Abstract
A unique synergistic effect on platinum drug cytotoxicity is noted in the presence of the tricyclic anti-depressant desipramine. Desipramine is used for treating neuropathic pain, particularly in prostate cancer patients. The clinically used drugs cisplatin (cis-[PtCl2(NH3)2]), oxaliplatin [1,2-diaminocyclohexaneoxalatoplatinum(II)], and the cationic trinuclear agent BBR3464 [{trans-PtCl(NH3)2}2-μ-(trans-Pt(NH3)2(H2N(CH2)6NH2)2)]4+, which has undergone evaluation in phase II clinical trials for activity in lung and ovarian cancers, were evaluated. Surprisingly, desipramine greatly augments the cytotoxicity of all the platinum-based chemotherapeutics in HCT116 colorectal carcinoma cell lines. Desipramine enhanced cellular accumulation of cisplatin, but had no effect on the accumulation of oxaliplatin or BBR3464, suggesting that enhanced accumulation could not be a consistent means by which desipramine altered the platinum-drug-mediated cytotoxicity. The desipramine/cisplatin combination resulted in increased levels of p53 as well as mitochondrial damage, caspase activation, and poly(ADP ribose) polymerase cleavage, suggesting that desipramine may synergize with cisplatin more than with other platinum chemotherapeutics partly by activating distinct apoptotic pathways. The study argues that desipramine may be a means of enhancing chemoresponsiveness of platinum drugs and the results warrant further investigation. The results emphasize the importance of understanding the differential pharmacological action of adjuvants employed in combinations with cancer chemotherapeutics.
Keywords: Anticancer drug, Albumin, DNA damage, Toxicity
Introduction
Cancer chemotherapy usually involves treatment with drug combination regimens. Platinum-based drugs play an important part as components of these regimens. Cisplatin (c-DDP) is curative in testicular cancer, whereas oxaliplatin (Eloxatin) is recommended with 5-fluorouracil (FOL-FOX) for treatment of metastatic colon cancer. The pharmacological action of any drug must be monitored in light of proposed combinations. The pharmacological parameters affecting platinum drug cytotoxicity and anti-tumor activity are generally accepted to be (1) the nature and extent of target (DNA) binding, (2) the extent of platinum drug cellular accumulation, and (3) metabolizing (destabilizing) interactions with sulfur-containing biomolecules such as human serum albumin (HSA) and glutathione. Reduced cellular accumulation is consistently being seen as a major cause of development of clinical resistance [1, 2]. Cellular accumulation can be affected by drug combinations and this point may be an important feature in designing potential combination regimens for new, emerging agents. In preclinical studies, the Raf kinase inhibitor BAY43-9006 reduces cellular accumulation of c-DDP and oxaliplatin [3], whereas, in contrast, the 20S proteasome inhibitor bortezomib enhances c-DDP accumulation by blocking the c-DDP-induced downregulation of the hCTR1 transporter in a concentration-dependent manner [4]. Interestingly, the concomitant administration of imatinib with c-DDP prevents c-DDP-induced nephrotoxicity by inhibiting c-DDP renal accumulation [5].
Adjuvant therapy with antidepressants is also common standard of care for cancer patients [6]. People with cancer are 3 times more likely than the general population and almost 2 times more likely than other hospitalized medical patients to develop major depression [7]. Untreated, this can lead to decreased compliance with medical care as well as increased psychological toll on the patient and the patient’s family. Patients will also most likely be treated with combination chemotherapy with a variety of anticancer drugs. There is therefore an important need to examine how the combination of an adjuvant drug interacts with the chemotherapeutic agent—the combination may be additive or antagonistic, where the drug interference can result in decreased efficacy of the treatment. Recent discussion on the negative effect of antidepressants on tamoxifen therapy in breast cancer patients highlights the necessity to understand the pharmacological action of drug combinations [8, 9]. The differential effects noted above on cellular platinum accumulation, and hence efficacy, could also extend to adjuvant therapies.
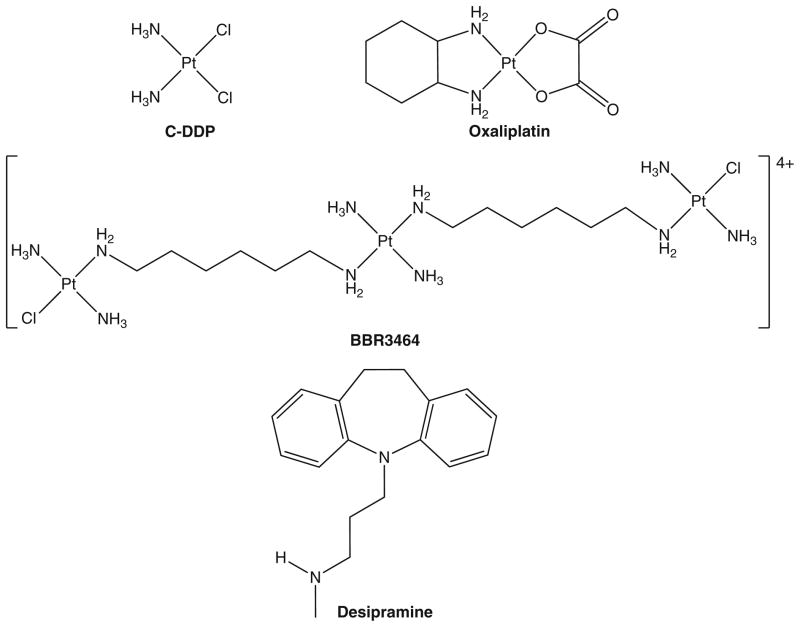
The choice of a specific antidepressant depends on a number of factors, including the nature of the depressive symptoms, medical problems present, and side effects of the specific drug. Low doses of tricyclic antidepressants can be especially useful as adjuvant pain medications in patients with neuropathic pain syndromes. Desipramine (Fig. 1) is one such tricyclic antidepressant and is important for treating neuropathic pain from cancer chemotherapy [10, 11]. It is also relevant as an inhibitor of organic cation transport accumulation pathways, a possible mechanism for cellular accumulation of c-DDP [1, 12]. As such, it was anticipated that desipramine might antagonize the effects of c-DDP and oxaliplatin (Fig. 1), the side effects of which are nephrotoxicity and neuropathy, respectively [13]. The tumor cell line selected for study was human colon HCT116. We also examined the preclinical drug BBR3464 ([{trans-PtCl(NH3)2}2-μ-(trans-Pt(NH3)2(H2N(CH2)6NH2)2)]4+) because a survey of cytotoxicity across the National Cancer Institute tumor panel showed enhanced sensitivity of colon cancers to this drug [14]. This article reports on the role of desipramine in modulating the biological effects of platinum drugs and the unexpected synergistic effect on cytotoxicity in the presence of the antidepressant.
