Abstract
Despite their identification more than 100 years ago by the French scientist Charles-Marie Benjamin Rouget, microvascular pericytes have proven difficult to functionally characterize, due in part to their relatively low numbers and the lack of specific cell markers. However, recent progress is beginning to shed light on the diverse biological functions of these cells. Pericytes are thought to be involved in regulating vascular homeostasis and hemostasis as well as serving as a local source of adult stem cells. To further define the properties of these intriguing cells, we have isolated pericytes from transgenic mice (Immortomouse®) harboring a temperature-sensitive mutant of the SV40 virus target T-gene. This Immortopericyte (IMP) conditional cell line is stable for long periods of time and, at 33°C in the presence of interferon gamma, does not differentiate. Under these conditions IMPs are alpha muscle actin-negative and exhibit a pluripotent phenotype, but can be induced to differentiate along both mesenchymal and neuronal lineages at 37°C. Alternatively, differentiation of wild type pericytes and IMPs can be induced directly from capillaries in culture. Finally, the addition of endothelial cells to purified IMP cultures augments their rate of self-renewal and differentiation, possibly in a cell-to-cell contact dependent manner.
Keywords: pericytes, stem cell, Immortomouse®, differentiation, brain, alpha muscle actin, neurosphere, osteogenesis, vascular niche, endothelial cells, neurospheres
Introduction
Microvascular pericytes are found in precapillary arterioles, capillaries, and post capillary venules of virtually every organ in the body. In the brain, pericytes are regulatory cells with primary roles in the maintenance of homeostasis and hemostasis (Shepro and Morel, 1993; Edelman et al., 2006; von Tell et al., 2006) at the blood brain barrier (BBB) (Balabanov and Dore-Duffy, 1998; Ballabh et al., 2004). Pericytes are known to migrate from their capillary location into the neurovascular space (Bernstein et al., 1982; Nadal et al., 1999; Dore-Duffy et al., 2000), where they may be induced to phenotypically or functionally differentiate. With the development of techniques to purify primary pericytes from the retina (Gitlin and D’Amore, 1983) and from CNS capillaries (Dore-Duffy, 2003), pericytes have been shown to differentiate along the mesenchymal or neuronal lineages depending on culture conditions (Canfield et al., 1996; Doherty et al., 1998, Dore-Duffy et al., 2006; Dore-Duffy, 2008). This multipotentiality also has been observed in dental pulp, fat, skin, liver, and muscle pericytes (Chungmeng and Tianmin, 2004; Herrera et al., 2006; Dellavelle et al., 2007; Saif et al 2009; Wang et al 2010; Lin et al 2010; Humphreys et al 2010; Zimmerlin et al 2010; Paquet-Fifield 2010). Pericytes may, therefore, represent a source of adult pluripotential stem cells (Dore-Duffy 2006; 2008; Cai et al 2010; Ergun et al 2010).
That pericytes rapidly differentiate in vitro has obscured interpretation of many studies, even those involving primary cell cultures. In particular, the identification of specific pericyte subsets remains difficult because pericyte stage-specific markers have not been identified. The generation of pericyte cell lines that exhibit an undifferentiated pericyte phenotype also has proven difficult because such cell lines exhibit phenotypic and functional properties of endothelial cells (ECs) and/or express markers associated with differentiated pericytes (Asashima et al., 2002; Berrone et al., 2009).
We have taken an alternate approach to generate stable pericyte cell populations using an in vivo source of cells that express a temperature-sensitive immortalizing oncogene. The Immortomouse® harbors a stably incorporated conditionally expressed transgene (Jat et al., 1991; Noble et al., 1992; Barber and Henderson, 1996; Dennis and Caplan, 1996b; Kanda et al., 1996; Walther et al., 1996), which encodes a variant of the tumor antigen (T-Ag) from the simian virus 40 A (SV40). A missense mutation of T-Ag (TsA) renders this protein functional at 33°C but inactive at body temperature (TsA58). In the Immortomouse®, coupling TsA58 to the H2Kb class-I promoter ensures expression in multiple tissues and confers cytokine regulated properties where expression is induced by interferon gamma (IFNγ) (Barald et al., 1997; Takacs-Jarrett et al., 1998; 2001). Thus, the expression of functional T-Ag is conditional on both temperature and the presence of IFNγ.
The Immortomouse® has been a source for conditionally immortalized cells from the endothelium (Kanda et al., 1996), renal mesangial cells (Barber and Henderson, 1996), Sertoli cells (Walther et al., 1996) mesenchymal cells (Dennis and Caplan, 1996b), cochlear hair cells (Kachar et al., 1986), skeletal muscle (Morgan et al., 1994; Ehler et al., 1995), epithelial cells (Whitehead et al., 1993; Kershaw et al., 1994; Whitehead and Joseph, 1994; Paradis et al., 1995), glial cells (Groves et al., 1993), and osteoclasts (Chambers et al., 1993). These immortalized cells continue to replicate at 33°C in the presence of IFNγ and do not exhibit differentiated phenotypes (Holley and Lawlor, 1997). Accordingly, we hypothesized that primary pericytes cultured from the Immortomouse® also would not differentiate at 33°C; however, we were uncertain whether they would replicate. In our hands, freshly isolated immortopericytes (IMPs) display low levels of cell division until exposed to environmental or exogenous stimuli.
Here we report the characterization of CNS capillary pericytes from the Immortomouse®. IMPs survive as undifferentiated cells for long periods of time at 33°C in presence of IFNγ and serum-containing culture medium and can be passaged, frozen, and thawed. However, IMPs proliferate slowly under these conditions and are largely quiescent. When IMPs are cultured at 37°C in the presence of serum and in the absence of IFNγ, they differentiate comparable to wild type mouse and rat pericytes. Furthermore, IMPs can be cultured for at least nine weeks before transfer to 37°C and retain their differentiation capacity. When cultured at 37°C in serum-free medium with N2 supplement and basic fibroblast growth factor (bFGF), IMPs differentiate along the neuronal lineage forming neurospheres comparable to wild type pericytes (Dore-Duffy et al., 2006; Dore-Duffy, 2008). IMPs do not differentiate in response to bFGF at 33°C. Spheres can also be generated directly from wild type and IMP capillaries in culture. The rate of sphere formation and the rate of differentiation is greater than that observed for spheres generated from primary pericytes, suggesting that ECs provide trophic factors that promote pericyte stem cell activity.
Methods
Animals
Male Immortomouse® mice (Charles River, Laboratories, Wilmington, MA) hemizygous for the interferon-inducible H2kb-tsA58 transgene were crossed with B6C3 F1 females (Taconic Farms, Germantown, NY) to yield mice for pericyte isolation and culture. The genotype of the mice was verified by PCR using primers, Immorto1, 5′-AGC GCT TGT GTC GCC ATT GTA TTC-3′ and Immorto2, 5′-GTC ACA CCA CAG AAG TAA GGT TCC-3′ (product = 1kb). An internal control for PCR, which amplifies a 0.2 kb fragment of the Plp1 gene used primers AG521, 5′-GCT GAT TTT TAA CCA CTC CAT GTC-3′ and AG522, 5′-CAA CTC ACC ATA CAT TCT GGC ATC-3′.
