, were gel purified using Qiagen kit (Germany) and used for direct sequencing (Applied Biosystems).
Table I.
Oligonucleotides employed to amplify or sequence rpoB and katG genes of M. tuberculosis isolates
In vivo experiments:
Mice - BALB/c mice were bred in the Experimental Animal Facility at National JALMA Institute for Leprosy and Other Mycobacterial Diseases, Agra. The adult (8 to 10 wk old or 20-22 g weight) mice were kept in sterile isolators in the biohazard animal unit of BSL-3 Laboratory. Permission was obtained from the Institute's Animal Ethical Committee for carrying out this study.
Bacteria and infection - M. tuberculosis H37Rv and the three MDR M. tuberculosis isolates were grown to mid-log phase in Middlebrook 7H9 medium (Difco Laboratory, USA), supplemented with 10 per cent; albumin dextrose catalase (ADC); (Difco Lab.) at 37°C. The turbidity of the cultures were adjusted to Mc Farland standard No.1 and the inoculum size was confirmed by titration and spotting serial dilutions on Middlebrook 7H11 plates supplemented with 10 per cent oleic acid albumin dextrose catalase (OADC) enrichment. The plates were incubated at 37°C for 4 wk prior to counting of colonies.
Culture was diluted in sterile saline containing 0.04 per cent tween 20 and clumping was disturbed by 20 repeated aspirations through a 29-gauge needle. Pulmonary infection of mice with M. tuberculosis was performed using aerosol generator as described previously20. Four groups of infected mice (6/group) were monitored regularly for clinical status.
Treatment: The treatment of the mice was started after one month of infection. Treatment by combination was given 5 days per week for 4 and 6 wk. All drugs except Ak, which is an injectable drug, were administered by gavage. The concentrations of drugs were as follows: mice infected with M. tuberculosis H37Rv, INH 5 mg/kg, RIF 10 mg/kg, EMB 15 mg/kg and PZA 25 mg/kg body weight; mice infected with M. tuberculosis ICC 2910, PAS 150 mg/kg, EMB, Cs, MXF and Ak 15 mg/kg body weight; mice infected with M. tuberculosis ICC 2903, PZA 25 mg/kg, EMB, MXF, and Ak 15 mg/kg body weight and mice infected with M. tuberculosis ICC 2908, PAS 150 mg/kg, Cs, MXF, Ak and ETH 15 mg/kg body weight. Mycobacterium w (M.w) was administered subcutaneously at 1 × 107 bacilli/mouse both alone and in combination with drugs7. A booster dose was given two wk after the first dose. Animals were not administered pyridoxin along with isoniazid.
Bacterial load in tissues: Bacterial loads in the lungs and spleen of infected as well as treated mice were evaluated at two days after completion of treatment and two week later. The mice were sacrificed and their organs were homogenized in 1 ml of normal saline containing 0.04 per cent of tween 20. Ten-fold serial dilutions of homogenates were plated in duplicates on to Middlebrook 7H11 agar plates supplemented with 10 per cent OADC and incubated at 37°C for one month. The colonies were counted after incubation period and expressed as log10 cfu/g tissue.
Statistical analysis: Student t-test was applied to find out the differences among different groups of animals.
Results & Discussion
In vitro evaluation of drugs and gene sequencing of MDR isolates: The susceptibility pattern of M. tuberculosis H37Rv, and three M. tuberculosis isolates (ICC 2910, ICC 2908 and ICC 2903) was evaluated against different drugs. M. tuberculosis isolate ICC 2910 was resistant to INH, RIF and STR. M. tuberculosis isolate ICC 2908 was resistant to INH, RIF, ETM, STR and OFL. M. tuberculosis ICC 2903 was resistant to INH and RIF, while M. tuberculosis H37Rv was susceptible to all drugs (Table II). Mutations in the hot-spot regions of various loci were characterized. Mutation in rpoB gene from TCG->TTG at 531 codon was found in both ICC 2910 and ICC 2903 isolates while in ICC 2908 mutation was found CAG->AAC at 526 codon. Mutation at these codons are correlated with high degree of rifampicin resistance21. A common mutation from CGG->CTG at 463 codon was found in katG in both ICC 2910 and ICC 2903 isolates (Table II).
Table II.
