Abstract
Objective:
Chronic granulomatous disease (CGD) is an inherited disorder of the Nicotinamide adenine dinucleotide phosphate reduced oxidase complex characterized by recurrent bacterial and fungal infections. Disseminated infection by combination of opportunistic agents is being increasingly reported in CGD patients. We presented in the retrospective review of medical records, the etiology, presentation, clinical characteristics the infections detected, predisposing condition and outcome of nocardiosis and actinomycosis involved in a group of pediatric patients diagnosed with CGD.
Materials and Methods:
The clinical presentation of CGD-related infections was reviewed retrospectively from the medical records of all 12 patients with CGD. We studied respectively 12 patients between 2001 and 2008, and we analyzed two pediatric patients with CGD who acquired Nocardia and Actinomyces infections, and their clinical and microbiological characteristics were described. The material for investigations was collected from scrapings, crusts, pus from subcutaneous abscesses or exudation from sinus tracts, surgical debridement, and biopsy specimens. The microbiological diagnosis was determined by biochemical tests, histology, microscopy, and culture of clinical samples.
Results:
The medical records of 12 diagnosed CGD patients with suspected nocardiosis or actinomycosis were reviewed. One patient was diagnosed with actinomycosis and one patient with nocardiosis. Patients consisted of seven males and five females with ranging ages of 3 to 18 years. Nocardiosis and actinomycosis isolated in the two patients were confirmed by histology and culture methods. Neutrophil oxidative burst were absent (NBT=0) in both patients. The most common manifestations of CGD due to fungal infections, actinomycosis, and nocardiosis were osteomyelitis (42.8%), pulmonary infections (28.6%), lymphadenopathy (14.3%), and skin involvement (14.3%) during their illness.
Conclusion:
Nocardiosis and actinomycosis in children indicate the need for evaluation for an underlying immunological deficiency. Early diagnosis remains critical for decreased morbidity and occasional mortality. Physicians caring for patients with CGD should maintain a high index of suspicion for nocardiosis and actinomycosis especially if work up for TB and fungal infections are negative.
Keywords: Chronic granulomatous disease, Nocardiosis, Actinomycosis
INTRODUCTION
Chronic granulomatous disease (CGD) is a primary immunodeficiency disorder and most cases are diagnosed at childhood. The X-linked forms more common, but autosomal recessive forms have also been described.[1] Patients with this disease show great susceptibility to fungal and bacterial infections.
CGD is characterized by recurrent life-threatening infections and excessive granuloma formation, involving the lungs, skin, soft tissues, and reticuloendothelial system.[2] Recurrent deep-seated infections with catalase-positive organisms such as Staphylococcus aureus or Aspergillus fumigatus are typical.
However, catalase-dependent virulence is not the complete story, because most pathogens in general are catalase-positive, but only a small subset is encountered in CGD patients: Staphylococcus aureus, Serratia marcescens, Burkholderia cepacia, Nocardia spp., and Aspergillus species. The frequency of infection with catalase negative organisms in CGD is extremely low. CGD, 5% of abscesses yielded streptococci but the number that were in pure culture is unknown.[3]
Nocardia infections are almost exclusively seen in immunocompromised patients and are important causes of infection in patients with CGD. Nocardia species have been implicated as the causes of pulmonary, cutaneous, ocular, and disseminated diseases in both immunocompetent and immunocompromised human hosts. Over the past several years the spectrum of disease caused by Nocardia species has changed due to the increase in the number of immunocompromised patients.[4] Infection with Nocardia poses a diagnostic challenge in patients with CGD because the signs and symptoms are often nonspecific, delay in diagnosis is common, and invasive procedures are frequently required to obtain appropriate tissue specimens.[5]
Actinomycosis is a chronic granulomatous condition that commonly manifests as cervicofacial, pulmonary, or abdominal disease caused by slowly progressive infection with oral and gastrointestinal communal Actinomyces israelii.[6]
This study describes the clinical characteristics of Nocardia and Actinomyces infections detected; in a group of pediatric patients diagnosed with CGD.
