Abstract
The drug of abuse γ-hydroxybutyrate (GHB) displays nonlinear renal clearance, which has been attributed to saturable renal reabsorption by monocarboxylate transporters (MCTs) present in the kidney. MCT1 is also present in red blood cells (RBCs); however, the significance of this transporter on the blood/plasma partitioning of GHB is unknown. The purpose of this research was to characterize the transport of GHB across the RBC membrane and assess GHB blood/plasma partitioning in vivo in the presence and absence of a competitive MCT inhibitor, l-lactate. In vitro experiments were performed using freshly isolated rat erythrocytes at pH values of 6.5 and 7.4. Inhibition with p-chloromercuribenzene sulfonate and 4,4′-diisothiocyanostilbene-2,2′-disulfonate were used to determine the contribution of MCT1 and band 3, respectively, on GHB uptake. For in vivo experiments, rats were administered GHB (400–1500 mg/kg) with and without l-lactate. In vitro experiments demonstrated that GHB is transported across the RBC membrane primarily by MCT1 at relevant in vivo concentrations. The Km for MCT1 was lower at pH 6.5 than that at pH 7.4, 2.2 versus 17.0 mM, respectively. The in vivo blood/plasma partitioning of GHB displayed linearity across all concentrations. l-Lactate coadministration increased GHB renal clearance but had no effect on the blood/plasma ratio. Unlike its MCT-mediated transport in the intestine and kidneys, GHB blood/plasma partitioning appears to be linear and is unaffected by l-lactate. These findings can be attributed, at least in part, to differences in physiologic pH at different sites of MCT-mediated transport.
Introduction
Endogenously produced via GABA metabolism, γ-hydroxybutyrate (GHB) is a short-chain fatty acid naturally present in several mammalian tissues, including the brain (Maitre, 1997). GHB is used therapeutically in the United States for the treatment of narcolepsy with cataplexy as sodium oxybate (Xyrem; Jazz Pharmaceuticals Inc., Palo Alto, CA) and for the treatment of alcohol withdrawal in Europe (Gallimberti et al., 2000). GHB has also recently been recognized as a common drug of abuse. Overdose cases involving GHB and its precursors, 1,4-butanediol and -butyrolactone, have been reported in the United States and other Western countries and have resulted in GHB-induced coma, respiratory depression, and fatality (Caldicott et al., 2004; Zvosec et al., 2010).
Even at low therapeutic doses, GHB displays nonlinear pharmacokinetics in humans (Palatini et al., 1993). In rats, this dose-dependence has been demonstrated to be due to saturable metabolism, saturable oral absorption, and saturable renal reabsorption (Lettieri and Fung, 1979; Arena and Fung, 1980; Morris et al., 2005). Saturable oral absorption and renal reabsorption have been explained by the concentration-dependent transport of GHB by monocarboxylate transporters (MCTs) (Morris et al., 2005; Wang et al., 2006; Lam et al., 2010). MCTs are proton-coupled transporters with ubiquitous expression, including expression at the intestine, kidney, brain, and at the red blood cell (RBC) membrane. Human and rodent RBCs primarily express MCT1 and have been used extensively for the characterization of this transporter (Poole and Halestrap, 1993; Garcia et al., 1995; Merezhinskaya and Fishbein, 2009). Although MCT-mediated transport has been demonstrated to govern several of the pharmacokinetic processes of GHB, the significance of transport by MCT1 at the RBC membrane is unknown. The blood/plasma (B/P) partitioning of GHB, including the linearity of this partitioning over relevant therapeutic and overdose concentrations, has not been evaluated.
Transport of other monocarboxylates, most notably lactate, across the RBC membrane has been well characterized. Lactate RBC transport has been demonstrated to be due to three distinct mechanisms: transport by MCT1, transport by the anion exchanger, AE1 or band 3, and passive diffusion, which is demonstrated to be minimal (Deuticke et al., 1982). At physiologic lactate concentrations, MCT-mediated transport has been identified as the primary mode of lactate transport across human and rat RBC membranes, with band 3-mediated transport being prominent at very high (>300 mM) lactate concentrations (Deuticke et al., 1978, 1982; Poole and Halestrap, 1993). GHB is a known MCT substrate; however, transport by band 3 has not been previously evaluated, and the relative contribution of these transporters to GHB transport across the RBC membrane is not known.
Increasing incidence of GHB abuse has been reported in the United States, with no pharmacological treatment currently identified to treat GHB overdose. Because of MCT-mediated renal reabsorption of GHB, increasing renal clearance via MCT inhibition represents a potential treatment strategy in GHB overdose cases. The coadministration of GHB with l-lactate and other MCT inhibitors has been demonstrated to be effective in our laboratory for increasing renal and total clearances of GHB in rats (Morris et al., 2005; Wang et al., 2008a,b). Because of ubiquitous MCT expression, which governs GHB pharmacokinetics, in vivo administration of MCT inhibitors has the potential to affect other pharmacokinetic parameters of GHB, along with that of renal clearance. There exists a lack of information regarding the in vivo blood/plasma partitioning of GHB, and, as such, the effect of MCT inhibitors on this partitioning is also unidentified. The purpose of this research was to characterize the transport of GHB at the RBC membrane and to determine GHB blood/plasma partitioning in vivo in the presence and absence of the competitive MCT inhibitor l-lactate.