MICs of antituberculosis agents against clinical isolates of M. tuberculosis and M. tuberculosis H37Rv, as determined by broth dilution method and analysis of rpoB and katG genes mutations
In vivo comparison of INH+RIF+ETM+PZA, immunotherapy alone and in combination against M. tuberculosis H37Rv: The activity of INH+RIF+ETM+PZA alone, with M. w and in combination of drugs with M. w against M. tuberculosis H37Rv murine model was examined in 4 and 6 wk treatment. In the beginning, the bacillary load (mean log10 cfu) in lungs and spleen were 4.86 ± 0.167 and 2.124 ± 0.152, respectively in untreated mice (Fig. 1a). After 4 wk of treatment, the mean cfu for the M.w vaccinated mice was significantly lower (P<0.05) than those for both lungs and spleens of early control groups. Although, the drug treated mice had better activities alone but in combination with M.w, a significant reduction in the number of M. tuberculosis organisms was observed in both lungs and spleen. The differences in organ cell counts between groups receiving drugs alone and in combination with M. w were significant different (P<0.05).
Fig. 1.
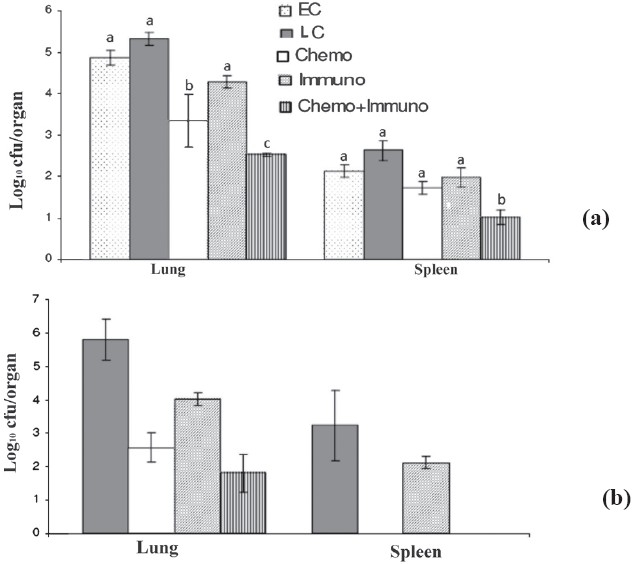
Mean (n=6) ± SD of M. tuberculosisstrain H37Rv in lungs and spleen of infected mice after once-daily treatment for 5 days per week for 4 (a) and 6 wk (b) with INH+RIF+ETM+PZA. EC, early control; LC, late control; Chemo, chemotherapy; Immuno, immuntherapy; Chemo + Immuno, chemotherapy + immunotherapy. a, b and c are statistically significant (P<0.05).
At 6 wk, drugs alone, or in M. w combination reduced the bacillary load in lung (from log10 2.56 ± 0.44 to 1.80 ± 0.56) (P<0.05) while the reduction in spleen was much less (no cfu seen). On the other hand, in immunotherapy group, the reduction in lungs and in spleen was much less compared to drug treated as well as drug combination with M. w group (P<0.05).
In vivo comparison of PAS+EMB+Cs+MXF+Ak, immunotherapy alone and in combination against M. tuberculosis ICC 2910: The activity of PAS+EMB+Cs+MXF+Ak, M. w alone, and in combination were studied against M. tuberculosis ICC 2910 (which is a INH, RIF, STR and OFL resistant strain). At the beginning of the treatment, the bacillary load was log10 5.22 ± 0.20 cfu in lung and log10 2.57 ± 0.43 cfu in spleen of untreated mice (Fig. 2). After 4 wk of immunization, Immuno + Chemo group was significantly different in terms of bacilli in the lungs when the counts were compared to early control groups but similar to Chemo as well as Immuno treated groups (Fig. 2a and b). After 4 and 6 wk, the mice treated with drugs in combination with M. w had comparatively better clearance of bacilli from lungs than drugs alone as well as immuno group; however, the values in lung and spleens were only statistically significant (P<0.05) after 6 wk as well as spleens drug treated and immunotherapy group compared with other groups as shown in (Fig. 2).
Fig. 2.