Fig. 1.
Structures of platinum compounds studied for cellular effects in the presence of desipramine
Materials and methods
Compound synthesis
BBR3464 and c-DDP were synthesized as previously described [15]. Oxaliplatin and desipramine were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture conditions
The HCT116 colorectal carcinoma cell lines were a kind gift from Bert Vogelstein (Johns Hopkins University, Baltimore, MD, USA). HCT116 cells were cultured in RPMI 1640 with 10% fetal bovine serum, 2 mmol/L L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 mmol/L sodium pyruvate (all from Biofluids, Rockville, MD, USA) in humidified air with 5% CO2. For the assays, cells were cultured in six-well plates at an initial density of 7.0 × 104 cells per milliliter. Different concentrations of drugs were added to each well as indicated. Total cells (adherent and nonadherent cells) were collected. BBR3464, c-DDP, and oxaliplatin concentrations were adjusted to achieve approximately 30–40% apoptosis after 72 h of treatment, allowing measurement of enhancement or inhibition in the presence of the antidepressant.
Propidium iodide DNA staining and analysis of apoptosis
Samples were fixed in a solution containing 35% 1× PBS (vol/vol), 12.48% fetal bovine serum (vol/vol), and 52.52% 70% ethanol (vol/vol). The samples were then washed with phosphate buffered saline (PBS), and stained with a solution containing 94% 1× PBS (vol/vol), 0.1 mg/mL RNase A, 0.0001 M EDTA, and 0.04 mg/mL propidium iodide (PI). The samples were stained for two hours. Samples were then analyzed for subdiploid DNA content using a FACScan flow cytometer (BD Biosciences, San Jose, CA, USA). It is noteworthy that this protocol differs significantly from the more common PI-based exclusion, which only differentiates live versus dead cells. Through fixation and RNase A treatment, we were able to detect intact versus fragmented DNA, revealing discrete stages of the cell cycle and the percentage of the population undergoing apoptosis.
Assessment of mitochondrial membrane potential
Alteration in mitochondrial membrane potential (Δψm) was assessed by staining with 3,3′-dihexyloxacarbocyanine iodide (Molecular Probes, Eugene, OR, USA]. 3,3′-Dihexyloxacarbocyanine iodide was added to 200 μL of cells at 40 nmol/L final concentration. Cultures were incubated for 30 min at 37 °C in a CO2 incubator. The cells were then washed twice with PBS and resuspended in 200 μL PBS for flow-cytometric analysis using a forward and side scatter gate sufficiently open to include apoptotic/dying cells.
Assessment of caspase activation
Staining for active caspases was performed with caspase kits (Immunochemistry Technologies, Bloomington, MN, USA), as specified by the manufacturer. Cells were incubated with a cleavable substrate that binds to the active caspases 3 and 7. Substrate cleavage results in increased fluorescence intensity, interpreted as caspase-positive cells. The percentage of caspase-positive cells was measured by flow cytometry.
Platinum accumulation assays
Cells were plated at 2.0 × 106 cells per milliliter. Platinum drug was added in different concentrations alone or 60 min after the addition of desipramine. After 8 or 16 h, cells were harvested and washed twice with PBS. The cell pellets were then dissolved in hot nitric acid, followed by the addition of hydrogen peroxide and hydrochloric acid, according to US Environmental Protection Agency procedure 3050b (all volumes reduced by one tenth), and diluted with Milli-Q water (Millipore, Billerica, MA, USA). Platinum analysis was performed with a Vista-MPX simultaneous inductively coupled plasma optical emission spectrometer (Varian, Palo Alto, CA, USA) at 265 nm. Standards and the blank were prepared in the same way as the samples.
Platinum drug binding to human serum albumin
A 0.33 mol/L solution of HSA and 1 mmol/L solutions of c-DDP, oxaliplatin, BBR3464, and desipramine were made up in PBS. The solutions were mixed in a stoichiometric 3:3:1 desipramine/platinum drug/HSA ratio. The controls were the cDDP, oxaliplatin, and BBR3464 bound to HSA in the absence of desipramine. Desipramine was added to the HSA solution and the mixture was incubated for 1 h at 37°C. The platinum drugs were then added and 150-μL aliquots were taken at 0 min, 30 min, 2 h, 6 h, 24 h, and 48 h. The samples were pipetted into filter tubes and centrifuged for 10 min at 14,000 rpm. The liquid that came through the filter was the free drug portion. The filter was then flipped over, placed in another tube, and centrifuged at 1,000 rpm for 5 min to give the portion with drug-bound protein. After the samples had been centrifuged, all portions were digested with acid according to US Environmental Protection Agency procedure 3050b (all volumes reduced by one tenth) as described previously [15] and were analyzed by inductively coupled plasma optical emission spectroscopy.
Measurement of platinum accumulation in DNA
Cells were plated at 2.0 × 106 cells per milliliter. Platinum drug was added in 160 μmol/L concentrations alone or 60 min after the addition of desipramine. After 36 h cells, were harvested and washed twice with PBS. The DNA was extracted using a DNeasy blood and tissue kit (QIAGEN, Valencia, CA, USA) according to the manufacturer’s instructions. The concentration and the purity of the extracted DNA were calculated using a Nanodrop spectrophotometer and the 260 nm/280 nm absorbance ratio. The samples were then digested with acid according to US Environmental Protection Agency procedure 3050b (all volumes reduced by one tenth) and were analyzed using an 820-MS axial simultaneous inductively coupled mass spectrometer (Varian, Palo Alto, CA, USA).
