Abstract
Cytochrome P450 2D6 (CYP2D6) is highly polymorphic. CYP2D6-2D7 hybrid genes can be present in samples containing CYP2D6*4 and CYP2D6*10 alleles. CYP2D7-2D6 hybrid genes can be present in samples with duplication signals and in samples with homozygous genotyping results. The frequency of hybrid genes in clinical samples is unknown. We evaluated 1390 samples for undetected hybrid genes by polymerase chain reaction (PCR) amplification, PCR fragment analysis, TaqMan copy number assays, DNA sequencing, and allele-specific primer extension assay. Of 508 CYP2D6*4-containing samples, 109 (21.5%) harbored CYP2D6*68 + *4-like, whereas 9 (1.8%) harbored CYP2D6*4N + *4-like. Of 209 CYP2D6*10-containing samples, 44 (21.1%) were found to have CYP2D6*36 + *10. Of 332 homozygous samples, 4 (1.2%) harbored a single CYP2D7-2D6 hybrid, and of 341 samples with duplication signals, 25 (7.3%) harbored an undetected CYP2D7-2D6 hybrid. Phenotype before and after accurate genotyping was predicted using a method in clinical use. The presence of hybrid genes had no effect on the phenotype prediction of CYP2D6*4- and CYP2D6*10-containing samples. Four of four (100%) homozygous samples containing a CYP2D7-2D6 gene had a change in predicted phenotype, and 23 of 25 (92%) samples with a duplication signal and a CYP2D7-2D6 gene had a change in predicted phenotype. Four novel genes were identified (CYP2D6*13A1 variants 1 and 2, CYP2D6*13G1, and CYP2D6*13G2), and two novel hybrid tandem structures consisting of CYP2D6*13B + *68×2 + *4-like and CYP2D6*13A1 variant 2 + *1×N were observed.
Introduction
Cytochrome P450 2D6 (CYP2D6) encodes an enzyme clinically relevant to the use of tamoxifen (Goetz et al., 2005, 2007) and the selection of psychiatric drugs (Black et al., 2007). CYP2D6 testing is sometimes done before tamoxifen treatment of breast cancer because patients who fail to metabolize tamoxifen to endoxifen via the CYP2D6 pathway may be at increased risk of breast cancer relapse (Goetz et al., 2007). Genotyping is also used to select psychotropic medications (Topic et al., 2000; Murphy et al., 2003; Kirchheiner et al., 2004; Malhotra et al., 2004).
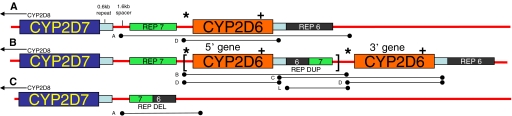
The CYP2D locus contains the CYP2D8 and CYP2D7 pseudogenes 5′ to the CYP2D6 gene (Fig. 1A). CYP2D6 genetic complexity is due to single nucleotide polymorphisms (SNPs), duplications and multiplications (Fig. 1B), deletions (Fig. 1C), and recombination events with the CYP2D7 pseudogene (Fig. 2, A–F). The allelic nomenclature of CYP2D6 is based upon the “star” (i.e., *) nomenclature method whereby alleles are named sequentially by the Human Cytochrome P450 (CYP) Allele Nomenclature Committee (http://www.cypalleles.ki.se/index.htm). Subfamilies of alleles are given the same number, but members of the family are given different suffixes. For example, CYP2D6*4 is a subfamily with A to N members, and each has different SNPs associated with the defining SNP for CYP2D6*4: 1846G>A. CYP2D6*4N has a gene conversion to CYP2D7 in exon 9, whereas CYP2D6*4-like, which is mentioned in this article, is different from CYP2D6*4N by the fact that it lacks a conversion to the CYP2D7 sequence in exon 9 (Kramer et al., 2009). Genotyping platforms are not designed to detect the exact allele present in the CYP2D6*4 subfamily so the suffix of A to N is often omitted in clinical genotyping results.
Fig. 1.
CYP2D locus structures for single, typical duplicated and deleted arrangements. A, single CYP2D6 arrangement. CYP2D8 (not shown) and CYP2D7 pseudogenes are located 5′ to the CYP2D6 gene. Similar 0.6-kb repeats follow the CYP2D7 and CYP2D6 sequences as do rep 7 and rep 6, respectively, which differ by just a few nucleotides in the 5′ and 3′ regions of 5 rep. Note that the 1.6-kb spacer is located 3′ to the CYP2D7 pseudogene, which is absent downstream of the CYP2D6 gene. B, typical CYP2D6 duplication arrangement. The first CYP2D6 gene is followed by rep dup, a hybrid containing a 5′ rep 6 sequence and a 3′ rep 7 sequence. Multiplications of the sequence shown between the brackets are known to exist. C, CYP2D6 deletion arrangement (CYP2D6*5), in which CYP2D7 is followed by a rep del that is a hybrid containing a 5′ rep 7 sequence and a 3′ rep 6 sequence. PCR fragments used in this investigation are depicted as lettered lines under the structures (Table 1). Probe locations for TaqMan copy number assays are designated as * and + for the 5′ flanking CYP2D6 assay and the CYP2D6 intron 6 assay, respectively.
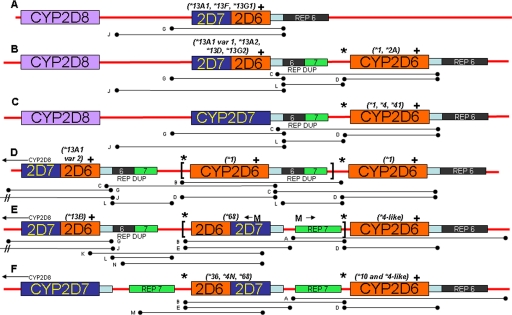
Fig. 2.
CYP2D locus structures for single and hybrid tandems of CYP2D7-2D6 and CYP2D6-2D7. A, single CYP2D7-2D6 arrangement (e.g., CYP2D6*13A1, CYP2D6*13F, and CYP2D6*13G1). B, CYP2D7-2D6 + CYP2D6 arrangement (e.g., CYP2D6*13A1, CYP2D6*13A2, CYP2D6*13D, and CYP2D6*13G2 with a tandem CYP2D6*1, CYP2D6*2A). Notice that the hybrid gene is followed by rep dup. C, CYP2D7 gene in a duplication arrangement that is followed by rep dup upstream of the tandem CYP2D6 gene (e.g., CYP2D6*1, CYP2D6*4, and CYP2D6*41). D, novel CYP2D7-2D6 + CYP2D6×N hybrid tandem multiplication arrangement. In this case, the CYP2D7-2D6 hybrid is a CYP2D6*13A1 variant 2 and the CYP2D6 alleles are CYP2D6*1. The absolute number of CYP2D6*1 alleles in tandem could not be determined. E, novel CYP2D7-2D6 hybrid (CYP2D6*13B) followed by rep dup, CYP2D6-2D7 hybrids (CYP2D6* 68×2) and a CYP2D6 gene (CYP2D6*4-like). The ←M and M→ designations show the relative locations of fragment M primers in this arrangement, which generates a product because the CYP2D6-2D7 hybrid is found in tandem in this arrangement. F, CYP2D6-2D7 + CYP2D6 arrangement (e.g., CYP2D6*36, CYP2D6*4N, CYP2D6*68 with a tandem, CYP2D6*10, and CYP2D6*4-like). Note that the CYP2D6-2D7 gene is followed by rep 7 rather than rep dup. Multiduplications have multiples of the sequence shown between the brackets and were observed in D and E. PCR fragments used in this analysis are depicted as lettered lines under the structures (Table 1). Probe locations for TaqMan copy number assays are designated as * and + for the 5′ flanking CYP2D6 assay and the CYP2D6 intron 6 assay, respectively. Note that CYP2D8 is thought to be present in all of the structures but is only shown in structures A to C for simplicity.