Antibodies and Chemicals
Affinity purified goat anti-mouse immunoglobulin and goat anti-rabbit IgG F(ab)2 fragments conjugated to Red 613 or FITC were purchased from CAPPEL (Durham, NC). Goat anti-mouse IgG, conjugated to AMCA was obtained from AbCam (Cambridge, MA). Affinity-purified rabbit anti-human von Willebrand factor (vWF) antibody (IgG) (1:500) was purchased from Dakopatts (Glostrup, Denmark) in either FITC conjugated or unconjugated forms and mouse anti-human vWF (IgG2a; 1:1000) was purchased from Boehringer Mannheim (Indianapolis, IN). Mouse anti-murine nestin (IgG) was purchased from Chemicon/Millipore (Temecula, CA). Rabbit anti-GFAP (1:200), and rabbit anti-neurofilament 200 (NFL-200) (1:200) were purchased from Sigma (St. Louis, MO). Mouse anti-BrdU was purchase from Becton Dickinson. Anti-mouse IgG conjugated to Cy3 was purchased from Sigma. Goat anti-mouse platelet derived growth factor beta receptor (PDGFβR) IgG was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit and goat anti-mouse NG2 chondroitin sulfate proteoglycan (IgG1) was purchased from Santa Cruz Biotechnology and mouse anti-rat O4 antigen (1:50) was purchased from Chemicon/Millipore. Goat anti-human C-terminus (C-20) CD146 (Mel-CAM) (IgG) was obtained from Santa Cruz Biotechnology. Rabbit anti-bovine IgM conjugated to FITC was obtained from Novus Biologicals (Littleton, CO). Mouse anti-GFAP (clone GA5) conjugated to Alexa-647 was obtained from Cell Signaling (Danvers, MA). Mouse anti-160kD neurofilament (NF-09) (IgG2a), which reacts to all species, and goat anti-mouse IgM, μ chain specific, F(ab)2 fragment were obtained from AbCam.
Primary pericyte isolation
Ten x 3-week-old homozygous mice carrying the H2Kb-tsA58 transgene were decapitated and the brain tissue immediately removed using sterile technique. Capillaries were isolated according to Joó and Karnushina (1973), as modified by Bowman et al., (1982), and further modified by Dore-Duffy et al (2003). Freshly isolated mouse capillaries were incubated overnight in collagenase and dispase at 37°C. Following incubation, capillaries were disrupted and single cell suspensions were grown in standard culture medium comprising 10% fetal calf serum in Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen, Carlsbad, CA). For cells grown at 33°C, the standard culture medium was supplemented with IFNγ (50 units/ml final volume, Pierce, Thermo-Fisher Scientific, Rockford, IL) to induce expression of the H2kb-tsA58 transgene. In BrdU-labeling experiments, cells were labeled with BrdU (BD Biosciences, Rockville, MD) in the culture medium (10 μM final) overnight. Cells were then fixed and labeled with anti-BrdU antibodies.
Cells were plated at 106 cells/ml for six hours at 37°C on uncoated plastic Petri dishes (Thermo-Fisher Scientific). Non-adherent cells were removed by vigorous washing and used for isolation of ECs (see below). The medium in adherent cultures was changed each day for two days and biweekly for additional culture periods. These cells were Griffonia symplicifolia agglutinin (GSA)− and Factor VIII− after culture at 33°C or 37°C (data not shown). They expressed platelet-derived growth factor beta receptor (PDGFβR) and displayed the morphological characteristics of wild type pericytes (Fig. 1A). Non-adherent cells were 95% Factor VIII+ and GSA+ (data not shown).
Figure 1. Characterization of IMPs.
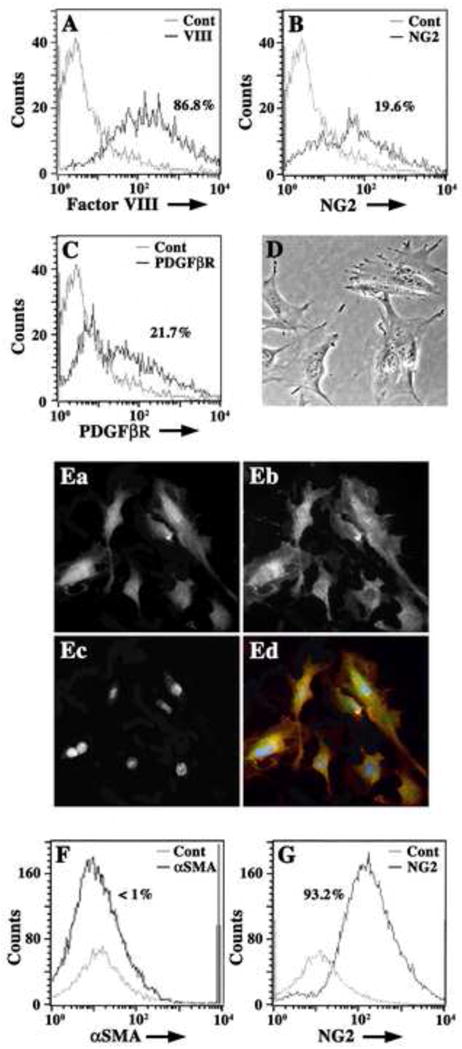
Freshly isolated Immortomouse® capillaries were enzymatically disrupted and single cell suspensions were analyzed by flow cytometry for the expression of pericyte (NG2, PDGFβR) and EC markers (Factor VIII) (A–C). In (A), a histogram overlay of isotype negative control antibody (grey line) and Factor VIII (black line) show that cell suspensions are predominately Factor VIII+ (86.8%) ECs. In (B), histogram overlays of negative isotype controls (grey line) and NG2+ cells (black line) show that cell suspensions also contain approximately 20% NG2+ cells (19.6%). In (C), histogram overlays of isotype negative controls (grey line) and PDGFβR+ cells (black line) show that 21.7% of cells express this pericyte marker. Cell suspensions plated at 5 × 105 cells/ml on plastic Petri dishes were incubated in standard culture medium for six hours at 37°C before non-adherent cells were washed off vigorously. Adherent cells are morphologically similar to pericytes (D) and are NG2+ (Ea; Ed, green) and PDGFβR+ (Eb; Ed, red) by dual immunocytochemistry. Nuclei are labeled with DAPI (Ec; Ed, blue). Adherent cells (for 48 hours) were suspended using RPMI-1640-EDTA and analyzed by flow cytometry for expression of αSMA and NG2. Histogram overlays from FACS analysis in (F) show negative control antibody (grey line) and αSMA+ cells (black line) and indicate that adherent cells are less than 1% αSMA+. Histogram overlays in (G) show control isotype antibody staining (grey line) and NG2 antibody staining (black line) and indicate that adherent cell suspensions are 93.2% NG2+. Histogram data are presented as cell number versus log fluorescence intensity (FITC) of individual cell markers.
Non-adherent cells derived from microvessel enzymatic digestion and subculture were counted using a hemocytometer and plated on collagen-coated dishes from 1 – 10 × 105 cells/ml in standard culture medium at 37°C. Cells were allowed to adhere overnight, then washed twice. These cells displayed the morphological characteristics of ECs and expressed several EC markers: Factor VIII, CD31, and the ligand for GSA (data not shown). Greater than 97% of cells were determined to be ECs.
