Abstract
Objectives:
The objectives of this report were to document the potential presence of Mayaro virus infection in Ecuador and to examine potential risk factors for Mayaro virus infection among the personnel of a military garrison in the Amazonian rainforest.
Materials and Methods:
The study population consisted of the personnel of a garrison located in the Ecuadorian Amazonian rainforest. The cross-sectional study employed interviews and seroepidemiological methods. Humoral immune response to Mayaro virus infection was assessed by evaluating IgM- and IgG-specific antibodies using ELISA.
Results:
Of 338 subjects studied, 174 were from the Coastal zone of Ecuador, 73 from Andean zone, and 91 were native to the Amazonian rainforest. Seroprevalence of Mayaro virus infection was more than 20 times higher among Amazonian natives (46%) than among subjects born in other areas (2%).
Conclusions:
Age and hunting in the rainforest were significant predictors of Mayaro virus infection overall and among Amazonian natives. The results provide the first demonstration of the potential presence of Mayaro virus infection in Ecuador and a systematic evaluation of risk factors for the transmission of this alphavirus. The large difference in prevalence rates between Amazonian natives and other groups and between older and younger natives suggest that Mayaro virus is endemic and enzootic in the rainforest, with sporadic outbreaks that determine differences in risk between birth cohorts of natives. Deep forest hunting may selectively expose native men, descendants of the Shuar and Huaronai ethnic groups, to the arthropod vectors of Mayaro virus in areas close to primate reservoirs.
Keywords: Alphavirus, Amazon, Ecuador, Mayaro virus
INTRODUCTION
Mayaro fever is a dengue-like acute viral disease that is usually self-limiting. Its agent is an alphavirus that belongs to the Togaviridae family within the Semliki Forest virus antigenic complex. The first isolation of Mayaro virus was made in August and September of 1954 in five febrile patients from Mayaro County, Trinidad Island.[1] Casals and Whitman characterized it as an arbovirus closely related to the Semliki Forest virus.[2]
The clinical manifestations of Mayaro virus disease have been recorded during outbreak studies and documented sporadic cases. After an incubation period of 7 to 12 days, the patient frequently presents with an abrupt fever accompanied by headache, arthralgias, myalgias, retro-orbital pain, vomiting, diarrhea, and maculopapular rash; some patients may develop severe and prolonged arthralgias.[3–6] Shorter incubation periods have also been observed,[6] Other symptoms reported less frequently include nausea, cough, sore throat, abdominal pain, nasal congestion and bleeding gums.[7] In 20% of the cases, swelling of small joints, especially in the wrists, fingers, ankles, and toes, has been reported. Usually the fever is between 39°C and 40.2°C.[8]
Presence of Mayaro virus antibodies in human populations has been reported in Bolivia, Brazil, Colombia, Panama, Peru, Surinam, Trinidad and Tobago, Venezuela, French Guiana and Mexico, suggesting the appearance and dissemination of this virus to other countries of the America continent.[3,6,9–11] Airborne virus infection of a laboratory worker has been also documented.[12] A Mayaro virus outbreak was reported in Belterra, Para, Brazil in 1978, with an attack rate of 20%.[13] Under apparently endemic and enzootic conditions, annual incidence rates between 1.6% to 7% were reported among Okinawan colonists in Bolivia in 1955[14] and among Dutch military troops detached in Surinam.[15]
Monkeys are suspected to be the principal reservoir for Mayaro virus, maintaining the enzoonosis in the rainforest, with periodic epizootics and epidemics.[16] Evidence of Mayaro infection has been found in Cebidae, Callithricidae, Saguinus, and Alouatta monkeys.[17–19] A recent serologic survey in French Guiana found a wide variety of non-flying mammals as reservoir for the virus, nevertheless their significance to the transmission of the virus is unknown.[20] In addition to this finding, it has been reported that Mayaro virus could also infect birds.[21]
Human beings acquire infection through infected mosquito bites. Epidemics begin with the onset of the rainy season and end near the onset of the dry season, correlating with the rise and fall in the mosquito population in the rainforest.[13] Mayaro virus has been isolated from several genera of mosquitoes, including Culex, Haemagogus, Mansonia, Aedes, Psorophora, and Sabethes.[9] Haemagogus spp. appears to be the main vector, especially H. janthinomys.[19] Many authors consider the possibility of urbanization of the disease since experimental studies have shown that the virus could infect Aedes aegypti, as population and land use increases.[7,21–23] The morbidity caused by the disease, specially the temporary incapacitating arthralgias, could cause an important social, economic and public health impact.