The recombinant events that occur with the CYP2D7 pseudogene may give rise to hybrid genes, which occur singly or in hybrid tandem arrangements. Single hybrids can be of the CYP2D6-2D7 variety, such as CYP2D6*61, which switches to the CYP2D7 sequence from intron 7 onward. As an alternative, single hybrids can be of the CYP2D7-2D6 variety. The home page of the Human Cytochrome P450 (CYP) Allele Nomenclature Committee has reclassified CYP2D7-2D6 hybrid genes that contain a CYP2D7-derived exon 1 (see http://www.cypalleles.ki.se/cyp2d6.htm for details), and this article uses this new classification for the first time (S. C. Sim, personal communication). A key to conversion to the new nomenclature is described under Materials and Methods. An example of a CYP2D7-2D6 hybrid is CYP2D6*13A1, in which the switch from CYP2D7 to CYP2D6 sequence occurs in intron 1 (Fig. 2A).
Hybrid genes can also occur in tandem arrangements. We recently described the CYP2D6*68 + *4-like and CYP2D6*4N + *4-like hybrid tandem arrangements (Kramer et al., 2009), and others described CYP2D6*36 + *10 (Chida et al., 2002; Gaedigk et al., 2006). These three structures are of the CYP2D6-2D7 + CYP2D6 hybrid tandem variety (Fig. 2F). Another hybrid tandem arrangement is the CYP2D7-2D6 + CYP2D6 variety (Fig. 2B), an example of which is CYP2D6*13A2 (EU530609) + *2A (Kramer et al., 2009; Gaedigk et al., 2010a).
The aim of this study was to determine the frequency of hybrid genes present in a large number of samples originally genotyped using the Luminex Tag-It Mutation Detection Kit for Cytochrome P450 2D6 (CYP2D6 ASPE kit) and to identify the impact of these variants on phenotype prediction. The CYP2D6 ASPE kit does not detect hybrid genes because the kit generates A and B amplicons for the 5′ and 3′ portions of the CYP2D6 gene, respectively. Amplicon A mix also contains additional primers that allow for the amplification of rep dup (Fig. 1B) and its subsequent detection by an ASPE primer. In the case of CYP2D7-2D6 + CYP2D6, a duplication signal is detected even though a hybrid gene is present in the 5′ position (Fig. 2, B and C). CYP2D6-2D7 and CYP2D7-2D6 genes will cause failure of A and/or B amplicon generation during the PCR step, depending upon the location of the switch from CYP2D6 to CYP2D7 sequence. Because there is a tendency for the CYP2D6 portions in the hybrid tandem gene to be identical to the CYP2D6 portion in the normal gene (Kramer et al., 2009), the kit will not detect partial failures in the ASPE step. In the case of a single CYP2D7-2D6 gene, total failure of PCR of the hybrid gene can occur, which can lead to a homozygous call on the basis of amplification of the nonhybrid allele. In this research, the samples studied included: 1) CYP2D6*4-containing samples, 2) CYP2D6*10-containing samples, 3) samples with homozygous genotypes, and 4) samples with duplication signal.
Materials and Methods
Samples and Initial Clinical Genotyping.
A total of 1390 deidentified samples submitted to the Nucleotide Polymorphism Laboratory of the Department of Laboratory Medicine and Pathology at the Mayo Clinic for clinical CYP2D6 genotyping using the Luminex Tag-It Mutation Detection Kit for Cytochrome P450 2D6 version 1 or 2 (hereafter named CYP2D6 ASPE kit version 1 or version 2, respectively; Luminex Corporation, Austin, TX) were studied with Mayo Clinic institutional review board approval. The samples were submitted from practices both internal and external to the Mayo Clinic system, but all were submitted to predict tolerability and response to either tamoxifen or psychotropic drugs. Samples were submitted from all over the world, and because they were deidentified, the ethnic and racial mixture of these samples could not be determined. To meet the criteria for study, a sample needed to have 1) at least one CYP2D6*4 allele (n = 508), 2) at least one CYP2D6*10 allele (n = 209), 3) a homozygous genotype (n = 332), or 4) a duplication signal (n = 341).
The CYP2D6 ASPE kit v2 detects the following CYP2D6 recombinants and alleles: gene duplication, gene deletion (*5), −1584C>G (*2A), 100C>T (*4 and *10), 124G>A (*12), 138insT (*15), 883G>C (*11), 1023C>T(*17), 1661G>C (*2, *4, *17, and others), 1707T>del (*6), 1758G>T/A (*8, *14), 1846G>A(*4), 2549A>del (*3), 2613–2615 del AGA (*9), 2850C>T (*2, *17, *41, and others), 2935A>C (*7), 2988G>A (*41), and 4180G>C (*2, *4, *17, and others). The CYP2D6 ASPE kit v1 lacked the 138insT, 1661G>C, 1758G>A, 2988G>A, and 4180G>C polymorphisms. All samples originally genotyped with the v1 kits that contained a CYP2D6*2 allele with duplication were retested using the v2 kit to identify CYP2D6*41 alleles. The 138insT and 1758G>A have a very low frequency, because we have found 4 and 2 alleles, respectively, in our experience of more than 11,000 samples; thus, we did not retest our samples for these. The 1661G>C and 4180G>C polymorphisms are part of several alleles and were added to the CYP2D6 ASPE kit v2 to help confirm allelic calls; thus, retesting for these was not done. In 240 of 285 heterozygous duplication samples, the duplicated allele was predicted on the basis of the mutant allelic ratios as established for the clinical assay.
DNA Extraction.
DNA was extracted from blood using a DNeasy Blood and Tissue Kit (QIAGEN, Valencia, CA) with the method described in the product literature.
PCR and Amplicon Verification.
CYP2D6-2D7 and CYP2D7-2D6 genes were detected by PCR using primers specifically designed to allow them to be amplified as described in Kramer et al. (2009) (Table 1; Figs. 1 and 2). The PCR primers used in this study were selected using Oligo Primer Analysis Software (version 6.71; Molecular Biology Insights Inc., Cascade, CO). PCR amplicon locations are shown in Figs. 1 and 2 as black bars under each structure. Specific primers were designed to prevent unintentional nonspecific annealing between the CYP2D6 gene, CYP2D7 pseudogene, and CYP2D8 pseudogene. All samples were screened for CYP2D6-2D7 genes using amplicon E and CYP2D7-2D6 genes using amplicon G, regardless of the original genotype.
TABLE 1.
