Abstract
Background:
Individually Andrographis paniculata Nees. (Acanthaceae), Phyllanthus niruri Linn.(Euphorbiaceae) and Phyllanthus emblica Linn. single plant extracts have been reported to have hepatoprotective activity. However, literature survey shows that no sufficient scientific data has been publish on pharmacological evaluation of these plants in combined form.
Method:
Hepatoprotective activity of the polyherbal hepatoprotaective formulation (PHF)-containing spray-dried aqueous extracts of Andrographis paniculata Nees. (Acanthaceae), Phyllanthus niruri Linn. (Euphorbiaceae) and Phyllanthus emblica Linn. (Euphorbiaceae), was screened against paracetamol, carbon tetrachloride (CCl4), and ethanol-induced hepatic damage in rats. PHF was evaluated by measuring levels of serum marker enzymes like SGOT, SGPT, ALP, direct bilirubin (DB), and lactate dehydrogenase (LDH). The histological studies were also studied support the biochemical parameters. Silymarin was used as standard drug.
Results:
Administration of PHF (100 and 200 mg/kg p.o.) significantly inhibited paracetamol, CCl4 and ethanol-induced elevation levels of SGPT, SGOT, ALP, DB and LDH. A comparative histopathological study of liver exhibited almost normal architecture as compared to toxicant group.
Conclusion:
Results suggests that the hepatoprotective effects of PHF might be useful for liver protection due to combined action of all plant extracts along with their phytoconstituents.
Keywords: Andrographis paniculata Nees, carbon tetrachloride, hepatoprotective activity, Marker enzymes, paracetamol, Phyllanthus niruri Linn
INTRODUCTION
Liver injuries induced by various hepatotoxins have been recognized as a major toxicological problem for years. However, there are a number of herbal formulations available on liver disorders in ayurvedic medicine. Various formulations like Liv-52,[1] Kamilari,[2] APCL-A Amalkadi Ghrita,[3] Panchagvya Ghrita, Himoliv,[4] and Livex[5] are well known for their hepatoprotective effects.
Large doses of paracetamol, widely used analgesic-antipyretic drug, is known to cause hepatotoxicity in human and laboratory animals. Increased lipid peroxidation in liver has been demonstrated as a frequent feature after poisoning with hepatotoxic substance.[6] Oxidative stress is one of the mechanism involved in carbon tetrachloride (CCl 4 )- induced hepatotoxicity, which also causes membrane disintegration, loss of membrane-associated enzymes, and necrosis. This is reflected through the biochemical parameter in liver and plasma.[7]
Excessive alcohol consumption is associated with the appearance of excess fat in the parenchymal cells with more complex acute change, which causes liver cell death. An acute inflammation with finally hepatic diseases, cirrhosis, is the leading cause of death.[6]
Andrographis paniculata showed protective effects in the activity of super oxide dismutase, catalase, glutathione peroxidase, glutathione reductase, as well as the level of glutathione.[8] Andrographolide, the active constituent isolated from the plant A. paniculata showed a significant dose-dependent protective activity against paracetamol and (CCl4) induced liver damage.[9]
Antihepatotoxic principles present in Phyllanthus niruri herbs are phyllanthin and hypophyllanthin which shows hepatoprotective activity. It also increases the life span of rats with hepatocellular carcinoma.[10] Antioxidant activities of the extracts were also demonstrable in vivo by the inhibition of CCl4 induced formation of lipid peroxides in the liver of rats by pretreatment with the extracts.[11] Dried 50% alcoholic extract of P. niruri (P.N.) and mixture of lignans isolated from P. niruri were screened for their hepatoprotective activity.[12]
A 50% hydro alcoholic fruit extract of Emblica officinalis shows hepatoprotective activity against anti-tuberculosis drugs induced liver toxicity.[13] Hepatocurative effect of E. officinalis against CYP 450 bioactivation hepatotoxicity is also reported. Hepatoprotective activity of E. officinalis and Chyavanprash in CCl4- induced hepatic toxicity is also described as well.[14] The formulation under the investigation was prepared in laboratory and subjected to the study of hepatoprotective activity. The herbal hepatoprotective formulation (HHF) consists of spray-dried aqueous extracts of A. paniculata Nees. (Acanthaceae), P.niruri Linn. (Euphorbiaceae) and P. emblica Linn. (Euphorbiaceae). In the indigenous medicine, all these plants are well known for their hepatoprotective activity.[15] Individual ingredients of the formulation were earlier investigated for their protective activity against different models of experimental hepatotoxicity. In the present experimental study on rats, a systematic research was undertaken to evaluate the possible effects of the formulation on the hepatotoxocity induced by diverse agents. The present communication substantiates the therapeutic utility of the formulation as hepatoprotective agents.