In late July through early August of 1997, an outbreak of hemorrhagic fever was reported in one of the Amazonian rainforest garrisons of the Ecuadorian Armed Forces. During the outbreak investigation, yellow fever was recognized as the cause of the epidemic. Yellow fever and Mayaro virus have been frequently associated, and we sought to investigate the potential presence and the risk factors of Mayaro virus infection in Ecuador.
MATERIALS AND METHODS
Study population
The jungle garrison under study consisted of one main post, three detachments, and five outposts, all located in southeastern Ecuador in the Amazonian rainforest near the Peruvian border. The geographic area, classified as humid tropical forest is situated 100 meters above sea level. The province has approximately 57,000 inhabitants, with an average population density of 1.92 inhabitants per square kilometer.
Study procedures
A cross-sectional seroepidemiological survey was conducted among the study population. After providing written informed consent, study subjects participated in a questionnaire interview concerning demographic variables, medical history, and potential risk factors. In a few cases, because of severity of the patients’ conditions, recent medical history data were obtained at the Military Hospital No. 1 of Quito (HG-1) rather than from patient interviews.
Blood samples were processed immediately after collection, and sera were stored frozen at -20°C until transported on dry ice to the U.S. Naval Medical Research Institute Detachment (NAMRID) in Lima, Peru for viral isolation and serologic testing. The Ecuadorian Armed Forces and the U.S. Navy Guidelines for the use of human subjects were followed. All procedures followed international guidelines for research in human subjects and were supervised by the Ecuadorian National Council against hemorrhagic fevers, complemented by health officers representing the Ecuadorian Armed Forces and the Ministry of Public Health.
Serology
Confluent monolayers of LLcMk2 or Vero cells were infected with prototype DEN-1, Oropouche (ORO) Peru 1992,[17] yellow fever 17D, or Venezuelan equine encephalitis (VEE) subtype I-AB virus, VR-69 (American Type Culture Collection [ATCC], Rockville, MD, U.S.A.) and Mayaro virus (MAY) TR467 strains. The resulting supernatant viral antigens were used to test sera for IgM antibody by a capture enzyme-linked immunosorbent assay (ELISA)[18] and for preparing lysate viral antigens for performing IgG antibody ELISA.[24,25] A capture ELISA using goat anti-human IgM (Tago, Camarillo, CA, U.S.A.) bound to 96-well Limbro microtiter plates was used to test for IgM antibodies.[24] An indirect ELISA employing viral infected or uninfected cell lysate bound to 96-well microtiter plates was used to test serum for IgG antibody.[26] Sera were tested initially at 1:100 dilutions, and reactive samples were further tested at 1:200 through 1:12,800 dilutions to determine the antibody endpoint titer. Mayaro virus specific IgM and IgG antibody positive and negative controls (run at 1:100 dilutions) were included in each test to validate the results. A horseradish peroxidase (HRP, Kirkegards and Perry, Gaithersburg, MD, U.S.A.) conjugated anti-mouse IgG and an enzyme substrate 2,2’-azino di (3-ethyl-benzthiazoline) sulfonate (ABTS) was used to detect IgM antibody. HRP-conjugated goat anti-human IgG and ABTS were used to detect IgG antibody. The absorbance was read with a spectrophotometer at 414-nm wavelength. The absorbance values for the mock antigens were subtracted from those of the viral antigen to yield correlated absorbance values. Serum dilutions with corrected absorbance values greater than the reference cut-off value, estimated as the mean absorbance of the 10 antibody-negative serum samples plus 3 standard deviations, were considered to indicate the sample was antibody positive. Samples with antibody titers of 1:200 or greater were classified as positive. All antibody titers were expressed as the reciprocal of the highest dilution yielding a positive result.
The laboratory procedures reported herein were conducted according to the principles set forth in U. S. federal guidelines (Guide for the Care Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Research Council, DHHS, Publication No. [NHI] 86-23, 1985).
Statistical analyses
Logistic regression analysis was used to evaluate the association between risk factors for arboviral infections and seroprevalence of alpahviruses antibodies. Odds ratio (OR) and 95% confidence intervals (CI) were calculated for each risk or protective factor and adjusted for the other factors in the model. The goodness of fit of the model was assessed using Hosmer and Lemeshow's test. In a second step, the analyses were restricted to personnel native to the Amazonian rainforest.
RESULTS
Alphavirus IgM antibodies were detected in three members of the military garrison who were ill very recently or were ill during the outbreak study. Fever, headache, arthralgias, and myalgias were the main symptoms reported. There were 338 study subjects, accounting for 97% of the active personnel of the garrison. Most were recruits assigned to one year of jungle training. Of these, 174 were from the coastal area of the country; 73 from the Andean zone, and 91 were native to the Amazonian rainforest, most from the Shuar and Huaorani indigenous rainforest ethnic groups.