PCR primers and amplicon characteristics
The primers shown were used to generate PCR amplicons for testing by allele-specific primer extension assay or DNA sequencing or for PCR fragment analysis. Refer to Figs. 1 and 2 for approximate annealing sites of these primers on the various genomic structures.
| PCR Product | Sequence | Arrangement Amplified | Size |
|---|---|---|---|
| base pairs | |||
| A | GTCCCACACCAGGCACCTGTACTGAATTAGTGGTGGTGGGTGTTTG | Nonduplicated gene and deletion arrangement | 15,629, 3471 |
| B | TCACCCCCAGCGGACTTATCACCACAGCCCTCAATAAGTGAA | 5′ gene of duplication arrangement | 12,052 |
| C | CACCACCCTCAGCCTCGTCTAGGTAGCCCTGGCCTATAGCTCCCTGACGCC | 3′ gene of duplication arrangement | 12,103 |
| D | TTGCCACATTATCGCCCGTGAAATAGGTAGCCCTGGCCTATAGCTCCCTGACGCC | Any normal arrangement of CYP2D6 | 8433 |
| E | TCACCCCCAGCGGACTTATCATACGGTGGGCTCCCTGCGAG | CYP2D6-2D7 gene | 6714 |
| G | CCTGTGTGGGCTTGGGGAGCTTGTGTGGTGAGGTGACGAGGCTGA | CYP2D7-2D6 gene | 5742 |
| J | ACCTGGACGCCTGACTTTATAGGTAGCCCTGGCCTATAGCTCCCTGACGCC | PCR fragment from CYP2D8-unique 3′ region to CYP2D6 3′ region | 9525 |
| K | GCCACCATGGTGTCTTTGCTTTCAAAGCTGACGACACGAGAGTGGCT | PCR fragment from exon 9 in CYP2D6 to exon 9 in CYP2D7 | 12,152 |
| L | TCAGCCTCGTCACCTCACCACAGGCCACAGCCCTCAATAAGTGAA | Any rep dup region | 5410 |
| M | CCTCAGGGATGCTGCTGTCTGAAAGCTGACGACACGAGAGTGGCT | PCR fragment from a unique region in rep 7 to a CYP2D7-specific exon 9 sequences and only amplifies if there are two rep 7 regions | 11,432 |
| N | CCTCAGGGACGCTGCTGTACAAAAGCTGACGACACGAGAGTGGCT | PCR fragment from a unique region in rep 6 to a CYP2D7-specific exon 9 sequence | 11,432 |
The PCR Master Mix was composed of 12-μl reactions containing 0.12 μl of LA Taq HS (5 U/μl; TaKaRa Bio Inc., Otsu, Shiga, Japan), 1.2 μl of 10× LA PCR buffer II (25 mM Mg2+), 2 μl of dNTP mixture (2.5 mM each), 6.48 μl of water, and 1 μl of betaine monohydrate (16.25 M; Fluka Biochemical, Steinheim, Germany), 0.5 μl of each primer, and 0.2 μl of genomic DNA (250 ng/μl).
Amplicons A to E, G, and J (Table 1) were generated as described by Kramer et al. (2009). Thermocycler parameters for amplicon K were as follows: 94°C for 1 min followed by 96°C for 10 s, 64°C for 30 s, 68°C for 11 min for 30 cycles, and a final extension at 72°C for 10 min and a 4°C hold. Thermocycler parameters for fragment L were as follows: 94°C for 1 min followed by 35 cycles of 96°C for 10 s and 68°C for 4 min, a final extension at 72°C for 10 min, and a 4°C hold. Thermocycler parameters for fragment M and N were as follows: 94°C for 1 min followed by 30 cycles of 96°C for 10 s and 68°C for 9 min, a final extension at 72°C for 10 min, and a 4°C hold.
PCR fragment sizes were analyzed using an Agilent Technologies DNA 12000 Kit (Agilent Technologies, Waldbronn, Germany) according to the manufacturer's protocol. Expected fragment sizes are shown in Table 1.
Genotyping by Allele-Specific Primer Extension Assay.
Genotyping of amplicons A to E, G, and J to M (Table 1) was performed using the CYP2D6 ASPE kit v2 as described in the product literature but without the original amplification step using the A and B primer sets. All samples were analyzed on the Luminex 100 IS device using IS 2.3 software (Luminex).
DNA Sequencing.
DNA sequencing was performed using an Applied Biosystems 3730xl DNA Analyzer and the ABI PRISM dRhodamine Terminator Cycle Sequencing Ready Reaction Kit with AmpliTaq DNA Polymerase, FS (Applied Biosystems, Foster City, CA). All CYP2D7-2D6 genes (amplicon G) and their associated rep dup regions (amplicon L) were sequenced bidirectionally using multiple overlapping amplicons. In addition, a unique structure containing CYP2D6*13B + *68×2 + *4-like was discovered (described below) and was sequenced from exon 9 of CYP2D6*13B through exon 9 of CYP2D6*68 using amplicons E, G, and K. The source of DNA for all sequencing was genomic DNA isolated from blood, and amplicons were generated per PCR section noted previously.
Real-Time PCR CYP2D6 Copy Number Assays.
The CYP2D6 copy number in samples containing both a CYP2D7-2D6 gene and a duplication signal was determined using two real-time (rt) PCR assays, which targeted different regions of the gene (Figs. 1 and 2). This assay was done only on the duplication samples because accurate copy number determination was needed to predict phenotype. Phenotype prediction for CYP2D6*4- and CYP2D6*10-containing samples is not affected by copy number as will be discussed below; thus, copy number was not determined for these samples. One assay, Hs04502391_cn (intron 6) was commercially available (Applied Biosystems). The other assay targeting the 5′ flanking region as described by Hosono et al. (2009) was synthesized and purchased from Applied Biosystems. Both assays were performed in triplicate with an internal control RNaseP TaqMan copy number reference and TaqMan Genotyping PCR Master Mix on a StepOnePlus rtPCR instrument as directed by the manufacturer (Applied Biosystems). Relative quantification was performed using CopyCaller software (Applied Biosystems) following the comparative ΔΔCT method. The confidence estimate (the probability that the calculated copy number is the correct assignment compared with other copy numbers that have nonzero probability of occurring) for a given sample was automatically generated. CopyCaller software uses the copy number and observed ΔCT values of a sample to calculate the confidence estimate.
Bioinformatics Tools.
DNA sequence data were compiled using Sequencher software (version 4.9; Gene Codes Corp., Ann Arbor, MI) and Mutation Surveyor (version 3.13; SoftGenetics LLC, State College, PA). GenBank entry M33388 served as a reference sequence for CYP2D6, and GenBank entry NC_000022, the reference sequence for chromosome 22, served as reference sequences for CYP2D7 and CYP2D8. The latter sequence was used because of sequencing variations in the original reference sequence for CYP2D7 and CYP2D8, M33387. Comparisons were performed using the Specialized BLAST program, bl2seq (http://blast.ncbi.nlm.nih.gov/) and CLUSTALW (http://www.ebi.ac.uk/Tools/clustalw/index/html) at default settings.
CYP2D6 Allelic Nomenclature.
Sequences were compared with the CYP2D6 alleles listed by the CYP2D6 nomenclature committee (http://www.cypalleles.ki.se/cyp2d6.htm). The home page of the Human Cytochrome P450 (CYP) Allele Nomenclature Committee has reclassified CYP2D7-2D6 hybrid genes that contain a CYP2D7-derived exon 1 (see http://www.cypalleles.ki.se/cyp2d6.htm for details), and this article uses this new classification for the first time. For this reason, all CYP2D7-2D6 and CYP2D7-2D6 + CYP2D6 alleles are designated CYP2D6*13A1-*13H according to this new nomenclature. The suffixes designate the switch location of the CYP2D7 to CYPD6 sequence. Examples of alleles discussed in this article include CYP2D6*13A1 and CYP2D6*13A2 (formerly CYP2D6*13 and CYP2D6*77, respectively), which switch from CYP2D7 to CYP2D6 in intron 1. CYP2D6*13B (formerly CYP2D6*79) switches in exon 2, CYP2D8*13D (formerly CYP2D6*78) switches in intron 4, CYP2D6*13F (formerly CYP2D6*16 and CYP2D6*66) switches in intron 7 to intron 8, and CYP2D6*13H (formerly CYP2D6*76) switches to CYP2D6 in exon 9.
Phenotype Prediction.