Individually single plant extract has been reported to have hepatoprotective activity. However, literature survey shows that no sufficient scientific data have been submitted on pharmacological evaluation of these plants in combined form. So it was decided to prepare and evaluate the formulation for its protective effect against the hepatotoxins like paracetamol, CCl4, and ethanol.
MATERIAL AND METHODS
Procurement of herbal extracts
The spray-dried extract of A. paniculata Nees. (Acanthaceae), P. niruri Linn. (Euphorbiaceae), and P. emblica Linn. (Euphorbiaceae) was procured from Tulsi Amrit Pvt. Ltd., Indore, India, as a gift sample.
Phytochemical analysis and HPTLC fingerprinting
All the plant extracts were identified according to the chemical test and fingerprinting data (Co-TLC with marker). HPTLC is one of the standardization parameter useful for qualitative and quantitative determination of phytoconstituents present in the herbal extract/formulation. HPTLC was performed on TLC plate precoated with silica gel 60 GF254 as stationary phase. Various pure solvents of varying polarity were tried in different proportions as mobile phase for the development of chromatogram. It was also identified by compounds like andrographolide from A. paniculata Nees, phyllanthin from P. niruri Linn, and gallic acid from P. emblica Linn.
Animals
Three-month-old Wistar albino rats of either sex weighing 180–220 g were used for the study. The animals were placed at random and allocated to treatment groups in polypropylene cages with paddy husk as bedding. Animals were housed at a temperature of 24 ± 2°C and relative humidity of 30–70%. A 12:12 light dark cycle was followed. All animals had free access of water ad libitum.
Study were reviewed and approved by the Institutional Animal and Ethics Committee of R.C. Patel Institute of Pharmaceutical Education and Research, Shirpur, India, and were in accordance with the guidelines of the Committee for the purpose of Control and Supervision of Experiments on Animals (CPCSEA), registration No.IAEC/09-07 and resolution number RCPCOP/IAEC/2006-07/09.
Drugs and chemicals
Ethanol, olive oil, CCl4, and gum acacia were obtained from Loba Chemie, Mumbai. Other drugs and chemicals include paracetamol (Sigma Chemicals), ALT (Alkaline aminotransferase) kit (Beacon Diagnostics Pvt. Ltd., Navsari, India), AST (Aspartate aminotransferase) kit (Beacon Diagnostics Pvt. Ltd., Navsari, India), alkaline phosphate (ALP) kit (Agappe Diagnostics Pvt. Ltd, Ernakulam, Kerla, India), lactate dehydrogenase (LDH) kit (Reckon Diagnostics Pvt. Ltd., India), Bilirubin kit (Accurex Biomedical Pvt. Ltd., Tarapur). Remi centrifuge machine, microplate reader (Power wave XS), and micropipette were used.
Herbal formulation[16]
The herbal hepatoprotective tablet formulation contains spray dried aqueous extract of Andrographis paniculata Nees (150 mg), Phyllanthus niruri Linn (200 mg) and Phyllanthus emblica Linn (200 mg), prepared by direct compression technique.
Acute toxicity study[17]
Swiss albino mice were divided into test group comprising of six animals in each group. The test was performed using increasing oral dose of herbal extracts from 500 to 5000 mg/kg b.w. The mice were observed continuously for 1 h and then half hourly for 4 h for any gross behavioral change and general motor activities like writhing, convulsion, response to tail pinching, gnawing, pupil size, fecal output, feeding behavior, etc., and further up to 72 h for any mortality. All the extracts did not cause any significant behavioral changes and no mortality was observed.