Materials and Methods
Chemicals and Reagents.
Sodium GHB used in these studies was provided by the National Institutes of Health National Institute on Drug Abuse (Bethesda, MD). [3H]GHB was purchased from American Radiolabeled Chemicals (St. Louis, MO). Deuterated GHB (GHB-d6) was obtained from Cerilliant (Round Rock, TX). Sodium l-lactate, silicone oil, and 4,4′-diisothiocyanostilbene-2,2′-disulfonate (DIDS) were purchased from Sigma-Aldrich (St. Louis, MO). p-Chloromercuribenzene sulfonate (pCMBS) was purchased from Toronto Research Chemicals, Inc. (North York, ON, Canada). All other chemicals were of analytical grade.
Animals and Animal Surgery.
Male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 270 to 330 g were used for in vivo experiments. Animals were housed under controlled temperature and humidity, with an artificial 12-h light/dark cycle, and food was available ad libitum. All animal protocols were approved by the Institutional Animal Care and Use Committee at the University at Buffalo. Animals were allowed to acclimate to their environment for 1 week before any surgical procedure. All surgeries were performed under anesthesia with ketamine/xylazine. Animals that were administered GHB alone underwent implantation of a jugular vein cannula, and those that were administered GHB and l-lactate underwent implantation of jugular and femoral vein cannulae. Cannulae were flushed daily with 40 IU/ml heparinized saline to maintain patency. All animals were allowed a minimum of 72 h for recovery from surgery before drug administration.
In Vivo Experimental Protocol.
To assess the in vivo B/P partitioning of GHB and the effect of l-lactate on the GHB B/P ratio, serial as well as sacrificial sampling studies were performed. In the first study, animals were sacrificed, and blood and plasma were collected at 10, 30, 60, 120, 150, 180, 210, 240, and 300 min after intravenous GHB administration at doses of 400, 600, and 800 mg/kg (n = 3 for each dose at each time point). Another set of animals was administered GHB at 600 mg/kg i.v. and l-lactate as a 330 mg/kg bolus, followed by a 121 mg/kg per hour infusion, and sacrificed at the same time points. In the second study, to assess individual B/P ratios across a wide concentration range, along with individual GHB renal clearances, animals were administered GHB at 1500 mg/kg i.v., and blood and plasma were serially collected at 3, 10, 20, 30, 60, 120, 150, 180, 210, 240, 270, 300, 330, and 360 min. Urine samples were also collected at determined intervals up to 480 min. Another set of animals was administered GHB at 1500 mg/kg i.v. and l-lactate as a 66 mg/kg bolus, followed by a 302.5 mg/kg per hour infusion, and samples were serially collected as for GHB alone. For serial sampling, n = 4 to 5 animals per group. In each study, l-lactate was administered 5 min after GHB administration. GHB was administered via the jugular vein cannula as a 200 or 300 mg/ml solution in water. l-Lactate infusions were administered using a PicoPlus syringe pump (Harvard Apparatus, Holliston, MA) as a 20 or 40 mg/ml solution in water via the femoral vein cannula.
Sample Analysis by Liquid Chromatography/Tandem Mass Spectrometry.
Sample Preparation.
GHB blood, plasma, and urine concentrations were determined using a previously validated liquid chromatography/tandem mass spectrometry (LC/MS/MS) assay (Felmlee et al., 2010a,b) with a slight modification for blood sample analysis. For preparation of blood and plasma standards, 50 μl of blank blood or plasma was added to 5 μl of GHB standard solution and 5 μl of GHB-d6. For samples, 50 μl of sample was added to 5 μl of double-distilled water and 5 μl of GHB-d6. Protein precipitation was then performed by the addition of 400 μl of acetonitrile, and samples were centrifuged at 10,000 rpm for 20 min. For blood samples, RBC lysis was first performed with the addition of 100 μl of double-distilled water followed by protein precipitation with 300 μl of acetonitrile and centrifugation. An aliquot of the resulting supernatant (200 μl) was removed and diluted with 800 μl of double-distilled water, and the diluted samples were extracted using an anion solid-phase extraction procedure previously described (Raybon and Boje, 2007) using Bond Elut SAX cartridges (100 mg of resin, 1-ml volume; Agilent Technologies, Santa Clara, CA). The samples were then dried under nitrogen gas and reconstituted with aqueous mobile phase. For urine samples, 50 μl of blank urine or urine sample was added to 10 μl of GHB-d6 and 10 μl of standard solution or double-distilled water. Protein precipitation was then performed by the addition of 930 μl of double-distilled water followed by 1 ml of acetonitrile and centrifugation at 10,000 rpm for 20 min. An aliquot of the resulting supernatant (1.25 ml) was used for analysis by LC/MS/MS.
