Abstract
We studied social cognition in 49 children 3 months after moderate to severe traumatic brain injury (TBI) and in 39 children with orthopedic injury (OI). Children underwent diffusion tensor imaging (DTI) and a mental attribution task showing two triangles. Mental state attributions increased when one triangle reacted to intentions of the other, but less so in the TBI than the OI group. DTI identified injury to white matter microstructure in the TBI group, but the relation of DTI to mental attributions did not differ between groups. Moderate to severe TBI produces white matter disconnections that may affect social cognitive networks.
The capacity to accurately process the intentions and emotions in oneself and others, which is often referred to as theory of mind (ToM) (Frith & Frith, 2003), is a social cognitive skill thought to underlie social interaction and facilitate development of social relationships, thereby contributing to social adjustment (Carrington & Bailey, 2009; Turkstra, Williams, Tonks, & Frampton, 2008; Yeates et al., 2007). Consistent with this view, ToM has been shown to be impaired following moderate to severe traumatic brain injury (TBI) due to closed head trauma (Dennis, Barnes, Wilkinson, & Humphreys, 1998; Dennis, Purvis, Barnes, Wilkinson, & Winner, 2001; Snodgrass & Knott, 2006) in children and adolescents. Social functioning often remains below age expectation in these patients despite recovery in cognitive domains over the first two years after injury (Dennis et al., 1998; Taylor et al., 2002; Yeates et al., 2007). The range of ToM tasks that have revealed deficits in children with TBI includes inferring the mental state of a protagonist by school-aged children with traumatic frontal lesions (Snodgrass & Knott, 2006), false belief tasks involving incongruous appearance-reality, false contents, and false location in 3- to 5-year-olds tested within 3 months post-moderate to severe TBI (Walz, Yeates, Taylor, Stancin, & Wade, 2009), interpreting complex emotions such as sarcasm and deceptive praise (Dennis et al., 2001), and processing the intentions and emotions communicated by adolescents in a video vignette (Turkstra, McDonald, & DePompei, 2001). Although some of the tasks used to date in children with TBI to investigate processing of mental states tend to stress aural comprehension and working memory of complex verbal material, others use visual presentation that involves a discrepancy between expectations and reality such as a milk container filled with crayons (Walz et al., 2009). Consequently, deficits in verbal processing and limitations in working memory (Levin et al., 2002, 2004) might explain at least in part the apparent social cognitive impairment in this population.
There is also a convergence between brain regions and networks implicated in ToM and the neuroanatomic distribution of focal lesions and white matter disconnections resulting from moderate to severe TBI in children (Yeates et al., 2007). Functional brain imaging of healthy participants has implicated one or more neural networks in mental attributions, most frequently involving medial prefrontal cortex (mPFC) (Carrington & Bailey, 2009) followed by temporoparietal junction (TPJ), and adjacent posterior temporal sulcus (pSTS) with variation across studies related to differences in tasks (Lombardo et al., 2009) and participant characteristics (Amodio & Frith, 2006; Gobbini, Koralek, Bryan, Montgomery, & Haxby, 2007; Lombardo et al., 2009). For example, Gobbini et al. (2007) also noted that the operculum, inferior parietal lobule (IPL), and TPJ are activated by story-related tasks such as false belief in which the participant takes the perspective of another person without observing actions, whereas tasks in which the participant infers the goals of others through observing their actions tend to activate pSTS. Nevertheless, there is overlap in the regions activated by story-based tasks and tasks involving processing of observed actions. Using a task wherein participants described the animated movements of two triangles presented under three conditions, including random movements devoid of interaction, goal directed movements involving interaction between the triangles without apparent reaction to one another’s mental state, and a ToM condition in which one triangle reacted to the intentions of the other (e.g., one triangle mocking the other), Castelli, Happe, Frith, and Frith (2000) found on positron emission tomography that movements in the ToM condition produced greater brain activation in mPFC, TPJ, basal temporal (temporal pole adjacent to amygdala and fusiform gyrus) region, and extrastriate cortex than a random condition in which the figures did not interact. Goal-directed interactions such as dancing that did not involve a triangle reacting to the mental state of the other, produced brain activation that was intermediate between the ToM and random conditions. Mental state attributions by the participants were correspondingly most frequent under the ToM condition and lowest in response to random movements.
Despite a growing literature on neural mechanisms of mental state attribution in healthy participants (Amodio & Frith, 2006; Carrington & Bailey, 2009; Castelli et al., 2000; de Lange, Spronk, Willems, Toni, & Bekkering, 2008; Gobbini et al., 2007; Lombardo et al., 2009; Pelphrey & Carter, 2008), little is known about the relation of brain imaging to mental state attribution in children following TBI. Studies to date are limited to clinical findings of computed tomography (CT) or structural magnetic resonance imaging (MRI) (Dennis et al., 2001; Snodgrass & Knott, 2006). Dennis found that frontal lesions on CT or MRI were associated with difficulty identifying complex emotions depicted in narratives presented verbally by an examiner and represented in drawings of the characters. All of the children with TBI studied by Snodgrass & Knott had frontal lesions on MRI and there was no analysis of ToM responses in relation to lesion characteristics. Consequently, there is a gap in knowledge concerning the relation of social cognition following TBI in children to specific regions of white matter injury, including white matter tracts connecting prefrontal, temporal, and posterior brain regions involved in social cognitive networks.