Passaging of cells
IMPs grown at 33°C or 37°C were washed twice with serum-free medium and once with in RPMI-1640 containing 10−4M EDTA. Cells were then incubated for 1–3 minutes at 37°C with RPMI-1640-EDTA. Petri dishes were shaken vigorously and loose cells were washed from the plates, centrifuged, washed thrice in RPMI-1640-EDTA, resuspended in fresh standard medium with or without IFNγ and replated at 5 × 105 cells/ml. Although IMPs were viable, their proliferation rate was low unless cultured at 37°C (n = 2). Passage efficiency was 90%. Pellets were resuspended in standard medium supplemented with 50% DMSO and frozen in liquid nitrogen. The viability of these cells after thawing was 70%.
Immunocytochemistry
Microvessels, primary cultured ECs and pericytes were allowed to dry onto alcohol-washed glass coverslips before fixation with 3% paraformaldehyde for ten minutes. Coverslips were blocked in PBS with 1% bovine serum albumin (BSA), permeabilized with 0.1% Triton X-100, and stained with antibodies against several cell type-specific markers, or with control antibodies. All primary and secondary antibodies were used at optimized concentrations and incubations were carried out at room temperature as previously reported (Dore-Duffy et al., 2007). Briefly, coverslips were incubated for 30 minutes, washed thrice with phosphate-buffered saline, pH7.4 (PBS) and incubated with fluorochrome-conjugated secondary antibodies [affinity purified anti-IgG F(ab)′ 2 fragments 1:100 dilution]. Secondary antibodies alone and/or isotype control antibodies were used as controls and yielded minimal staining. In dual and triple labeling experiments, species-matched immunoglobulins were not used. Immunofluorescence was examined using an Orthoplan-2 (Zeiss, Germany) fluorescence microscope equipped with 25X and 60X oil objectives. Percentages of positive microvessels or cells were calculated after counting a minimum of 300 cells or microvessels. Coverslips were photographed using a 35 mm CONTAX 165 MT camera (Kyocera Corp., Japan).
Limiting dilution cultures
Following capillary digestion and enrichment (see above), IMPs were counted using a hemocytometer, diluted in standard culture medium to 1–5 cells/ml at the highest dilution and allowed to adhere to uncoated Petri dishes overnight. Cultures were inspected daily to determine cell densities (cells /mm2) using a 10X ocular grid eyepiece on an inverted microscope.
Flow Cytometry and Cell Sorting
Single cell suspensions in PBS were incubated with primary and/or secondary antibodies directed against cell type-specific markers. To remove ECs, cells were incubated with GSA–fluorescein isothiocyanate (FITC) conjugate (diluted 1:100) for 60 minutes at 4°C and GSA-FITC+ cells were removed as previously described (Dore-Duffy, 2003). For analysis, suspensions were single or dual-labeled with GSA-FITC and/or antibodies directed against mouse NG2, alpha-smooth muscle actin (αSMA), , PDGFβR or Factor VIII (see below). Cell suspensions were fixed and analyzed using a Becton-Dickinson flow cytometer. Isotype-matched nonspecific antibodies were used to label cells as negative controls and values for percentages were subtracted. Flow cytometry analysis was performed using FACS Diva software (BD Biosciences).
Culture of bFGF responsive cells
The viability of cells was determined by trypan blue exclusion. Viable cells (200/cm2) were plated in 35 mm uncoated Petri dishes or 16-well dishes in stem cell medium [(DMEM/F-12 with N2 supplement, no serum containing 20 ng/ml murine recombinant bFGF (Gibco, Grand Island, NY)]. The number of free-floating spheres was counted after varying days in culture depending on the experimental protocol. Some spheres were cultured on poly-L-ornithine (Poly-O) coated glass coverslips for immunocytochemistry. Others were used to establish self-renewal. Pericyte spheres were transferred to conical test tubes in bFGF-supplemented stem cell medium, diluted to 1 sphere/tube and mechanically dissociated by vigorous up-and-down pipetting. One cell was added per microwell using limiting dilution and incubated in bFGF containing stem cell medium. About 10% of these cells survived. Cells produced new spheres which were transferred to Poly-O coated slides for immunocytochemical labeling.
Statistical analyses
Quantitative data are expressed as mean ± S.D. and generally from at least three independent observations or experiments. Data were analyzed by linear regression (Figs 2, 5C and 7H) followed by One-way Analysis Of Variance (ANOVA) to compare the slopes of the curves and Bonferroni post hoc multiple comparisons testing. For Fig. 2, the regression analysis followed Log-transformation of the ordinate data. All other statistical analyses used One-Way ANOVA with Bonferroni post hoc testing. All tests were implemented in Prism 5.0d software for Mac (Graphpad Software Inc, La Jolla, CA).
Figure 2. IMPs proliferate at low and high densities.
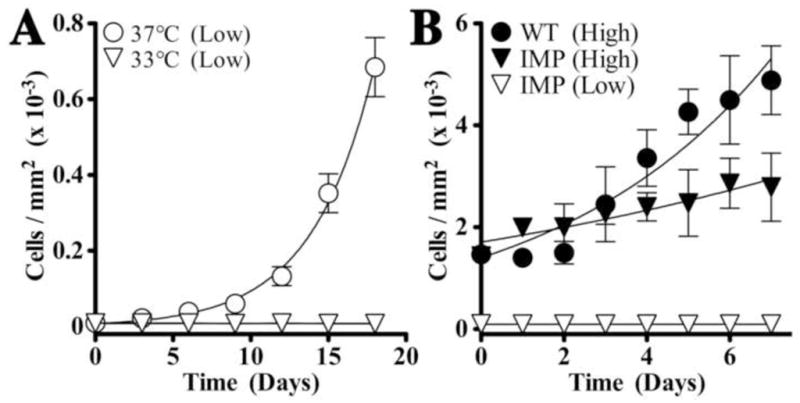
IMPs and wild type pericytes were seeded at two densities: 5 × 102 (A, Low) or 5 × 103 (B, High) cells/well in 96-well plates and cultured at 33°C in standard culture medium containing 50 U/ml IFNγ or at 37°C in standard medium without IFNγ. The cells were counted daily using a 10X ocular grid. As many grids were counted as needed to cover the surface of the well with a minimum of five grids counted per well (mean ± SD, n = 3 experiments). (A) In low density cultures, IMPs do not proliferate at 33°C and only proliferate when transferred to 37°C. (B) Regression analysis indicates that in high density cultures at 33°C, IMPs proliferate (compared with low density cultures; P < 0.05); however, this rate of growth is significantly slower than for wild type pericytes grown under the same conditions (P < 0.05).
Figure 5. IMPs differentiate along the mesenchymal lineage only at 37°C.