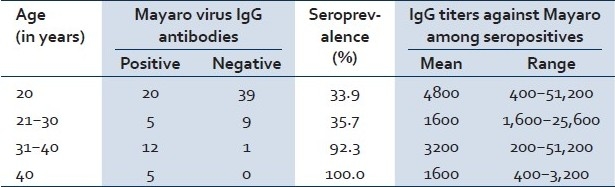
A high seroprevalence of 46% was found in personnel native to the Amazonian rainforest. Two variables were significantly associated with higher alphavirus seroprevalence: age of 30 years or older (OR = 7.9; CI = 2.2–29.1) and history of hunting (OR = 6.2; CI = 1.2–32.4). Clearing of the forest (OR = 1.5; CI = 0.5–4.3) and going into the rain forest (OR = 1.8; CI = 0.4–7.4) showed weak, but not statistically significant, associations with alphavirus seroprevalence. Contact with domestic animals was not associated with the presence of alphavirus antibodies. Use of bed nets did not show a protective effect in reducing alphavirus seroprevalence (OR = 1.1; CI = 0.2–5.9); use of repellents had a weak, but nonsignificant, protective effect (OR = 0.7; CI = 0.2–2.2; [Table 1]). Amazonian native personnel younger than 21 years of age showed a seroprevalence of 33.9%. The seroprevalence increased to 35.7% in the 21 to 30 year age group, increased sharply to 92.3% among persons 30 to 40 years of age, and was 100% for those over 40 years of age [Table 2].
Table 1.
Correlates of Mayaro virus seroprevalence (IgG) among military personnel, July–August 1997
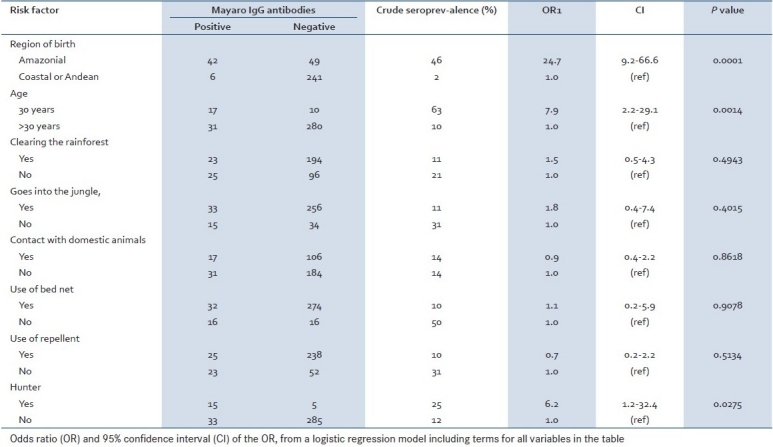
Table 2.
Mayaro IgG antibody prevalence by age strata among personnel native to the Amazonian rainforest
DISCUSSION
The results from this study suggest the presence of alphavirus infection in Ecuador. While the technique used, ELISA, is not enough to determine the specific alphavirus circulating in the area, the clinical and epidemiological data suggest the potential presence of Mayaro virus. The possibility of other heterologous alphavirus antibodies cross-reacting with Mayaro virus when ELISA or HI test are used is high, especially with Venezuelan Equine Encephalitis Virus (VEEV) and Chikungunya.[27] However, Chikungunya infections have never been reported in the Amazon basin and the clinical manifestations of VEEV are characterized by central nervous system manifestations but not by arthralgias, as found in our study.[28] Moreover, only 3% of healthy persons from this enzootic region had VEEV-neutralizing antibodies suggesting that humans are exposed but do not develop apparent infection with VEEV due to poor infectivity and/or avirulence of South American strains .[29] We report an alphavirus seroprevalence of 46% among this rainforest population. In the hypothetical case that VEEV may have been circulating in this area, the possible 3% seroprevalence is negligible compared to the 46% found in this study. Therefore, the clinical and epidemiological aspects indicate that the possible alphavirus circulating in the area is Mayaro Virus, representing this paper, the first report to document the potential transmission of Mayaro virus in Ecuador.