Experimental procedure
Paracetamol induced hepatotoxicity
Healthy albino rats of either sex weighing between 200 and 250 g were provided with rat feed in the same quantity and water ad libitum throughout the experiment. Animals were randomly divided into five groups, six animals. Animal of Group I served as normal received distilled water, Group II was control, received vehicle. Animal of Group III received standard silymarin 50 mg/kg p.o whereas Groups IV Group V were treated with HHF at 100 and 200 mg/kg orally for 5 days.[18] On the third day, paracetamol suspension (5% gum acacia) was administered in a dose of 3 g/kg b.w, orally to all groups except normal. Blood was collected after 48 h.
Carbon tetrachloride induced hepatotoxicity
Adult rats of either sex weighing 150-200 g were divided into the five groups of six rats. Group I received distilled water and olive oil 1 ml/kg i.p., Group II served as control received CCl 4 1 ml/kg i.p.; Group III received silymarin 50 mg/kg p.o.; while Groups IV and V received 200 and 400 mg/kg HHF orally for 5 days. On third day, hepatotoxicity was induced in all groups by CCl4 with olive oil 1 ml/kg, 1:1, i.p.[19] except normal group. Blood was collected after 48 h.
Ethanol-induced hepatotoxicity
Animals were divided into the five groups of six rats. Group I received normal feed and distilled water, Group II served as control, received ethanol (36.6% v/v) 30 ml/kg/day p.o., in three divided doses.[20] Group III received standard silymarin 50 mg/kg along with ethanol 30 ml/kg/day, in three divided doses, while Groups IV and V received HHF at the dose of 200 and 400 mg/kg with 36.6% ethanol 30 ml/kg/day in three divided doses for 20 days. All the animals received their respective treatment for 20 days by oral administration. Blood was collected on the 21st day.
Assessment of liver function assay
Rats of all groups were anesthetized with ether. Then blood was withdrawn from all groups of rats by puncturing retro-orbital plexus and allowed to coagulate for 45 min at room temperature. Serum was separated by centrifugation (Remi centrifuge machine) at 2500 rpm at 30°C for 15 min. Serum samples were immediately subjected to biochemical estimation of serum glutamate pyruvate transaminase (SGPT), serum glutamate oxaloacetate transaminase (SGOT), ALP, LDH, total bilirubin (TB), and direct bilirubin (DB) by microplate reader (Power wave XS), according to the colorimetric methods.[21]
Histopathological studies
The rats were sacrificed and liver was rapidly excised followed by fixing it for 48 h in 10% formalin, and was dehydrated by passing successively in different mixtures of ethyl alcohol–water (50%, 80%, and 95%) and finally in absolute alcohol, cleared in xylene and embedded in paraffin. Thick sections (4–5 mm) were prepared and then stained with hematoxylin and eosin dye for microscopic observation of cell necrosis, fatty change.
Statistical analysis
Results of the biochemical estimations were reported as mean ± S.E.M, and statistical analysis was performed by one-way ANOVA and Dunnet's test. At 95% confidence interval, P<0.05 and P<0.01 were considered to be significant.
RESULTS
Phytochemical analysis
All the extracts showed the presence of glycosides, flavonoids, alkaloids proteins, carbohydrates, and phenolic compounds. According to TLC results, chloroform:methanol (9:1) solvent system for andrographolide scanned over the wavelength 229 nm, toluene:ethyl acetate:formic acid (5:3.5:1.5) for phyllanthin at 254 nm, and toluene:ethyl acetate:formic acid (2.5: 5.5: 2 v/v/v) for gallic acid at 254 nm. Results of HPTLC fingerprinting showed the presence of andrographolide at Rf 0.45, phyllanthin at Rf 0.73, and gallic acid at Rf 0.68 in HHF of A. paniculata Ness, P. niruri Linn, and P. Linn, respectively.
DISCUSSION
Hepatic cells participate in metabolic activities and contain host of enzymes. In tissue, asparate aminotransferase (AST) and alkaline aminotransferase (ALT) were found to be in higher concentrations in cytoplasm, and AST exists in mitochondria. In liver injury, transport function of the hepatocytes gets disturbed, resulting in the leakage of plasma membrane and thereby causing an increased enzyme level in serum.[22] The elevated activities of these enzymes are indicative of cellular leakage and the functional integrity of the cell membranes in liver. ALP is excreted by liver via bile in the liver injury due to hepatotoxins, which results in a defective excretion of bile by the liver and is reflected in their increased levels in serum. In drug-induced liver toxicity, the level of LDH, and TB and DB get elevated. The present study was carried out to find out the effect of the formulation on the paracetamol-, CCl4-, and ethanol-induced hepatotoxicity.