Sample analysis.
The same LC/MS/MS method was used for GHB blood, plasma, and urine analyses. LC/MS/MS equipment consisted of an Agilent 1100 series HPLC system including an online degasser, binary pump, and autosampler (Agilent Technologies), which was connected to a PE Sciex API 3000 triple-quadrupole tandem mass spectrometer with a turbo ion spray (Applied Biosystems, Foster City, CA). Seven microliters of sample was injected onto an XTerra MS C18 column (250 × 2.1 mm i.d., 5-μm particle size) (Waters, Milford, MA). GHB was eluted with a flow rate of 0.2 ml/min, 100 to 90% A over 5 min, 90 to 10% A from 5 to 7.5 min, and 10 to 100% A from 7.5 to 12 min. Mobile phase A was made up of 0.1% formic acid in 95:5 water/acetonitrile. Mobile phase B was made up of 0.1% formic acid in 5:95 water/acetonitrile. The limit of quantification for blood and plasma was 1 and 4 μg/ml for urine, respectively. Mass spectrometer conditions and calibration ranges have been described previously (Felmlee et al., 2010a). l-Lactate plasma concentrations were determined by a YSI 1500 Sport Lactate Analyzer (YSI, Inc., Yellow Springs, OH).
In Vitro Uptake.
Freshly isolated erythrocytes from male Sprague-Dawley rats (Harlan Laboratories) were used for in vitro experiments. Blood was withdrawn from animals sacrificed under isoflurane anesthesia. After removal of the plasma and buffy coat, erythrocytes were washed three times in uptake buffer for removal of extracellular lactate. Uptake buffer consisted of 137 mM NaCl, 5.4 mM KCl, 2.8 mM CaCl2, 1.2 mM MgCl2, and 10 mM HEPES at the specified pH values. RBCs were then made into a 40% suspension in uptake buffer. For uptake, an oil-stop technique was used similar to those previously described (Jarvis and Young, 1980; Endres et al., 2009). To initiate transport, 50 μl of the RBC suspension was spiked into 200 μl of uptake buffer containing various concentrations of [3H]GHB. Transport was stopped by transferring 150 μl of this resulting suspension to centrifuge tubes containing 350 μl of ice-cold stop buffer (137 mM NaCl, 5.4 mM KCl, 2.8 mM CaCl2, 1.2 mM MgCl2, and 10 mM HEPES at pH 7.4) over 200 μl of silicone oil (specific gravity 1.05). Tubes were then centrifuged and the oil and buffer were aspirated off. Red blood cells were washed three times with ice-cold uptake buffer for removal of extracellularly bound [3H]GHB. Cells were then lysed with 1 ml of double-distilled water, and 500 μl of the lysate was transferred to vials with 3 ml of scintillation fluid for measurement of radioactivity. Radioactivity was measured using a 1900CA Tri-Carb liquid scintillation analyzer (PerkinElmer Life and Analytical Sciences, Waltham, MA). Total protein content of the RBC lysate was determined by spectrophotometry. Initial time-dependent uptake was used to determine the linear range of GHB uptake at each pH value. For determination of the transporters involved in GHB transport, specific inhibitors were used. To inhibit MCT1, the RBC suspension was incubated in 1 mM pCMBS for 1 h before addition of [3H]GHB. For band 3 inhibition, at pH 7.4 the RBC suspension was incubated in 10 μM DIDS for 5 min before addition of [3H]GHB. Although DIDS is also capable of inhibiting MCTs, this concentration has been demonstrated to completely inhibit anion transport by band 3 (Halestrap, 1976), while still much lower than the IC50 value for rat MCT1 (rMCT1) (Poole and Halestrap, 1991). To determine an inhibition constant for l-lactate, uptake of 10 mM GHB was assessed in the presence of various l-lactate concentrations at pH 7.4. Unless otherwise specified, in vitro experiments were performed at room temperature in triplicate and repeated three times.
Data and Statistical Analysis.
In vivo blood and plasma GHB concentrations were plotted against one another, and the B/P ratios were determined by linear regression. Total clearance (CL) was calculated as dose/AUC, where AUC represents the plasma AUC0-∞, determined by noncompartmental analysis using WinNonlin 5.2 (Pharsight Corporation, Mountain View, CA). Renal clearance (CLR) was determined as Ae/AUC, where Ae represents the total amount excreted in the urine. Metabolic clearance (CLm) was calculated as CL − CLR. The B/P ratios and clearance values for animals administered GHB at 1500 mg/kg with and without l-lactate were compared to determine any statistically significant effect of l-lactate administration on these GHB pharmacokinetic parameters. Statistical comparisons were performed using Student's t tests or Mann-Whitney rank sum tests using SigmaPlot 10.0 (Systat Software, Inc., San Jose, CA). Using WinNonlin, in vitro GHB concentration-dependent uptake was fitted with eq. 1, and inhibition by l-lactate was fitted with eq. 2:
Results
In Vivo GHB B/P Partitioning.