Western blotting
Whole-cell lysates were blotted with a mouse monoclonal antibody against human p53 (BD Bioscience), monoclonal antibody against poly(ADP ribose) polymerase (PARP) (Trevigen, Gaithersburg, MD, USA), and actin (Sigma), and were subsequently resolved with secondary antibody conjugated with horseradish peroxidase. Blots were treated with ECL Western blotting detection reagents from GE Healthcare, Amersham, Buckinghamshire, England. Band intensity was measured by densitometry with an Eagle Eye II system (Stratagene, La Jolla, CA, USA).
Colony formation assay
Cells were cultured (250–1,500 cells per well in a six-well plate) and 12 h after plating, cells were treated with drugs for 48 h. Afterward, the drug-containing medium was carefully removed, the cells were washed once, and fresh medium lacking drugs was added. Cells for colony formation assays were cultured for an additional 10–14 days, after which the medium was removed and cells were fixed with methanol, stained with crystal violet, and counted manually.
Statistical analysis
Statistical analysis was performed using the t test for two data points using SysStat9 (SPSS, Chicago, IL, USA). p < 0.05 was considered to be significant. The combination index (CI) was calculated with Calcusyn from Biosoft (Cambridge, UK) for synergy. The proportion of dead cells was entered into the program, and the degree of synergism was determined from the CI value according to the Chou and Talaly [16] algorithms. A CI value of less than 1 shows synergism, whereas a CI value greater than 1 shows antagonism. When the CI value remains close to 1, additivity is indicated. The results given are the mean and the standard error.
Results
Effect of desipramine on platinum-drug-induced cytotoxicity: synergistic effects of desipramine
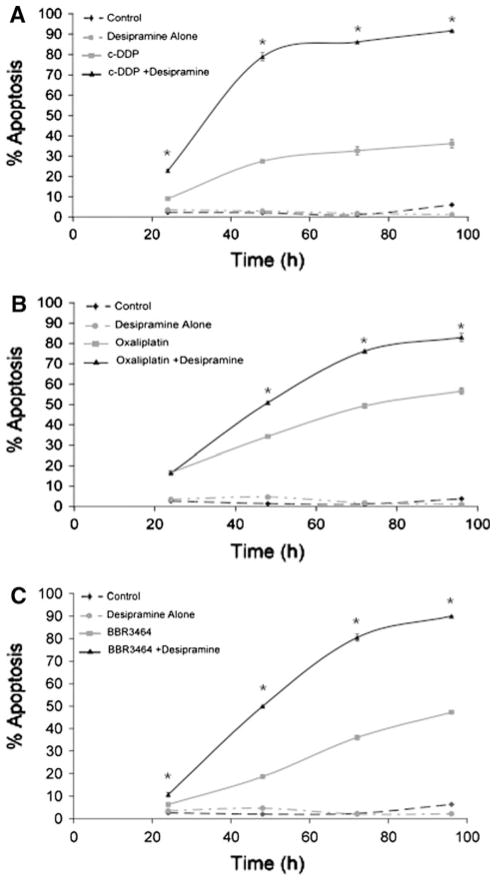
Desipramine surprisingly augmented the cytotoxicity of all three platinum drugs studied (Fig. 2). The enhancement of apoptosis in the presence of desipramine is time- and concentration-dependent. The highest percentage of apoptosis was observed with desipramine at a concentration of 20 μmol/L in combination with BBR3464 or c-DDP, but for oxaliplatin, the percentage of apoptosis peaked with 40 μmol/L desipramine (Fig. S1). At this latter concentration, desipramine alone has very little effect on HCT116 survival, and this concentration was therefore used for time-dependence studies. To determine the concentration dependence, cells were treated with platinum drugs and different concentrations of desipramine for 72 h, and the percentage of apoptosis was measured by PI DNA staining. The concentrations of the platinum drugs were chosen to give approximately 30–40% apoptosis as single agents after 72 h. In the case of c-DDP, the enhancement reached a maximum at 48 h after treatment, where desipramine increased the c-DDP-induced apoptosis from 27% to approximately 80% (Fig. 2a). For oxaliplatin, a quantitatively smaller but significant increase in apoptosis from 49 to 76% was observed after 72 h of treatment in the presence of the antidepressant (Fig. 2b). The BBR3464/desipramine combination at 72 h also showed a significant increase to approximately 80% in comparison with that induced by either desipramine or BBR3464 alone.
Fig. 2.
Time-course dependence of the effect of desipramine on platinum-drug-induced apoptosis in HCT116 colorectal carcinoma cells. Subdiploid cell content was detected by propidium iodide DNA staining. HCT116 cells were cultured with 10 μmol/L c-DDP, 30 μmol/L oxaliplatin, or 50 μmol/L BBR3464, respectively, for the indicated times in the absence and presence of 40 μmol/L desipramine. Platinum drug concentrations were adjusted to achieve approximately 20–30% apoptosis after 48 h, allowing measurement of enhancement or inhibition. Platinum drugs were added to the medium after 1 h of treatment with desipramine. Each point represents the average (± standard error of the mean, SEM) of three independent experiments. Asterisks p < 0.05 when comparing cells treated with and without desipramine, by Student’s t test. All points after 48 h have p < 0.05 for platinum drug with desipramine versus platinum drug alone
The combined apoptosis data were subjected to statistical analysis (using Calcusyn, see “Materials and methods”), and the CI was calculated for evidence of synergy (Table 1). CI < 1 is indicative of synergy and below 0.3 there is strong synergy. When CI > 1, antagonism is indicated and a CI of approximately 1 is considered indicative of an additive rather than a synergistic response to the combination. The data showed strong synergy with desipramine for all platinum compounds, with the strongest synergy being observed for BBR3464. Again, note that all platinum drug concentrations were first adjusted to achieve approximately 30–40% apoptosis after 72 h of treatment, allowing measurement of enhancement or inhibition. Significantly low CI values were obtained independent of the concentration of either agent used. The CI increased somewhat with c-DDP concentration but always within the mathematical index of synergy. Interestingly, at constant c-DDP and BBR3464 concentrations, the CI value was dependent upon the desipramine concentration. We noted synergy with desipramine concentrations of 5 and 20 μmol/L, plateauing thereafter for both platinum drugs, but a stronger synergy with BBR3464 (Table 1). In contrast, the behavior of oxaliplatin was somewhat different. The lower concentrations of desipramine (5–20 μmol/L) yielded additive rather than synergistic effects, but above 40 μmol/L (the concentration which gave an optimal increase in apoptosis, Fig. S1), the CI dropped dramatically and was essentially independent of oxaliplatin concentration.