Evidence of transmission of Mayaro virus has been reported in Trinidad and Tobago, Brazil, Bolivia, Surinam, Guiana, Colombia, Venezuela, and Peru[3,6,16] under similar circumstances; Mayaro virus isolates or antibodies were detected during the investigation of yellow fever outbreaks. We observed an overall seroprevalence of 48 of 338 (14%) in garrison personnel, largely restricted to Amazonian natives (42/91, 46%). Serological surveys of other populations in northern South America have found antibodies against Mayaro virus in rates that range from 1% to 60%.[11,13,30–32]
Le Duc et al. carried out a study in Belterra, Brazil in 1977 and 1978. Following an attack, rate of 20% among susceptible hosts with an estimated baseline seroprevalence of 10%, the overall Mayaro virus seroprevalence after the outbreak was about 30%.[13] Azevedo et al. reported a 34% seroprevalence after another outbreak in a settlement located in Santa Barbara municipality, northern Brazil.[33]
Average seroprevalence rates of 12% in Amazonas State, 4% in Para State, 5% in Mato Grosso State, 9% in Goias State, and 5% in Paraiba State were described in a national serum survey of Brazilian military recruits.[31] Our average seroprevalence is similar to that described in Brazilian recruits from the Amazonas State. When our analysis was restricted to the Amazonian natives, seroprevalence increased dramatically to 46%.
High seroprevalence was observed by Black et al. in a seroepidemiological survey carried out among indigenous tribes living in the lower Amazon Basin in Brazil: 47% of the Tiriyo tribe, 46% of the Xikrin tribe, 49% of the Mekranoti tribe, 20% of the Kuben KK tribe, and 37% of the Gorotire tribe were found to be seropositive for Mayaro virus antibodies. The seroprevalence was 42% for all indigenous tribes.[30] Talarmin et al. also found a significant higher seroprevalence in ethnic groups living in rain forest areas of French Guiana. The seroprevalence of Noir-Marrons and Amerindians observed during the serological survey was 23.7% and 19.4%, respectively.[32]
Because the personnel from the Andean and Coastal region were mobilized to the Amazonian rainforest, the 2% seroprevalence rate among them may have reflected the cumulative incidence rate under endemic and enzootic conditions and after an average of one year of residence in the garrison and its detachments. This rate is similar to the 1.6 to 5.3% annual infection rate reported in the Dutch military troops on patrol in Surinam.[15]
Hunting was significantly associated with a high seroprevalence of Alphavirus antibodies. Subjects with a history of hunting were six times more likely to be anti-body positive than were non hunters (OR = 6.2; CI = 1.2–032.4). During the initial 1957 report of Mayaro fever in Trinidad, Causey, and Maroja stated: “This brief series suggests that exposure to the virus occurred at a time and place when the men were segregated from women and children, namely, during the day and at work”.[34] Similarly, during the construction of the Transamazon Highway, the hunting activity of the colonists was the suspected cause that resulted in high rates of arboviral infections, including Mayaro disease.[35] This observation, hunting as a risk factor for Mayaro virus infection, is also suggested by other authors when trying to explain the higher seroprevalence observed with increasing age.[32] Game constitutes the main source of protein among Shuar and Huaroni ethnic groups, contributing an estimated of 20% of the diet. Meat sources include mammals, such as peccary, agouti, and howler, squirrel, capuchin, and black monkeys. Monkeys are usually stalked with blowguns and darts poisoned with curare. The hunter, usually with male companions, waits until the monkeys are calling among themselves before stepping stealthily to the base of the monkeys’ tree and blowing the darts. Shotguns or carbine rifles are used to hunt ground-dwelling game. Hunting monkeys in the most remote parts of the rainforest usually takes several days. Because one man can only hunt a fraction of a monkey troop, hunting expeditions can involve up to 12 men working together. The hunting team consists of young men supervised by the old master of ceremony, the “wea,” who inhales tobacco and guides the hunters during the hunting ritual session called “kusupan.”[36,37]