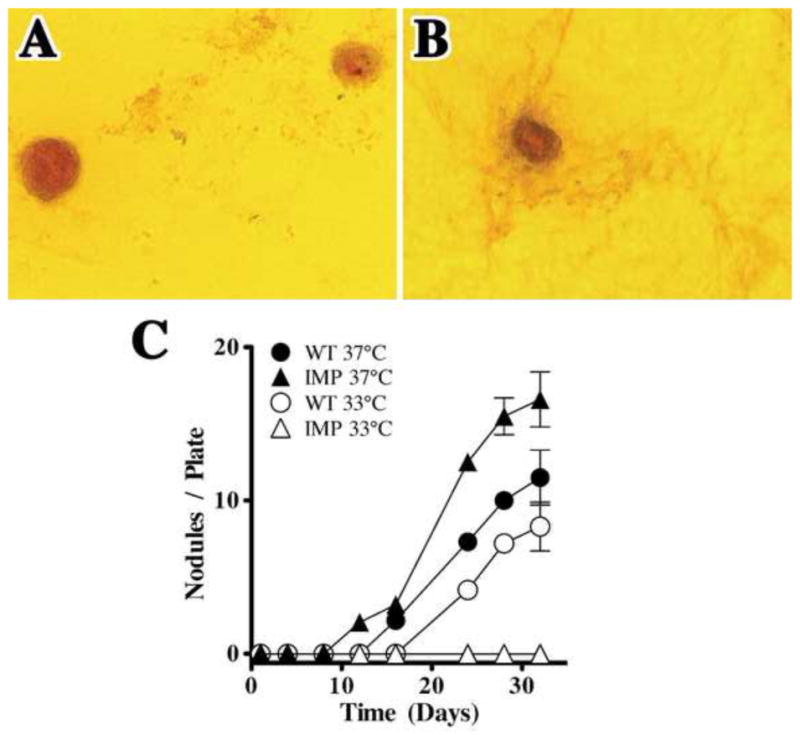
Wild type pericytes and IMPs were seeded at 1 × 105 cells/ml and cultured at 33°C or 37°C in Nunclon 4-well Petri dishes (1.9 cm2) in standard culture medium. The number of alizarin red+ nodules per plate was quantified at several time points. (A) Wild type pericytes form alizarin red bone nodules by 21 – 28 days at 37°C or 33°C (not shown). (B) IMPs also form nodules, but only at 37°C. In (C), quantification (mean ± SD, n = 3) shows the number of nodules/plate in wild type pericyte and IMP cultures. In contrast to IMPs, regression analysis indicates that nodule formation in wild type pericyte cultures is independent of growth at 33°C versus 37°C (P > 0.05). The number of nodules in IMP cultures grown at 37°C is greater than for wild type pericyte cultures (P < 0.05).
Figure 7. IMP neurospheres can be generated directly from isolated capillaries.
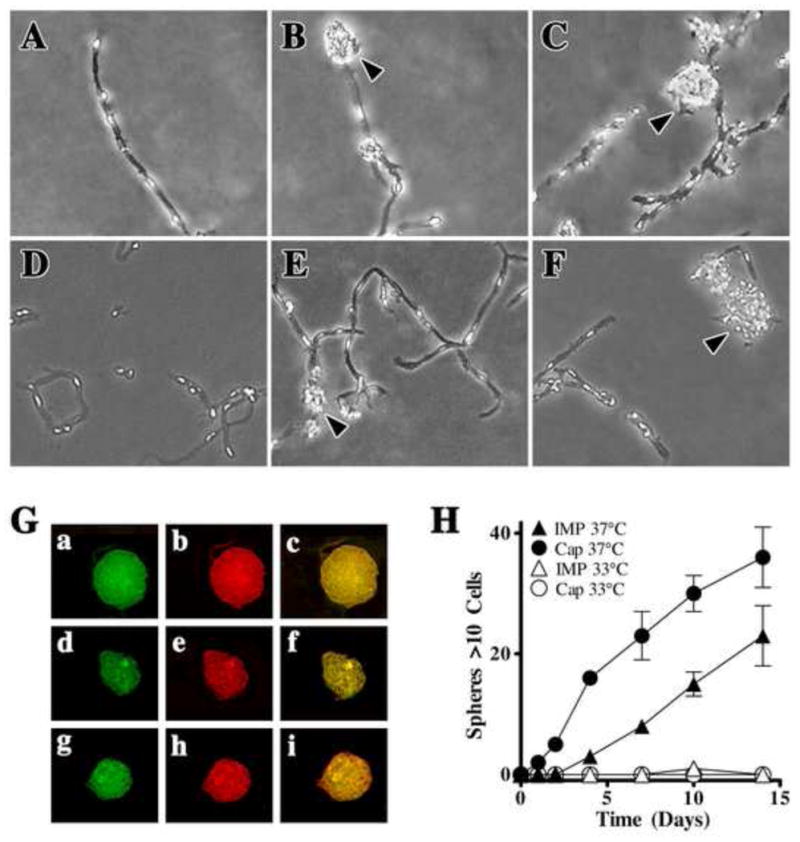
Freshly isolated CNS capillaries from wild type mouse pericytes (A–C) or from the Immortomouse® (D–F) were incubated in stem cell medium containing 20 ng/ml bFGF at 37°C. Phase-bright cells along the capillaries appear to protrude after 24 hr, and small multicellular aggregates (arrowheads) are visible by two days (B,E). After three to five days, large sphere-like cell aggregates (arrowheads) are either attached to the vessel or are floating in the media (C,F). (G) Capillary-generated IMP spheres were collected and allowed to adhere to Poly-O-coated coverslips, fixed, and dual stained for NG2 (green) and GFAP (red) (Ga-c), NG2 (green) and NF (red) (Gd-f) or NG2 (green) and O4 (red) (Gg-i). (H) Regression analysis indicates that the rate of IMP neurosphere formation from Immortomouse® capillaries (Cap) exceeds that observed in IMP cultures (IMP) grown at 37°C (P < 0.05). Similar to IMP cultures, cultured IMP capillaries do not generate neurospheres at 33°C.
Results
Morphology and immunocytochemical characterization of IMPs
Immortomouse® capillaries were digested overnight and cell suspensions were analyzed using FACS for the presence of NG2- and PDGFRβ-expressing cells (both antigens are expressed by pericytes) and Factor VIII+ ECs (Fig. 1A–C). Capillary-derived cell suspensions are 86.8% ECs (Fig. 1A) and contain approximately 20% NG2+ cells (Fig. 1B) and 21.7% PDGFβR+ cells (Fig. 1C). Pericyte enriched cell populations were obtained by cell sorting (Dore-Duffy, 2003) and allowed to adhere at 33°C in the presence of IFNγ (Fig. 1D). Cells displaced from Petri dishes (i.e. ECs) were cultured on poly O-coated coverslips, fixed, and stained for expression of Factor VIII or the ligand for GSA (data not shown). Adherent cells were dual stained for NG2 (Ozerdem et al., 2001, 2002) and PDGFβR (Fig. 1E).
Greater than 97% of the non-adherent cells are Factor VIII+ and less than two percent stain with NG2 in some preparations (data not shown). Adherent Immortomouse® cells are morphologically indistinguishable from normal pericytes (Fig. 1D) and greater than 90% are PDGFβR+ and NG2+ by immunocytochemistry (Fig. 1E). Five-day-old cultures grown at 33°C are αSMA− (Fig. 1F) and 93% NG2+ (Fig. 1G). IMPs are viable for at least nine weeks with little change in morphology and these cells can be frozen and thawed. They are viable at a plating efficiency of 90% from subculture and 70% after freezing.