Table 1.
Analysis of combinational therapy of platinum drugs and desipramine in vitro
| PI DNA staining (72 h) HCT116 WT |
BBR3464a (μmol/L) | Des (μmol/L) | Apop (%) | CI | c-DDP (μmol/L) | Des (μmol/L) | Apop (%) | CI | Ox (μmol/L) | Des (μmol/L) | Apop (%) | CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 15 | 40 | 58 | 0.031 | 5 | 40 | 83 | 0.174 | 15 | 40 | 70 | 0.13 |
| 25 | 40 | 62 | 0.034 | 10 | 40 | 86 | 0.303 | 30 | 40 | 79 | 0.103 | |
| 50 | 40 | 81 | 0.008 | 15 | 40 | 88 | 0.422 | 45 | 40 | 87 | 0.051 | |
| B | 50 | 5 | 41 | 0.46 | 10 | 5 | 50 | 0.922 | 30 | 5 | 52 | 1.035 |
| 50 | 10 | 48 | 0.233 | 10 | 10 | 67 | 0.602 | 30 | 10 | 54 | 0.917 | |
| 50 | 20 | 87 | 0.003 | 10 | 20 | 84 | 0.343 | 30 | 20 | 48 | 1.508 | |
| 50 | 40 | 81 | 0.008 | 10 | 40 | 86 | 0.303 | 30 | 40 | 79 | 0.103 | |
| 50 | 50 | 87 | 0.003 | 10 | 50 | 86 | 0.303 | 30 | 50 | 86 | 0.046 |
Cytotoxicity was determined after 72 h of exposure to platinum drugs using propidium iodide DNA staining assay as described in “Materials and methods.” The combination index was calculated by the Calcusyn program, and a value less than 1 indicated synergy. The table shows data from three experiments per data point
PI propidium iodide, WT wild type, A constant desipramine concentration was used with different platinum drug concentrations; B constant platinum drug concentration was used with different desipramine concentrations, Des desipramine, Apop apoptosis, CI combination index, c-DDP cisplatin, Ox oxaliplatin
As NO3 salt
Mechanistic studies on the effect of desipramine on platinum drug cellular accumulation and DNA binding
To examine the possible mechanism(s) of the desipramine effect, several assays were employed, including measurement of HSA interactions, total cellular platinum accumulation, and platinum–DNA binding, as well as the effects of the platinum drug/antidepressant combination on downstream signaling pathways.
Interactions with human serum albumin
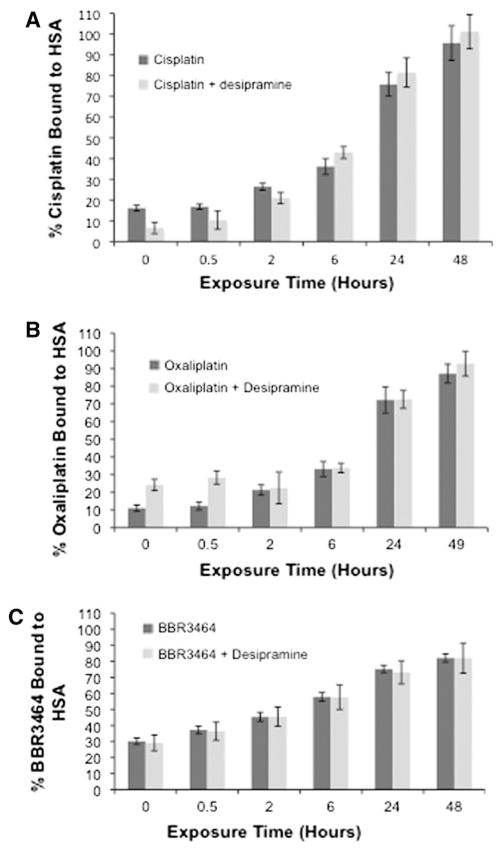
Serotonin-specific reuptake inhibitors, which have become first-line therapy for treatment of depression in cancer patients, are strongly protein bound, and therefore consideration must be given to their interaction with anticancer agents [17]. Binding to HSA may also affect the cytotoxicity and cellular accumulation of platinum drugs as well as their structural integrity [18–20]. In a cell-free assay, the presence of equimolar concentrations of desipramine did not affect the binding of any of the platinum drugs to HSA (Fig. 3). There are slight differences between c-DDP and oxaliplatin at early time points, but overall the antidepressant drug does not affect binding of platinum drugs. Simultaneous binding of both types of drug to albumin may well still occur—the binding sites may simply be different.