To evaluate the effects of moderate to severe pediatric TBI on mental state attribution, we presented silent animations of two moving triangles under conditions that differed in degree of apparent social interaction (Abell, Happe, & Frith, 2000). This approach exploits the finding that observers infer intentions and feelings in the movement of interacting shapes presented in animated, nonverbal sequences (Heider & Simmel, 1944). Interpretation of these movements as representing the actions of persons has been attributed to the kinetic properties of the stimuli rather than their shape or the background (Castelli et al., 2000; Heider & Simmel, 1944). Presenting the same animations as in the present study, Abell et al. (2000) found that about one-third of the mental state attributions by school aged children with autism were inappropriate to the condition. For example, in response to viewing non-interactive, random movements by two triangles, autistic children frequently reported mental state attributions. In contrast, the proportion of mental state attributions in responses by healthy adults and typically developing children corresponded to the condition, that is, highest when one of the triangles appeared to react to the mental state of the other, intermediate for interactive movements (e.g., dancing), and lowest for the random condition wherein the triangles “floated” devoid of any interaction.
To investigate mental state attributions in relation to the microstructural integrity of white matter of brain regions and circuitry implicated in social cognition, we acquired diffusion tensor imaging (DTI) in children with moderate to severe TBI. We postulated that mental attributions in response to viewing these social animations would be reduced as a function of TBI severity and disruption of white matter microstructure involved in neural networks mediating social cognition (Carrington & Bailey, 2009).
METHOD
This is a prospective investigation of children age 7 to 17 years who had moderate to severe TBI and a comparison group of children who sustained orthopedic injury (OI) whom we recruited during or shortly after their hospitalization and studied at approximately three months post-injury. With increasing emphasis on peer relations during the transition from school age childhood to adolescence, we focused on this age range while limiting the youngest age to 7 years to facilitate compliance with un-sedated brain imaging.
Participants
Forty-nine children who had sustained moderate to severe TBI were recruited prospectively from trauma centers in Dallas, Houston, and Miami (Table 1). Severe TBI was defined by a lowest-post-resuscitation Glasgow Coma Scale (GCS) (Teasdale & Jennett, 1974) score of 8 or less, while moderate TBI corresponded to a GCS score of 9–12 or a GCS score of 13–15 associated with presence of a brain lesion on CT performed within 24 hours post-injury. For comparison to a neurologically intact group whose demographic variables and risk factors were similar to the TBI patients, 39 children with OI were recruited from the same trauma centers. Eligibility criteria in both groups included right handedness (Oldfield, 1971), no pre-injury psychosis as determined by a structured psychiatric interview (Kaufman et al., 1997) with the parent, no previous neurologic disorder or hospitalization for TBI, and no history of mental deficiency. Socioeconomic level was measured using a social composite index (SCI) (Yeates et al., 1997). Of the pediatric patients and families whom we approached, 78% agreed to participate in this study.
TABLE 1.
Demographic Features and Mechanism of Injury in the TBI and OI Groups
|
OI (N = 39) |
TBI (N = 49) |
p-Value | |||
|---|---|---|---|---|---|
| Mean | STD | Mean | STD | ||
| Age at injury (years)† | 12.07 | 2.19 | 13.4 | 2.79 | 0.017 |
| Socioeconomic status (Yeates et al., 1997) | 0.22 | 0.86 | 0.02 | 0.8 | 0.251 |
| Injury-study interval (months) | 3.79 | 0.6 | 3.76 | 0.68 | 0.837 |
| Glasgow Coma Scale score (Teasdale & Jennett, 1974) | NA | NA | 8.37 | 4.43 | NA |
| n | Percentage | n | Percentage | p-Value | |
| Gender | |||||
| Male | 26 | 66.67 | 32 | 65.31 | 0.894 |
| Female | 13 | 33.33 | 17 | 34.69 | |
Groups significantly differ. OI = orthopedic injury; TBI = traumatic brain injury.
Procedure
Mental state attributions from observing movement were investigated using Abell et al.’s (2000) computer animations of a large red triangle and a small blue triangle within a white framed background on a computer screen. The three conditions included (1) Random Movement (RM) in which the triangles moved around the screen in a random fashion (e.g., floating), but did not interact with one another, (2) Goal Directed (GD), wherein the triangles moved purposefully and interacted reciprocally (e.g., dancing), but without reacting to the other’s mental state, and (3) ToM, which showed one triangle reacting to the other’s mental state (e.g., one triangle coaxed the other to come out of the box). The GD and ToM conditions thus tested the child’s ability to interpret social movements with complexity increasing from simple, purposeful action to reacting to the mental state of another agent. The task had two levels of cueing: in Uncued, the child was presented one animation of 24–63 seconds duration in each of the three conditions (RM, GD, ToM) and asked to describe what he/she saw happening on the screen. In Cued, the child was again presented one animation in each of the three conditions, but this time was prompted to describe what was happening on the screen as if the movements were by humans. The Uncued presentation always preceded the Cued presentation, but condition was randomized within each level of cueing. Responses were recorded, transcribed, and coded for two scores, accuracy and the type of attribution. The attribution score indicated whether the child attributed mental states to the animated triangles. A score of zero meant that the child described the movements strictly as physical action (“bumping into each other”), a score of one denoted that the child described the triangles as interacting with each other without mentioning mental states (“dancing”), and a score of two denoted that the child recognized the triangles as reacting to one another as inferred from the child’s use of a mental attribution such as “pretending,” “fooling,” or “coaxing.” Mental state attributions were predicted to be more common in the ToM condition (Abell et al., 2000). To evaluate whether the child attended to, accurately perceived, and had sufficient language ability to describe the animations, response accuracy was scored according to the criteria of Abell et al. (2000) (0, 1, 2 points) wherein a score of zero was given if the child completely failed to describe the animation, a score of one indicated a partially correct description (“two triangles moving around”), and a score of two was given if the child gave an exact description of the animation sequence. Thus the accuracy measure served as an attention and language control measure for the attribution score.
Inter-rater agreement in scoring for accuracy was established for two examiners using data of 104 children with moderate to severe TBI and orthopedically injured children. The responses were scored for accuracy independently by two examiners, yielding an agreement of 93% or higher on each level of cueing of each condition. For attribution, the agreement was evaluated within 16 patients, and agreement was 88% and higher for each level of cueing for each condition.