MATERIALS AND METHODS
Twelve patients with diagnosis of CGD were respectively studied over an 8 year period from 2001 to 2008. The clinical presentation of CGD-related nocardiosis and actinomycosis was reviewed retrospectively from the medical records of all 12 patients with CGD. The infections were described according to the site of infection and the infectious agent. A severe infection was defined as an episode of infection requiring hospitalization and intravenous antimicrobial treatment or surgical treatment.
The material for investigations was collected from scrapings, crusts, pus from subcutaneous abscesses or exudation from ear, cranial, facial, liver, lymph node, and biopsy specimens. Specimens for systemic infections were abscess and bronchial alveolar lavage. Diagnosis was made by direct examination, culture, biochemical tests, and histology. Specimens were cultured on Sabouraud's dextrose agar, blood agar and brain heart infusion agar (B.B.L), and fluid thioglycolate, anaerobic culture media, and biochemical properties. Duplicate cultures on each medium were incubated at 35 and 25°C regularly examined up to 4 weeks and identified using standard methods. For diagnosis of Nocardia and Actinomyces the preparations were stained using Kynion, and Gram stain. Actinomyces and Nocardia were identified by culture, staining, and biochemical tests. Biochemical tests were performed at the Actinomyces Reference Laboratory of the Centers for Disease Control and Prevention (CDC) by the methods of Berd.[7]
RESULTS
Case 1
The patient was a 14-year-old boy who was a known case of CGD, with osteomyelitis due to Nocardia asteroides of the proximal femur with right hip pain, inability to walk, and overlying cellulitis. The mycological and bacteriological diagnosis was determined by microscopy, culture, and biochemical tests of clinical samples.
The isolate had been identified as N. asteroides by standard methods,[8] the strain was negative on cellobiose, positive on glycerol, and negative on hypoxanthine and sorbitol. It was identified as a strain of N. asteroides [Figure 1].
Figure 1.
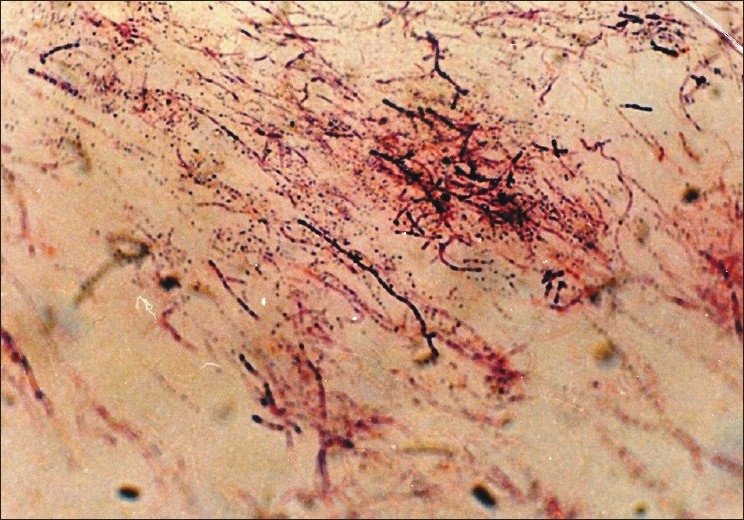
Nocardia asteroides. Filamentous gram-positive organism in sulfur granule
Our patient survived after treatment with trimethoprim-sulfamethoxazole (TMP-SMX) and ceftriaxone.
Case 2
A 12-year-old girl with CGD presented with 1 week of painful red fluctuant swelling over the upper right neck and fever. She had noted a gradually increasing mobile mass over the right neck. CGD had been diagnosed at age 2 complicated by multiple pneumonias and liver abscesses. She had an erythematous, warm, tender, tense, fluctuant mass in the right submandibular area without other lymph node enlargement. She was admitted with a high grade fever (40°C orally). The patient had received one or two different antibiotics prior to the isolation of A. israelii like the other patiens.
The biopsy of the submandibular mass indicated chronic granuloma. Because of the possibility of actinomycosis, a culture and smear were requested. Gram stain of the purulent discharge of the lymph node revealed irregular gram-positive filamentous microorganisms. After 4 weeks, microorganisms grown on anaerobic culture media and biochemical testing confirmed A. israelii (positive glucose, mannitol and sucrose fermentation but negative hemolysis, catalase, starch hydrolysis, DNase indole and urease) [Figure 2]. Aspiration of her lymph node yielded A. israelii in pure culture. Leukocyte count was 8200/mm3 and sedimentation rate 86 mm/h. She was treated with intravenous levofloxacin with gradual improvement. Levofloxacin and ceftriaxone were given for a total of 4 weeks with complete resolution.