Fig. 3.
c-DDP, oxaliplatin, and BBR3464 binding (with standard deviations) to human serum albumin (HSA) in the presence and absence of desipramine. The platinum drugs and desipramine are in a 1:1 molar ratio. The drugs and HSA are in a 1:3 molar ratio. The BBR3464 concentration is divided by 3 to reflect the trinuclear nature of the drug
Cellular platinum accumulation
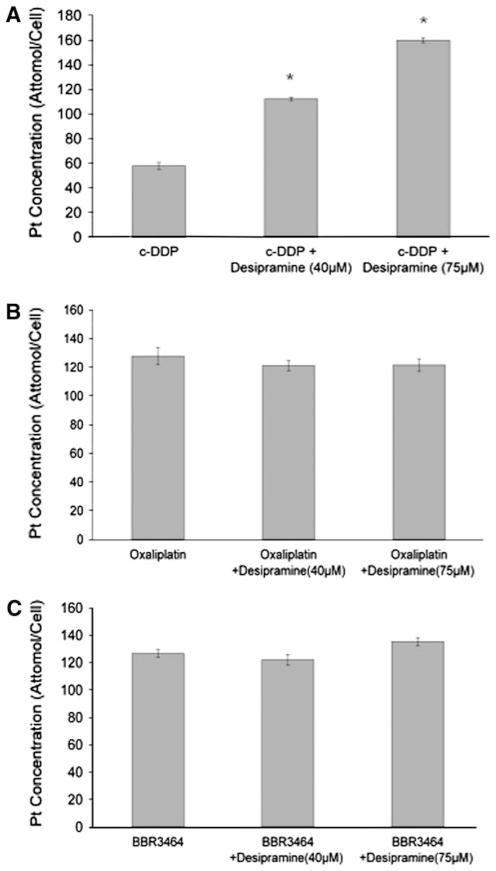
Platinum drug cellular accumulation in HCT-116 colon cancer cells was measured in cells treated with platinum drug with and without desipramine (Fig. 4). Desipramine surprisingly enhanced the cellular accumulation of c-DDP more than twofold, but in contrast, neither oxaliplatin nor BBR3464 showed any difference in cellular accumulation in the presence of the tricyclic antidepressant (4). The somewhat higher accumulation of oxaliplatin relative to c-DDP in human colon cancer cells at equimolar concentrations was confirmed [21]. Because of the more rapid accumulation of BBR3464 at early time points, accumulation for this agent was measured after 8 h, whereas a 16-h time point was used for the mononuclear drugs. The overall results did confirm the higher cellular accumulation of the 4+ charged compound relative to the neutral c-DDP and oxaliplatin at early time points [15, 22].
Fig. 4.
The effect of desipramine on platinum compound cellular accumulation. HCT116 cells were treated with equimolar concentrations (20 μmol/L) of c-DDP and oxaliplatin for 16 h and of BBR3464 for 8 h, with the indicated concentrations of desipramine. Cellular platinum content was measured by inductively coupled plasma optical emission spectroscopy as described in “Materials and methods.” Each point represents the average (±SEM) of three independent experiments. Asterisks p < 0.05 as compared with drug alone as determined by the t test
Cellular Pt–DNA binding
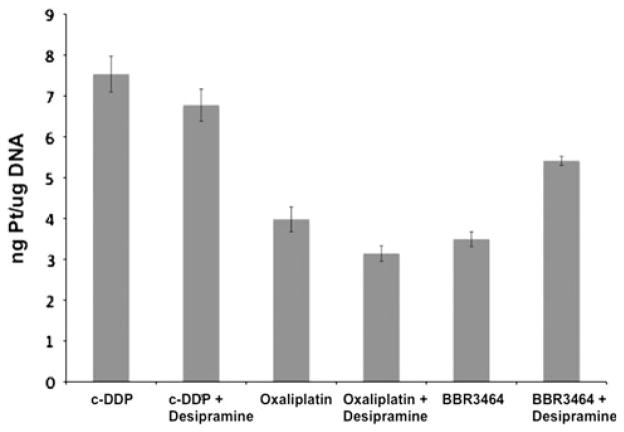
Another important parameter for platinum cytotoxicity is the extent of DNA binding. No enhancement of Pt–DNA binding was found for either c-DDP or oxaliplatin (Fig. 5). Some increase was found for BBR34364 but, by itself, is unlikely to explain the enhancement of cytotoxicity by the antidepressant drug. Since cellular accumulation in the presence of desipramine is only enhanced in the case of c-DDP (Fig. 4a), there appears to be no direct correlation between enhanced accumulation and the levels of Pt–DNA adducts.
Fig. 5.
Nanograms of platinum per microgram of DNA (with standard deviations) after 36 h exposure to the platinum drugs and 40 μmol/L desipramine. The concentration of the platinum drugs was chosen so that the platinum drug to cell ratio was 4 × 10−13 mol/cell
Cellular and signaling effects of desipramine/platinum drug combinations
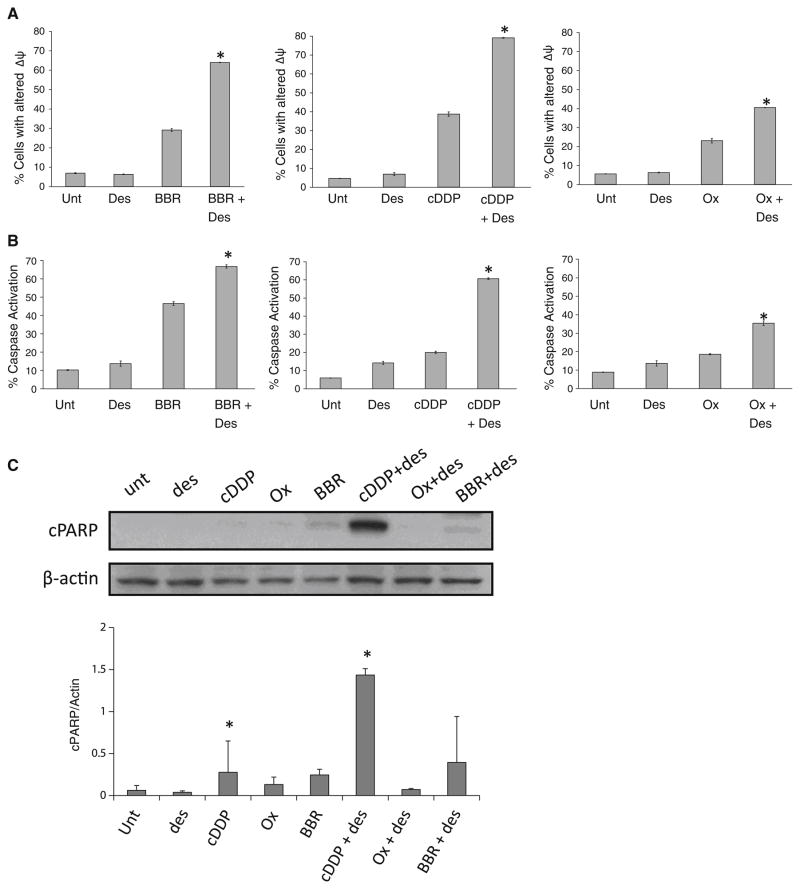
To extend the findings on apoptosis, we examined the impact of desipramine on specific apoptotic pathways, beginning with changes in mitochondrial membrane potential (Δψm), which can lead to caspase enzyme activation. Desipramine/platinum drug treatment significantly altered Δψm, increasing the percentage of cells showing reduced Δψm by approximately 2 orders of magnitude compared with those treated with c-DDP, oxaliplatin, or BBR3464 alone (Fig. 6a). In a similar manner, desipramine also increased caspase 3 activation in HCT116 carcinoma cells treated with c-DDP, oxaliplatin, or BBR3464 addition (Fig. 6b). In agreement with our apoptosis findings, the effects on Δψm and caspase activation were most overt in the desipramine/c-DDP combination, and were least when desipramine was combined with oxaliplatin. Caspase activation can lead to the cleavage of survival/repair proteins, including PARP. To complete the analysis of the mitochondrial and caspase pathways, we examined cellular levels of cleaved PARP by Western blot analysis. Constant platinum drug concentrations of 10 μmol/L were used for all platinum drugs. As shown in Fig. 6c, desipramine significantly enhanced PARP cleavage in HCT116 cells treated with c-DDP, but did not alter PARP under other conditions.
