Abstract
Background:
Miraziridine A, a natural peptide isolated from a marine sponge, is a potent cathepsin B inhibitor with a second-order rate constant of 1.5 × 104 M-1 s-1. In the present study, miraziridine A was isolated from the Red Sea sponge Theonella swinhoei on the basis of chromatographic and spectrometric techniques. We conclude that T. swinhoei from the Red Sea represents an alternative source of the aziridinylpeptide miraziridine A to the previously identified Theonella mirabilis from Japan. We confirmed that the metabolite is produced by marine sponges from different geographical locations.
Context:
Marine sponges have been proven to be a rich source of secondary metabolites exhibiting a huge diversity of biological activities, including antimicrobial, antitumor and immunomodulatory activities. Theonella species (order Lithistida, Demospongiae) have been shown to be a source of anti-protease and anti-HIV secondary metabolites.
Aims:
To identify the protease inhibitor mirazirine A in the marine sponge Theonella swinhoei.
Material and Methods:
The marine sponge Theonella swinhoei was collected by SCUBA diving in the Red Sea in Eilat (Israel). Sponge material was lyophilized and further extracted successively with cyclohexane, dichloromethane and methanol to obtain three crude extracts. LC-MS analysis was performed to confirm the presence of Miraziridine A in the dichloromethane fraction.
Results:
In the present study, miraziridine A was isolated from the Red Sea sponge T. swinhoei on the basis of chromatographic and spectrophotometric techniques.
Conclusions:
We conclude that T. swinhoei from the Red Sea represents an alternative source of the aziridinylpeptide miraziridine A to the previously identified Theonella mirabilis from Japan.
Keywords: Anti-protease, cathepsin L, miraziridine A, T. swinhoei
INTRODUCTION
Marine sponges have been proven to be a rich source of secondary metabolites exhibiting a huge diversity of biological activities, including antimicrobial, antitumor and immunomodulatory activities.[1] An example is agelasphin 9b, a glycosphingolipid isolated from the sponge Agelas mauritianus which is a potent NKT cell stimulator.[2] According to the Marinlit database, around 7,400 compounds have thus far been isolated from marine sponges,[3] and around 200 metabolites are reported every year.[4] Theonella species (order Lithistida, Demospongiae) have been shown to be a source of anti-protease and anti-HIV secondary metabolites.[5,6] For example, the marine sponge Theonella aff. mirabilis, has been reported to contain the protease inhibitor miraziridine A [Figure 1],[7] and the papuamides A and B with anti-HIV properties.[8] Miraziridine A is a secondary metabolite of particular interest due to its three structural elements (i) (2R,3R)-aziridine-2,3-dicarboxylic acid, (ii) (3S,4S)-4-amino-3-hydroxy-6-methylheptanoic acid (statine) and (iii) (E)-(S)-4-amino-7-guanidino-hept-2-enoic acid (vinylogous arginine residue), which are responsible for inhibition of three different classes of proteases, such as serine (e.g., trypsin), cysteine (e.g., cathepsins B and L) and aspartyl proteases (e.g., pepsin).[9]
Figure 1.
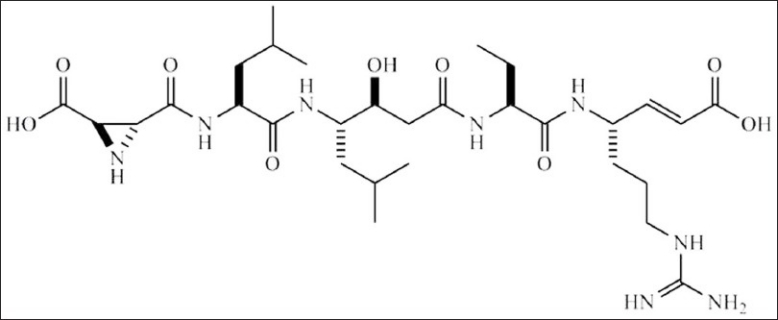
Miraziridine A.
The sponge T. swinhoei (abbreviated as “Ts”) has been found to contain antifungals including cyclolithistide A, theonegramides and theopalauamide, as well as paltolides and cytotoxic polytheonamides.[10]
MATERIAL AND METHODS
General experimental procedures
Sponge biomass was lyophilized with a Christ ALPHA II-12 freeze dryer. LC-MS was performed on an Agilent 1100 LC/MSD trap with a HPLC system 1100, Agilent, using a Phenomenex Jupiter 4 μ Proteo 90A RP C18 column (4.6 × 150 mm). Column chromatography was performed on Sephadex HL-20. Solvents used for extraction and column chromatography were glass distilled prior to use and solvents used for LC-MS were HPLC grade.