Phenotype prediction was based on the genotype and the activity listed on the CYP2D6 Allele Nomenclature Web page (http://www.cypalleles.ki.se/cyp2d6.htm) and the literature cited below. Phenotype prediction was binned into four categories in a manner similar to that reviewed by Ingelman-Sundberg (2005) and Kirchheiner et al. (2004). It should be noted that other methodologies for phenotype prediction have been described, and this is a controversial area of pharmacogenomics (Steimer et al., 2004; Gaedigk et al., 2008). In particular, there is controversy about where to classify the CYP2D6*2A versus other CYP2D6*2 alleles. There is evidence that the CYP2D6*2 alleles (except CYP2D6*2A) have reduced function, although this is somewhat substrate-dependent (Raimundo et al., 2000, 2004; Bapiro et al., 2002; Yu et al., 2002; Abduljalil et al., 2010). However, the c.1584C>G polymorphism found in CYP2D6*2A increases protein production, possibly through increased induction, which compensates for the reduced function caused by the other polymorphisms found in the CYP2D6*2 alleles, resulting in a function similar to and possibly greater than that of CYP2D6*1 (Løvlie et al., 2001; Zanger et al., 2001).
The classification used here is as follows: an ultrarapid metabolizer (UM) had more than two normally functioning alleles (CYP2D6*1 or CYP2D6*2A). An extensive metabolizer (EM) had two normally functioning alleles or one normally functioning allele and two reduced function alleles (e.g., CYP2D6*2, CYP2D6*10, CYP2D6*17, and CYP2D6*41) or two normally functioning alleles and a reduced function allele. An intermediate metabolizer (IM) had one normally functioning allele and either a reduced function allele or a null allele (e.g., CYP2D6*4, CYP2D6*5, CYP2D6*6, or single CYP2D7-2D6 gene). Samples with two or three reduced function alleles were also considered intermediate metabolizers. A poor metabolizer had only null alleles or a null allele plus a reduced function allele.
Results
Summary of Hybrid Genes Found in CYP2D6*4, CYP2D6*10, Duplication, and Homozygous Samples.
Overall, 8.5% of the 1390 samples studied had CYP2D6*4N + *4-like or CYP2D6*68 + *4-like, 3.2% had CYP2D6*36 + *10, 0.3% had a single CYP2D7-2D6 gene, and 1.8% had a CYP2D7-2D6 + CYP2D6, CYP2D7-CYP2D6 + CYP2D6×N, or CYP2D7-2D6 + CYP2D6-2D7×2 + CYP2D6 hybrid tandem arrangement (described below) (Table 2). However, this set of samples was enriched for CYP2D6*4, CYP2D6*10, homozygous samples, and samples with duplication signals. Therefore, the overall frequency is expected to be different in the general population, which should contain a random variety of alleles.
TABLE 2.
Frequency of hybrid genes per sample type
The frequency of hybrids genes in CYP2D6*4 and CYP2D6*10 samples is reported for both heterozygous or homozygous and heterozygous alone. We did not determine whether one or both chromosomes contained a hybrid tandem in homozygous samples. For this reason, to determine the risk that a CYP2D6*4 or CYP2D6*10 allele is actually in a hybrid tandem arrangement, we determined the percentage of heterozygous samples for each, which had a hybrid tandem. The “Any homozygous genotype” row included any sample with an initial homozygous genotype except those with duplications. Finally, the frequency of hybrid genes in samples originally genotyped as having duplications is shown. The percentage of hybrid alleles in the 1390 samples is also shown by category, although note that the sample set was not from a random population but was purposefully enriched for samples with CYP2D6*4, CYP2D6*10, duplication, and homozygous genotypes; thus, frequency of hybrid genes in a general population is likely to be different.
| Category | n | % |
|---|---|---|
| Heterozygous or homozygous CYP2D6*4 | 508 | |
| Containing CYP2D6*4N + *4-like | 9 | 1.8 |
| Containing CYP2D6*68 + *4-like | 109 | 21.5 |
| Percentage of total samples (n = 1390) with CYP2D6*4N + *4-like or CYP2D6*68 + *4-like (n = 118) | 8.5 | |
| Heterozygous CYP2D6*4 | 482 | |
| Containing CYP2D6*4N + *4-like | 7 | 1.5 |
| Containing CYP2D6*68 + *4-like | 83 | 17.2 |
| Heterozygous or homozygous CYP2D6*10 | 209 | |
| Containing CYP2D6*36 + *10 | 44 | 21.1 |
| Percentage of total samples (n = 1390) with CYP2D6*36 + *4-like (n = 44) | 3.2 | |
| Heterozygous CYP2D6*10 | 193 | |
| Containing CYP2D6*36 + *10 | 28 | 14.5 |
| Any homozygous genotype | 332 | |
| Containing CYP2D7-2D6 hybrid genes | 4 | 1.2 |
| Percentage of total samples (n = 1390) with CYP2D7-2D6 hybrid gene (n = 4) | 0.3 | |
| Any duplication sample | 341 | |
| Containing CYP2D7-2D6 + CYP2D6 hybrid tandem present | 25 | 7.3 |
| Percentage of total samples (n = 1390) with CYP2D7-2D6 + CYP2D6 or novel structure (n = 25) | 1.8 |
Of samples with either a heterozygous or homozygous CYP2D6*4 allele (n = 508), 9 (1.8%) had CYP2D6*4N + *4-like and 109 (21.5%) had CYP2D6*68 + *4-like. Of samples with a heterozygous or homozygous CYP2D6*10 allele (n = 209), 44 (21.1%) had CYP2D6*36 + *10 (Fig. 2F). For homozygous CYP2D6*4 and CYP2D6*10 samples, we did not determine whether one or both chromosomes contained a hybrid tandem arrangement. To determine the risk that an individual CYP2D6*4 or CYP2D6*10 allele is actually in a hybrid tandem arrangement, we also calculated the percentage of heterozygous samples that had a hybrid tandem. Of CYP2D6*4 heterozygotes (n = 482), 7 (1.5%) harbored CYP2D6*4N + *4-like and 83 (17.2%) harbored CYP2D6*68 + *4-like. Of CYP2D6*10 heterozygous samples (n = 193), 28 (14.5%) harbored CYP2D6*36 + *10.
Of samples homozygous for any allele (n = 332), 4 (1.2%) had a CYP2D7-2D6 gene. Of samples with a duplication signal (n = 341), 25 (7.3%) contained a CYP2D7-2D6 + CYP2D6 hybrid tandem or similar arrangement as discussed below.
Characteristics of 5′ Genes in CYP2D7-2D6 + CYP2D6 Hybrid Tandem.
Table 3 shows the actual 5′ genes present in the CYP2D7-2D6 + CYP2D6 hybrid tandem we observed. DNA sequence analysis was done for all of the 5′ genes found in this research. In seven samples, the DNA sequence analysis revealed that the 5′ gene was actually a CYP2D7 that converted to a CYP2D6 sequence in the 0.6-kb repeat located just downstream of the CYP2D7 sequence and upstream of the rep dup sequence (Figs. 1A and 2C). Four 2D7 + CYP2D6*1 (cases 1, 10, 20, and 22), two 2D7 + *41 (case 4 and 16), and one 2D7 + CYP2D6*4 (case 8) were observed.
TABLE 3.