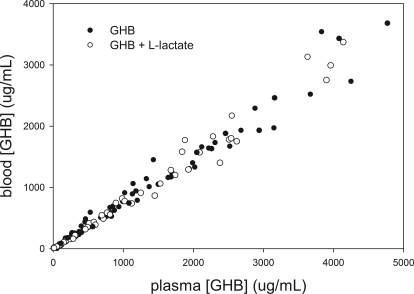
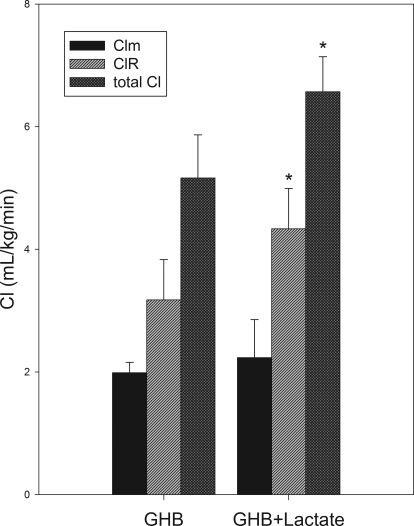
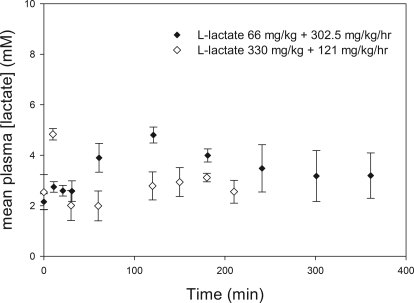
The in vivo B/P partitioning is shown in Fig. 1, and resulting B/P ratios are shown in Table 1. This partitioning appears linear over the entire in vivo range of concentrations. Concomitant l-lactate administration resulted in a B/P ratio that is similar to that for GHB alone. Comparing animals in which individual B/P ratios could be obtained from serial sampling, the mean B/P ratios were similar to those obtained using pooled data from both sampling strategies, indicating that these ratios were representative of the entire dataset. From this set of experiments, the mean B/P ratio in animals administered GHB with l-lactate was not significantly different from that of animals administered GHB alone. However, in the same animals, this dose of l-lactate significantly increased renal and total clearances of GHB compared with GHB alone (Fig. 2). Lactate concentrations achieved with both l-lactate regimens are shown in Fig. 3.
Fig. 1.
In vivo blood/plasma partitioning of GHB in the presence and absence of l-lactate. Data include those of both serial and sacrificial sampling in rats after intravenous GHB administration in doses of 400 to 1500 mg/kg with and without one of two l-lactate doses, 330 mg/kg followed by 121 mg/kg per hour or 66 mg/kg followed by 302.5 mg/kg per hour (n = 74 total).
TABLE 1.
B/P partitioning in rats administered GHB (±) l-lactate
Using a Student's t test, no significant difference was determined between animals administered GHB at 1500 mg/kg compared to those administered GHB at 1500 mg/kg + l-lactate (P > 0.05). Data presented as mean (S.D.).
| Regimen | B/P Ratio |
|---|---|
| GHB 400-1500 mg/kg† | 0.75 |
| GHB 600 or 1500 mg/kg + l-lactate† | 0.76 |
| GHB 1500 mg/kg†† | 0.74 (0.07) |
| GHB 1500 mg/kg + l-lactate 66 mg/kg + 302.5 mg/kg per hour†† | 0.75 (0.07) |
Ratio determined using data from all animals, including serial and sacrificial sampling studies (n = 74 rats total) and both l-lactate doses (330 mg/kg + 121 mg/kg per hour and 66 mg/kg + 302.5 mg/kg per hour). No S.D. was determined because sacrificial sampling did not allow determination of individual B/P ratios across a concentration range.
Mean of individual B/P ratios determined from serial sampling study only (n = 4–5 in each group). These values were used for statistical comparison.
Fig. 2.
Clearance of GHB in rats administered GHB with and without l-lactate. GHB was administered intravenously at 1500 mg/kg followed by l-lactate as a 66 mg/kg bolus and 302.5 mg/kg per hour infusion. Data are presented as mean ± S.D. (n = 4–5/group). Clearances between groups administered GHB alone and with l-lactate were compared using Student's t tests or Mann-Whitney rank sum tests. *, P < 0.05.
Fig. 3.