Animal material, extraction, and identification of miraziridine A
The marine sponge T. swinhoei was collected by SCUBA diving in the Red Sea in Eilat (Israel) at a depth of 3 m in December 2004 (GPS: 29°30’ 07’’ N; 34°55’ 02’’ W). Sponge material was frozen and transported to the laboratory and subsequently stored at -80 °C. Sponge biomass was lyophilized to obtain 7.3 g of the dried material and further extracted successively with cyclohexane, dichloromethane and methanol to obtain three crude extracts, TsCY (87 mg), TsDCM (98 mg) and TsMeOH (470 mg). Prepared extracts were subjected to reverse phase LC-MS, using H2O (0.1% formic acid)-MeCN (0.1% formic acid) gradient (60% H2 O for 5 min, 60-5% H2 O for 20 min and 5% H 2 O for 15 min), and the peaks were detected at 254 nm. Crude dichloromethane extract containing the [M+H]+ peak for miraziridine A was subjected to column chromatography on sephadex (eluent: methanol) to generate three sub-fractions (TsDCM-1, TsDCM-2 and TsDCM-3). The three subfractions obtained were subsequently analyzed by LC-MS; TsDCM-3 contained the [M+H] + peak for miraziridine A. Furthermore, TsDCM-3 and synthetic miraziridine A, synthesized according to Schaschke,[9] were subjected to LC-MS, H2 O/MeCN gradient (100% H2O for 3 min, 100-0% for 32 min, 0-100% for 10 min), and detection at 220 and 254 nm.
Synthesis of miraziridine A
Synthesis of miraziridine A was previously described by Schaschke.[9]
Enzyme assay
Cathepsin L protease inhibition assay was performed according to Vicik et al.[11] Briefly, the fluorometric assay was done at 25°C in a 20 mM Tris-HCl buffer pH 6.0, in a total volume of 200 μl. The final substrate concentration was 6.25 μM. The final enzyme concentration was 53 ng ml-1. The substrate (Cbz-Phe-Arg-AMC) and inhibitor stock solutions were prepared in dimethyl sulfoxide (DMSO) (final concentration in the assay 10%) and were diluted with assay buffer. The sub-fraction containing miraziridine A was tested in duplicates at a final concentration of 100 μg ml-1. The protease inhibition assay was carried out on a Cary Eclipse fluorescence spectrophotometer (Varian, Darmstadt, Germany) using a microplate reader (excitation 365 nm, emission 460 nm).
RESULTS AND DISCUSSION
In this study, the sponge T. swinhoei was collected offshore Israel in the Red Sea and extracts were prepared through sequential extraction of the freeze-dried sponge (7.3 g) with cyclohexane (CY), dichloromethane (DCM) and methanol (MeOH). LC-MS analysis of the three preparations (CY: 87 mg, DCM: 98 mg and MeOH: 470 mg) showed that the crude dichloromethane extract yielded an ion peak at m/z 670.1 [M+H]+, which indicated the presence of miraziridine A. The TsDCM extract was further partitioned using sephadex HL-20 to obtain three sub-fractions (TsDCM-1, -2, -3) which were subsequently analyzed by LC-MS; it was observed that the sub-fraction TsDCM-3 contained the pentapeptide. When comparing the mass spectra of synthetic compound 1, synthesized according to Schaschke,[9] and of TsDCM-3, we observed a molecular ion peak at m/z 670.1 [M+H]+ in both the solution of synthetic miraziridine A and in the sub-fraction TsDCM-3. Coelution experiments were pursued showing only one peak at a retention time of 16.2 min. Additionally, a fluorometric assay was performed to test the cathepsin L inhibition by the crude extract TsDCM-3 at a concentration of 100 μg ml-1, showing 60% inhibition.
Miraziridine A, previously found in the sponge Theonella aff. mirabilis and in this study identified in the sponge T. swinhoei, has already served as a model to synthesize protease inhibitors.[12] A recent study showed that 75% of the metabolites reported between 1985 and 2008 were obtained from marine invertebrates, mostly from sponges. Thus marine sponges remain a significant source of bioactive metabolites, leading to more active synthetic derivatives, many of them currently in clinical use.[13] For example, Halaven, a synthetic analogue of halichondrin B, a product isolated from the marine sponge Halichondria okadai, was recently approved by the U.S. FDA to be used in the treatment of advanced breast cancer.[14] In this study we confirmed that the protease inhibitor miraziridine A is produced by marine sponges from different geographical locations. Moreover we highlight marine sponges as a source of novel protease inhibitors.