DTI Acquisition
Imaging was acquired on a 1.5 T Philips scanner (Cleveland, OH) at each performance site. Transverse multislice spin echo, single shot, echo planar imaging (EPI) sequences were used (10150.5 ms TR, 90 ms TE, 2.7 mm slices, 0 mm gap). A 256 mm field of view (FOV) was used with a measured voxel size of 2.69 × 2.69 × 2.7 mm and a reconstructed voxel size of 2.00 × 2.00 × 2.7 mm. Diffusion was measured along 15 directions (number of b-value = 2, low b-value = 0 and high b-value = 860 sec/mm2). To improve signal to noise ratio, high-b images were acquired twice and averaged in most cases. The imaging protocol also included FLAIR and gradient echo magnetic resonance imaging (MRI) sequences to identify focal lesions in gray and white matter. Acquisition time was 5 minutes 45 seconds, and 55 slices were acquired. MRI findings were reviewed and lesions were coded for neuroanatomic site and pathology by the project neuroradiologist who had no access to the behavioral data.
DTI analysis
Selection of sites for analysis was guided by evidence for participation of distributed networks involving frontotemporal and parietal regions in social cognition (Adolphs, 2009; Amodio & Frith, 2006; Carrington & Bailey, 2009), including interhemispheric transfer of information. Regions of interest (ROIs) included the corpus callosum (genu, body, and splenium), left and right total prefrontal white matter, medial prefrontal white matter (mPFWM) (including Brodmann area 10), total temporal white matter, uncinate fasciculus, cingulum bundle, and inferior longitudinal fasciculus. These ROIs and circuitry have been implicated in networks mediating the processing of others’ intentions and emotions (Adolphs, 2009; Amodio & Frith, 2006; Carrington & Bailey, 2009).
Quantitative DTI fiber tractography analysis of ROI
Operators who analyzed the DTI were not given any information concerning the outcome data. The Philips diffusion affine registration tool (Netsch & van Muiswinkel, 2004) was used to remove shear and eddy current distortion and head motion prior to calculating FA maps with Philips fiber tracking 4.1V3 Beta 4 software. ROIs were drawn manually using the protocols previously published or described below, then the automated Philips three-dimensional fiber tracking tool (Hoogenraad, 2002) was utilized to determine fiber tracks passing through ROIs. Mean fractional anisotropy (FA) and mean diffusivity (MD) of the fiber system, which were automatically generated by the software, was used as the quantitative measure for DTI variables. The algorithm for fiber tracking is based on the Fiber Assignment by Continuous Tracking (FACT) method (Mori, Crain, Chacko, & van Zijl, 1999). For each of the ROIs listed below, we used standard parameters where tracking terminated if the FA in the voxels decreased below 0.2 or if the angle between adjacent voxels along the track was larger than 6.75 degrees. For ROIs such as prefrontal white matter that were not individual tracts, we used fiber tractography to measure the mean FA and mean MD for the fibers coursing through the region.
Protocols
Protocols for the corpus callosum, total prefrontal white matter, mPFWM, uncinate fasciculus, inferior longitudinal fasciculus, and cingulum bundle have been previously published (Levin et al., 2008; Wakana et al., 2007; Wilde et al., 2006, 2009).
Intra-rater and inter-rater agreement
To examine intra-rater agreement, DTI analysis of each region was performed twice for each patient; intra-class correlation coefficients (ICC) exceeded 0.95 for all DTI indices. Inter-rater agreement was also assessed by measurement of each protocol by two different raters in five cases in each group; ICCs again exceeded 0.98.
Statistical Analysis
Two groups (OI vs. TBI) of patients were compared on demographic variables using Fisher’s Exact test or Chi-Square test for categorical variables (gender, race, mechanism of injury) and t test for continuous variables (age at injury, SCI). The response variables for measurement of accuracy and attribution were ordinal and the design involved doubly repeated measures, that is, each participant had three conditions (RM, GD, and ToM) and two levels of cueing (uncued and cued). Therefore, generalized linear mixed models (GLMM) were employed to detect group, condition and cueing differences on accuracy and attribution. GLMM was also used to test relations of DTI data to the variables. For both analyses, demographic variables, such as age at injury, SCI and gender, were controlled if they had a significant effect on the response variables. Multinomial distributions were assumed for accuracy and attribution scores, with the “best” outcome modeled (“accurate-2 points” for accuracy scores and “mentalizing-2 points” for attribution scores). GLMM is similar to general linear mixed models, except that the distributions of the dependent variables are from the exponential family, and are related to the predictor variables through a nonlinear link function. Generalized Linear Mixed models utilizing GLIMMIX procedure in SAS were used for the analysis of the measurement of accuracy and attribution. Age at injury was centered at its grand mean for the purpose of interpretation of the intercept. In this exploratory study, Bonferroni correction was not used for the multiple tests. Figures were plotted from the model derived data.
RESULTS
Demographic Features and Mechanism of Injury
As seen in Table 1, the TBI group was older than the OI patients, but no other between-group differences were present in demographic features or time since injury. Motor vehicle crashes were a more frequent cause of moderate to severe TBI, whereas sports-related injuries were more common in the OI patients. Age, gender, and socioeconomic level were not related to mental state attributions.