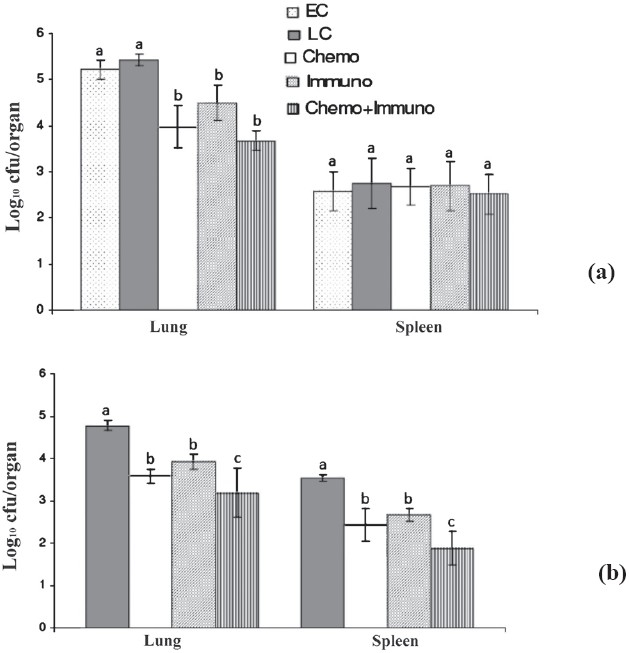
Mean (n=6) + SD of M. tuberculosis MDR strain ICC 2910 in lungs and spleen of infected mice after once-daily treatment for 5 days per week for 4 (a) and 6 wk (b) with PAS+EMB+Cs+Ak. EC, early control; LC, late control; Chemo, chemotherapy; Immuno, immuntherapy; Chemo + Immuno, chemotherapy + immunotherapy. a ,b and c are statistically significant (P<0.05).
In vivo comparison of EMB+PZA+MXF+Ak, immunotherapy alone and in combination against M. tuberculosis ICC2908: The activity of drug treated (EMB+PZA+MXF+Ak), immunotherapy alone and in combination was studied in M. tuberculosis ICC 2903 (which is resistant to INH and RIF) and in the beginning of treatments, the mean load was log10 4.68 ± 0.167 cfu and log10 2.042 ± 0.29 cfu in lungs and spleen respectively of untreated mice. After 4 wk of immunization with M. w, the bacillary load decreased significantly (P<0.05) in lungs and spleens compared to respective early control groups. EMB+PZA+MXF+Ak treated group also showed significant (P<0.05) reduction in bacillary load both in lung and spleen but in combination with M. w, the reduction in the number of M. tuberculosis organisms was more (Fig. 3a). Two additional weeks of treatment further reduced significantly the numbers of the mean cfu in both organs of the mice of drug treated, and drug and immunothery group. The mean cfu values for the M. w combination group were significant lower (P<0.05) than those for drug alone in both organs (Fig. 3b).
Fig. 3.
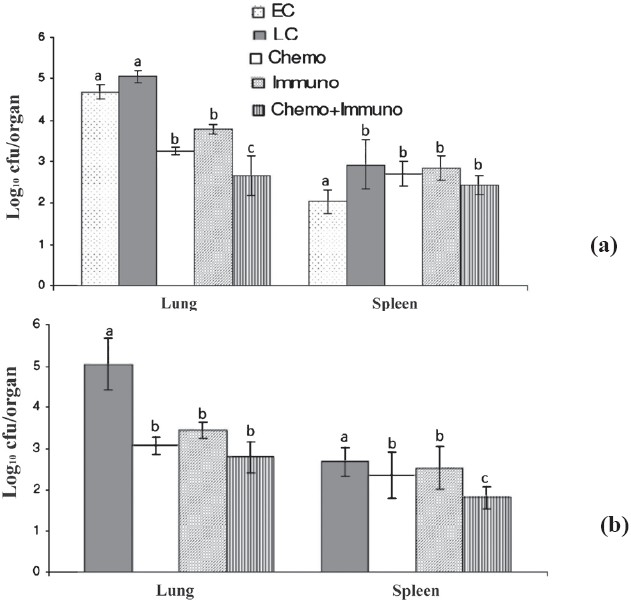
Mean (n=6) ± SD of M. tuberculosis MDR strain ICC 2903 in lungs and spleen of infected mice after once-daily treatment for 5 days per week for 4 (a) and 6 wk (b) with EMB+PZA+MXF+Ak. EC, early control; LC, late control; Chemo, chemotherapy; Immuno, immuntherapy; Chemo + Immuno, chemotherapy + immunotherapy. a,b and c are statistically significant (P<0.05%).