Interestingly, protease inhibitors have been reported as well from microbial sources. For example, (2S,3S)-aziridine-2,3-dicarboxylic acid[15] and leupeptin[16] have been reported from actinomycete strains, and circinamide from cyanobacteria isolates.[17] (2S,3S)-Aziridine-2,3-dicarboxylic acid and circinamide contain an aziridine moiety, to which the inhibitory activity of miraziridine A is mainly attributed.[12] Moreover, the actinomycete Kibdelosporangium sp. was found to produce the aziridine-containing metabolite azinomycin A.[18] Actinomycetes and cyanobacteria constitute parts of the microbial consortia present in marine sponges that can account for nearly half of the sponge's biomass.[19,20] This fact leads us to consider that protease inhibitors such as miraziridine A can be the result of the symbiotic interactions between microbes and sponge.
Furthermore, protozoa, e.g., plasmodia, express a broad spectrum of proteases essential for survival of the parasite,[21] and it is known that cysteine and aspartic acid protease inhibitors are synergistic against plasmodia.[22] It will be interesting in the future to resynthesize miraziridine A and perform further bioactivity testing, where compound 1 would be tested alone or in combination with aspartic acid inhibitors. It will be also interesting to synthesize miraziridine A-derived molecules. For example, the shortening of the molecule by combining only two of the three protease-inhibiting building blocks (aziridine and statine, aziridine and vinylogous arginine, or vinylogous arginine and statine) will represent an alternative to produce potent protease inhibitors.
CONCLUSIONS
The presence of the aziridinylpeptide miraziridine A in the dichloromethane extract of T. swinhoei was documented in this study, thus confirming its presence in marine sponges of geographically disparate oceanic regions. In the future, the use of miraziridine A alone or in combination with aspartic acid inhibitors against certain parasitic infections, as well as the synthesis of novel protease inhibitors derived from miraziridine A will be investigated. We showed marine sponges as a potential source of novel protease inhibitors.
ACKNOWLEDGMENT
We gratefully acknowledge M. Ilan (Tel-Aviv University, Israel) for sponge collection from Israel and C. Heindl (University of Würzburg, Germany) for technical assistance in the laboratory. Financial support was provided by a doctoral fellowship (Graduate College “Immunmodulation GK 520” funded by the DFG) to P.T. and the DFG (SFB 630) to T.S. (TP A4) and U.H. (TP A5).
Footnotes
Source of Support: German Research Society, financial support
Conflict of Interest: None declared.
REFERENCES
- 1.Molinski TF, Dalisay DS, Lievens SL, Saludes JP. Drug development from marine natural products. Nat Rev Drug Discov. 2009;8:69–85. doi: 10.1038/nrd2487. [DOI] [PubMed] [Google Scholar]
- 2.Natori T, Koezuka Y, Higa T. Agelasphins, Novel Alpha-Galactosylceramides from the Marine Sponge Agelas Mauritianus. Tetrahedron Lett. 1993;34:5591–2. [Google Scholar]
- 3.MarinLit Database. Department of Chemistry, University of Canterbury. 2008. [cited in 2008]]. Available from: http://www.chem.canterbury.ac.nz/marinlit/marinlit.shtml .