Plasma l-lactate concentrations after concomitant l-lactate and GHB administration. l-Lactate was administered 5 min after GHB administration, time 0. Closed symbols represent data from serially sampling for up to 360 min in rats administered intravenous GHB at 1500 mg/kg + l-lactate at 66 mg/kg followed by 302.5 mg/kg per hour (n = 4 total). Open symbols represent data from rats sacrificed at up to 210 min after intravenous administration of 600 mg/kg GHB + 330 mg/kg l-lactate followed by 121 mg/kg per hour (n = 3 at each time point). Data are presented as mean ± S.D.
In Vitro GHB Uptake.
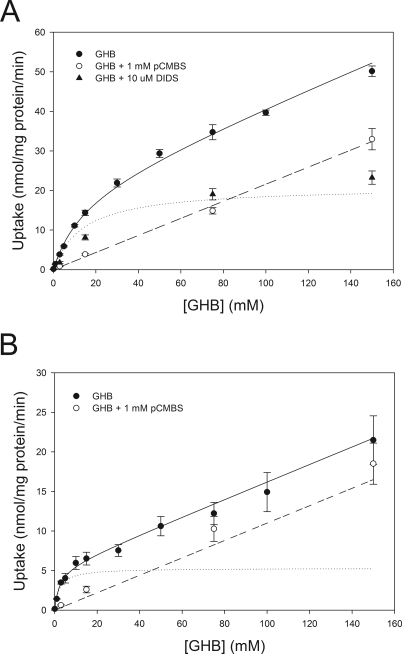
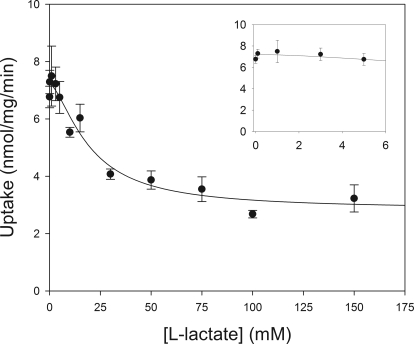
Based on time-dependent experiments, 5 and 15 s were used for concentration-dependent experiments at pH values of 6.5 and 7.4, respectively. Concentration-dependent uptake at each pH is shown in Fig. 4. At both pH values, incubation of the cell suspension with pCMBS resulted in complete inhibition of the saturable transport component, indicating that all saturable transport within this range of GHB concentrations is due to MCT-mediated transport. Inhibition of band 3 with DIDS at pH 7.4 resulted in inhibition of the linear transport component, indicating that this component is due to band 3-mediated transport. The resulting transport with DIDS inhibition was similar to that simulated with the Vmax and Km for MCT-mediated transport, suggesting that total transport can be accounted for by these two transporters. The contribution of MCT-mediated transport, determined by transport inhibited by pCMBS at pH 7.4, is given in Table 2. At the lower GHB concentrations, relevant to in vivo GHB concentrations, incubation with pCMBS resulted in inhibition of ∼75% of total transport, indicating that MCT-mediated transport is the primary mechanism responsible for RBC membrane transport of GHB in vivo. Total transport and transport in the presence of pCMBS at pH 7.4 were simultaneously fitted with nonlinear regression, resulting in values of 17.0 mM, 20.9 nmol/mg per minute, and 0.24 μl/mg per minute for Km, Vmax, and P at pH 7.4, respectively. Fitting the total and pCMBS-inhibited transport at pH 6.5 resulted in a much lower Km value of 2.2 mM, with Vmax and P values of 5.3 nmol/mg per minute and 0.11 μl/mg per minute, respectively. Fitting GHB transport in the presence of l-lactate resulted in an IC50 value for l-lactate of 19.1 mM (Fig. 5). No significant inhibition was observed at l-lactate concentrations at or below 5 mM.
Fig. 4.
Concentration-dependent uptake of GHB in rat RBCs. A, uptake at pH 7.4. B, uptake at pH 6.5. Closed circles, total transport. Open circles, transport in the presence of 1 mM pCMBS. Closed triangles, transport in the presence of 10 μM DIDS. Data are presented as mean ± S.E.M. Total transport and transport in the presence of pCMBS were simultaneously fitted by nonlinear regression represented by the solid (total transport) and dashed (pCMBS) lines, respectively. Dotted line, simulated MCT-mediated transport using fitted Km and Vmax values.
TABLE 2.
Contribution of MCT-mediated transport to GHB uptake in rat RBCs at pH 7.4
| GHB (mM) | Inhibition of MCT-Mediated Transport by 1 mM pCMBS (Mean Percentage Inhibition Compared with Control) |
|---|---|
| % | |
| 3 | 76.5 |
| 15 | 70.2 |
| 75 | 52.1 |
| 150 | 27.8 |
Fig. 5.
Concentration-dependent inhibition of GHB RBC uptake by l-lactate. Closed circles, uptake of 10 mM GHB at pH 7.4 at the respective l-lactate concentration. Solid line, fitting with eq. 2. Results are from two experiments performed in triplicate. Data are presented as mean ± S.E.M. Inset displays effect of measured in vivo l-lactate concentrations (≤5 mM) on GHB transport compared to control (0 mM).