Paracetamol-induced hepatotoxicity
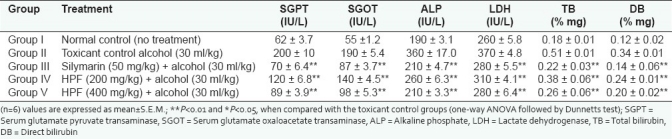
Paracetamol is a common antipyretic agent which is safe in therapeutic dosage but can produce fetal hepatic necrosis in human, rats, and mice with toxic doses. It is mainly metabolized in liver to excretable glucuronide and sulfate conjugates. Paracetamol is metabolized to a minor electrophilic metabolite, N-acetyl-p-benzoquinoneimine (NAPQI), which during paracetamol overdose depletes glutathione and initiates covalent binding to cellular proteins and initiates cell damage.[23] Due to liver injury, the transport function of hepatocytes gets disturbed resulting in the leakage of plasma membrane, thereby causing an increased enzyme level in the serum. In the present study, oral administration of paracetamol (3 g/kg) caused a significant rise in SGPT, SGOT, ALP, LDH, TB, and DB levels. Pretreatment of rats with herbal formulation at a dose of 100 and 200 mg/kg b.w for the 5 days resulted in significant (P<0.01) decrease in the level of SGPT, SGOT, ALP, LDH, DB, and TB [Table 1]. Silymarin (reference standard) significantly (P<0.01) reduced these levels to normal. The results revealed that hepatoprotective herbal formulation was potent and found to be equipotent with the standard.
Table 1.
Effect of polyherbal formulation and silymarin on serum parameters in paracetamol-induced hepatic damage in rat
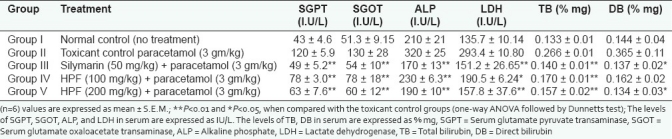
Hepatoprotective effect of herbal formulation was further confirmed by the histopathological study of liver. Liver section of paracetamol-treated rats showed gross necrosis of the centrilobular hepatocytes characterized by lymphocytic infiltration and portal trides. Herbal formulation treated animals show protection against liver damage by minimal necrosis in centrilobular and regeneration of hepatocytes [Figure 1].
Figure 1a.
Histopathology of liver tissues. Histopathological study of paracetamol-induced hepatotoxicity
Figure 1b.
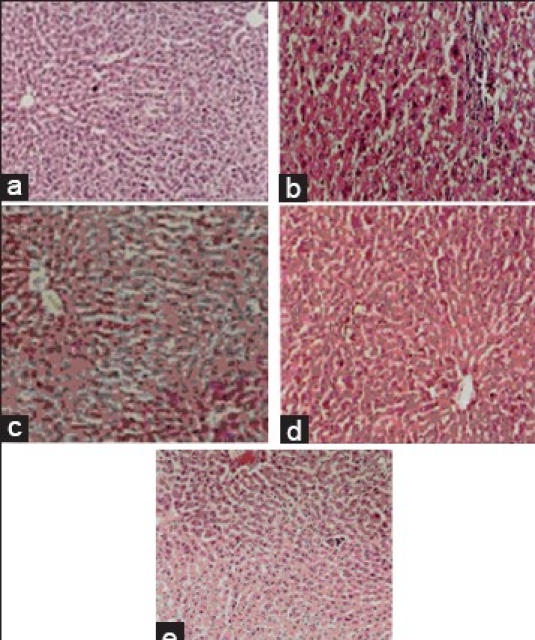
Histopathology of liver tissues. Histopathological study of carbon tetrachloride induced toxicity
Carbon tetrachloride induced hepatotoxicity
It is well established that hepatotoxicity by CCl4 is due to enzymatic activation to released CCl3 radical in free state, which in turn disrupts the structure and function of lipid and protein macromolecule in the membrane of the cell.[3] The stabilization of serum bilirubin, SGPT, SGOT, ALP, and LDH levels by herbal formulation is a clear indication of the improvement of the functional status of liver cells. The increased levels of SGPT, SGOT, ALP, LDH, TB, and DB are the conventional indicator of liver injury. In the present study, a significant (P<0.01) reduction in the level of SGPT, SGOT, ALP, LDH, TB, and DB was observed in the groups of animals treated with 200 and 400 mg/kg, b.w of herbal formulation. Silymarin significantly (P<0.01) reduced these levels to normal [Table 2]. The results reveal the hepatoprotective effect of herbal formulation compared with the CCl4 -treated group. This finding can further corroborated with histophathological studies. The histophathological examination clearly reveals that the hepatocytes, central vein, are almost normal in herbal formulation 200 and 400 mg/kg, b.w, treated group in contrast to the toxicant group [Figure 2].