IMPs proliferate slowly at 33°C
IMPs were seeded on Petri dishes and maintained in culture in the presence of IFNγ at 33°C (permissive conditions) to drive TsA58 synthesis and preserve its biological activity or at 37°C in the absence of IFNγ (non-permissive conditions). IMPs grown in low density at 33°C remain viable but are essentially quiescent (Fig. 2A). A similar lack of growth is observed in cultures containing two to three cells/well in limiting dilution experiments with little increase in cell number for most wells even after 3 weeks (data not shown). However, when plated at 37°C at low density IMP cultures proliferate (Fig. 2A). In a second set of experiments at 33°C, we compared the growth of IMPs plated at low or high densities to wild type pericytes (Fig. 2B). In contrast to low density cultures, IMPs proliferate in high density cultures. However, regression analysis indicates that this rate is slower than for wild type pericytes grown under similar culture conditions (P < 0.05). Together, these data indicate that IMPs remain viable at 33°C in culture, even in the absence of proliferation.
IMP expression of αSMA and CD146
Fresh capillary-derived IMPs are αSMA−. Wild type pericytes express αSMA heterogeneously at the capillary level and induce expression of this marker in nearly 100% of pericytes after 5 days in culture, which may represent a stage in the differentiation of this lineage (Veerbek et al 1994; Leibner et al 2000; Sieczkiewicz and Herman 2004; Dore-Duffy 2008). To determine if IMPs would remain αSMA− after prolonged culture periods we cultured fresh isolated cells for 9 weeks at 33°C then transferred to 37°C. Cells were dual-stained with antibodies to detect expression of αSMA and Factor VIII or αSMA and NG2 using flow cytometry. Under permissive conditions for five days, IMPs do not express Factor VIII (Fig. 3B). When grown at 33°C for nine weeks, IMPs remain αSMA− and express NG2 (Fig. 3C). However, when nine-week IMP cultures are transferred to 37°C, they become αSMA+ by five days in culture, suggesting that IMPs do not differentiate at 33°C but are capable of differentiating to αSMA+ cells at 37°C (Fig. 3D).
Figure 3. IMPs express αSMA.
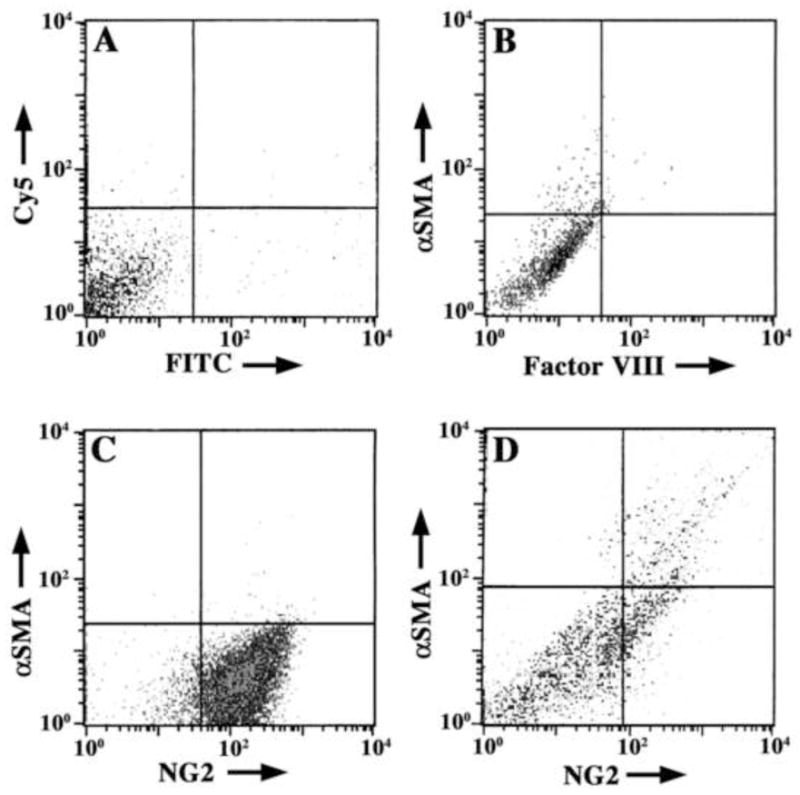
(A–C) IMPs were seeded at 104 cells/ml and grown in standard culture medium. Thereafter, they were fixed, dual stained and analyzed for antibody binding by flow cytometry. The horizontal and vertical lines indicate intensity thresholds, above which cells are considered positive for labeling with one or both primary antibody markers. (A) Secondary antibody negative controls (Cy5 and FITC) show that non-specific labeling of IMPs is low and confined to the lower left quadrant of the plot. (B) Freshly isolated IMPs grown for five days at 33°C and labeled with primary antibodies against αSMA and Factor VIII followed by secondary antibodies. Most cells are αSMA− and NG2−. (C) IMPs cultured at 33°C for nine weeks in the presence of IFNγ are αSMA− and NG2+. (D) IMPs grown for nine weeks at 33°C before transfer to 37°C for five to seven days (data at day five are shown) begin to express αSMA. These data suggest that IMPs do not differentiate to the αSMA phenotype during culture at 33°C; however, they retain the potential to differentiate for long periods of time in continuous culture. Data are presented as log-log plots of fluorescence intensity from FITC (abscissa) and Cy5 (ordinate) for each cell marker pair.
A number of studies have indicated that at least a subset of pericytes express CD146 and that this marker is associated with pluripotent activity (Dennis and Caplan 1996a; Paquet-Fifield et al, 2009; Sakai et al, 2010; Maier et al 2010). Accordingly, wild type pericytes and IMPs were analyzed for CD146 expression using immunocytochemistry. Dual labeling with anti-CD146 and anti-PDGFβR antibodies show that a subset of freshly isolated CNS microvascular pericytes from both rat (Fig. 4A) and mouse (Fig. 4B) express CD146 at low levels and occasional presumptive EC contaminants are strongly CD146+ (arrowhead, Fig. 4B). A greater proportion of IMPs are CD146dim and PDGFβR+ (Fig. 4C,D) than wild type pericytes (55% versus 32% in two experiments with 100–200 cells counted from five coverslips), but further studies are needed to determine if expression of this marker is dependent on the stage of pericyte differentiation.
Figure 4. Wild type pericytes and IMPs express CD146.