Discussion
Although saturable, carrier-mediated oral absorption and renal reabsorption of GHB have been demonstrated and attributed to MCT-mediated transport, the linearity and effect of MCTs on the distribution of GHB have not been fully investigated. In situ experiments indicate a role of MCT1 in the distribution of GHB into the brain (Bhattacharya and Boje, 2004); however, the significance of this transport in vivo has not been determined. The intracellular RBC space can contribute considerably to drug distribution. Here, we demonstrate the significance of MCT-mediated transport of GHB into RBCs in vitro and the distributive effects of this transport in vivo. Our in vivo experiments demonstrate linearity in GHB B/P partitioning along with a lack of effect of l-lactate administration on this parameter. These results are surprising considering the nonlinearity of MCT-mediated renal clearance at these GHB doses and the ability of l-lactate to increase this clearance, which was demonstrated in this and other studies (Morris et al., 2005; Wang et al., 2008a). To further investigate these findings, we performed in vitro experiments to determine the contribution of MCT-mediated transport in RBCs at relevant in vivo GHB concentrations and the effect of changes in pH at the transport site. Results of these in vitro experiments demonstrate that at relevant in vivo concentrations (<40 mM), the majority of GHB transport across the RBC membrane is MCT-mediated. These in vitro experiments also demonstrate a decrease in the affinity of GHB for MCT1 as the pH level increases, indicated by a much lower Km value at pH 6.5 compared to that at pH 7.4. A similar trend for l-lactate transport has been noted previously (Deuticke, 1982), and our experiments confirm this effect of pH on MCT-mediated transport of GHB. Likewise, although inhibition of GHB transport by 2 mM l-lactate in rat kidney membrane vesicles expressing rMCT1 has been demonstrated at pH 5.5 (Wang et al., 2006), our current experiments at pH 7.4 demonstrate no inhibition of GHB transport at this and other relevant in vivo l-lactate concentrations. These in vitro findings can explain our results observed in in vivo experiments. Considering a decrease in GHB and l-lactate affinity for MCT1 at physiologic blood pH compared to that of the lower pH in the renal tubule, nonlinearity and inhibition could be expected to occur in the kidney at doses in which these effects are not observed at the RBC membrane.
The resulting Km for GHB of 17.0 mM determined at pH 7.4 is considerably higher than that previously determined (4.6 mM) for rMCT1 in our laboratory (Wang et al., 2006). This reported Km value of 4.6 mM was determined at pH 6.0, and, as the current study demonstrates, this value would be expected to be lower than that at pH 7.4. The reported value is similar to our currently reported value of 2.2 mM at pH 6.5 and is more relevant to MCT-mediated transport at the kidney rather than that in the blood. The Ki value for l-lactate has also been previously determined to be 2.2 mM for inhibition of its own transport in rat RBCs at pH 7.4 (Poole et al., 1990); this Ki value is much lower than our currently reported value of 19.1 mM. Although the reported Ki value was assessed as physiologic blood pH, this value was determined at a very low temperature (6°C) because of rapid lactate transport kinetics. The values of Ki and Km for MCT substrates have been observed to increase with temperature; severalfold differences have been reported as temperature is increased from 5–20°C (Deuticke, 1982; Poole and Halestrap, 1993). The IC50 value determined at a higher temperature in our studies is expected to be much greater than the previously reported Ki value. Our in vitro results, at a more relevant temperature, demonstrate no inhibition of GHB transport at the l-lactate concentrations reached in vivo, which is consistent with a lack of inhibition at the RBC membrane in our in vivo studies.
Along with the effect of pH, other factors may contribute to the observed in vivo results, including the presence of sodium-coupled monocarboxylate transporters (SMCTs; SLC5A8 and SLC5A12) in the kidney, which transport both GHB and l-lactate (Ganapathy et al., 2008). Previous experiments in this laboratory demonstrated that the Km and IC50 values of GHB and l-lactate for SMCT1 are lower than that for MCT1 (Cui and Morris, 2009); therefore, transport by SMCTs in the kidney likely plays a role in the nonlinearity of GHB renal clearance and inhibition by l-lactate. Expression of SMCTs on RBCs has not been demonstrated, and this may also contribute to the observed differences in transport at the RBC membrane and in the kidney. Whereas blood/plasma partitioning displayed linearity, GHB plasma concentrations that were reached in our in vivo experiments were greater than our reported Km value of 17.0 mM at pH 7.4; therefore, it is assumed that there are additional effects at the RBC membrane that prevent saturation of GHB transport at this site. Bidirectional MCT-mediated transport and trans-stimulation may contribute to the observed linearity at this membrane, because this phenomenon has been demonstrated to occur with monocarboxylates in RBCs (Halestrap, 1976). Trans-stimulation may prevent saturation at steady-state in vivo, although this effect would not be observed in our unidirectional uptake experiments. Indeed, the Km and Vmax of l-lactate measured under equilibrium exchange have been shown to be much higher than that determined by unidirectional uptake or efflux (Deuticke, 1982); therefore, this is likely also true for GHB. In addition, because of rapid transport kinetics, in vitro experiments were performed at a temperature lower than the physiologic temperature of 37°C. Considering the discussed effects of temperature on MCT-mediated transport, the actual in vivo Km for GHB uptake may in fact be higher than our determined value at pH 7.4. Along with MCT-mediated transport, in vitro experiments also indicate that band 3 plays a role in GHB RBC transport. Although this transport contributes at high GHB concentrations, it is unlikely that this transport alone would explain the lack of saturation at the RBC membrane in vivo, because this effect would also be expected to occur in vitro, and it was not observed in our studies.