In vivo comparison of PAS+Cs+MXF+Ak+ETH, immunotherapy alone and in combination M. tuberculosis ICC 2908: The activity of PAS-Cs-MXF-Ak-ETH or M. w alone and in combination was studied after 4 and 6 wk in mice infected with M. tuberculosis ICC 2908, resistant to INH, RIF, ETM, STR and OFL. Four and six wk treatment with PAS-Cs-MXF-Ak-ETH reduced the mean cfu more in both lung and spleen of mice than those mice given no treatment (P<0.05), but in the M. w combination treated group the reduction was statistically more in lung as well than the drug treated group (P<0.05). Treatment alone with M. w also significantly (P<0.05) reduced the mean cfu in the lung and spleen compared with untreated mice. However, difference in cell counts between drug treatment alone and M. w combination treated groups was not significant (Fig. 4).
Fig. 4.
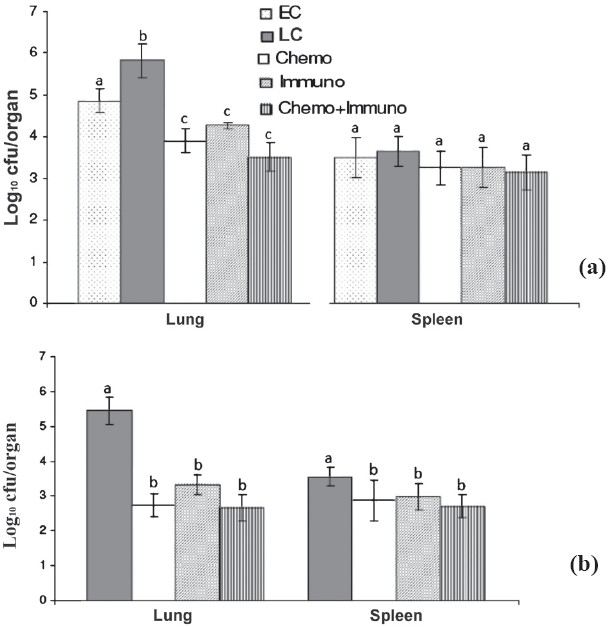
Mean (n=6) + SD of M. tuberculosis MDR strain ICC 2908 in lungs and spleen of infected mice after once-daily treatment for 5 days per week for 4 (a) and 6 wk (b) with PAS+ MXF+Cs+Ak+Ethio. EC, early control; LC, late control; Chemo, chemotherapy; Immuno, immuntherapy; Chemo + Immuno, chemotherapy + immunotherapy. a,b and c are statistically significant (P<0.05).
Chemotherapy of tuberculosis caused by multi drug resistant strains is limited to relatively inefficacious and toxic drugs22. Besides direct bactericidal activity, long-term effectiveness is one of the most important features which needs to considered while developing newer drugs for chemotherapy23. In vitro susceptibility data coupled with evaluation of agents against Mycobacterium isolates in the murine system are expected to provide important information for clinical trials24.
Vaccination of mice with killed Mycobacterium w has been shown to protect the animals against subsequent tuberculosis10,11,25. Thus Mycobacterium w, as immunomodulator with chemotherapy was included in this study as an important tool to treat MDR-TB in murine model.
The regimen containing Ak, PZA, MXF, and ETH is currently the most potent one against MDR-TB in the mouse model10. By using this regimen, lung and spleen cultures have been reported to be negative in a significantly shorter treatment duration (9 months) than the conventional TB regimen consisting of streptomycin, INH, and ethambutol, which failed to render the organ culture negative even after 12 months of treatment26. However, use of this regimen in humans is less preferred due to its poor efficacy than RIF-INH-PZA, and hepatotoxicity associated with ETH- pyrazinamide combination27.
In the chemotherapy group of mice infected with H37Rv, after 6 wk a 3 log reduction in cfu in lungs was seen while spleens were culture negative. On the other hand, the different regimens used against MDR strains significantly reduced the bacterial load from the lungs (ranging from 1.2 to 2 log) but the reduction was not significant in spleen (ranging from 0.3 to 0.8 log). Among the different chemotherapy regimens used in MDR infected mice PAS-Cs-MXF-Ak regimen (which is used against INH, RIF, ETM, STR and OFL resistant strain) was most effective.
To validate the effect of M. w immunotherapy, M. w alone and in combination with drugs against susceptible and MDR isolates of M. tuberculosis were tested in murine model. In M. w treated mice infected with H37Rv, after 6 wk of therapy, a reduction was seen in lung as well as spleen (1.8 log and 1.1 log cfu, respectively) though it was less compared to the chemotherapy. On the other hand, in MDR isolates, reduction of 1 to 1.6 log cfu in lungs and 0.2 to 0.9 log cfu in spleen of vaccine treated groups was observed. These results suggested that the immunotherapy though killed the tuberculosis bacilli, but the rate of killing was much slower compared to chemotherapy.