Characteristics of duplication samples found to contain CYP2D7-2D6 genes
In the original CYP2D6 genotype column, parentheses around the star alleles designate samples in which the duplicated allele could not be determined in the clinical assay. Correspondingly, this led to some ambiguity in the original phenotype prediction for a subset of samples. Samples originally genotyped as a CYP2D6*2 that were later found to be CYP2D6*41 are designated with a #. This occurred because of limitations of the CYP2D6 ASPE kit version 1. Subsequent testing was done with the CYP2D6 ASPE kit version 2, which does detect CYP2D6*41. TaqMan copy number assay probe locations for these structures are found in Figs. 1 and 2.
| Case No. | Original CYP2D6 Genotype | Copy Number per TaqMan Results |
Actual CYP2D6 Genotype | Predicted Phenotype Comparing Original Genotype to Actual Genotype | |
|---|---|---|---|---|---|
| CYP2D6 Promoter Assay | CYP2D6 Intron 6 Assay | ||||
| 1 | *1/*1×N | 2 | 2 | *1/2D7 + *1 | UM to EM |
| 2 | *2A×N/*17 | 2 | 3 | *13A2 + *2A/*17 | EM to IM |
| 3 | *1/*2A×N | 2 | 3 | *1/*13A2 + *2A | UM to EM |
| 4 | (*2A/*41)×N# | 2 | 2 | 2D7 + *41/*2A | EM or IM to IM |
| 5 | *1×N/*2A | 2 | 2 | *13G2 + *1/*2A | UM to EM |
| 6 | *2A/*2A×N | 2 | 3 | *2A/*13A2 + *2A | UM to EM |
| 7 | *1×N/*5 | 1 | 2 | *13A1 variant 1 + *1/*5 | EM to IM |
| 8 | *2A×N/*4 | 2 | 2 | *2A/2D7 + *4 | EM to IM |
| 9 | *2A/*2A×N | 2 | 3 | *2A/*13A2 + *2A | UM to EM |
| 10 | *1×N/*2A | 2 | 2 | 2D7 + *1/*2A | UM to EM |
| 11 | *2A/*41×N# | 2 | 3 | *13A2 + *2A/*41 | EM or IM to IM |
| 12 | *2A×N/*4 | 2 | 3 | *13A2 + *2A/*4 | EM to IM |
| 13 | (*2A/*41)×N# | 2 | 3 | *13D + *2A/*41 | EM or IM to IM |
| 14 | (*2A/*4)×N | 2 | 3 | *13A2 + *2A/*4 | EM or IM to IM |
| 15 | *1/*1×N | 5–6a | 5–6b | *1/*13A1 variant 2 + *1×N | UM (no change) |
| 16 | *41/*41×N | 2 | 2 | *41/2D7 + *41 | IM (no change) |
| 17 | *2A/*2A×N | 2 | 3 | *2A/*13A2 + *2A | UM to EM |
| 18 | (*1/*2A)×N | 2 | 3 | *1/*13D + *2A | UM to EM |
| 19 | (*1/*4)×N | 4c | 3 | *1/*13B + *68×2 + *4 | EM or IM to IM |
| 20 | (*1/*41)×N# | 2 | 2 | 2D7 + *1/*41 | EM or IM to IM |
| 21 | (*1/*2A)×N | 2 | 3 | *1/*13A2 + *2A | UM to EM |
| 22 | (*1/*6)×N | 2 | 2 | 2D7 + *1/*6 | EM or IM to IM |
| 23 | *2A/*2A×N | 2 | 3 | *2A/*13A2 + *2A | UM to EM |
| 24 | (*2A/*4)×N | 3 | 3 | *13A2 + *2A/*68 + *4 | EM or IM to IM |
| 25 | (*2A/*41)×N | 2 | 3 | *13A2 + *2A/*41 | EM or IM to IM |
Confidence estimates for the copy number assays were all >0.99 with the following exceptions:
0.56;
<0.50;
0.85.
Twelve CYP2D6*13A2 + *2A, one CYP2D6*13A1 (variant 1) + *1 (case 7), one CYP2D6*13A1 (variant 2) + CYP2D6*1×N (case 15), two CYP2D6*13D + *2A (cases 13 and 18), one CYP2D6*13G2 + *1 (case 5), and one CYP2D6*13B + *68×2 + *4 (case 19) were also observed. GenBank entry numbers for CYP2D6*13A1 variant 1 and variant 2 are HQ670230 and HQ670231, respectively. CYP2D6*13A1 variant 1 has intron 4 c.667–149G, CYP2D6*13A1 has a T and variant 1 has an intron 5 c.844–49G, and CYP2D6*13A1 has a T. CYP2D6*13A1 variant 2 has only the intron 4 c.667–149G. (Note that locations are derived from the CYP2D6 reference sequence M33388.) An additional novel allele was identified, which was assigned CYP2D6*13G2 by the nomenclature committee, GenBank entry number HQ670229). In this sample, the conversion from CYP2D7 to CYP2D6 occurred in intron 7 on the basis of sequence data and comparison to CYP2D7 and CYP2D6 sequence data, although it also contains one CYP2D7-like SNP in exon 8.
To confirm the presence of a rep dup in each of these samples, DNA sequence analysis was performed using amplicon L (Table 1; Figs. 1B and 2, B–D). In every case, the first 53 nucleotides of rep dup contained the five nucleotides specific to rep 6 and the last 39 nucleotides of rep dup contained the 4 nucleotides that define rep 7 (Steen et al., 1995; Soyama et al., 2006). Absence of the CYP2D7 pseudogene 5′ to all CYP2D7-2D6 hybrids was confirmed by the presence and size of fragment J (Fig. 2, B–E), which cannot be generated if CYP2D7 is present because of the size of the amplicon.
Table 4 summarizes the frequency with which a given duplicated allele was found to have an associated CYP2D7-2D6 gene. A CYP2D7-2D6 hybrid tandem was present in 7 of 91 (7.7%) samples involving a CYP2D6*1 allele. Likewise, a hybrid tandem was present in 14 of 98 (14.3%) of samples involving a CYP2D6*2A, 2 of 25 (8%) alleles involving a CYP2D6*4 allele, and 2 of 4 (50%) samples involving a CYP2D6*41 allele. Of note, there were 20 samples in which a CYP2D6*2×N (not CYP2D6*2A×N) was predicted to be duplicated, and none of them were found to have a hybrid tandem. Although these numbers are small, the risk of an undetected hybrid tandem being present in a sample appears to be greatest in those samples in which the CYP2D6*1, CYP2D6*2A, CYP2D6*4, and CYP2D6*41 is predicted to be duplicated using the CYP2D6 ASPE kits as described here.
TABLE 4.
Frequency of CYP2D7-2D6 genes in tandem to specific CYP2D6 alleles
This table shows the frequency for which a predicted duplicated allele was found to have a CYP2D7-2D6 + CYP2D6 arrangement. Star alleles predicted to be duplicated using the CYP2D6 ASPE kits are shown in column 1.
| Predicted Duplicated CYP2D6 Allele | n | CYP2D7-2D6 Gene Present | % |
|---|---|---|---|
| *1 | 91 | 7 | 7.7 |
| *2 | 20 | 0 | 0.0 |
| *2A | 98 | 14 | 14.3 |
| *4 | 25 | 2 | 8.0 |
| *9 | 1 | 0 | 0.0 |
| *10 | 1 | 0 | 0.0 |
| *41 | 4 | 2 | 50.0 |
| Total | 240 | 25 |
Characteristics of Single CYP2D7-2D6 Genes.
DNA sequencing was performed on all homozygous samples that were found to contain a single CYP2D7-2D6 gene (Fig. 2A). Of these, three were found to be CYP2D6*13F and one was found to have a novel allele named CYP2D6*13G1 (JN618990).
Gene Copy Numbers of CYP2D7-2D6 + CYP2D6 Hybrid Tandem.