- 4.Laport MS, Santos OC, Muricy G. Marine sponges: Potential sources of new antimicrobial drugs. Curr Pharma Biotechnol. 2009;10:86–105. doi: 10.2174/138920109787048625. [DOI] [PubMed] [Google Scholar]
- 5.Fusetani N, Fujita M, Nakao Y, Matsunaga S, Van Soest RW, Tokaramide A. a new cathepsin B inhibitor from the marine sponge Theonella aff. mirabilis. Bioorg Med Chem Lett. 1999;9:3397–402. doi: 10.1016/s0960-894x(99)00618-6. [DOI] [PubMed] [Google Scholar]
- 6.Plaza A, Bifulco G, Masullo M, Lloyd JR, Keffer JL, Colin PL, et al. Mutremdamide A and koshikamides C-H, peptide Inhibitors of HIV-1 entry from different Theonella species. J Organ Chem. 2010;75:4344–55. doi: 10.1021/jo100076g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakao Y, Fujita M, Warabi K, Matsunaga S, Fusetani N. Bioactive marine metabolites. Part 104. Miraziridine A, a novel cysteine protease inhibitor from the marine sponge Theonella aff. mirabilis. J Am Chem Soc. 2000;122:10462–3. [Google Scholar]
- 8.Ford PW, Gustafson KR, McKee TC, Shigematsu N, Maurizi LK, Pannell LK, et al. Papuamides A-D, HIV-inhibitory and cytotoxic depsipeptides from the sponges Theonella mirabilis and Theonella swinhoei collected in Papua New Guinea. J Am Chem Soc. 1999;121:5899–909. [Google Scholar]
- 9.Schaschke N. Miraziridine A: natures blueprint towards protease class-spanning inhibitors. Bioor Med Chem Lett. 2004;14:855–7. doi: 10.1016/j.bmcl.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 10.Plaza A, Keffer JL, Lloyd JR, Colin PL, Bewley CA. Paltolides A--C, anabaenopeptin-type peptides from the palau sponge Theonella swinhoei. J Nat Prod. 2010;73:485–8. doi: 10.1021/np900728x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vicik R, Busemann M, Gelhaus C, Stiefl N, Scheiber J, Schmitz W, et al. Aziridide-based inhibitors of cathepsin L: Synthesis, inhibition activity, and docking studies. Chem Med Chem. 2006;1:1126–41. doi: 10.1002/cmdc.200600106. [DOI] [PubMed] [Google Scholar]
- 12.Konno H, Kubo K, Makabe H, Toshiro E, Hinoda N, Nosaka K, et al. Total synthesis of miraziridine A and identification of its major reaction site for cathepsin B. Tetrahedron. 2007;63:9502–13. [Google Scholar]
- 13.Hu GP, Yuan J, Sun L, She ZG, Wu JH, Lan XJ, et al. Statistical research on marine natural products based on data obtained between 1985 and 2008. Marine Drugs. 2011;9:514–25. doi: 10.3390/md9040514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narayan S, Carlson EM, Cheng H, Du H, Hu Y, Jiang Y, et al. Novel second generation analogs of eribulin, Part I: Compounds containing a lipophilic C32 side chain overcome P-glycoprotein susceptibility. Bioorg Med Chem Lett. 2011;21:1630–3. doi: 10.1016/j.bmcl.2011.01.111. [DOI] [PubMed] [Google Scholar]
- 15.Naganawa H, Usui N, Takita T, Hamada M, Umezawa H. S-2,3-dicarboxy-aziridine: A new metabolite from a Streptomyces. J Antibiotics (Tokyo) 1975;28:828–9. doi: 10.7164/antibiotics.28.828. [DOI] [PubMed] [Google Scholar]
- 16.Hozumi M, Ogawa M, Sugimura T, Takeuchi T, Umezawa H. Inhibition of tumorigenesis in mouse skin by leupeptin, a protease inhibitor from Actinomycetes. Cancer Res. 1972;32:1725–8. [PubMed] [Google Scholar]
- 17.Shin HJ, Matsuda H, Murakami M, Yamaguchi K. Circinamide, a novel papain inhibitor from the cyanobacterium Anabaena circinalis (NIES-41) Tetrahedron. 1997;53:5747–54. [Google Scholar]
- 18.Ogasawara Y, Liu HW. Biosynthetic studies of aziridine formation in azicemicins. J Am Chem Soc. 2009;131:18066–8. doi: 10.1021/ja907307h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hentschel U, Usher KM, Taylor MW. Marine sponges as microbial fermenters. FEMS Microbiol Ecol. 2006;55:167–77. doi: 10.1111/j.1574-6941.2005.00046.x. [DOI] [PubMed] [Google Scholar]
- 20.Taylor MW, Radax R, Steger D, Wagner M. Sponge-associated microorganisms: Evolution, ecology, and biotechnological potential. Microbiol Mol Biol Rev. 2007;71:295–347. doi: 10.1128/MMBR.00040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breuning A, Degel B, Schulz F, Buchold C, Stempka M, Machon U, et al. Michael acceptor based antiplasmodial and antitrypanosomal cysteine protease inhibitors with unusual amino acids. J Med Chem. 2010;53:1951–63. doi: 10.1021/jm900946n. [DOI] [PubMed] [Google Scholar]
- 22.Semenov A, Olson JE, Rosenthal PJ. Antimalarial synergy of cysteine and aspartic protease inhibitors. Antimicrob Agents Chemother. 1998;42:2254–8. doi: 10.1128/aac.42.9.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]