Fig. 6.
Effect of desipramine on downstream signaling pathways of p53 activated by platinum drugs. a Effect on platinum-drug-induced mitochondrial damage. HCT116 cells were cultured with 50 μmol/L BBR3464, 10 μmol/L c-DDP, or 30 μmol/L oxaliplatin in the absence or presence of 40 μmol/L desipramine for 72 h. Reduction in mitochondrial membrane potential was assessed by staining with 3,3′-dihexyloxacarbocyanine iodide as described in “Materials and methods.” b Effect of desipramine on platinum-drug-induced caspase activation. HCT116 cells were cultured as in a. Cells were stained for active caspase 3/caspase 7 as described in “Materials and methods.” The data shown are the percentage of the population displaying active caspase 3/caspase 7. Each point represents the average (±SEM) of three independent experiments. c The effect of desipramine on poly(ADP ribose) polymerase (PARP) expression in platinum-drug-treated samples. HCT116 cells were precultured for 1 h with 40 μmol/L desipramine before the addition of 10 μmol/L platinum drugs for 24 h. The graph shows averages and standard deviations from three measurements of PARP expression normalized to actin loading control. Asterisks p < 0.05 when comparing cells treated with and without desipramine, by Student’s t test
Platinum-drug-mediated p53 expression
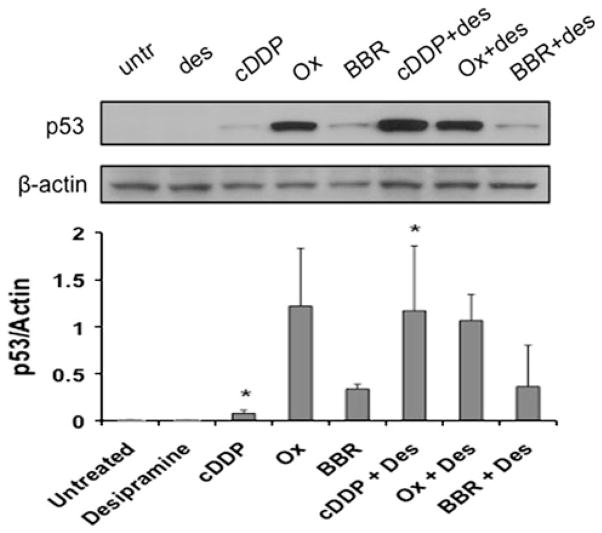
Many apoptotic signaling pathways converge at the transcription factor p53. p53 causes cell death in part by inducing mitochondrial damage that activates the death effector caspase enzymes [23]. Since all platinum drugs have been argued to elicit apoptosis in certain cell lines via a p53-dependent pathway [24, 25], p53 levels were measured by Western blot analysis and quantified by densitometry (Fig. 7). Interestingly, p53 levels in the presence of desipramine alone were very low—in agreement with the observation that although desipramine induces apoptosis in a mitochondrial-dependent manner, HCT116 cells are not especially sensitive to this agent [26]. All three platinum drugs induced measureable levels of p53, with oxaliplatin having the most overt effect under these conditions. As with PARP cleavage (Fig. 6c), desipramine only enhanced p53 stabilization in combination with c-DDP, and had little or no effect when combined with BBR3464 or oxaliplatin.
Fig. 7.
Effect of desipramine on p53 levels induced by platinum drugs. HCT116 cells were cultured with 50 μmol/L BBR3464, 10 μmol/L c-DDP, or 30 μmol/L oxaliplatin in the absence or presence of 40 μmol/L desipramine. p53 expression was detected by Western blotting. The graph shows averages and standard deviations from three measurements of PARP expression normalized to actin loading control. Asterisks p < 0.05 when comparing cells treated with and without desipramine, by Student’s t test
Discussion
These results complement the published reports of interference of tamoxifen efficacy in breast cancer treatment by antidepressants [8, 9, 27]. Tamoxifen is standard adjuvant treatment for women with estrogen-receptor-positive breast cancer and is a preventative agent for women at high risk of developing breast cancer. Studies have shown that three antidepressants—paroxetine, fluoxetine, and bupropion—may interfere with tamoxifen treatment by inhibiting the action of CYP2D6, the principal metabolic enzyme that converts tamoxifen into the active form endoxifen [8, 9]. Given the seriousness of these findings, it is imperative to examine the generality of these findings across the anti-cancer armamentarium. The results presented here show that the tricyclic antidepressant desipramine augments the cytotoxicity of platinum anticancer agents. The reasons for this interesting result, with potential clinical importance, have been explored by examining the comparative pharmacological action and cellular biology of the platinum drugs in the presence and absence of the antidepressant. Desipramine does not interact with either c-DDP or [PtCl(dien)]Cl (where dien is diethylenetriamine) as monitored by 1HNMR spectroscopy experiments.