The CYP2D6 copy number was determined for all samples that gave a duplication signal and were determined to have a CYP2D7-2D6 + CYP2D6 arrangement (Table 3). rtPCR assays were performed, which used one primer pair plus a TaqMan probe to target the intron 6 region of CYP2D6 and another primer pair plus TaqMan probe to target the 5′ flanking region of the CYP2D6 promoter region (Figs. 1 and 2). CYP2D7-2D6 genes were detected on the basis that the copy number varied between the two regions. In particular, CYP2D7-2D6 genes have a CYP2D7 promoter region so the 5′ flanking probe will not bind there in distinction to any CYP2D6 genes present in a sample. CYP2D7-2D6 genes that switch to CYP2D6 sequence upstream of intron 6 have a binding site for the intron 6 probe. Therefore, early crossing CYP2D7-2D6 genes (e.g., CYP2D6*13A1, CYP2D6*13A1 variant 1 and variant 2, CYP2D6*13A2, CYP2D6*13D, and CYP2D6*13B), which convert to the CYP2D6 sequence upstream of intron 6 were detected with the intron 6 probe but not with the 5′ CYP2D6 probe. The CYP2D7-2D6 gene, which was not detected by the intron 6 probe, was CYP2D6*13G2 because of its conversion to CYP2D6 in intron 7. Neither probe detected instances in which the 5′ CYP2D7 pseudogene converted to the CYP2D6 trailing sequence in the 0.6-kb repeat region just downstream of CYP2D7 and upstream of the rep dup sequence.
CYP2D6-2D7 hybrids (cases 19 and 24) were detected with the CYP2D6 5′ probe because they have a CYP2D6 promoter sequence (Fig. 2F). The intron 6 assay did not yield an amplification product from the CYP2D6*68 hybrid because this allele switched to the CYP2D7 sequence in intron 1. Copy numbers for each assay were as expected for all but two samples (Table 3). Five to six copies of CYP2D6 were observed with both the intron 6 and the CYP2D6 promoter probes for case 15, indicating that CYP2D6*1 is multiplied, but the total number could not be determined. The product literature states that decreased confidence estimates are observed with increased copy numbers. In addition, case 19 had three and four copies with the intron 6 and CYP2D6 promoter assay, respectively, suggesting two copies of CYP2D6*68. Cases 15 and 19 are discussed in detail below.
Novel CYP2D7-2D6 + CYP2D6×N Hybrid Tandem.
We could not reliably determine the number of CYP2D6 genes by rtPCR in case 15 (Table 3). We expected that the intron 6 assay would show one more copy than the CYP2D6 promoter assay, because the CYP2D6*13A1 gene would not be detected by the TaqMan probe in the promoter region. The presumed reason for this finding is that reliability of rtPCR to detect copy numbers higher than 5 is poor (see product literature). The structure of this sample was supported by CYP2D6 ASPE kit analysis of fragments A and B (Table 1; Fig. 2D), both of which were genotyped as CYP2D6*1. This is the first time that a CYP2D7-2D6 hybrid gene has been reported as part of a multiplied CYP2D6 arrangement.
Novel CYP2D7-2D6 + CYP2D6-2D7×2 + CYP2D6 Hybrid Tandem.
The results from PCR fragment analysis (Fig. 3), the CYP2D6 ASPE kit, sequence analysis (amplicons E, G, and K), and copy number assays were compiled to generate the structure of case 19 (Table 3; Fig. 2E), a novel CYP2D7-2D6 + CYP2D6-2D7×2 + CYP2D6 hybrid tandem. The presence of CYP2D6*13B followed by CYP2D6*68 on the same chromosome was confirmed by the amplification of fragment K (Fig. 3, lane 7), which spans from exon 9 in CYP2D6 to exon 9 in CYP2D7. This amplicon was sequenced using multiple amplicons and primers to ensure continuity between the CYP2D6*13B and CYP2D6*68 alleles. In addition, four copies of the CYP2D6 promoter region were observed with the rtPCR CYP2D6 promoter copy number assay, consistent with two CYP2D6*68 alleles plus CYP2D6*4 on one chromosome and CYP2D6*1 on the other chromosome, whereas three copies of CYP2D6 intron 6 were found using the intron 6 copy number assay. This occurred because CYP2D6*13B contains CYP2D6 intron 6 as does CYP2D6*4 on the same chromosome and CYP2D6*1 on the other chromosome, but CYP2D6*68, which converts to CYP2D7 in intron 1, is not detected by the intron 6 copy number assay. The presence of tandem CYP2D6*68 alleles was confirmed by PCR amplification using forward primers located in the rep 7 (fragment M) and rep 6 (fragment N) regions of the gene with a reverse primer located in exon 9 of CYP2D7 (Fig. 3, lane 10). These amplifications would occur only if both rep dup and rep 7 each were followed by a CYP2D6*68 (CYP2D6-2D7) arrangement (Fig. 2E). Original genotyping of case 19 yielded a CYP2D6*1/*4 genotype. This genotype is consistent with the arrangement shown in Fig. 2E because the locations of the Luminex kit primers allows PCR amplification of both the normal CYP2D6 (Fig. 1A) as well as the 3′ most CYP2D6 structure in the novel arrangement (Fig. 2E). It should be noted that although CYP2D6*13B has been observed alone (Gaedigk et al., 2010b), this is the first time it has been reported in tandem with other genes.
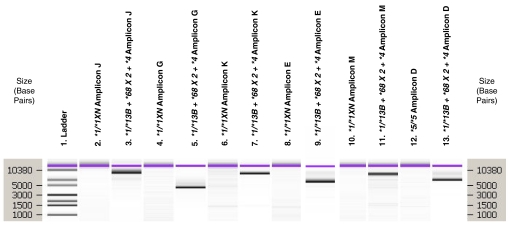
Fig. 3.
Amplicon analysis of novel CYP2D6*13B + *68×2 + *4. PCR fragment analysis for case 19 (Fig. 2E). PCR primers and expected fragment sizes for products D, E, G, J, K, and M are listed in Table 1. Fragment analysis was performed using the Agilent Technologies DNA 12000 kit. The ladder in lane 1 shows the size in base pairs of the molecular weight marker. For each amplicon, a negative control is shown first followed by amplicons generated for case number 19. Amplicon J (lanes 2 and 3) primed from the trailing sequence of CYP2D8 to the beginning of rep 6 or rep dup. When the CYP2D7 pseudogene was present, no amplicon was generated because of the lengthy span of DNA. In this case (lane 3), a fragment was generated indicating that CYP2D7 was absent. Amplicon G (lanes 4 and 5) uniquely amplified the CYP2D7-2D6 gene (CYP2D6*13B) and was used in sequencing. Amplicon K (lanes 6 and 7) primed from the CYP2D6 exon 9 sequence in CYP2D6*13B to the CYP2D7 exon 9 sequence in CYP2D6*68. This amplicon was sequenced using multiple amplicons to ensure continuity between the CYP2D6*13B and CYP2D6*68 alleles. Amplicon M (lanes 8 and 9) primes from a unique forward region in rep 7 to the exon 9 sequence in CYP2D7 found in CYP2D6*68. Amplification would only occur with a complete rep 7 (not rep dup) upstream of CYP2D6*68. Amplicon E (lanes 10 and 11) uniquely amplified CYP2D6-2D7 genes (CYP2D6*68) and was used to generate an amplicon that was genotyped using the CYP2D6 ASPE v2 kit, thus verifying the CYP2D6*68 allele. Amplicon D (lanes 12 and 13) amplified CYP2D6 gene in any normal arrangement (e.g., Fig. 1A), any CYP2D6 gene 3′ allele in a duplication arrangement (Fig. 1B), and any CYP2D6 gene in a tandem (Fig. 2, B–F). This amplicon generated the genotype of heterozygous CYP2D6*4 from the tandem gene in Fig. 2E and the CYP2D6*1 allele on the other chromosome using the CYP2D6 v2 kit.
Impact of Hybrid Genes on Predicted Phenotype.
CYP2D6-CYP2D7 + CYP2D6 hybrid tandems.