Table 2.
Effect of polyherbal formulation and silymarin on serum parameters in CCl4-induced hepatic damage in rats
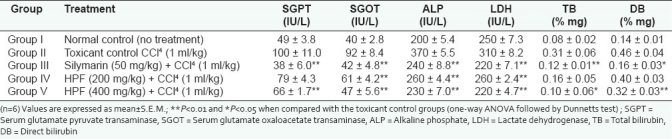
Figure 2.
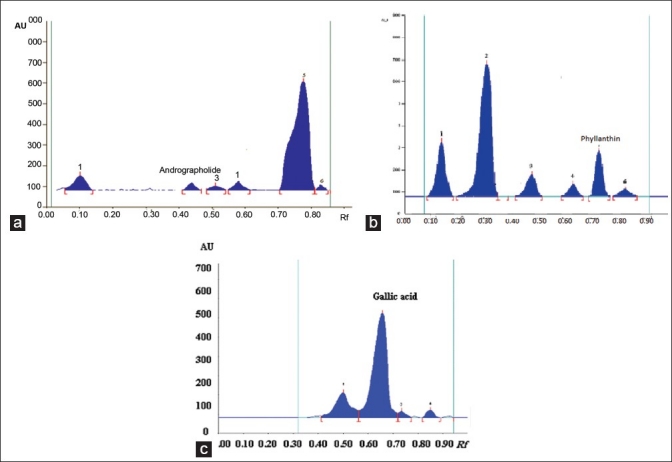
HPTLC finger printing of poly herbal hepatoprotective formulation (PHF) shows the presence of (a) Andrographolide, (b) Phyllanthin, (c) gallic acid
Alcohol-induced hepatotoxicity
Oxidative stress is one of the major factors in the etiology of ethanol injury mainly by Kupffer cell derived from ROS .Chronic consumption of ethanol causes injury to the liver cells. Increased level of serum albumin, AST, ALT, and ALP in alcohol-treated rats can be attributed to the damaged structural integrity of the hepatic cells because of the enzymes ALP located in the cytoplasm and released in the circulation after cellular damage. Alcohol consumption causes both plasma and organelle membrane damage.[24] Alcohol consumption is known to cause fatty infiltration and cirrhosis. It enhanced lipid peroxidation produced during the microsomal metabolism of ethanol. It will directly generate free radicals such as O2 (superoxide), H2O2, OH (hydroxy free radicals), and CH3CHOH (hydroxy ethyl free radicals) through activation of cytochrome P-450 2E 1 enzymes. Oral administration of ethanol at a dose of 30 ml/kg/day significantly increased the SGOT, SGPT, ALP, LDH, TB, and DB. Treatment with herbal formulation at the dose of 200 and 400 mg/kg/day along with alcohol showed significantly (P<0.01) reduced levels of SGOT, SGPT, ALP, LDH, TB, and DB as compared with control (alcohol treated) [Table 3]. This shows that herbal formulation containing A. paniculata Nees, P. niruri Linn, and P. emblica extracts to an extent preserves the structural integrity of the liver from the adverse effects of ethanol. Silymarin significantly (P<0.01) reduced these levels to normal.
Table 3.
Effect of polyherbal formulation and silymarin on serum parameters in ethanol-induced hepatic damage in rats
The histopathological studies were performed to find out fatty changes, normal hepatic architecture, hepatocellular necrosis, and lymphocytic infiltration. Normal group showed no change, whereas rats treated with ethanol showed moderate to marked fatty changes microvesicular to vacuolar [Figure 1c]. Herbal formulation treated group produced a marked degree of protection against ethanol-induced alterations, similar to those from normal rats.