It is worthy of note that desipramine has differential effects on cellular accumulation of the platinum agents yet enhances the cytotoxicity of all the platinum agents studied. Only the cellular accumulation of c-DDP is affected by the presence of desipramine (accumulation was increased 2.7-fold by desipramine), which may explain the more rapid kinetics of apoptosis seen (48 h for c-DDP vs. 72 h for BBR3464 and oxaliplatin) (Fig. 4). It is plausible that increased accumulation of c-DDP is due to a reduced recycling of a potential c-DDP importer or perhaps inhibition of an efflux transporter. Desipramine inhibits the serotonin transporter and affects trafficking of transporters such as β-adrenoreceptors [12, 28–30]. There is no consistent effect upon Pt–DNA binding with desipramine treatment—only BBR3464 showed measurable increases in the number of DNA adducts in the presence of desipramine. It is noteworthy that the cellular pharmacological action of BBR3464 differs from that of both c-DDP and oxaliplatin, further emphasizing the distinctness of the trinuclear structure in comparison with mononuclear drugs [31, 32].
The kinetics of DNA binding and indeed cellular uptake of all three platinum agents are different, and it is difficult therefore to examine identical time points for both accumulation and DNA platination assays. Nevertheless, the results are consistent in that, unlike the tamoxifen case, there is no direct correlation of cellular pharmacological action (plasma protein binding, cellular accumulation, and/or cellular Pt–DNA binding) with the enhancement of cytotoxicity by desipramine. Nevertheless, a biological explanation for these results could lie in modulation of Pt–DNA damage repair, and this feature is under further investigation. Interestingly, p53 levels were significantly enhanced in the desipramine/c-DDP combination over those for the platinum agent alone. These findings suggest that desipramine may synergize with c-DDP more than with other platinum chemotherapeutics partly by activating distinct apoptotic pathways. Desipramine itself induces apoptotic cell death through both mitochondrial and nonmitochondrial pathways in different colon carcinoma cells [26, 33]. Notably, p53 induction in HCT116 cells by desipramine is also not as significant as that caused by platinum agents, and apoptotic cell death is considered to occur primarily through disturbance of mitochondrial function, although the sensitivity of HCT116 cells in general is less than that of HT29 cells [26]. The molecular mechanism of desipramine apoptosis is unlikely to be a consequence of direct DNA modification, [26, 33], and thus complementary signaling pathways may be activated in the presence of both drugs. In this scenario, the role of p53 may lie in enhancement of two pathways—one a consequence of induction through direct DNA modification and the second a multiple effect on signaling pathways through the presence of p53, adding to the cellular stress.
The viability of HCT116 cells is not significantly altered in the presence of up to 50 μmol/L desipramine, [26], suggesting that the results obtained here at the concentrations used may have genuine pharmacological significance. The optimal concentration of desipramine was within the range of clinically relevant doses achieved in patients, although there is some discrepancy in literature values—free desipramine concentration in the serum has been reported at approximately 9.5 μmol/L, [34], but toxicity has been noted at 3.8 μmol/L [35].
In conclusion, an unexpected result is the synergistic effect on cytotoxicity shown by desipramine. The tricyclic organic compound, a safe and effective antidepressant used as an adjuvant for cancer treatment, greatly augments the cytotoxicity of platinum-based chemotherapeutics. In the case of c-DDP, these effects correlated to some extent with enhanced activation of the p53 mitochondrial death pathway. The study is of high clinical relevance and argues that desipramine may be a means of enhancing chemoresponsiveness to platinum-based anticancer agents and that desipramine warrants further investigation for its clinical utility.
Finally, it is worthy of note that the mechanistic study of drug combinations—either adjuvant agents or emerging clinical candidates—may help to distinguish the cellular pharmacological action and cancer biology of new potential platinum agents in comparison with the clinical drugs. Especially cellular accumulation may be affected by combination treatments, and comparative study of new platinum clinical candidates such as BBR3464 may further help to distinguish their profile from the profiles of the clinically used drugs.
These types of studies may help to identify unique agents above and beyond data provided by cytotoxicity assays.
Supplementary Material
Acknowledgments
We thank Fréderic Frezard and Paul Dent for helpful discussions. This work was supported by grants from the National Institutes of Health, R01-CA78754 (N.P.F.) and R01-AI59638 (J.R.).
Abbreviations
- c-DDP
Cisplatin
- CI
Combination index
- HSA
Human serum albumin
- PARP
Poly(ADP ribose) polymerase
- PBS
Phosphate-buffered saline
- PI
Propidium iodide
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00775-011-0836-1) contains supplementary material, which is available to authorized users.
Contributor Information
Peyman Kabolizadeh, Department of Chemistry, Virginia Commonwealth University, 1001 W. Main St., Richmond, VA 23284, USA.
Brigitte J. Engelmann, Department of Chemistry, Virginia Commonwealth University, 1001 W. Main St., Richmond, VA 23284, USA
Nicholas Pullen, Department of Biology, Virginia Commonwealth University, Richmond, VA, USA.