Hunting, therefore, selectively places Shuar and Huaorani native males in close contact with Hemagogus mosquitoes, which breed in tree holes and feed on blood from monkeys. In South America, the monkeys, in turn, serve as reservoirs of Mayaro virus in the transmission cycle. During the Belterra outbreak, Hoch et al. collected blood samples from 585 mammals representing 47 species. Only primates had demonstrable antibodies against Mayaro virus. Antibodies against Mayaro virus were detected in the single Cebidae monkey studied and in 32 of the 119 (27%) Callithricidae.[38] In 1994 and 1995 in Guiana, Talarmin et al. tested sera from 106 howler monkeys (Alouatta seniculos) finding 66.6% positive for Mayaro virus antibodies. The gender of the monkey was not correlated with seroprevalence, but seroprevalence increased with increased age and weight of the monkeys. Sera were also collected from 44 marmoset monkeys (Saguinus midas) that showed a lower seroprevalence of 18.2%.[32] In 1982, a study carried out by Seymour et al. in Panama, found howler monkeys (Alouatta villosa) and agoutis (Dasyprocta punctata) to have antibodies against Mayaro virus.[39] A recent serological survey for Mayaro virus conducted in French Guiana on 28 non-flying mammalian rainforest species found a wide variety of animals as reservoirs for Mayaro virus. However, primates presented the highest seroprevalence.[20]
Human subjects aged 30 years or more were eight times more likely to be seropositive than younger subjects (OR = 7.9; CI = 2.2–29.1). When the analysis was restricted to native personnel [Table 2] seroprevalence increased sharply to over 90% at age 30 years or more. In the study carried out in French Guiana, Talarmin et al. observed that seroprevalence increased significantly with age: <10 years, 0%; 10–19 years, 5.5%; 20–29 years, 5.9%; 30–39 years, 8%; 40–49 years, 13%; and 50 years, 20%.[32] This steady rise in the increase of Mayaro virus antibody prevalence by age can be interpreted as evidence of transmission under endemic and enzootic conditions. On the other hand, in our study group, seroprevalence showed a sudden increase in the 30 years age group. Considering that age closely reflects years of residence among native rainforest population, it is plausible that the sharply higher prevalence in the 30 years age group is indicative that a major epidemic or epizootic event occurred sometime in the past two decades (when the younger birth cohort of native men were not yet hunting), followed by the maintenance of endemic levels linked to an enzoonosis. Consequently, our results can be interpreted as evidence that hunter cohorts may be exposed sporadically to alphavirus, most likely Mayaro virus, epizootic in the deep Amazonian rainforest. The epidemic and epizootic behavior of Mayaro virus transmission was demonstrated by the Belterra, Para, Brazil study carried out in 1978.[19]
Use of repellents or bed nets was not associated with protection against the disease. This is congruent with the diurnal behavior of H. janthinomys, the most likely vector in the Amazon Basin, whose activity peaks at approximately 1300 hours, decreases around 1600 hours, and ceases by 1800 hours.[19]
Those who go to the jungle and clear the forest showed an increased risk; however, the point estimates showed a wide confidence interval that included the null value. Haemagogus spp. is mainly a tree-hole breeder, although it occasionally can be found in bamboo stumps.
The presence of a high seroprevalence of alphavirus, most likely Mayaro virus, antibodies was found during the investigation of a yellow fever outbreak. The detection of Mayaro virus during yellow fever outbreaks is common.[16] Yellow fever virus and Mayaro virus have isolated simultaneously in H. janthinomys.[19] Concomitant identification of yellow fever and Mayaro virus has been reported among Alouatta, Cebidae, and Callithricidae monkeys.[19,39]
Although Mayaro virus has not been reported in Ecuador, a syndrome often referred to as “jungle flu” has been described by civilian and military physicians in the Ecuadorian rainforest.[40] This clinical syndrome was described in this study as flu-like symptoms with fever, arthralgia, myalgia, and headache. As mentioned before, these clinical symptoms are compatible with a potential infection with Mayaro virus. This study constitutes the first systematic evaluation of risk factors associated with the transmission of Mayaro virus, and its findings suggest a simple model of transmission in rainforest. While this data suggest further evidence that Mayaro viral infection is common among rural/forest dwelling humans throughout South America, there is the possibility of other heterologous alphavirus antibodies cross-reacting with Mayaro virus when ELISA or HI test are used, especially Venezuelan Equine Encephalitis Virus (VEEV) and Una virus.[27] Although the status of Una virus infection in the Ecuadorian Amazon has not been investigated, there are reports of the presence of its subtype Una virus in Argentina, Brazil and Paraguay.[41,27] A recent study of acute undifferentiated febrile illnesses in selected clinics from Ecuador, Peru, Bolivia, and Paraguay, reported the presence of Mayaro virus in Bolivia and Peru.[42] Guayaquil, a coastal region of Ecuador, was selected for this study. It is plausible that Forshey et al. did not find Mayaro virus infections in this area of Ecuador because the virus is commonly found in rainforest areas. A limitation of our study is that neutralization tests were not performed to provide more conclusive results related to viral specificity of infection.