When immunotherapy (M. w) was given along with chemotherapy, there was reduction of 4 log cfu in lungs of H37Rv infected mice whereas the spleens were cultures negative after 6 wk of treatment. With MDR isolates, this combination made a reduction of 1.6 to 2.3 log cfu in lungs while in spleens the reduction ranged from 0.8 to 1.7 log cfu. Thus immunotherapy with chemotherapy resulted in better reduction in cfu in both lungs and spleen compared to chemotherapy/immunotherapy alone.
The organisms used in this study were found to be differentially resistant to anti-TB drugs. It is likely that quantitative susceptibility testing of isolates would provide information useful in designing treatment regimens from individuals infected with resistant organisms. Furthermore, individuals may benefit from immunization with M. w, as a significant decrease in numbers of M. tuberculosis lesions and bacillary load in lungs and spleens was observed in immunized mice.
In conclusion, Mycobacterium w, as immunomodulator with chemotherapy could be an important tool to treat MDR-TB in mouse model. Immunotherapy alone had limited role in the treatment as was evident in the study. The findings point towards a beneficial therapeutic effect of Mycobacterium w as an adjunct to chemotherapy.
Acknowledgments
The authors acknowledge the financial support from Department of Biotechnology, Government of India and Indian Council of Medical Research, New Delhi, for running the BSL-3 Laboratory for animal experiments.
References
- 1.Dye C, Lonnroth K, Jaramillo E, Williams BG, Raviglione M. Trends in tuberculosis incidence and their determinants in 134 countries. Bull World Health Organ. 2009;87:683–91. doi: 10.2471/BLT.08.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nunes EA, De Capitani EM, Coelho E, Joaquim OA, Figueiredo IRO, Cossa AM, et al. Patterns of anti-tuberculosis drug resistanceamong HIV infected patients in Maputo, Mozambique, 2002-2003. Int J Tuberc Lung Dis. 2005;9:494–500. [PubMed] [Google Scholar]
- 3.Global tuberculosis control. Geneva: WHO; 2010. WHO. [Google Scholar]
- 4.RNTCP Report: TB India 2011. [accessed on August 20, 2011]. Available from: http://www.tbcindia.org .
- 5.Fine PEM. Variation in protection by BCG: implication of and for heterologous immunity. Lancet. 1995;346:1339–45. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 6.Ly LH, McMurray DN. Tuberculosis: Vaccine in pipeline. Expert Rev Vaccines. 2008;7:635–50. doi: 10.1586/14760584.7.5.635. [DOI] [PubMed] [Google Scholar]
- 7.Gupta UD, Katoch VM, Mcmurray DN. Current status of TB vaccines. Vaccine. 2007;25:3742–51. doi: 10.1016/j.vaccine.2007.01.112. [DOI] [PubMed] [Google Scholar]
- 8.Katoch VM. Vaccines for Mycobacterial diseases: A review. Punjab Univ Res Bull. 2000;52:1–12. [Google Scholar]
- 9.Ahmed S, Jaber AA, Mokaddas E. Frequency of embB codon 306 mutations in ethambutol-susceptible and -resistant clinical Mycobacterium tuberculosis isolates in Kuwait. Tuberculosis. 2007;87:123–9. doi: 10.1016/j.tube.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Singh IG, Mukherjee R, Talwar GP. Resistance to inoculation of Mycobacetrium tuberculosis H37Rv in mice of different inbred strains following immunization with a leprosy vaccine based on Mycobaceterium w. Vaccine. 1991;9:10–4. doi: 10.1016/0264-410x(91)90309-t. [DOI] [PubMed] [Google Scholar]
- 11.Talwar GP, Zaheer SA, Mukherjee R, Walia R, Misra R, Sharma AK, et al. Immuotherapeutic effects of vaccine based on a saprophytic cultivable Mycobacterium w, in multi-bacillary leprosy patients. Vaccine. 1990;8:121–9. doi: 10.1016/0264-410x(90)90134-8. [DOI] [PubMed] [Google Scholar]
- 12.Katoch K, Katoch VM, Natrajan M, Sreevatsa, Gupta UD, Sharma VD, et al. 10-12 years follow-up of highly bacillated BL/LL leprosy patients on combined chemotherapy and immunotherapy. Vaccine. 2004;22:3649–57. doi: 10.1016/j.vaccine.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 13.Talwar GP. Continuing challenges in tuberculosis research. Indian J Tuberc. 1992;39:67–9. [Google Scholar]