Group Differences in Accuracy and Attribution Scores
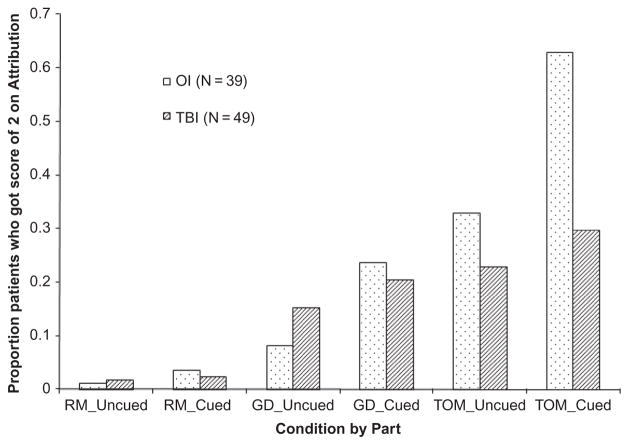
As shown in Table 2, the groups did not significantly differ in the accuracy of their responses (F(1,411) = 1.28, p = .259). However, OI patients had higher mental state attribution scores than TBI patients depending on cueing (F(1,432) = 5.67, p = .018) and condition (F(2,432) = 3.92, p = .021). The interaction of group with condition reflected that OI patients’ mental state attributions were increased in the ToM condition relative to the GD and RM conditions to a greater extent than in the TBI group. Figure 1 shows the effect of condition by group. However, cueing the participant to respond as if the triangles represented human movements effectively increased mental state attributions across conditions in the OI group (t(432) = −4.43), p < .0001), but not in the TBI patients (t(432) = −1.46, p = 0.146) (Figure 1).
TABLE 2.
Group Differences on Raw Means of Accuracy and Attribution Scores
| Cueing Level | Condition |
Accuracy |
Attribution |
|||||
|---|---|---|---|---|---|---|---|---|
| Score | OI (%) | TBI (%) | p-Value | OI (%) | TBI (%) | p-Value | ||
| Uncued | Random | 0 | 79.49 | 71.43 | ||||
| 1 | 2.86 | 0 | 0.432 | 17.95 | 26.53 | 0.722 | ||
| 2 | 97.14 | 100 | 2.56 | 2.04 | ||||
| Goal directed | 0 | 22.5 | 10.2 | |||||
| 1 | 15.79 | 26.67 | 0.231 | 72.5 | 81.63 | 0.272 | ||
| 2 | 84.21 | 73.33 | 5 | 8.16 | ||||
| Theory of mind | 0 | 10 | 12.24 | |||||
| 1 | 40.54 | 36.96 | 0.739 | 50 | 55.1 | 0.764 | ||
| 2 | 59.46 | 63.04 | 40 | 32.65 | ||||
| Cued | Random | 0 | 48.72 | 60.42 | ||||
| 1 | 5.41 | 6.52 | 1 | 38.46 | 25 | 0.399 | ||
| 2 | 94.54 | 93.48 | 12.82 | 14.58 | ||||
| Goal directed | 0 | 12.5 | 10.64 | |||||
| 1 | 24.32 | 21.28 | 0.74 | 62.5 | 68.09 | 0.861 | ||
| 2 | 75.68 | 78.82 | 25 | 21.28 | ||||
| Theory of mind | 0 | 2.56 | 10.42 | |||||
| 1 | 27.03 | 50 | 0.034 | 38.46 | 62.5 | 0.007 | ||
| 2 | 72.97 | 50 | 58.97 | 27.08 | ||||
The p-values were from Chi-square or Fisher’s Exact test; OI = orthopedic injury; TBI = traumatic brain injury.
FIGURE 1.
Percentage of patients with attribution score = 2 (highest score for mental state attribution) plotted by group (orthopedic injury (OI) vs. traumatic brain injury (TBI)), condition (random, goal directed, theory of mind), and cueing (uncued, cued).
Relation of language skills to attribution scores
With childhood TBI resulting in language difficulties (Chapman et al., 1992; Hanten et al., 2009) and the scoring for the Triangles task dependent on language output, we analyzed whether deficient language skills could account for the between-group differences in mental state attribution scores. We calculated Spearman correlations to test the relation of mental attribution scores for each condition with language measures that were also obtained from the participants at 3 months post-injury. Subtest raw scores of the Woodcock Johnson Tests of Achievement–III (Woodcock & Mather, 2001) were available for Letter-Word Identification, a test of feature detection and analysis (for letters) and recognition of visual word forms from a phonological lexicon, as well as access of pronunciations associated with visual word forms. In addition, we analyzed the Expressive Language subscale of the Vineland Adaptive Behavior Scales (Sparrow, Balla, & Cicchetti, 1984), which is a parent interview addressing different aspects of child function. Correlations between these language variables and mental state attributions were all <0.30 and not significant for either group under any of the three conditions of the task with or without cueing.
Relation of working memory to attribution scores
To examine the possibility that working memory limitations contributed to the capacity of children with TBI to process and describe the mental states represented by the movements of triangles, we correlated mental state attribution scores with performance on the Sternberg Item Recognition Task (Sternberg, 1966). With a high rate of response accuracy typically obtained on the Sternberg task, we measured working memory by subtracting reaction time to respond to a memory probe letter under a memory load of only a single letter from a memory load 6 letters (maximum load) and also from a memory load of 4 letters (intermediate memory load). In support of the validity of analyzing reaction times on the Sternberg task, all of the TBI and OI patients had 95% correct or better performance under the memory loads of 1 and 4 letters. With a load of 6 letters, all of the TBI patients had at least 95% correct and 95% of the OI group performed at this level. Shorter residual reaction times reflect more efficient working memory so a negative correlation with the number of mental state attributions would be expected if processing the intentions of others depended on working memory efficiency. Under the Random condition without cueing, mental state attributions were significantly correlated with working memory in the TBI group under memory loads of 6 letters, r = −0.293, p = .049 and marginally under 4 letters, r = −0.280, p = .059. However, correlation coefficients in the OI group were not significant. With cueing in the Random condition, correlations were not significant for either group. Under the Goal Directed condition without cueing, correlations were significant for the memory load of 6 items in the TBI group, r = −0.306, p = .038, but not in the OI group. With cueing in the Goal Directed Condition, a significant positive correlation was obtained for memory load 4 in the TBI group, r = 0.413, p = .005, indicating an association between less efficient working memory and mental state attributions. However, there was no correlation in the OI group. In the TOM condition without cueing, mental state attribution scores in the TBI group were not correlated with working memory whereas the correlation was significant in the OI group for memory load 6 (r = −0.439, p = .046) and marginal for memory load 4 (r = −0.274, p = .087) performance. However, when cueing for human movement was provided, mental attribution scores were not significantly correlated with working memory in either group.