Figure 2.
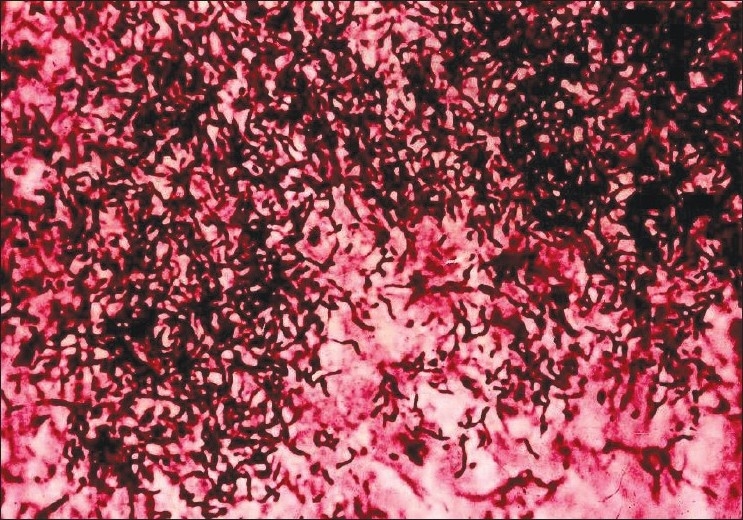
Actinomyces israelii, brunching filamentous Gram stain of the purulent discharge of the lymph node in aspiration of the lymph node
DISCUSSION
CGD is a rare inherited disease of the phagocyte NADPH oxidase system that causes defective production of toxic oxygen metabolites, impaired bacterial and fungal killing, and recurrent life-threatening infections, mostly by catalase-producing organisms.[6]
Co-pathogens were also responsible for the association of multiple antibiotics. The most frequent isolates included were Aspergillus sp. (25%) and Fusarium sp. (16.6%), one of which have been described previously.[9]
In our region, nocardiosis is an uncommon infection, probably under diagnosed in many cases. In fact, only this case was isolated in samples referred to Pasteur Institute of Iran in the study period.
Nocardia species are found in soil around the world.[10] Systemic nocardiosis occurs more frequently in immunocompromised patients, including patients with CGD.[11,12] Patients with CGD are unable to generate “respiratory burst” after stimulation of neutrophils and monocytes and are therefore unable to kill certain microorganisms. “Respiratory burst” results in the generation of superoxide (O2), hydrogen peroxide (H2O2), and hypochlorite (OCl). Leukocytes from patients with CGD have severely diminished hydrogen peroxide production. Although CGD is rare, occurring in about 1 in one million individuals, it is an important model of defective neutrophil oxidative metabolism.[9] N. asteroides is the predominant species and the one most commonly associated with disseminated disease.[4]
Among the isolates received from clinical laboratories, 35% of strains were N. asteroides, the most common etiological agent of nocardiosis in humans and animals.[13] In the period from 1987 to 1990, from Institute Pasteur of Paris, it was estimated that between 150 and 250 cases of nocardiosis are diagnosed in France each year.[14] A total of 63 clinical isolates were referred to the NRC and identified as N. asteroides in (66.7%). N. asteroides accounted for 71.4% of pulmonary infections, 80.0% of central nervous system infections and 80.0% of systemic infections. Patients infected with N. asteroides died in 17.6% of cases, compared with 57.1% of patients infected with N. farcinica. Corticosteroid therapy represented a significant factor in mortality. Isolates of N. asteroides revealed variable resistance, whereas isolates of N. farcinica were resistant to most antimicrobial agents. Only amoxicillin/clavulanic acid, imipenem, cefoxitin, kanamycin, amikacin, minocycline, and vancomycin showed activity against both species.[14]
Nocardiosis is still difficult to diagnose because of the bacteria's slow growth. Serodiagnostic tests are not yet available for clinical use to identify active disease, and treatment may be delayed.[15] Infection with Nocardia poses a diagnostic challenge in patients with CGD because the signs and symptoms are often nonspecific, delay in diagnosis is common, and invasive procedures are frequently required to obtain appropriate tissue specimens.[6]
Nocardia infections in patients with CGD are not usually fatal if treated properly, and prophylaxis with IFN-γ and a sulfonamide may protect against dissemination.[16] Lerner[15] concluded that if cultures are repeatedly positive, especially in immunocompromised patients, therapy should be administered. Our patient had experienced a complete recovery from his Nocardia infection after treatment with trimethoprim-sulfamethoxazole (TMP-SMX) and ceftriaxone. The treatment of choice for Nocardia infections is TMP-SMX, imipenem, amicacin, and linzilid while the treatment for Actinomyces is penicillin G, ampicillin/amoxicillin. In the series of 22 episodes of Nocardia infection in 19 patients with CGD reported by Dorman and colleagues.[5] 45% of the patients were receiving TMP-SMX at the time of diagnosis and 30% were receiving IFN-γ.[17] The results confirm that Nocardia species are important causes of infection in patients with CGD.