CONCLUSIONS
Indigenous people who grew up in the Amazonian rainforest showed increased seroprevalence rates of alphavirus virus infection in relation to populations from the Coastal and Andean zones of the country. This increased seroprevalence among Amazonian birth cohorts along with the clinical and epidemiological data demonstrates the potential presence of Mayaro virus epidemics epizootics going on periodically in the deep Amazonian rainforest with the maintenance of endemicity linked to a constant enzoonosis. Our findings suggest the potential transmission of Mayaro virus in Ecuador, especially among the native population of the Amazonian basin, most of whom are descendants of the ethnic Shuar and Huaorani people. Hunting places these ethnic groups in close contact with arthropod vectors and primate reservoirs, which are the key elements that participate in this jungle model of transmission taking place deep in the rainforest. The high seroprevalence of alphavirus antibodies observed among natives of the Amazonian rainforest identifies a possible Mayaro focus of jungle transmission in this tropical area of Ecuador. Experimental infections of Aedes mosquitoes suggest the possibility of the appearance of Mayaro virus urban model of transmission, as has been observed in Asia with the Chikungunya virus, an alphavirus closely related to Mayaro virus.[43] It is necessary to develop and implement epidemiological sentinel centers for the surveillance of vector-borne diseases in Central and South America.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Anderson CR, Downs WG, Wattley GH, Ahin NW, Reese AA. Mayaro virus: A new human disease agent: II. Isolation from blood of patients in Trinidad, B.W.I. Am J Trop Med Hyg. 1957;6:1012–6. doi: 10.4269/ajtmh.1957.6.1012. [DOI] [PubMed] [Google Scholar]
- 2.Casals J, Whitman L. Mayaro virus: A new human disease agent: I. Relationship to other Arbor Viruses. Am J Trop Med Hyg. 1957;6:1004–11. doi: 10.4269/ajtmh.1957.6.1004. [DOI] [PubMed] [Google Scholar]
- 3.Pinheiro FP, LeDuc JW. Mayaro virus disease. In: Monath T, editor. The arboviruses, epidemiology and ecology. Boca Raton: CRC Press; 1998. pp. 138–48. [Google Scholar]
- 4.Taylor SF, Patel PR, Herold TJ. Recurrent arthralgias in a patient with previous Mayaro fever infection. South Med J. 2005;98:484–5. doi: 10.1097/01.SMJ.0000145879.14102.F4. [DOI] [PubMed] [Google Scholar]
- 5.Tesh RB. Arthritides caused by mosquito-borne viruses. Annu Rev Med. 1982;33:31–40. doi: 10.1146/annurev.me.33.020182.000335. [DOI] [PubMed] [Google Scholar]
- 6.Torres JR, Russell KL, Vasquez C, Barrera R, Tesh RB, Salas R, et al. Family cluster of Mayaro fever, Venezuela. Emerg Infect Dis. 2004;10:1304–6. doi: 10.3201/eid1007.030860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tesh RB, Watts DM, Russell KL, Damodaran C, Calampa C, Cabezas C, et al. Mayaro virus disease: An emerging mosquito-borne zoonosis in tropical South America. Clin Infect Dis. 1999;28:67–73. doi: 10.1086/515070. [DOI] [PubMed] [Google Scholar]
- 8.Pinheiro FP, Freitas RB, da Rosa JF, Gabbay YB, Mello WA, LeDuc JW. Vol. 30. Belterra, Brazil: Series An Outbreak of Mayaro Virus Disease; 1981. An Outbreak of Mayaro Virus Disease in Belterra, Brazil: I. Clinical and Virological Findings; pp. 674–81. [DOI] [PubMed] [Google Scholar]
- 9.Groot H, Kerr JA, Sanmartin C, Vidales H. Antibodies to yellow fever and other arthropod-borne viruses in human residents of San Vicente de Chucuri, Santander, Colombia. Am J Trop Med Hyg. 1959;8:175–89. doi: 10.4269/ajtmh.1959.8.175. [DOI] [PubMed] [Google Scholar]
- 10.Navarrete-Espinoza J, Gómez-Dantés H. Arbovirus causing hemorrhagic fever at IMSS. Rev Med Inst Mex Seguro Soc. 2006;44:347–53. [PubMed] [Google Scholar]