In conclusion, the contribution of MCT-mediated transport of GHB at the RBC membrane has been demonstrated to be significant at relevant in vivo concentrations, although this transport does not appear to be saturable with in vivo GHB administration. Likewise, whereas intravenous l-lactate administration inhibits GHB transport in the kidney, this MCT inhibitor appears to have no effect on GHB distribution into RBCs at relevant plasma concentrations. The difference in pH at these transport sites likely contributes to these observations, although other effects may be involved. This work emphasizes the need to consider physiologic conditions, particularly pH, when translating in vitro MCT transport kinetics to in vivo effects on drug pharmacokinetics.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA023223]; and by a fellowship from Pfizer Global Research and Development (to B.L.M.).
A portion of this work was previously presented as an abstract as follows: Morse BL, Felmlee MA, and Morris ME (2010) Erythrocyte transport of γ-hydroxybutyrate in vitro and in vivo. American Association of Pharmaceutical Sciences Annual Meeting & Exposition; 2010 Nov 14–18; New Orleans, LA. American Association of Pharmaceutical Scientists, Arlington, VA.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
- GHB
- γ-hydroxybutyrate
- MCT
- monocarboxylate transporter
- RBC
- red blood cell
- pCMBS
- p-chloromercuribenzene sulfonate
- DIDS
- 4,4′-diisothiocyanostilbene-2,2′-disulfonate
- GHB-d6
- deuterated GHB
- AE
- anion exchanger
- AUC
- area under the curve
- B/P
- blood/plasma
- CL
- clearance
- CLm
- metabolic clearance
- CLR
- renal clearance
- HPLC
- high-performance liquid chromatography
- LC/MS/MS
- liquid chromatography/tandem mass spectrometry
- rMCT1
- rat MCT1
- SMCT
- sodium-coupled monocarboxylate transporter.
Authorship Contributions
Participated in research design: Morse, Felmlee, and Morris.
Conducted experiments: Morse and Felmlee.
Contributed new reagents or analytic tools: Morse and Felmlee.
Performed data analysis: Morse.
Wrote or contributed to the writing of the manuscript: Morse, Felmlee, and Morris.
References
- Arena C, Fung HL. (1980) Absorption of sodium gamma-hydroxybutyrate and its prodrug gamma-butyrolactone: relationship between in vitro transport and in vivo absorption. J Pharm Sci 69:356–358 [DOI] [PubMed] [Google Scholar]
- Bhattacharya I, Boje KM. (2004) GHB (gamma-hydroxybutyrate) carrier-mediated transport across the blood-brain barrier. J Pharmacol Exp Ther 311:92–98 [DOI] [PubMed] [Google Scholar]
- Caldicott DG, Chow FY, Burns BJ, Felgate PD, Byard RW. (2004) Fatalities associated with the use of gamma-hydroxybutyrate and its analogues in Australasia. Med J Aust 181:310–313 [DOI] [PubMed] [Google Scholar]
- Cui D, Morris ME. (2009) The drug of abuse gamma-hydroxybutyrate is a substrate for sodium-coupled monocarboxylate transporter (SMCT) 1 (SLC5A8): characterization of SMCT-mediated uptake and inhibition. Drug Metab Dispos 37:1404–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuticke B. (1982) Monocarboxylate transport in erythrocytes. J Membr Biol 70:89–103 [DOI] [PubMed] [Google Scholar]
- Deuticke B, Beyer E, Forst B. (1982) Discrimination of three parallel pathways of lactate transport in the human erythrocyte membrane by inhibitors and kinetic properties. Biochim Biophys Acta 684:96–110 [DOI] [PubMed] [Google Scholar]
- Deuticke B, Rickert I, Beyer E. (1978) Stereoselective, SH-dependent transfer of lactate in mammalian erythrocytes. Biochim Biophys Acta 507:137–155 [DOI] [PubMed] [Google Scholar]