The CYP2D6*4, CYP2D6*4N, and CYP2D6*4-like alleles have the 1846A variation, which results in a splicing defect that encodes an enzyme with no activity. The CYP2D6*68 converts to the CYP2D7 sequence from intron 1 forward and is also expected to produce a null allele because of the presence of the CYP2D6*10 polymorphism c.100C>T plus the presence of an exon 4 c.631dupG resulting in a frameshift that causes a stop codon 43 bases downstream on the insertion (p.Glu211GlyfsX43) although no in vivo or in vitro research has been reported for this allele. The CYP2D6*10 allele is described as having reduced function and the CYP2D6*36 allele is described as having poor function (Gaedigk et al., 2006). Thus, the presence of a CYP2D6*36, *4N, and *68 in a tandem does not affect the predicted phenotype of the sample.
Single CYP2D7-2D6 samples.
All CYP2D7-2D6 genes carry a T-insertion in exon 1 that causes a frameshift and a premature termination (c.137_138insT, p.Leu47AlafsX207). These hybrids are nonfunctional (Gaedigk et al., 2010b). Four (100%) of four samples originally genotyped as having a homozygous genotype, which were found to have a CYP2D7-2D6 gene on one chromosome (Fig. 2A), had a change in phenotype prediction. These samples were originally genotyped as CYP2D6*1/*1 but were found to actually be heterozygous for CYP2D6*13G1 (n = 1) and CYP2D6*13F (n = 3), resulting in a change in phenotype prediction from EM to IM.
CYP2D7-2D6 + CYP2D6 hybrid tandem.
Table 3 shows the change in phenotype of the samples with CYP2D7-2D6 + CYP2D6 hybrid tandems (Fig. 2, B and C) observed in this research. Detection of the hybrid gene resulted in a change of predicted phenotype in 23 (92%) of 25 samples containing a hybrid. In 9 of the samples (cases 4, 11, 13, 14, 19, 20, 22, 24, and 25), the duplicated allele could not be predicted at the time of original genotyping and the original phenotype was specified “EM or IM.” After the correct genotype was determined, the predicted phenotype was confirmed to be IM. Thus, in these cases, more precise phenotype prediction could be determined using the methods described here. If these 5 cases are also included in the “no change” group, then 18 samples (56%) had a change in phenotype.
Discussion
The intent of this study was to determine the frequency of CYP2D6-2D7 and CYP2D7-2D6 genes in samples containing CYP2D6*4 alleles, CYP2D6*10 alleles, homozygous samples of any variety, and samples with duplication signals that were originally genotyped with a commercially available ASPE kit. Furthermore, we wanted to determine the impact of these undetected genes on predicted phenotype.
The main findings of this research are that hybrid genes 1) are relatively common in clinical samples containing CYP2D6*4 alleles, CYP2D6*10 alleles, and duplication signals, 2) are uncommon in homozygous samples, and 3) frequently affect phenotype prediction in samples originally genotyped as homozygous and, separately, those that were originally genotyped as having a duplication. However, hybrid tandems do not change the predicted phenotype in CYP2D6*4- and CYP2D6*10-containing samples.
Our results involving duplication samples support those described by Gaedigk et al. (2010a), in which three CYP2D6*13A2 + *2 and one CYP2D6*13D + *2 were found in 32 duplication-positive white samples (12.5%) and one CYP2D6*13H + *1 was found in 59 duplication-positive African-American samples (1.6%) for an overall frequency of 5.4% compared with our 7.3%. The difference between these two studies is probably due to a difference in the ethnic mix of patients, although ethnic and racial data are not available for our samples. We also saw more CYP2D6*13A2 + *2A (n = 12) versus CYP2D6*13D + *2A (n = 2) (Table 3). Of interest, no hybrid genes were observed with other CYP2D6*2 variants. We also identified seven samples with duplication signals, which contained a CYP2D7 gene with a CYP2D6-like region downstream of exon 9 similar to that described by Gaedigk et al. (2010b). In particular, cases 1, 10, 20, and 22 had CYP2D7 + *1, cases 4 and 16 had CYP2D7 + *41, case 8 had CYP2D7 + *4, and all cases had sequence-confirmed rep dup 3′ to the CYP2D7 hybrid allele.
In our collection of 341 samples originally genotyped with duplications, a hybrid tandem gene was present in 25 (7.3%), and this had an impact on phenotype prediction for 92% of these samples. Furthermore, a single CYP2D7-2D6 gene was only found in 4 of 332 samples originally genotyped as homozygous, but it changed predicted phenotype in 100% of cases. Although the need to test for the rare single CYP2D7-2D6 gene in a clinical setting can be debated in the absence of actual evidence that a given patient has genotype-phenotype discordance, the authors argue that the frequency of hybrid tandems in duplication samples and their impact on phenotype prediction warrants their analysis.
The CYP2D6 ASPE kit that was used to originally genotype our samples was not designed to detect hybrid genes; thus, it is not surprising that none were detected by this kit during original clinical genotyping. Hybrid genes will also not be detected in a sequence-based analysis unless primers are designed to specifically allow for the PCR amplification of these hybrids. Furthermore, microarray-based assays, such as the AmpliChip CYP450 Test (Roche Diagnostics, Basel, Switzerland), will not detect hybrid genes unless the initial PCR amplification step uses primers that amplify the hybrid gene and then hybrid gene-specific probes are designed into the array.
Another feature that can affect phenotype is gene multiplication. In this study, 2 of 25 (8%) samples tested had otherwise undetected multiplications. This allowed for more accurate phenotype prediction in case 15 (CYP2D6*1/*13A1 variant 2 + *1×N), and the rtPCR copy number assays allowed for the identification of two unique CYP2D7-2D6 hybrid arrangements (case 15 and 19). These findings show that duplication samples are challenging to accurately genotype, and it appears that use of an array of analyses, such as we have done here, is necessary to be sure of completely accurate genotyping.
One limitation of this study is that no ethnic or racial data were available for these samples because, per the institutional review board requirement for this type of study, all samples needed to be deidentified. Likewise, no phenotypic data are available for these individual samples.
In conclusion, to be detected, CYP2D7-2D6 genes must be specifically tested for using methods comparable to those outlined here. CYP2D7-2D6 genes were found frequently in samples originally genotyped to have duplications using a CYP2D6 ASPE kit. Their detection resulted in a change in phenotype prediction in a high percentage (92%) of cases in which a duplication call was made. Furthermore, phenotype changed in 100% of rarer cases in which a single CYP2D7-2D6 gene was present in our samples. CYP2D6-2D7 genes must also be specifically tested for to be detected, but they have no effect on phenotype prediction because they were found in tandem with CYP2D6*4 and CYP2D6*10 alleles, which are null or have reduced function, respectively, and the hybrid genes (CYP2D6*36, CYP2D6*4N, and CYP2D6*68) have poor phenotype activity.
Two new structures, CYP2D6*13B + (*68×2) + *4 and CYP2D6*13A1 variant 2 + (*1×N), were described as were two new alleles, CYP2D6*13G1 and CYP2D6*13G2, and two variations of the CYP2D6*13A1 allele.
The information presented here is crucial for detection of hybrid genes regardless of the platform used to genotype CYP2D6. Probe sets for microarray detection must be designed with knowledge of hybrid genes or probes will not bind in a fashion that will allow their detection. In addition, CYP2D6 full gene sequencing and high throughput genotyping using next-generation DNA analyzers will need to use the DNA sequences and genomic structures presented here as scaffolds for interpretation of sequence results.
This research was supported by National the Institutes of Health National Center for Research Resources [Clinical and Translational Science Award UL1-RR024150]; and the Mayo Clinic.