Relation of attribution scores to social functioning and behavioral adjustment
To address whether mental state attributions were related to everyday social functioning, we correlated attribution scores with the Interpersonal Relationships subscale score in the Socialization Domain of the Vineland Adaptive Behavior Scale and with the Social Skills raw score obtained from the parent form of the Behavioral Assessment System for Children (BASC, Reynolds & Kamphaus, 1998) and the Personal Adjustment composite raw score obtained by self-report on the BASC. All of these Spearman correlation coefficients were less than 0.30 and none were significant in either group.
Relation of Severity of TBI to Mental State Attributions
Within the TBI group, there was no significant relation of GCS score to the mental attribution score (F(1,222) = 0.36, p = .550).
Between group differences in DTI findings
Analyses revealed significantly lower FA in the TBI group than the OI patients for all regions examined except for left and right inferior longitudinal fasciculus and right cingulum bundle (Table 3). Significantly higher MD (p < .05) was also present in the TBI group in all ROIs, with the exception of the left and right cingulum bundle (Table 4).
TABLE 3.
Group Differences on FA Using Quantitative FT DTI
| Region |
OI (N = 39) |
TBI (N = 49) |
t | p | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |||
| Genu of CC | 0.473 | 0.023 | .422–.515 | 0.429 | 0.031 | .362–.486 | 6.57 | <.0001 |
| Body of CC | 0.457 | 0.023 | .408–.492 | 0.423 | 0.034 | .326–.475 | 4.78 | <.0001 |
| Right Temporal | 0.428 | 0.026 | .356–.489 | 0.401 | 0.029 | .328–.461 | 4.02 | 0.0002 |
| Left Temporal | 0.424 | 0.023 | .371–.457 | 0.4 | 0.029 | .321–.469 | 3.61 | 0.0006 |
| Right Uncinate | 0.381 | 0.022 | .324–.423 | 0.342 | 0.041 | .234–.406 | 4.61 | <.0001 |
| Left Uncinate | 0.379 | 0.029 | .305–.430 | 0.348 | 0.029 | .289–.400 | 3.92 | 0.0002 |
| Right Frontal | 0.386 | 0.017 | .341–.425 | 0.358 | 0.026 | .284–.406 | 5.15 | <.0001 |
| Left Frontal | 0.386 | 0.018 | .339–.413 | 0.355 | 0.024 | .290–.392 | 5.97 | <.0001 |
| Right mPFWM | 0.385 | 0.026 | .305–.428 | 0.365 | 0.035 | .281–.423 | 2.6 | 0.0116 |
| Left mPFWM | 0.382 | 0.031 | .277–.434 | 0.358 | 0.032 | .267–.414 | 3.1 | 0.0029 |
| Right Dorsolateral Frontal | 0.38 | 0.025 | .303–.430 | 0.357 | 0.024 | .282–.402 | −2.7 | 0.0088 |
| Left Dorsolateral Frontal | 0.382 | 0.024 | .309–.413 | 0.359 | 0.021 | .304–.391 | −2.47 | 0.0161 |
| Right Cingulum Bundle | 0.378 | 0.032 | .310–.457 | 0.366 | 0.035 | .288–.441 | 1.41 | 0.1627 |
| Left Cingulum Bundle | 0.413 | 0.029 | .373–.491 | 0.392 | 0.032 | .330–.469 | 2.8 | 0.0067 |
| Right ILF | 0.406 | 0.028 | .348–.448 | 0.404 | 0.033 | .304–.449 | 0.23 | 0.8179 |
| Left ILF | 0.409 | 0.032 | .354–.471 | 0.403 | 0.029 | .344–.453 | 0.75 | 0.4561 |
FA = fractional anisotropy. FT = fiber tractography. DTI = diffusion tensor imaging. OI = orthopedically injured. TBI = traumatic brain injury. SD = standard deviation. CC = corpus callosum. mPFWM = medial prefrontal white matter. ILF = inferior longitudinal fasciculus.
TABLE 4.