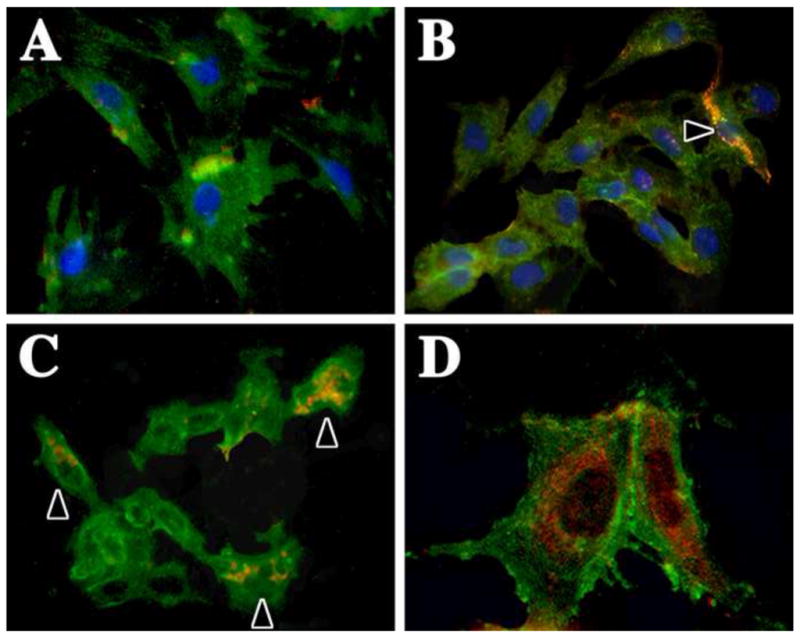
Primary mouse pericytes and IMPs were grown on glass coverslips in standard culture medium at 37°C. Cells were fixed in paraformaldehyde, permeabilized with Triton X-100 and dual stained to detect expression of PDGFβR (green) and CD146 (MCAM) (red). (A) Wild type rat pericytes are heterogeneous for CD146 expression and express this protein at low levels. (B) Mouse wild type pericytes express CD146 at low levels, while occasional presumptive ECs cells express this protein strongly (arrowhead). (C, D) A greater proportion of IMPs are CD146+ (arrowheads in C) as shown at low (C) and high (D) magnification. Nuclei in (A and B) are labeled with DAPI (blue).
IMPs are pluripotent
1) Mesenchymal differentiation and bone nodule formation
To further investigate IMPs in culture, we examined their differentiation along both mesenchymal and neuronal lineages. Primary pericytes differentiate in vitro in serum-containing medium along the mesenchymal lineage and become αSMA+ after five to seven days in culture. These cells ultimately form muscle, fat and Alizarin red+ bone nodules in culture (Brighton et al., 1992; Diaz-Flores et al., 1992; Schor et al., 1992; Canfield et al., 1996; Doherty et al., 1998; Ozerdem et al., 2002; Collett and Canfield, 2005). We have compared nodule formation in wild type pericyte cultures (21–28 days in culture) with IMPs maintained at 33°C or 37°C for 21–28 days (Fig. 5A). IMPs form nodules when cultured at 37°C (Fig. 5B) but not at 33°C (Fig. 5C). Only wild type pericytes form nodules at both 33°C and 37°C (Fig. 5C). These results suggest that pericytes isolated from the Immortomouse® can differentiate at 37°C along the mesenchymal lineage.
2) Neurosphere formation in response to bFGF
We previously reported (Dore-Duffy et al., 2006, 2008) that rat CNS pericytes differentiate along the neuronal lineage in response to bFGF and questioned if IMPs respond to bFGF under permissive and non-permissive conditions. At 33°C in the presence of IFNγ, four-day-old IMP cultures do not form neurospheres in response to bFGF but do form spheres at 37°C (Fig. 6A) that can be labeled with antibodies against BrdU and nestin (Fig. 6B) or PDGFβR and NG2 (Fig. 6C). Furthermore, IMPs self-renew (Table 1) and spheres differentiate into glial fibrillary acidic protein (GFAP)+ astrocytes (Fig. 6D), O4+ oligodendrocyte progenitors (Fig. 6E), and neurofilament (NF)+ neurons (Fig. 6F) within two weeks at 37°C. Together, these results indicate that IMPs do not differentiate at 33°C, but can differentiate at 37°C and are comparable to wild type pericytes in their pluripotency.
Figure 6. IMPs form neurospheres at 37°C in the presence of bFGF.
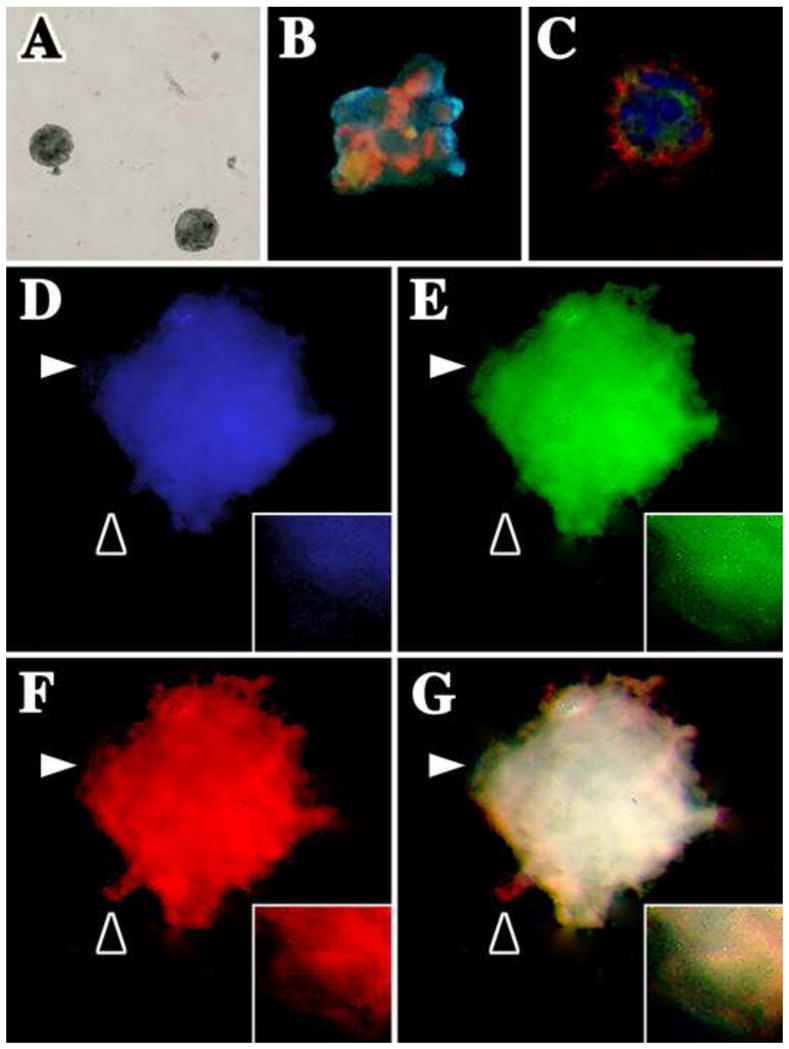
IMPs were cultured at low density in stem cell culture medium containing 20 ng/ml bFGF. (A) IMP-derived neurospheres are formed within two weeks. Spheres were pulse-labeled with 10 μM BrdU overnight and fixed and dual labeled with antibodies against (B) BrdU (green) and nestin (red) or (C) NG2 (green) and PDGFβR (red). Nuclei are labeled with DAPI (blue). (D–G) IMPs differentiate into each of the major cell types in the CNS: oligodendrocytes, astrocytes and neurons. Three-week-old neurospheres were triple-labeled with antibodies against GFAP (blue), O4 antigen (green) and neurofilament (red). (G) The three-channel overlay shows that all antigens generally overlap (white); however, insets in D–F show high magnification images (captured at the white arrowheads) to demonstrate that the staining patterns are not identical. Black arrowheads also highlight non-uniform labeling for the three antibodies.
