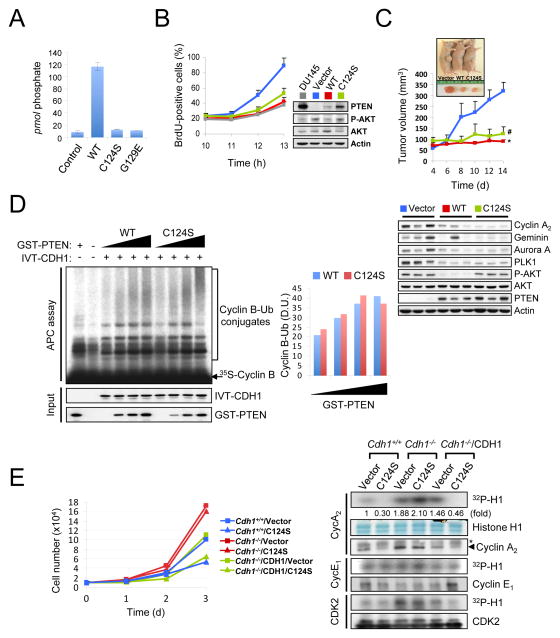
Figure 6. PTEN regulates APC-CDH1 independently of its phosphatase activity.
(A) diC8-PtdIns(3,4,5)P3 (40 μM) was incubated without (control) and with PTEN (WT), PTEN(C124S) or PTEN(G129E) immunoprecipitates (1 μg) at 37°C for 30 min and then the free phosphate was measured using the Green Reagent. Error bars represent the SEM from three different experiments. (B) PC3 cells complemented with wild-type or phosphatase-inactive PTEN(C124S) were nocodazole synchronized, released for the indicated times and pulsed with BrdU 30 min before harvesting. The proportion of BrdU+ cells was measured (left) and cell lysates were subjected to western blot (right). Error bars represent SEM from 3 different experiments. (C) PC3 cells complemented with wild-type or phosphatase-inactive PTEN(C124S) were injected subcutaneously into nude mice. Tumor volume was monitored (top) and tissue lysates at 2 week after injection were subjected to immunoblotting (bottom). Error bars represent SEM (n=6 mice/group). (D) Immunopurified APC/C from PC3 cells, released 3hrs post nocodazole synchronization, were subjected to in vitro ubiquitination assay using 35S-labeled Cyclin B in the presence of in vitro-translated (IVT)-CDH1 (2 μl) and different amounts of wild-type or PTEN(C124S) proteins (0, 50, 100, 200 ng). Right panel illustrates the density unit (D.U.) of Cyclin B-Ub conjugates analyzed by the ImageJ 1.38× software. (E) Growth curves (left) and in vitro kinase assay (right) of immortalized wild-type and Cdh1−/− MEFs, infected with a retroviral combination of human CDH1 and PTEN(C124S) (with selection) as indicated, and followed over a 3-day period. Error bars represent SEM from three different experiments. The relative kinase activities were normalized with histone H1 inputs. Asterisk indicates heavy chain of IgG. “see also Figures S5 and S6”. P value was determined by Student’s t test (*P<0.01; #P<0.05).