- 14.Patel N, Deshpande MM, Shah M. Effect of an immunomodulator containing Mycobacterium w on sputum conversion in pulmonary tuberculosis. J Indian Med Assoc. 2002;100:191–3. [PubMed] [Google Scholar]
- 15.Kim BJ, Lee KH, Park BN, Kim SJ, Bai GH, Kim SJ, et al. Differentiation of mycobacterial species by PCR-restriction analysis of DNA (342 base pairs) of the RNA polymerase gene (rpoB) J Clin Microbiol. 2001;39:2102–9. doi: 10.1128/JCM.39.6.2102-2109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Telenti A, Marchesi F, Balz M, Bally F, Böttger EC, Bodmer T. Rapid identification of Mycobacteria to the species level by PCR and restriction enzyme analysis. J Clin Microbiol. 1993;31:175–8. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katoch VM, Parashar D, Chauhan DS, Singh DS, Sharma VD, Ghosh S. Rapid identification of mycobacteria by gene amplification restriction analysis technique targeting 16S-23S ribosomal DNA spacer and flanking regions. Indian J Med Res. 2007;125:155–62. [PubMed] [Google Scholar]
- 18.Palomino JC, Martin A, Camacho M, Guerra H, Swings J, Portaels F. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2002;46:2720–2. doi: 10.1128/AAC.46.8.2720-2722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin A, Camacho M, Portaels F, Palomino JC. Resazurin microtiter assay plate testing of Mycobacterium tuberculosis susceptibilities to second-line drugs: rapid, simple, and inexpensive method. Antimicrob Agents Chemother. 2003;47:3616–9. doi: 10.1128/AAC.47.11.3616-3619.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain R, Dey B, Dhar N, Rao V, Singh R, Gupta UD, et al. Modulation of cytokine milieu in lungs by recombinant BCG over expressing Ag85C confers enhanced and long lasting protection against tuberculosis. PLoS Pathogen. 2008;3:1–11. doi: 10.1371/journal.pone.0003869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musser JM. Antimicrobial agent resistance in mycobacteria: molecular genetic insights. Clin Microbiol Rev. 1995;8:496–514. doi: 10.1128/cmr.8.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chambers HF, Turner J, Schecter GF, Kawamura M, Hopewell PC. Imipenem for treatment of tuberculosis in mice and humans. Antimicrob Agents Chemother. 2005;49:2816–21. doi: 10.1128/AAC.49.7.2816-2821.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenaerts AM, Chase SE, Chmielewski AJ, Cynamon MH. Evaluation of rifapentine in long-term treatment regimens for tuberculosis in mice. Antimicrob Agents Chemother. 1999;43:2356–60. doi: 10.1128/aac.43.10.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klemens SP, Destefano MS, Cynamon MH. Therapy of mutidrug-resistant lesions from studies with mice. Antimicrob Agents Chemother. 1993;37:2344–7. doi: 10.1128/aac.37.11.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta A, Geetha N, Mani J, Upadhyay P, Katoch VM, Natarajan M, et al. Immunogenicity and protective efficacy of Mycobacterium w against M. tuberculosis in mice immunized with live versus heat killed M. w by the aerosol or parenteral route. Infect Immun. 2009;77:223–31. doi: 10.1128/IAI.00526-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lounis N, Veziris N, Chauffour A, Pernot CT, Andries K, Jarlier V. Combination of R207010 with drugs used to treat multidrug-resistant tuberculosis have the potential to shorten treatment duration. Antimicrob Agents Chemother. 2006;50:3543–7. doi: 10.1128/AAC.00766-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wada M. The adverse reaction of anti-tuberculosis drugs and its management. Nippon Rinsho. 1998;56:3091–5. [PubMed] [Google Scholar]
- 28.Siddiqi N, Shamim M, Hussain S, Choudhary RK, Ahmed N, Prachee, et al. Molecular characterization of multidrug-resistant isolates of tuberculosis from patients in north India. Antimicrob Agents Chemother. 2002;46:443–50. doi: 10.1128/AAC.46.2.443-450.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]