Table 1.
ECs enhance pericyte neurosphere formation
| Spheres / culture* | Differentiation (days in culture) | |
|---|---|---|
| Wild type pericytes | 26 ± 6 | 10 – 14 |
| IMPs | 29 ± 4 | 10 – 12 |
| Wild type pericytes + ECs** | 34 ± 5*** | 5 |
| IMPs ± ECs | 36 ± 7*** | 3 – 5 |
| IMPs ± ECCM§ | 24 ± 5 | 15 |
Mean ± SD; n = 3 experiments with 3 wells per determination.
ECs added back at a ratio of 1:4.
Significantly different from wild type pericyte and IMP cultures (P < 0.01).
Conditioned medium from three-day old primary EC cultures plated at 106 cells/ml.
ECs augment sphere formation
1) Pericytes differentiate directly from cultured capillaries
Freshly isolated capillaries from the Immortomouse® or from wild type mice were cultured in stem cell medium containing bFGF and incubated under permissive or non-permissive conditions. At 37°C, both wild type (Fig. 7A–C) and IMPs (Fig. 7D–F) initially form spheres in association with capillaries which subsequently detach and are released into the medium. Capillary-generated IMP spheres differentiate within five days in culture and contain NF+, GFAP+, and O4+ cells (Fig. 7G). This differentiation is much faster than that observed for spheres derived from primary purified IMPs or wild type pericytes (Fig. 7H) (Dore-Duffy, 2006), suggesting that the native capillary niche and/or EC–derived trophic factor(s) enhance self-renewal and differentiation (Fig. 7E).
2) Cultured pericytes respond to ECs
To further explore the possibility that ECs provide trophic support for pericytes, we examined pericyte self-renewal and differentiation with fresh EC growth medium or EC-conditioned medium. Primary purified pericytes or IMPs were co-cultured with ECs at a ratio of 4:1 to recapitulate the typical ratio observed in mouse CNS. Table 1 shows that the co-cultures enhance both the number and rate of pericyte derived-sphere formation. In contrast, conditioned medium from primary EC cultures grown at high or low densities do not significantly enhance neurosphere formation. Table 2 indicates that EC/pericyte co-cultures also increase both the rate of self-renewal and differentiation of pericytes. Together, these results support a trophic role for ECs in vitro that may also occur in the vascular niche. That EC-derived conditioned medium obtained from growth at two cell densities did not modify pericyte proliferation or differentiation, which suggests that cell-to-cell contact or cell contact-mediated release of substance(s) is necessary to modify pericyte stem cell behavior. Additional experiments will be required to confirm these observations and to demonstrate the mechanism.
Table 2.
Pericyte and IMP self-renewal at 37°C
| # Primary Spheres | # Secondary Spheres* | # Differentiated Spheres** | |
|---|---|---|---|
| Wild type pericytes | 10 | 29 ± 10 | 15 ± 11 |
| Wild type pericytes + ECsa | 25 | 49 ± 22*** | 35 ± 21*** |
| IMPs | 31 | 56 ± 19 | 25 ± 11 |
| IMPs + ECs | 45 | 71 ± 30*** | 42 ± 25*** |
Primary spheres were disrupted after two weeks (mean ± SD).
Mean number of differentiated spheres in secondary sphere preparation after two weeks (n = 2 experiments, > 10 primary spheres disrupted per experiment).
Significantly different from wild type pericyte and IMP cultures (P < 0.01).
5 × 104 ECs were added to 104 pericytes, the density of cells resuspended in stem cell medium was equal.
Discussion
The Immortomouse®, which expresses the tsSV40 T-Ag protein, has been extremely useful for generating stable cell lines from a number of tissues (Holley and Lawlor, 1997). In general, immortalized cells proliferate under permissive conditions and do not differentiate until they are cultured at 37°C in the absence of IFNγ. In our study, we have isolated brain pericytes (IMPs) from these animals. IMPs express T-Ag and retain the typical morphological appearance of wild type cultured pericytes (Gitlin and D’Amore, 1983; Shepro and Morel, 1993; Balabanov and Dore-Duffy, 1998; Edelman et al., 2006; von Tell et al., 2006). Similar to these cells, IMPs exhibit numerous blunted projections and are not contact inhibited when seeded at high density and grown at 37°C. The cells express the classic pericyte markers, NG2 and PDGFβR, and do not express endothelial markers such as αSMA, GSA and factor VIII.
Freshly isolated primary wild type pericytes cultured at 37°C undergo an initial lag period of 1–3 days during which proliferation is quite low (Gitlin and D’Amore, 1983; Balabanov et al., 1996). Thereafter, the rate of proliferation increases (data not shown). Similarly, cultured primary IMPs are initially quiescent at 37°C in low density cultures (Fig. 2A) and after longer incubation at 37°C, but not 33°C, these cultures proliferate. In high density cultures at 33°C, IMPs also proliferate after an initial lag, but they do so at a slower rate than wild type pericytes grown under similar conditions (Fig. 2B). These data suggest that the overall characteristics of proliferation of IMPs are similar to wild type cells except for a slower growth rate.
In the Immortomouse®, the activities of p53 and the Rb proteins are repressed by T-Ag expression from the transgene, which enables many cell types to proliferate indefinitely at 33°C (as reviewed, Fanning, 1992). Surprisingly, IMPs grown under permissive conditions proliferate slowly, and only in high density cultures, which suggests that cell cycle regulation in these cells may differ from other Immortomouse®-derived cell types. For example, IMPs may express additional cell cycle regulators that are only partially repressed or resistant to T-Ag inhibition. Alternatively, rapid proliferation of IMPs first may require differentiation or a lineage restriction step that is blocked by T-Ag. It is currently unclear what this step may involve but in view of the behavior of wild type pericytes, which only proliferate in culture after becoming αSMA+, the expression of this protein may be a marker of differentiation.
Alterations in cell cycle regulation or maintenance of quiescence have been described for both adult and embryonic stem cells (Dennis and Caplan 1996a; Paquet-Fifield et al, 2009; Sakai et al, 2010), although we cannot exclude the possibility that pericytes are heterogeneous cells, some of which may be mitotic while others are quiescent (Doherty and Canfield, 1999). Alternatively, pericytes have been reported to differentiate along the mesenchymal lineage and the slow rate of replication that we observe may stem from proliferation of a precursor population(s) derived from differentiated pericytes (Doherty and Canfield, 1999). However, we consider this explanation to be unlikely because IMPs do not differentiate into αSMA+ mesenchymal precursors under permissive conditions.