Sarah et al[5] reported that patients receiving prophylaxis with IFN-γ and/or a sulfonamide were significantly less likely to have disseminated nocardiosis than were patients receiving neither of these medications. The long-term use of IFN-γ as prophylaxis for patients with CGD has been widespread since the 1990s.[18] Despite many years of studies, the utility of IFN-γ therapy remains controversial. For patients with CGD who have nocardiosis, aggressive management with antibiotics, as well as surgery, is usually successful.[19]
Physicians caring for patients with CGD should maintain a high index of suspicion for nocardiosis, especially in those receiving chronic steroid therapy. Early diagnosis remains critical for decreased morbidity and occasional mortality. Nocardiosis in patients with CGD are not usually fatal if treated properly and prophylaxis with IFN-γ and a sulfonamide may protect against dissemination. Nocardiosis can become a severe infection and mainly affects profoundly immunocompromised patients. Differential diagnosis often delays the time to diagnosis, which worsens the outcome.[20] Nocardia infections in children indicate the need for evaluation for an underlying immunological deficiency.[3] Nocardiosis can be difficult to recognize, which leads to misdiagnosis and consequently underestimation of its incidence.[21] Nocardia species should be considered as possible causative agents in immunocompromised patients with infection.[22] Another rare finding in this study was lymph node abscess due to A. israelii. Actinomycosis is caused by Actinomyces, a gram-positive anaerobe that is a normal inhabitant of the oral cavity.[23,24] Actinomycosis is a chronic granulomatous condition that commonly manifests as cervico-facial, pulmonary, or abdominal disease, caused by slowly progressive infection with oral and gastrointestinal communal Actinomyces species.[6] The organism has been classified as a bacterium and placed in the Actinomycetaceae family. Unusual organisms, uncommon in normal hosts, are being more frequently found in these patients.[3,25]
Bassiri and colleagues reported actinomycosis in 5 of 71 cases (5.7%) of fungal and actinomycosis infections in immunocompromised patients in Iran between 1994 and 2001.[21]
The main step in the pathogenesis of actinomycosis is disruption of the mucosal membrane leading to suppuration and abscess formation. The abscess then expands into adjacent tissue with no regard to tissue planes. Actinomycosis of the lymph nodes is rare and may clinically simulate lymphoma or other malignancies.[26] Actinomycosis is a catalase-negative infection important to consider in CGD. Appropriate diagnosis significantly impacts the patient management and decreases the morbidity associated with this infection. Our data are consistent with these observations and emphasize the crucial importance of making a microbiologic diagnosis in every case before starting anti-infective therapy. The initial diagnosis primarily depends on clinical judgment. In patients with poor dental hygiene and cervical or extensive lymph-adenopathy, actinomycosis should be included in the differential diagnosis. Diagnosis of actinomycosis is very difficult because the microorganism cannot be detected in most instances,[6] so the diagnosis is sometimes based only on clinical findings and the response to antibiotics. The treatment of actinomycosis includes both prolonged antibiotic therapy and surgical debridement. Treatment of actinomycosis is usually simple in immunocompetent individuals, but is more complicated in those with CGD because of delayed diagnosis and an increased risk of chronic invasive or debilitating disease.