Group Differences on MD Using Quantitative FT DTI
| Region |
OI (N = 39) |
TBI (N = 49) |
t | p | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |||
| Genu of CC | 0.847 | 0.043 | .749–.919 | 0.9 | 0.067 | .732–1.011 | −3.95 | 0.0002 |
| Body of CC | 0.898 | 0.065 | .761–1.058 | 0.951 | 0.111 | .755–1.292 | −2.42 | 0.0188 |
| Right Temporal | 0.857 | 0.055 | .739–.977 | 0.9 | 0.078 | .761–1.146 | −2.5 | 0.0151 |
| Left Temporal | 0.835 | 0.057 | .712–.974 | 0.869 | 0.057 | .752–1.015 | −2.31 | 0.0241 |
| Right Uncinate | 0.807 | 0.044 | .713–.885 | 0.846 | 0.063 | .706–1.005 | −2.79 | 0.007 |
| Left Uncinate | 0.82 | 0.055 | .707–.953 | 0.848 | 0.058 | .720–1.015 | −1.83 | 0.0726 |
| Right Frontal | 0.821 | 0.045 | .701–.899 | 0.855 | 0.056 | .725–.983 | −2.79 | 0.0069 |
| Left Frontal | 0.816 | 0.043 | .722–.902 | 0.849 | 0.049 | .727–.925 | −3 | 0.0038 |
| Right mPFWM | 0.83 | 0.053 | .707–.923 | 0.863 | 0.063 | .716–1.020 | −2.28 | 0.0261 |
| Left mPFWM | 0.826 | 0.053 | .717–.944 | 0.856 | 0.049 | .739–.937 | −2.38 | 0.0201 |
| Right Dorsolateral Frontal | 0.813 | 0.46 | .696–.886 | 0.847 | 0.056 | .731–.990 | −2.7 | 0.0088 |
| Left Dorsolateral Frontal | 0.81 | 0.045 | .722–.890 | 0.838 | 0.045 | .723–.909 | −2.47 | 0.0161 |
| Right Cingulum Bundle | 0.765 | 0.045 | .655–.821 | 0.777 | 0.052 | .657–.894 | −1 | 0.3205 |
| Left Cingulum Bundle | 0.765 | 0.042 | .668–.844 | 0.78 | 0.05 | .656–.867 | −1.3 | 0.1966 |
| Right ILF | 0.823 | 0.052 | .704–.919 | 0.849 | 0.052 | .730–1.010 | −1.98 | 0.0519 |
| Left ILF | 0.813 | 0.051 | .710–.901 | 0.858 | 0.061 | .734–.988 | −3.13 | 0.0027 |
FT = fiber tractography. DTI = diffusion tensor imaging. MD = mean diffusivity. OI = orthopedically injured. TBI = traumatic brain injury. SD = standard deviation. CC = corpus callosum. mPFWM = medial prefrontal white matter. ILF = inferior longitudinal fasciculus.
Relation of DTI findings to mental state attributions
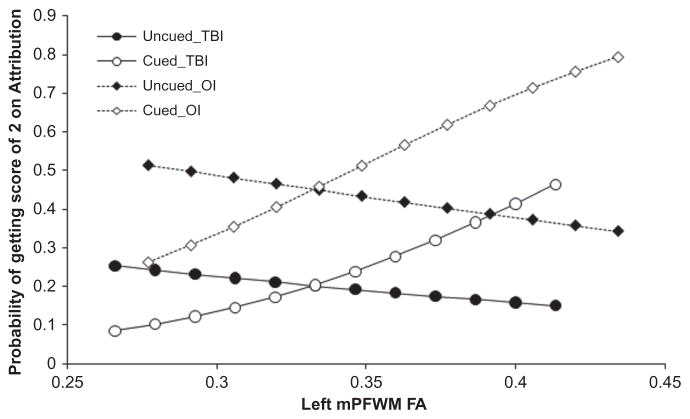
A significant interaction of left mPFWM FA with cueing (F(1,316) = 8.61, p = .004) indicated that when patients were cued, mental attributions increased with FA, t(316) = 2.17, p = .031, but not in the absence of cueing. Figure 2 depicts this cue-dependent effect of left mPFWM FA. Attributions significantly increased with left dorsolateral frontal FA for both groups and all three conditions with and without cueing t(317) = 2.16, p = .031. Left cingulum bundle FA was positively related to mental attributions, t(326) = 2.79, p = .006, and this relation was also consistent in both groups for all conditions with and without cueing.
FIGURE 2.
Probability of mental state attributions by left medial pre-frontal white matter (mPFWM) fractional anisotropy (FA), condition, and cueing plotted for traumatic brain injury (TBI) and orthopedic injury (OI) groups.
Brain Lesions Identified on MRI
The numbers of patients with hemispheric lesions included prefrontal (n = 34), temporal (n = 27), parietal (n = 23), and occipital (n = 6). In addition, 6 participants with TBI had lesions in the amygdala. However, Spearman correlation coefficients between the presence of lesions in each of these sites and the attribution score fell below 0.30 and were not significant for any site.
DISCUSSION
Social functioning is often below age expectation in children and adolescents following moderate to severe TBI (Taylor et al., 2002; Yeates et al., 1997). In contrast to recovery of cognitive functions over one to two years after injury, social relations with peers are often persistently impaired suggesting that TBI compromises development of age-appropriate social skills. It has been hypothesized that deficient social cognitive skills such as processing the intentions (Snodgrass & Knott, 2006) and complex emotions of others such as irony and deception (Dennis et al., 1998, 2001) and finding solutions to interpersonal dilemmas (Janusz, Kirkwood, Yeates, & Taylor, 2002; Yeates et al., 2007) contribute to a decline in social functioning and adversely impact social adjustment following moderate to severe TBI.
In the present study we exploited the finding that observing the movement of interacting geometric shapes in a silent cartoon can evoke mental state attributions (Heider & Simmel, 1944). Using these animated, nonverbal sequences, Abell et al. (2000) showed that children with autism exhibited difficulty inferring the intentions and emotions that are reported by healthy participants. Support for the construct validity of this task in the present study is provided by the higher attribution scores in the ToM and GD conditions relative to the RM condition, a finding that replicates previous reports in other populations (Castelli et al., 2000). Although we found that groups of TBI and OI patients did not significantly differ in accuracy of interpreting the movements of triangles, children and adolescents with moderate to severe TBI were less likely to respond with mental state attributions in the cued condition.
We acknowledge limitations of this study, chiefly the cross-sectional design and three month follow-up interval. Movements by triangles were also less complex than the multimodality stimuli inherent in typical social interactions. It is also conceivable that language deficits might have contributed to the between-group difference in mental attribution scores. However, we found that the correlations between language test variables and mental attribution scores were consistently <0.30 and were not significant. Moreover, accuracy in describing the movements of the triangles was also dependent on expressive language and did not differ between the TBI and OI groups, supporting our interpretation that reduced mental state attributions by children with TBI was not secondary to a language deficit. Although limitations of working memory could have contributed to the between group difference in attribution scores under the cued condition, the pattern of correlations with working memory as measured by the Sternberg Item Recognition task did not support this possibility. Correlations of working memory with attribution scores were not significant with cueing under the ToM condition in either group and within the TBI group, these correlations were as likely to be significant in non-cued as with cued in the Random and Goal Directed conditions.