Jennifer K. Stewart, Department of Biology, Virginia Commonwealth University, Richmond, VA, USA
John J. Ryan, Department of Biology, Virginia Commonwealth University, Richmond, VA, USA
Nicholas P. Farrell, Email: npfarrell@vcu.edu, Department of Chemistry, Virginia Commonwealth University, 1001 W. Main St., Richmond, VA 23284, USA
References
- 1.Hall MD, Okabe M, Shen DW, Liang XJ, Gottesman MM. Annu Rev Pharmacol Toxicol. 2008;48:495–535. doi: 10.1146/annurev.pharmtox.48.080907.180426. [DOI] [PubMed] [Google Scholar]
- 2.Howell SB, Safaei R, Larson CA, Sailor MJ. Mol Pharmacol. 2010;77:887–894. doi: 10.1124/mol.109.063172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heim M, Scharifi M, Zisowsky J, Jaehde U, Voliotis D, Seeber S, Strumberg D. Anticancer Drugs. 2005;16:129–136. doi: 10.1097/00001813-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Jandial DD, Farshchi-Heydari S, Larson CA, Elliott GI, Wrasidlo WJ, Howell SB. Clin Cancer Res. 2009;15:553–560. doi: 10.1158/1078-0432.CCR-08-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanihara Y, Masuda S, Katsura T, Inui K. Biochem Pharmacol. 2009;78:1263–1271. doi: 10.1016/j.bcp.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Pirl WF, Roth AJ. Oncology (Williston Park) 1999;13:1293–1301. (discussion 1301–1292, 1305–1296) [PubMed] [Google Scholar]
- 7.Massie MJ. J Natl Cancer Inst Monogr. 2004:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 8.Desmarais JE, Looper KJ. J Clin Psychiatry. 2009;70:1688–1697. doi: 10.4088/JCP.08r04856blu. [DOI] [PubMed] [Google Scholar]
- 9.Kelly CM, Juurlink DN, Gomes T, Duong-Hua M, Pritchard KI, Austin PC, Paszat LF. BMJ. 2010;340:c693. doi: 10.1136/bmj.c693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kishore-Kumar R, Max MB, Schafer SC, Gaughan AM, Smoller B, Gracely RH, Dubner R. Clin Pharmacol Ther. 1990;47:305–312. doi: 10.1038/clpt.1990.33. [DOI] [PubMed] [Google Scholar]
- 11.Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. N Engl J Med. 1992;326:1250–1256. doi: 10.1056/NEJM199205073261904. [DOI] [PubMed] [Google Scholar]
- 12.Koepsell H, Lips K, Volk C. Pharm Res. 2007;24:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- 13.O’Dwyer PJ, Stevenson JP, Johnson SW. Helvetica Chimica Acta, Zurich. 1999. Clinical status of cisplatin, carboplatin, and other platinum-based antitumor drugs. In: Lippert B (ed) Cisplatin: chemistry and biochemistry of a leading anticancer drug; pp. 26–69. [DOI] [Google Scholar]
- 14.Manzotti C, Pratesi G, Menta E, Di Domenico R, Cavalletti E, Fiebig HH, Kelland LR, Farrell N, Polizzi D, Supino R, Pezzoni G, Zunino F. Clin Cancer Res. 2000;6:2626–2634. [PubMed] [Google Scholar]
- 15.Harris AL, Ryan JJ, Farrell N. Mol Pharmacol. 2006;69:666–672. doi: 10.1124/mol.105.018762. [DOI] [PubMed] [Google Scholar]
- 16.Chou T, Talalay P. Trends Pharmacol Sci. 1983;4:450–454. [Google Scholar]
- 17.Hiemke C, Hartter S. Pharmacol Ther. 2000;85:11–28. doi: 10.1016/s0163-7258(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 18.Colmenarejo G, Alvarez-Pedraglio A, Lavandera JL. J Med Chem. 2001;44:4370–4378. doi: 10.1021/jm010960b. [DOI] [PubMed] [Google Scholar]
- 19.Moller C, Tastesen HS, Gammelgaard B, Lambert IH, Sturup S. Metallomics. 2010;2:811–818. doi: 10.1039/c0mt00046a. [DOI] [PubMed] [Google Scholar]
- 20.Timerbaev AR, Hartinger CG, Aleksenko SS, Keppler BK. Chem Rev. 2006;106:2224–2248. doi: 10.1021/cr040704h. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, Lovejoy KS, Shima JE, Lagpacan LL, Shu Y, Lapuk A, Chen Y, Komori T, Gray JW, Chen X, Lippard SJ, Giacomini KM. Cancer Res. 2006;66:8847–8857. doi: 10.1158/0008-5472.CAN-06-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabolizadeh P, Ryan J, Farrell N. Biochem Pharmacol. 2007;73:1270–1279. doi: 10.1016/j.bcp.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Haupt S, Berger M, Goldberg Z, Haupt Y. J Cell Sci. 2003;116:4077–4085. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- 24.Siddik ZH. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor PM, Jackman J, Bae I, Myers TG, Fan S, Mutoh M, Scudiero DA, Monks A, Sausville EA, Weinstein JN, Friend S, Fornace AJ, Jr, Kohn KW. Cancer Res. 1997;57:4285–4300. [PubMed] [Google Scholar]
- 26.Arimochi H, Morita K. Pharmacology. 2008;81:164–172. doi: 10.1159/000111144. [DOI] [PubMed] [Google Scholar]
- 27.Harv Ment Health Lett. 2010;26:6–7. No authors. [Google Scholar]
- 28.Burgi S, Baltensperger K, Honegger UE. J Biol Chem. 2003;278:1044–1052. doi: 10.1074/jbc.M209972200. [DOI] [PubMed] [Google Scholar]
- 29.Deupree JD, Reed AL, Bylund DB. J Pharmacol Exp Ther. 2007;321:770–776. doi: 10.1124/jpet.106.118935. [DOI] [PubMed] [Google Scholar]
- 30.Yau JL, Noble J, Thomas S, Kerwin R, Morgan PE, Lightman S, Seckl JR, Pariante CM. Neuropsychopharmacology. 2007;32:2520–2529. doi: 10.1038/sj.npp.1301389. [DOI] [PubMed] [Google Scholar]
- 31.Mangrum JB, Farrell NP. Chem Commun. 2010;46:6640–6650. doi: 10.1039/c0cc01254h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrell N. Met Ions Biol Syst. 2004;42:251–296. [PubMed] [Google Scholar]
- 33.Arimochi H, Morita K. Eur J Pharmacol. 2006;541:17–23. doi: 10.1016/j.ejphar.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 34.Hursting MJ, Clark GD, Raisys VA, Miller SJ, Opheim KE. Clin Chem. 1992;38:2468–2471. [PubMed] [Google Scholar]
- 35.Gillman PK. Br J Pharmacol. 2007;151:737–748. doi: 10.1038/sj.bjp.0707253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.