[6] The treatment choice for actinomycosis is penicillin G 18-24mil units IV/d × 2-6 weeks, then amoxicillin 500-750 mg PO three times a day/four times a day × 6-12 months and oral therapy alone may be adequate. Multiple infections in patients with CGD are sustained by different strains of the same few species of bacteria.[16] In reported series by Guide and colleagues, 60–90% of patients had some underlying immunosuppressive condition such as chronic steroid use, solid organ transplantation, lymphoreticular malignancy, CGD, or HIV infection.[16] Typical sites of dissemination include the lungs, skin, brain, and musculoskeletal system. Early diagnosis, aggressive management of infections, continuing intensive surveillance and monitoring of compliance to anti-infective regimens have had a positive effect on the morbidity and mortality rates of CGD. However, the problem of invasive fungal infections remains critical for these patients and only a longer follow-up will show whether the new advances in antimicrobial therapy will allow a long-term survival or will only delay the fatal outcome for a few years.[19] Our study does not provide evidence justifying long-term prophylaxis with IFN-γ in CGD patients
CONCLUSION
The physician should keep actinomycosis in mind as a possible diagnosis and perform the appropriate investigation, if the pathogenic findings of a mass are in favor of granulomatous inflammatory process or micro-abscess formation, and if work up for TB, and or fungal infections are negative. Actinomycosis should be vigorously sought and promptly treated in patients with CGD presenting with uncommon and prolonged clinical signs of infection.
Footnotes
Source of Support: Pasteur Institute of Iran.
Conflict of Interest: None declared.
REFERENCES
- 1.Lekstrom-Himes JA, Gallin JI. Immunodeficiency Diseases Caused by Defects in Phagocytes. N Engl J Med. 2000;343:1703–14. doi: 10.1056/NEJM200012073432307. [DOI] [PubMed] [Google Scholar]
- 2.Johnston RB, Newman SL. Chronic granulomatous disease. Pediatr Clin North Am. 1977;24:365–76. doi: 10.1016/s0031-3955(16)33424-1. [DOI] [PubMed] [Google Scholar]
- 3.Winkelstein JA, Marino MC, Johnston RB, Jr, Boyle J, Curnutte J, Gallin JI, et al. Chronic granulomatous disease.Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–69. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 4.McNeil M M, Brown J M. The medically important aerobic actinomycetes: Epidemiology and microbiology. Clin Microbiol Rev. 1994;7(Suppl 3):357–417. doi: 10.1128/cmr.7.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorman SE, Guide SV, Conville PS, DeCarlo ES, Malech HL, Gallin JI, et al. Nocardia infection in Chronic Granulomatous Disease. Clin Infect Dis. 2002;35:390–4. doi: 10.1086/341416. [DOI] [PubMed] [Google Scholar]
- 6.Reichenbach J, Lopatin U, Mahlaoui N, Beovic B, Siler U, Zbinden R, et al. Actinomyces in chronic granulomatous disease: An emerging and unanticipated pathogen. Clin Infect Dis. 2009;49(Suppl 11):1703–10. doi: 10.1086/647945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berd D. Laboratory identification of clinically important aerobic actinomycetes. Appl Microbiol. 1973;25:665–81. doi: 10.1128/am.25.4.665-681.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra SK, Gordon RE, Barnett DA. Identification of Nocardiae and streptomycetes of medical importance. JClinMicrobiol. 1980;11:728–36. doi: 10.1128/jcm.11.6.728-736.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.BassiriJahromi S, Khaksar AA, VaziriKashani M, Arshi S. Disseminated infection due to Fusarium sp In a patient with chronic granulomatous disease. Med J IslamRepub Iran. 1998;12(Suppl 1):93–6. [Google Scholar]
- 10.Sorrell TC, Mitchell DH, Iredell JR. Principles and practice of infectious diseases. 6th ed. Philadelphia, Pa: Churchill Livingstone Elsevier; 2005. Nocardia species. chap 252. [Google Scholar]