Our results extend previous studies (Dennis et al., 1998, 2001; Snodgrass & Knott, 2006; Walz et al., 2009) of social cognition in children following TBI by demonstrating altered processing of mental states relative to children who sustained traumatic injury to their limbs without brain insult. Moreover, cueing participants to respond as if the observed movements were human had less of an impact on performance by children and adolescents with moderate to severe TBI than the OI comparison group. In contrast, response to cueing was more robust by the TBI group under the GD condition than when the triangles reacted to one another’s intentions in the ToM condition. This dissociation suggests that serious TBI may result in difficulty understanding and/or verbalizing visual representation of complex interpersonal interactions by others.
Consistent with previous studies (Ewing-Cobbs et al., 2008; Levin et al., 2008; Wilde et al., 2006) using DTI to evaluate the microstructure of white matter in children with TBI, quantitative tractography analysis of the DTI data acquired at approximately 3 months after moderate to severe TBI disclosed that the integrity of white matter was disrupted in nearly all ROIs including areas implicated in social cognitive networks. In the left mPFWM we found that the relation of FA to mental state attributions differed depending on cueing. Attributions increased with left mPFWM FA when the children were cued to respond as if the movements were human, but this relation was not seen in the uncued condition. Although mental attributions also increased with left dorsolateral frontal and left cingulum bundle FA, these relations did not depend on cueing.
Anterior rostral mPFC, including BA 10 and the subregion inferior and rostral to anterior cingulate cortex, has been selectively activated by various ToM tasks differing in modality in fMRI and PET studies of mental state attribution (Amodio & Frith, 2006; Carrington & Bailey, 2009; Castelli et al., 2000). Anterior rostral mPFC has also been identified in self-monitoring of mental states, suggesting at least partial overlap in the neural substrate for processing one’s own and others’ mental states (Amodio & Frith, 2006; Castelli et al., 2000; Lombardo et al., 2009). Review of functional brain imaging studies of ToM disclosed that mPFC and orbitofrontal cortex were the most frequently activated regions across studies (Carrington & Bailey, 2009). Although we found between group differences in both FA and MD in prefrontal and temporal regions implicated in ToM and in white matter tracts, the relation to mental state attribution did not depend on group. Based on our DTI findings, we suggest that axonal injury resulted in disconnection of mPFC and orbitofrontal cortex from other prefrontal subregions, posterior cortical regions and amygdala that together constitute a social cognitive network. In contrast, we did not find any relation of focal lesions identified on MRI to mental attribution scores. Severity of impaired consciousness, as measured by the post-resuscitation GCS score, was also not related to mental attribution scores within this sample of children with moderate to severe TBI. We interpret the dissociation of DTI findings from the GCS scores as implicating the relevance of white matter connections within social cognitive networks to mental state attributions. In contrast, severity of impaired consciousness is determined by various pathophysiological variables, including secondary brain insult and mass lesions requiring surgery in addition to diffuse and focal white matter injury.
Using fMRI and the same animated sequence of moving triangles that we employed, Castelli et al. (2000) reported that relative to the RM condition, the ToM condition activated mPFC with anterior cingulate cortex and other regions implicated in social cognition including the basal temporal area adjacent to amygdala, and lateral occipital gyrus. Integrating fMRI with DTI could identify alteration in the pattern of mental attribution-related brain activation after TBI in children and elucidate its relation to integrity of white matter connections. Future studies could also address in greater depth the relation of mental state attributions to psychosocial adjustment, especially peer relations.
Contributor Information
Harvey S. Levin, Department of Physical Medicine and Rehabilitation, Baylor College of Medicine, Houston, Texas
Elisabeth A. Wilde, Departments of Physical Medicine and Rehabilitation, Neurology, and Radiology, Baylor College of Medicine, Houston, Texas
Gerri Hanten, Department of Physical Medicine and Rehabilitation, Baylor College of Medicine, Houston, Texas.
Xiaoqi Li, Department of Physical Medicine and Rehabilitation, Baylor College of Medicine, Houston, Texas.
Zili David Chu, Department of Radiology, Baylor College of Medicine, Houston, Texas, and Department of Pediatric Radiology, Texas Children’s Hospital, Houston, Texas.
Ana C. Vásquez, Department of Physical Medicine and Rehabilitation, Baylor College of Medicine, Houston, Texas
Lori Cook, Center for BrainHealth, University of Texas at Dallas, Dallas, Texas.
Ragini Yallampalli, Department of Physical Medicine and Rehabilitation, Baylor College of Medicine, Houston, Texas.