- 11.Tavares-Neto J, Freitas-Carvalho J, Nunes MR, Rocha G, Rodrigues SG, Damasceno E, et al. Serologic survey for yellow fever and other arboviruses among inhabitants of Rio Branco, Brazil, before and three months after receiving the yello fever 17D vaccine. Revista da Sociedade Brasileira de Medicina Tropica. 2004;37:1–6. doi: 10.1590/s0037-86822004000100001. Brazil. [DOI] [PubMed] [Google Scholar]
- 12.Junt T, Heraud JM, Lelarge J, Labeau B, Talarmin A. Determination of natural versus laboratory human infection with Mayaro virus by molecular analysis. Epidemiol Infect. 1999;123:511–3. doi: 10.1017/s0950268899003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeDuc JW, Pinheiro FP, da Rosa AP. An outbreak of Mayaro virus disease in Belterra, Brazil: II.Epidemiology. Am J Trop Med Hyg. 1981;30:682–8. doi: 10.4269/ajtmh.1981.30.682. [DOI] [PubMed] [Google Scholar]
- 14.Schaeffer M, Gajdusek DC, Lema AB, Eichenwald H. Epidemic jungle fevers among Okinawan colonists in the Bolivian rain forest: I. Epidemiology. Am J Trop Med Hyg. 1959;8:372–96. doi: 10.4269/ajtmh.1959.8.372. [DOI] [PubMed] [Google Scholar]
- 15.Karbaat J, Jonkers AH, Spence L. Arbovirus infections in Dutch military personnel stationed in Surinam: A preliminary study. Trop Geogr Med. 1964;16:370–6. [PubMed] [Google Scholar]
- 16.Pinheiro FP, Travassos da Rosa A. Arboviral zoonoses of Central and South America. In: Beran GW, Zoonoses Viral, editors. Handbook series of zoonoses. 2nd ed. Boca Raton: CRC Press; 1994. pp. 201–25. [Google Scholar]
- 17.Chavez R, Colan E, Philips I. Fiebre de Oropuche en Iquitos: Reporte preliminar de 5 casos. Rev Farmacol Tera (Lima) 1992;2:12–14. [Google Scholar]
- 18.Duermeyer W, Wielaard F, van der Veen J. A new principle for the detection of specific IgM antibodies applied in an ELISA for hepatitis A. J Med Virol. 1979;4:25–32. doi: 10.1002/jmv.1890040104. [DOI] [PubMed] [Google Scholar]
- 19.Hoch AL, Peterson NE, LeDuc JW, Pinheiro FP. An outbreak of Mayaro virus disease in Belterra, Brazil. III. Entomological and ecological studies. Am J Trop Med Hyg. 1981;30:689–98. doi: 10.4269/ajtmh.1981.30.689. [DOI] [PubMed] [Google Scholar]
- 20.de Thoisy B, Gardon J, Salas RA, Morvan J, Kazanji M. Mayaro virus in wild mammals, French Guiana. Emerg Infect Dis. 2003;9:1326–9. doi: 10.3201/eid0910.030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Figueiredo LT. Emergent arboviruses in Brazil. Rev Soc Bras Med Trop. 2007;40:224–9. doi: 10.1590/s0037-86822007000200016. [DOI] [PubMed] [Google Scholar]
- 22.Aitken TH, Anderson CR. Virus transmission studies with trinidadian mosquitoes: Part II. Further observations. Am J Trop Med Hyg. 1959;8:41–5. doi: 10.4269/ajtmh.1959.8.41. [DOI] [PubMed] [Google Scholar]
- 23.Coimbra TL, Santos CL, Suzuki A, Petrella SM, Bisordi I, Nagamori AH, et al. Mayaro virus: Imported cases of human infection in Sao Paulo State, Brazil. Rev Inst Med Trop Sao Paulo. 2007;49:221–4. doi: 10.1590/s0036-46652007000400005. [DOI] [PubMed] [Google Scholar]
- 24.Chu YK, Rossi C, Leduc JW, Lee HW, Schmaljohn CS, Dalrymple JM. Serological relationships among viruses in the Hantavirus genus, family Bunyaviridae. Virology. 1994;198:196–204. doi: 10.1006/viro.1994.1022. [DOI] [PubMed] [Google Scholar]
- 25.LeDuc JW, Ksiazek TG, Rossi CA, Dalrymple JM. A retrospective analysis of sera collected by the hemorrhagic fever commission during the Korean Conflict. J Infect Dis. 1990;162:1182–4. doi: 10.1093/infdis/162.5.1182. [DOI] [PubMed] [Google Scholar]
- 26.Meegan JM, Yedloutschnig RJ, Peleg BA, Shy J, Peters CJ, Walker JS, et al. Enzyme-linked immunosorbent assay for detection of antibodies to Rift Valley fever virus in ovine and bovine sera. Am J Vet Res. 1987;48:1138–41. [PubMed] [Google Scholar]
- 27.Travassos da Rosa A, Vasconcelos PF, Travassos da Rosa JF. Brazil: Instituto Evandro Chagas; 1998. An overview of arboviruses in Brazil and neighbouring countries; pp. 19–31. [Google Scholar]