Figure 1c.
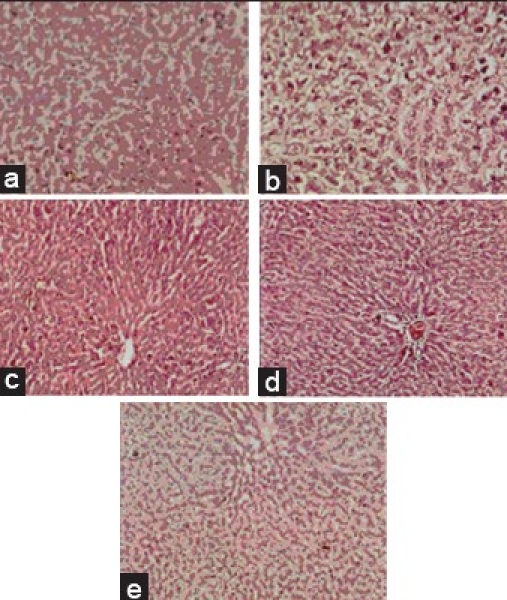
Histopathology of liver tissues. Histopathological study of ethanol-induced hepatotoxicity
CONCLUSION
Our finding confirmed the hepatoprotective effect of the herbal formulation and revealed that the herbal formulation has preventive action on paracetamol-, CCl4-, and ethanol-induced hepatotoxicity in a dose-dependent manner. Herbal formulation significantly reduced the levels of SGPT, SGOT, ALP, LDH, TB, and DB. These findings are also confirmed by histopathological observation. Traditionally these plants are reported to have hepatoprotective activity. The resulting hepatoprotective activity of all three plants could be attributed to phytochemicals like phyllanthin, embellin, and andrographolide.
ACKNOWLEDGMENT
Authors are thankful to the Head of the Department of Pharmacology Dr. P.H. Patil, R.C. Patel Institute of Pharmaceutical Education and Research, Shirpur, for providing the necessary facility and for the support and guidance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chauhan BL, Mohan AR, Kulkarni RD, Mitra SK. Bioassay for evaluation of the hepatoprotective effect of Liv-52, a Polyherbal formulation, on ethanol metabolism in chronic alcohol exposed rats. Indian J Pharmacol. 1994;26:117–20. [Google Scholar]
- 2.Rajesh MG, Latha MS. Preliminary evaluation of the antihepatotoxic activity of kamilari, a Polyherbal formulation. J Ethanopharmacol. 2004;91:99–104. doi: 10.1016/j.jep.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Achliya GS, Wadodkar SG, Dorle AK. Evaluation of hepatoprotective effect of Amalkadi Ghrita against carbon tetrachloride-induced hepatic damage in rats. J Ethanopharmacol. 2004;90:1–4. doi: 10.1016/j.jep.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya D, Pandit S, Mukherjee R, Pal N, Sur TK. Hepatoprotective activity of Himoliv, a polyherbal formulation in rats. Indian J Physio Pharmacol. 2003;47:435–40. [PubMed] [Google Scholar]
- 5.Venkateshwaran PS, Millman I, Blumbergb S. Effect of an extract from Phyllanthus niruri on Hepatitis B and woodchuck hepatitis viruses: In-vitro and in-vivo studies. Proc Nat Acad Sci USA. 1987;84:274–8. doi: 10.1073/pnas.84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapur V, Pillai KK, Hussian SZ, Balani DK. Hepatoprotecitve activity of Jigrine on liver damage caused by Alcohol-Carbon tetrachloride and paracetamol in rats. Indian J Pharmacol. 1994;26:35–40. [Google Scholar]
- 7.Sotelo F, Martinez FD, Muriel D, Santillan RL, Castillo D, Yahuaca P, et al. Evaluation of the effectiveness of Rosmarinus officinalis (Lamiaceae) in the alleviation of carbon tetrachloride induced acute hepatotoxicity in rats. J Ethnopharmacol. 2002;81:145–54. doi: 10.1016/s0378-8741(02)00090-9. [DOI] [PubMed] [Google Scholar]
- 8.Trivedi NP, Rawa UM. Hepatoprotective and antioxidant property of Andrographis paniculata (Nees) in BHC induced liver damage in mice. Indian J Exp Biol. 2001;39:41–6. [PubMed] [Google Scholar]
- 9.Rana AC, Avadhoot Y. Hepatoprotective effects of Andrographis paniculata against carbon tetrachloride-induced liver damage. Arch Pharm Res. 1991;14:93–5. doi: 10.1007/BF02857822. [DOI] [PubMed] [Google Scholar]