Jill V. Hunter, Department of Radiology, Baylor College of Medicine, Houston, Texas, and Department of Pediatric Radiology, Texas Children’s Hospital, Houston, Texas
References
- Abell F, Happe F, Frith U. Do triangles play tricks? Attribution of mental states to animated shapes in normal and abnormal development. Cognitive Development. 2000;15:1–16. [Google Scholar]
- Adolphs R. The social brain: neural basis of social knowledge. Annual Review of Psychology. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Carrington SJ, Bailey AJ. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Human Brain Mapping. 2009;30(8):2313–2335. doi: 10.1002/hbm.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Happe F, Frith U, Frith C. Movement and mind: A functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12(3):314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Chapman SB, Culhane KA, Levin HS, Harward H, Mendelsohn D, Ewing-Cobbs L, et al. Narrative discourse after closed head injury in children and adolescents. Brain and Language. 1992;43(1):42–65. doi: 10.1016/0093-934x(92)90020-f. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Spronk M, Willems RM, Toni I, Bekkering H. Complementary systems for understanding action intentions. Current Biology. 2008;18(6):454–457. doi: 10.1016/j.cub.2008.02.057. [DOI] [PubMed] [Google Scholar]
- Dennis M, Barnes MA, Wilkinson M, Humphreys R. How children with head injury represent real and deceptive emotion in short narratives. Brain and Language. 1998;61:450–483. doi: 10.1006/brln.1997.1886. [DOI] [PubMed] [Google Scholar]
- Dennis M, Purvis K, Barnes MA, Wilkinson M, Winner E. Understanding of literal truth, ironic criticism, and deceptive praise following childhood head injury. Brain and Language. 2001;78(1):1–16. doi: 10.1006/brln.2000.2431. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Swank P, Kramer L, Cox CS, Jr, Fletcher JM, et al. Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. Neuroimage. 2008;42(4):1305–1315. doi: 10.1016/j.neuroimage.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society B: Biological Sciences. 2003;358(1431):459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV. Two takes on the social brain: a comparison of theory of mind tasks. Journal of Cognitive Neuroscience. 2007;19(11):1803–1814. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- Hanten G, Li X, Newsome MR, Swank P, Chapman SB, Dennis M, et al. Oral reading and expressive language after childhood traumatic brain injury: Trajectory and correlates of change over time. Topics in Language Disorders. 2009;29(3):236–248. [Google Scholar]
- Heider F, Simmel M. An experimental study of apparent behavior. American Journal of Psychology. 1944;57:243–259. [Google Scholar]
- Hoogenraad F. Multi-center evaluation of in-vivo fibertracking. Best, The Netherlands: Philips Medical Systems; 2002. [Google Scholar]
- Janusz JA, Kirkwood MW, Yeates KO, Taylor HG. Social problem-solving skills in children with traumatic brain injury: Long-term outcomes and prediction of social competence. Child Neuropsychology. 2002;8(3):179–194. doi: 10.1076/chin.8.3.179.13499. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Chang CC, Zhang L, Schachar R, Ewing-Cobbs L, et al. Working memory after traumatic brain injury in children. Annals of Neurology. 2002;52(1):82–88. doi: 10.1002/ana.10252. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Zhang L, Swank PR, Ewing-Cobbs L, Dennis M, et al. Changes in working memory after traumatic brain injury in children. Neuropsychology. 2004;18(2):240–247. doi: 10.1037/0894-4105.18.2.240. [DOI] [PubMed] [Google Scholar]
- Levin HS, Wilde EA, Chu Z, Yallampalli R, Hanten GR, Li X, et al. Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. Journal of Head Trauma Rehabilitation. 2008;23(4):197–208. doi: 10.1097/01.HTR.0000327252.54128.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek SA, Suckling J, et al. Shared neural circuits for mentalizing about the self and others. Journal of Cognitive Neuroscience. 2009;22(7):1623–1635. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Netsch T, van Muiswinkel A. Quantitative evaluation of image-based distortion correction in diffusion tensor imaging. IEEE Trans Med Imaging. 2004;23(7):789–798. doi: 10.1109/TMI.2004.827479. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Carter EJ. Brain mechanisms for social perception: lessons from autism and typical development. Annals of the New York Academy of Sciences. 2008;1145:283–299. doi: 10.1196/annals.1416.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus EW. Manual. Circle Pines, MN: American Guidance Service, Inc; 1998. Behavior Assessment System for Children. [Google Scholar]
- Snodgrass C, Knott F. Theory of mind in children with traumatic brain injury. Brain Injury. 2006;20(8):825–833. doi: 10.1080/02699050600832585. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti D. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153(736):652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short-and long-term outcomes after traumatic brain injury in children: behavior and achievement. Neuropsychology. 2002;16(1):15–27. doi: 10.1037//0894-4105.16.1.15. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Turkstra LS, McDonald S, DePompei R. Social information processing in adolescents: data from normally developing adolescents and preliminary data from their peers with traumatic brain injury. Journal of Head Trauma Rehabilitation. 2001;16(5):469–483. doi: 10.1097/00001199-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Turkstra LS, Williams WH, Tonks J, Frampton I. Measuring social cognition in adolescents: implications for students with TBI returning to school. NeuroRehabilitation. 2008;23(6):501–509. [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz N, Yeates KO, Taylor HG, Stancin T, Wade SL. First-Order Theory of Mind skills shortly after traumatic brain injury in 3- to 5-year-old children. Developmental Neuropsychology. 2009;34(4):507–519. doi: 10.1080/87565640902964490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, Chu Z, Bigler ED, Hunter JV, Fearing MA, Hanten G, et al. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. Journal of Neurotrauma. 2006;23(10):1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- Wilde EA, McCauley SR, Chu Z, Hunter JV, Bigler ED, Yallampalli R, et al. Diffusion tensor imaging of hemispheric asymmetries in the developing brain. Journal of Clinical and Experimental Neuropsychology. 2009;31(2):205–218. doi: 10.1080/13803390802098118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, Mather N. Woodcock-Johnson Tests of Achievement. Itasca, IL: Riverside; 2001. [Google Scholar]
- Yeates KO, Bigler ED, Dennis M, Gerhardt CA, Rubin KH, Stancin T, et al. Social outcomes in childhood brain disorder: a heuristic integration of social neuroscience and developmental psychology. Psychological Bulletin. 2007;133(3):535–556. doi: 10.1037/0033-2909.133.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Drotar D, Wade SL, Klein S, Stancin T, et al. Preinjury family environment as a determinant of recovery from traumatic brain injuries in school-age children. Journal of the International Neuropsychological Society. 1997;3(6):617–630. [PubMed] [Google Scholar]