- 11.Southwick FS. Nocardiosis. In: Goldman L, Ausiello D, editors. Cecil Medicine. 23 ed. Philadelphia, Pa: Saunders Elsevier; 2007. chap 351. [Google Scholar]
- 12.Castelli L, Zlotnik H, Ponti R, Vidotto V. First reported Nocardia otitidiscaviarum infection in an AIDS patient in Italy. Mycopathologia. 1994;126:131–6. doi: 10.1007/BF01103766. [DOI] [PubMed] [Google Scholar]
- 13.Idriss ZH, Cunningham RJ, Wilfert CM. Nocardiosis in children: Report of three cases and review of the literature. Pediatrics. 1975;55(Suppl 4):479–84. [PubMed] [Google Scholar]
- 14.Boiron P, Provost F, Chevrier G, Dupont B. Review of nocardial infections in France 1987 to 1990. Eur J ClinMicrobiol Infect Dis. 1992;11:709–14. doi: 10.1007/BF01989975. [DOI] [PubMed] [Google Scholar]
- 15.Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22(Suppl 6):891–903. doi: 10.1093/clinids/22.6.891. [DOI] [PubMed] [Google Scholar]
- 16.Guide SV, Stock F, Gill VJ, Anderson VL, Malech HL, Gallin JI, et al. Reinfection, rather than persistent infection, in patients with chronic granulomatous disease. J Infect Dis. 2003;187:845–53. doi: 10.1086/368388. [DOI] [PubMed] [Google Scholar]
- 17.Dorman SE, Malech HL, Gallin JI, Holland SM. Nocardia infections in chronic granulomatous disease [abstract 105]. In: Programs and abstracts of the 36th Annual Infectious Diseases Society of America Meeting (Denver, CO) Clin Infect Dis. 1998;27:940. [Google Scholar]
- 18.Wynne SM, Kwon-Chung KJ, Shea YR, Filie AC, Varma A, Lupo P, et al. Invasive infection with Trichosporoninkin in 2 siblings with chronic granulomatous disease. J Allergy ClinImmunol. 2004;114:1418–24. doi: 10.1016/j.jaci.2004.07.066. [DOI] [PubMed] [Google Scholar]
- 19.Martire B, Rondelli R, Soresina A, Pignata C, Broccoletti T, Finocchi A, et al. Clinical features, long-term follow-up and outcome of a large cohort of patients with Chronic Granulomatous Disease: An Italian multicenter study G.Ugazio, Alessandro Plebani, Domenico De Mattia and IPINET. ClinImmunol. 2008;126(Suppl 2):155–64. doi: 10.1016/j.clim.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Matulionyte R, Rohner P, Uçkay I, Lew D, Garbino J. Secular trends of Nocardia infection over 15 years in a tertiary care hospital. J ClinPathol. 2004;57:807–12. doi: 10.1136/jcp.2004.016923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.BassiriJahromi S, Khaksar AA. Deep-Seated Fungal Infections in Immunocompromised Patients in Iran. Iran J Allergy, Asthma Immunol. 2005;4(Suppl 1):27–32. [PubMed] [Google Scholar]
- 22.Shetty AK, Arvin AM, Gutierrez KM. Nocardiafarcinica pneumonia in chronic granulomatous disease. Pediatrics. 1999;104(4 Suppl 1):961–4. doi: 10.1542/peds.104.4.961. [DOI] [PubMed] [Google Scholar]
- 23.Howard BJ, Keiser JF, Smith TF, Weissfeld AS, Tilton RC. 2nd ed. United States: St Louis, Mo, Mosby-Yearbook; 1994. Miscellaneous pathogenicorganisms. Clinical and Pathogenic Microbiology; pp. 467–9. [Google Scholar]
- 24.Goussard P, Gie R, Kling S, Beyers N. Thoracic actinomycosis mimicking primary tuberculosis. Pediatr Infect Dis J. 1999;18:473–5. doi: 10.1097/00006454-199905000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Kottilil S, Malech HL, Gill VJ, Holland SM. Infections with Haemophilus species in chronic granulomatous disease: Insights into the interaction of bacterial catalase and H2O2 production Original Research Article. ClinImmunol. 2003;106(Suppl 3):226–30. doi: 10.1016/s1521-6616(02)00048-7. [DOI] [PubMed] [Google Scholar]
- 26.Amrikachi M, Krishnan B, Finch CJ, Shahab I. Associated Lymphadenopathy Mimicking. Arch Pathol Lab Med. 2000;124:1502–5. doi: 10.5858/2000-124-1502-AAAAAA. [DOI] [PubMed] [Google Scholar]