- 28.Hassing RJ, Leparc-Goffart I, Tolou H, van Doornum G, van Genderen PJ. Cross-reactivity of antibodies to viruses belonging to the Semliki forest serocomplex. Euro Surveill. 2010;15:19588. [PubMed] [Google Scholar]
- 29.Aguilar PV, Robich RM, Turell MJ, O’Guinn ML, Klein TA, Huaman A, et al. Endemic eastern equine encephalitis in the Amazon region of Peru. Am J Trop Med Hyg. 2007;76:293–8. [PubMed] [Google Scholar]
- 30.Black FL, Hierholzer WJ, Pinheiro FD, Evans AS, Woodall JP, Opton EM, et al. Evidence for persistence of infectious agents in isolated human populations. Am J Epidemiol. 1974;100:230–50. doi: 10.1093/oxfordjournals.aje.a112032. [DOI] [PubMed] [Google Scholar]
- 31.Niederman JC, Henderson JR, Opton EM, Black FL, Skvrnova K. A nationwide serum survey of Brazilian military recruits, 1964: ii. Antibody patterns with arboviruses, polioviruses, measles and mumps. Am J Epidemiol. 1967;86:319–29. doi: 10.1093/oxfordjournals.aje.a120742. [DOI] [PubMed] [Google Scholar]
- 32.Talarmin A, Chandler LJ, Kazanji M, de Thoisy B, Debon P, Lelarge J, et al. Mayaro virus fever in French Guiana: Isolation, identification, and seroprevalence. Am J Trop Med Hyg. 1998;59:452–6. doi: 10.4269/ajtmh.1998.59.452. [DOI] [PubMed] [Google Scholar]
- 33.Azevedo RS, Silva EV, Carvalho VL, Rodrigues SG, Nunes-Neto JP, Monteiro H, et al. Mayaro fever virus, Brazilian Amazon. Emerg Infect Dis. 2009;15:1830–2. doi: 10.3201/eid1511.090461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Causey OR, Maroja OM. Mayaro virus: A new human disease agent: III.Investigation of an epidemic of acute febrile illness on the River Guama in Para, Brazil, and isolation of Mayaro virus as causative agent. Am J Trop Med Hyg. 1957;6:1017–23. [PubMed] [Google Scholar]
- 35.Dixon KE, Llewellyn CH, Travassos da Rosa AP, Travassos da Rosa JF. A multidisciplinary program of infectious disease surveillance along the Transamazon highway in Brazil: epidemiology of arbovirus infections. Bull Pan Am Health Organ. 1981;15:11–25. [PubMed] [Google Scholar]
- 36.Drown F, Down M. New York: Harper and Brother; 1961. Mission to the head-hunters; pp. 80–3. [Google Scholar]
- 37.Harner MJ. New York: Doubleday Natural History Press; 1972. The Jivaro: People of the sacred waterfalls; pp. 57–60. [Google Scholar]
- 38.Simpson D. Arbovirus infections. In: Cook GC, editor. Mason's Tropical Disease. Philadelphia: WB Sauders; 1996. pp. 143–56. [Google Scholar]
- 39.Seymour C, Peralta PH, Montgomery GG. Serologic evidence of natural TogavirusiInfections in Panamanian sloths and other vertebrates. Am J Trop Med Hyg. 1983;32:854–61. doi: 10.4269/ajtmh.1983.32.854. [DOI] [PubMed] [Google Scholar]
- 40.Izurieta R, Macaluso M, Watts D, Tesh RB, Mason W, Guerra B, et al. Buenos Aires, Argentina: International Society for Infectious Diseases; 2000. Hunting in the rainforest and Mayaro infection: A model of transmission for an emerging alphavirus in Ecuador.In Abstracts of the 9th International Congress of Infectious Diseases; pp. 95–8. [Google Scholar]
- 41.Díaz LA, del Pilar Díaz M, Almirón WR, Contigiani MS. Infection by UNA virus (Alphavirus; Togaviridae) and risk factor analysis in black howler monkeys (Alouatta caraya) from Paraguay and Argentina. Trans R Soc Trop Med Hyg. 2007;101:1039–41. doi: 10.1016/j.trstmh.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Forshey BM, Guevara C, Laguna-Torres VA, Cespedes M, Vargas J, Gianella A, et al. Arboviral etiologies of acute febrile illnesses in western South America, 2000-2007. PLoS Negl Trop Dis. 2010;4:787. doi: 10.1371/journal.pntd.0000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith GC, Francy DB. Laboratory studies of a Brazilian strain of Aedes albopictus as a potential vector of Mayaro and Oropouche viruses. J Am Mosq Control Assoc. 1991;7:89–93. [PubMed] [Google Scholar]