- 10.Syamasundar K, Singh B, Thakur R. Antihepatotoxic principles of Phyllanthus Niruri herbs. J Ethanopharmacol. 1985;14:41–4. doi: 10.1016/0378-8741(85)90026-1. [DOI] [PubMed] [Google Scholar]
- 11.Harish R, Shivanandappa T. Antioxidant and Hepatoprotective potential of Phyllanthus niruri. Food Chem. 2006;95:180–5. [Google Scholar]
- 12.Nahid T, Chattervedi S, Agrawal SS, Ahmed N. Hepatoprotective studies on Paracetamol induced liver cell damage in albino mice. Exp Med. 2005;12:211–2. [Google Scholar]
- 13.Tasduq A, Kaiser P, Gupta DK, Kapahi BK, Jyotsna S, Maheshwari HS, et al. Protective effect of a 50% hydro alcoholic fruit extract of Emblica officinalis against anti-tuberculosis drugs induced liver toxicity. Phyto Res. 2005;19:193–7. doi: 10.1002/ptr.1631. [DOI] [PubMed] [Google Scholar]
- 14.Jose JK, Kuttan R. Hepatoprotective activity of Emblica officinalis and chyavanprash. J Ethanopharmacol. 2000;72:135–40. doi: 10.1016/s0378-8741(00)00219-1. [DOI] [PubMed] [Google Scholar]
- 15.Handa SS, Sharma A. Hepatoprotective activity of andrographolide against galactosamine and paracetamol intoxication in rats. Indian J Med Res. 1990;92:284–92. [PubMed] [Google Scholar]
- 16.Shrikumar S, Ravi TK. Approaches towards development and promotion of herbal drugs. Pharmacog Rev. 2007;1:180–4. [Google Scholar]
- 17.Ghosh MN. 3rd ed. Kolkata: Hilton and Company; 2005. Fundamentals of experimental Pharmacology; pp. 190–7. [Google Scholar]
- 18.Chattopadhayay RR, Bandyopadhyay M. Possible Mechanism of hepatoprotective activity of Azadirachta indica leaf extract against paracetamol induced hepatic damage in rats: Part III. Indian J Pharmacol. 2005;37:184–5. [Google Scholar]
- 19.Janbaz KH, Gilani AH. Studies on preventive and curative effects of berberine on chemical-induced hepatotoxicity in rodents. Fitoterapia. 2000;71:25–33. doi: 10.1016/s0367-326x(99)00098-2. [DOI] [PubMed] [Google Scholar]
- 20.Grange LL, Wang M, Watkins R, Ortiz D, Sanchez ME, Konst J, et al. Protective effects of the flavonoid mixture, silymarin, on fetal rat brain and liver. J Ethnopharmacol. 1999;65:53–61. doi: 10.1016/s0378-8741(98)00144-5. [DOI] [PubMed] [Google Scholar]
- 21.Reitman S, Frankel S. Colourimetric method for the determination of serum oxaloacetic and glutamic pyruvic trasaminase. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 22.Rajagopal SK, Manickam P, Periyasamy V, Namasivayam N. Activity of Cassia auriculata leaf extract in rats with alcoholic liver injury. J Nutr Biochem. 2003;14:252–8. doi: 10.1016/s0955-2863(03)00053-6. [DOI] [PubMed] [Google Scholar]
- 23.Dienstag JL, Isselbacher KJ. 16th ed. New York: Mc-Graw Hill; 2005. Liver And Biliary tract disease. Harrison's Principle of internal Medicine; pp. 1808–80. [Google Scholar]
- 24.Jafri MA, Subhani MJ, Javed K, Singh S. Hepatoprotective activity of leaves of Cassia occidentalis against paracetamol and ethyl alcohol intoxication in rats. J Ethanopharmacol. 1999;66:355–61. doi: 10.1016/s0378-8741(99)00037-9. [DOI] [PubMed] [Google Scholar]