- Endres CJ, Moss AM, Ke B, Govindarajan R, Choi DS, Messing RO, Unadkat JD. (2009) The role of the equilibrative nucleoside transporter 1 (ENT1) in transport and metabolism of ribavirin by human and wild-type or Ent1(−/−) mouse erythrocytes. J Pharmacol Exp Ther 329:387–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee MA, Roiko SA, Morse BL, Morris ME. (2010a) Concentration-effect relationships for the drug of abuse gamma-hydroxybutyric acid. J Pharmacol Exp Ther 333:764–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee MA, Wang Q, Cui D, Roiko SA, Morris ME. (2010b) Mechanistic toxicokinetic model for gamma-hydroxybutyric acid: inhibition of active renal reabsorption as a potential therapeutic strategy. AAPS J 12:407–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimberti L, Spella MR, Soncini CA, Gessa GL. (2000) Gamma-hydroxybutyric acid in the treatment of alcohol and heroin dependence. Alcohol 20:257–262 [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Thangaraju M, Gopal E, Martin PM, Itagaki S, Miyauchi S, Prasad PD. (2008) Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J 10:193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CK, Brown MS, Pathak RK, Goldstein JL. (1995) cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J Biol Chem 270:1843–1849 [DOI] [PubMed] [Google Scholar]
- Halestrap AP. (1976) Transport of pyruvate and lactate into human erythrocytes. Evidence for the involvement of the chloride carrier and a chloride-independent carrier. Biochem J 156:193–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis SM, Young JD. (1980) Nucleoside transport in human and sheep erythrocytes. Evidence that nitrobenzylthioinosine binds specifically to functional nucleoside-transport sites. Biochem J 190:377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam WK, Felmlee MA, Morris ME. (2010) Monocarboxylate transporter-mediated transport of gamma-hydroxybutyric acid in human intestinal Caco-2 cells. Drug Metab Dispos 38:441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettieri JT, Fung HL. (1979) Dose-dependent pharmacokinetics and hypnotic effects of sodium gamma-hydroxybutyrate in the rat. J Pharmacol Exp Ther 208:7–11 [PubMed] [Google Scholar]
- Maitre M. (1997) The gamma-hydroxybutyrate signalling system in brain: organization and functional implications. Prog Neurobiol 51:337–361 [DOI] [PubMed] [Google Scholar]
- Merezhinskaya N, Fishbein WN. (2009) Monocarboxylate transporters: past, present, and future. Histol Histopathol 24:243–264 [DOI] [PubMed] [Google Scholar]
- Morris ME, Hu K, Wang Q. (2005) Renal clearance of gamma-hydroxybutyric acid in rats: increasing renal elimination as a detoxification strategy. J Pharmacol Exp Ther 313:1194–1202 [DOI] [PubMed] [Google Scholar]
- Palatini P, Tedeschi L, Frison G, Padrini R, Zordan R, Orlando R, Gallimberti L, Gessa GL, Ferrara SD. (1993) Dose-dependent absorption and elimination of gamma-hydroxybutyric acid in healthy volunteers. Eur J Clin Pharmacol 45:353–356 [DOI] [PubMed] [Google Scholar]
- Poole RC, Cranmer SL, Halestrap AP, Levi AJ. (1990) Substrate and inhibitor specificity of monocarboxylate transport into heart cells and erythrocytes. Further evidence for the existence of two distinct carriers. Biochem J 269:827–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole RC, Halestrap AP. (1991) Reversible and irreversible inhibition, by stilbenedisulphonates, of lactate transport into rat erythrocytes. Identification of some new high-affinity inhibitors. Biochem J 275:307–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole RC, Halestrap AP. (1993) Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol 264:C761–C782 [DOI] [PubMed] [Google Scholar]
- Raybon JJ, Boje KM. (2007) Pharmacokinetics and pharmacodynamics of gamma-hydroxybutyric acid during tolerance in rats: effects on extracellular dopamine. J Pharmacol Exp Ther 320:1252–1260 [DOI] [PubMed] [Google Scholar]
- Wang Q, Darling IM, Morris ME. (2006) Transport of gamma-hydroxybutyrate in rat kidney membrane vesicles: Role of monocarboxylate transporters. J Pharmacol Exp Ther 318:751–761 [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang X, Morris ME. (2008a) Effects of l-lactate and d-mannitol on gamma-hydroxybutyrate toxicokinetics and toxicodynamics in rats. Drug Metab Dispos 36:2244–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang Q, Morris ME. (2008b) Pharmacokinetic interaction between the flavonoid luteolin and gamma-hydroxybutyrate in rats: potential involvement of monocarboxylate transporters. AAPS J 10:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvosec DL, Smith SW, Porrata T, Strobl AQ, Dyer JE. (2010) Case series of 226 hydroxybutyrate-associated deaths: lethal toxicity and trauma. Am J Emerg Med 29:319–332 [DOI] [PubMed] [Google Scholar]