Drs. Black and O'Kane and the Mayo Clinic have licensed intellectual property to AssureRx.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
- SNP
- single nucleotide polymorphism
- ASPE
- allele-specific primer extension assay
- PCR
- polymerase chain reaction
- rt
- real-time
- UM
- ultrarapid metabolizer
- EM
- extensive metabolizer
- IM
- intermediate metabolizer
- kb
- kilobase
- CT
- the cycle number at which fluorescence crosses an arbitrary line in rtPCR.
Authorship Contributions
Participated in research design: Black, Walker, and O'Kane.
Conducted experiments: Walker and Harmandayan.
Contributed new reagents or analytic tools: Black, Walker, and O'Kane.
Performed data analysis: Black, Walker, O'Kane, and Harmandayan.
Wrote or contributed to the writing of the manuscript: Black, Walker, O'Kane, and Harmandayan.
References
- Abduljalil K, Frank D, Gaedigk A, Klaassen T, Tomalik-Scharte D, Jetter A, Jaehde U, Kirchheiner J, Fuhr U. (2010) Assessment of activity levels for CYP2D6*1, CYP2D6*2, and CYP2D6*41 genes by population pharmacokinetics of dextromethorphan. Clin Pharmacol Ther 88:643–651 [DOI] [PubMed] [Google Scholar]
- Bapiro TE, Hasler JA, Ridderström M, Masimirembwa CM. (2002) The molecular and enzyme kinetic basis for the diminished activity of the cytochrome P450 2D6.17 (CYP2D6.17) variant. Potential implications for CYP2D6 phenotyping studies and the clinical use of CYP2D6 substrate drugs in some African populations. Biochem Pharmacol 64:1387–1398 [DOI] [PubMed] [Google Scholar]
- Black JL, 3rd, O'Kane DJ, Mrazek DA. (2007) The impact of CYP allelic variation on antidepressant metabolism: a review. Expert Opin Drug Metab Toxicol 3:21–31 [DOI] [PubMed] [Google Scholar]
- Chida M, Ariyoshi N, Yokoi T, Nemoto N, Inaba M, Kinoshita M, Kamataki T. (2002) New allelic arrangement CYP2D6*36×2 found in a Japanese poor metabolizer of debrisoquine. Pharmacogenetics 12:659–662 [DOI] [PubMed] [Google Scholar]
- Gaedigk A, Bradford LD, Alander SW, Leeder JS. (2006) CYP2D6*36 gene arrangements within the CYP2D6 locus: association of CYP2D6*36 with poor metabolizer status. Drug Metab Dispos 34:563–569 [DOI] [PubMed] [Google Scholar]
- Gaedigk A, Fuhr U, Johnson C, Bérard LA, Bradford D, Leeder JS. (2010a) CYP2D7-2D6 hybrid tandems: identification of novel CYP2D6 duplication arrangements and implications for phenotype prediction. Pharmacogenomics 11:43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaedigk A, Jaime LK, Bertino JS, Jr, Berard A, Pratt VM, Bradford LD, Leeder J. (2010b) Identification of novel CYP2D7-2D6 hybrids: non-functional and functional variants. Front Pharmacol doi:10.3389/fphar.2010.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. (2008) The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther 83:234–242 [DOI] [PubMed] [Google Scholar]
- Goetz MP, Knox SK, Suman VJ, Rae JM, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL, et al. (2007) The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat 101:113–121 [DOI] [PubMed] [Google Scholar]
- Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL, Flockhart DA, et al. (2005) Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 23:9312–9318 [DOI] [PubMed] [Google Scholar]
- Hosono N, Kato M, Kiyotani K, Mushiroda T, Takata S, Sato H, Amitani H, Tsuchiya Y, Yamazaki K, Tsunoda T, et al. (2009) CYP2D6 genotyping for functional-gene dosage analysis by allele copy number detection. Clin Chem 55:1546–1554 [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M. (2005) Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J 5:6–13 [DOI] [PubMed] [Google Scholar]
- Kirchheiner J, Nickchen K, Bauer M, Wong ML, Licinio J, Roots I, Brockmöller J. (2004) Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry 9:442–473 [DOI] [PubMed] [Google Scholar]
- Kramer WE, Walker DL, O'Kane DJ, Mrazek DA, Fisher PK, Dukek BA, Bruflat JK, Black JL. (2009) CYP2D6: novel genomic structures and alleles. Pharmacogenet Genomics 19:813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løvlie R, Daly AK, Matre GE, Molven A, Steen VM. (2001) Polymorphisms in CYP2D6 duplication-negative individuals with the ultrarapid metabolizer phenotype: a role for the CYP2D6*35 allele in ultrarapid metabolism? Pharmacogenetics 11:45–55 [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Murphy GM, Jr, Kennedy JL. (2004) Pharmacogenetics of psychotropic drug response. Am J Psychiatry 161:780–796 [DOI] [PubMed] [Google Scholar]
- Murphy GM, Jr, Kremer C, Rodrigues HE, Schatzberg AF. (2003) Pharmacogenetics of antidepressant medication intolerance. Am J Psychiatry 160:1830–1835 [DOI] [PubMed] [Google Scholar]
- Raimundo S, Fischer J, Eichelbaum M, Griese EU, Schwab M, Zanger UM. (2000) Elucidation of the genetic basis of the common ‘intermediate metabolizer’ phenotype for drug oxidation by CYP2D6. Pharmacogenetics 10:577–581 [DOI] [PubMed] [Google Scholar]
- Raimundo S, Toscano C, Klein K, Fischer J, Griese EU, Eichelbaum M, Schwab M, Zanger UM. (2004) A novel intronic mutation, 2988G>A, with high predictivity for impaired function of cytochrome P450 2D6 in white subjects. Clin Pharmacol Ther 76:128–138 [DOI] [PubMed] [Google Scholar]
- Soyama A, Saito Y, Ohno Y, Komamura K, Kamakura S, Kitakaze M, Tomoike H, Ozawa S, Sawada J. (2006) Diverse structures of chimeric CYP-REP7/6-containing CYP2D6 and a novel defective CYP2D6 haplotype harboring single-type *36 and CYP-REP7/6 in Japanese. Drug Metab Pharmacokinet 21:395–405 [DOI] [PubMed] [Google Scholar]
- Steen VM, Molven A, Aarskog NK, Gulbrandsen AK. (1995) Homologous unequal cross-over involving a 2.8 kb direct repeat as a mechanism for the generation of allelic variants of human cytochrome P450 CYP2D6 gene. Hum Mol Genet 4:2251–2257 [DOI] [PubMed] [Google Scholar]
- Steimer W, Zöpf K, von Amelunxen S, Pfeiffer H, Bachofer J, Popp J, Messner B, Kissling W, Leucht S. (2004) Allele-specific change of concentration and functional gene dose for the prediction of steady-state serum concentrations of amitriptyline and nortriptyline in CYP2C19 and CYP2D6 extensive and intermediate metabolizers. Clin Chem 50:1623–1633 [DOI] [PubMed] [Google Scholar]
- Topić E, Stefanović M, Ivanisević AM, Blazinić F, Culav J, Skocilić Z. (2000) CYP2D6 genotyping in patients on psychoactive drug therapy. Clin Chem Lab Med 38:921–927 [DOI] [PubMed] [Google Scholar]
- Yu A, Kneller BM, Rettie AE, Haining RL. (2002) Expression, purification, biochemical characterization, and comparative function of human cytochrome P450 2D6.1, 2D6.2, 2D6.10, and 2D6.17 allelic isoforms. J Pharmacol Exp Ther 303:1291–1300 [DOI] [PubMed] [Google Scholar]
- Zanger UM, Fischer J, Raimundo S, Stüven T, Evert BO, Schwab M, Eichelbaum M. (2001) Comprehensive analysis of the genetic factors determining expression and function of hepatic CYP2D6. Pharmacogenetics 11:573–585 [DOI] [PubMed] [Google Scholar]