In their capillary location, less than 10% of wild type pericytes express αSMA; however, those pericytes that are located at pre-capillary arteriolar junctions and on post-capillary venules are αSMA+ under normal conditions (Dore-Duffy, 2008). Expression of αSMA may be induced in vivo or in vitro under a number of pathological conditions and is thought to reflect differentiation and/or a mechanism by which focal capillary blood flow can be regulated (Dennis and Caplan, 1996a; Peppiatt et al 2006; Dore-Duffy, 2008; Paquet-Fifield et al, 2009; Kutcher and Herman 2009; Sakai et al, 2010). In primary cultures, αSMA is induced in 100% of pericytes by five to seven days at 37°C depending on the plating density. Thus, induction of αSMA may be associated with functional and perhaps phenotypic differentiation. IMPs cultured for long periods at 33°C do not express αSMA and are quiescent at normal density. They are pluripotent and differentiate along both the neuronal and mesenchymal lineages when cultured at 37°C.
Pericytes have long been known to exhibit stem cell properties (Shepro and Morel, 1993; as reviewed, Dore-Duffy, 2008) and to differentiate into fibroblasts (Katenkamp and Stiller, 1975). The mesenchymal potential of these cells has been described by a number of laboratories, including the elegant studies by Canfield and colleagues (Schor et al., 1995; Doherty and Canfield, 1999). We have extended these studies by showing that CNS capillary pericytes are adult pluripotential stem cells (Dore-Duffy, 2006). Subsequent studies have confirmed these findings and have demonstrated that pericytes from other organs serve as a local source for progenitor cells (Crisan et al, 2007; Dore-Duffy, 2008). For example, liver pericytes (Ito cells, stellate cells) are liver cell progenitors (Beltrami et al., 2007; Jones et al., 1995; Suematsu and Aiso, 2001) and skin pericytes are the source of regenerating skin tissue in adults (Lardon et al. 2002; Dore-Duffy et al, 2006). More recent studies have identified CD146 in pluripotent skin pericytes (Paquet-Fifield et al, 2009). Purified pericytes also demonstrate high myogenic potential in culture and in vivo (Rajkumar et al., 2005; Crisan et al., 2007; Dellavalle et al., 2007; Kadoya et al., 2008). Davidoff et al., (2004) reported that cells of testicular blood vessels (vascular smooth muscle cells or pericytes) are the progenitors of Leydig cells and express nestin, as do stem cells of the nervous system. Recent studies have shown that the pulp of human teeth contains a population of cells with stem cell properties that may originate from pericytes (Lovschall et al., 2007).
Although ostensibly quiescent cells, pericytes have a self-renewal capacity that is comparable to primitive mesenchymal cells and, with appropriate signals, give rise to proliferative and committed progenitor cell lineages (Jones et al., 1995). Currently, it is unclear if vascular pericytes represent a progenitor population that has retained the ability to transdifferentiate to a mesenchymal stem cell associated with induction of αSMA, or if pericytes are primitive quiescent cells in the vascular location (Lovschall et al., 2007; Polchert et al., 2008; Trivedi and Hematti, 2008).
Primary pericytes form neurospheres in response to bFGF (Dore-Duffy et al 2006). When these aggregates are disrupted, the cells express markers for neurons, immature astrocytes, oligodendrocytes, pericytes, and other cell types expressing marker combinations suggestive of immature progenitors such as 04 and GFAP. We do not know if these cells are maintained in neurospheres grown for prolonged periods or whether they differentiate to single subsets on incubation. In the current study, we have not disrupted IMP-derived neurospheres or used confocal microscopy to determine if apparent marker co-localization in Figs 6 and 7 reflects dual expression of markers in immature progenitors. However, co-localization is not observed in all cells within the neurosphere. Whichever the case, our data show that IMPs appear to represent pre-αSMA+ cells that are morphologically and phenotypically comparable to freshly isolated pericytes and retain stem cell activity.
The current study reveals important properties of IMPs over primary wild type pericytes, which are that they can be maintained in culture indefinitely without differentiating and that they can be frozen and thawed without changes in morphology or marker expression. These properties have significant experimental advantages. For example, signaling experiments can be conveniently timed for studying synchronized pericyte differentiation by growing IMPs at the permissive and non-permissive temperatures as necessary. In addition, culture conditions that are conducive to proliferation without differentiation can be determined to enable expansion and characterization of this enigmatic cell type.
Here we show that IMPs in their capillary location in ex vivo cultures can be induced to proliferate and differentiate. Similar to IMPs, wild type capillary pericytes generate neurospheres that bud or migrate from vessels at significantly higher rates than those derived from single cells. Furthermore, co-culturing primary pericytes with ECs enhances neurosphere formation. These data suggest a trophic role for ECs within the vascular niche that cannot be substituted by EC conditioned medium, although we cannot exclude the possibility that growth factors secreted by ECs are too dilute in the conditioned medium to exert biological activity. Also, we cannot discount the possibility that EC-pericyte interactions involve indirect signaling mechanisms through matrix proteins. For example, perlecan is known to augment neurogenesis (Girós et al, 2007; Kerever et al, 2007). In addition, a recent study (Arai and Lo, 2009) shows that ECs stimulate the formation of oligodendrocyte progenitors in the absence of cell-to-cell contact. Currently, it is unclear why our results do not confirm the previous study, although that work was performed using EC cell lines rather than primary cell cultures.
In conclusion, we report the characterization of mouse brain IMPs which neither differentiate nor proliferate when cultured at 33°C in the presence of IFNγ and exhibit several characteristics of quiescent stem cells. At 37°C in the presence of serum, IMPs differentiate along the mesenchymal lineage (comparable to wild type pericytes) and form bone nodules. IMPs can be induced to differentiate along the neuronal lineage at 37°C in serum-free medium in the presence of bFGF and ECs augment their generation and rate of differentiation. A major advantage of IMPs over wild type pericytes is their utility in the timing of pericyte stem cell experiments and for studying the regulation of quiescence and self-renewal. Thus, our results indicate that large-scale purification of IMPs will be a convenient source of adult multipotent stem cells. Continued investigation of IMPs will enable us to determine the regulatory networks governing pericyte pluripotential stem cell activity and the transition from quiescence.
Highlights.
We characterize the morphology and cell type specific markers of immortalized pericytes from the Immortomouse
Immortalized pericytes (IMPs) are alpha-smooth muscle actin negative when grown for long periods at 33 degrees C and express NG2 and PDGF receptor
IMPs retain the potential to differentiate along mesodermal and neural cell lineages under appropriate culture conditions
Generation of neurospheres in IMP cultures is enhanced by the presence of endothelial cells
Acknowledgments
A.G. acknowledges technical assistance from Kevin Olson. This work is supported by grants to P.D.D. from NINDS, NIH (NS47672) and the National Multiple Sclerosis Society (CA1042A8, PP1517) and to A.G. from NINDS, NIH, (NS43783) and the National Multiple Sclerosis Society (RG2891 and RG4078).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009;29:4351–5. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asashima T, Iizasa H, Terasaki T, Hosoya K, Tetsuka K, Ueda M, Obinata M, Nakashima E. Newly developed rat brain pericyte cell line, TR-PCT1, responds to transforming growth factor-beta1 and beta-glycerophosphate. Eur J Cell Biol. 2002;81:145–52. doi: 10.1078/0171-9335-00227. [DOI] [PubMed] [Google Scholar]
- Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neuroscience Res. 1998;53:637–44. doi: 10.1002/(SICI)1097-4547(19980915)53:6<637::AID-JNR1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Balabanov R, Washington R, Wagnerova J, Dore-Duffy P. CNS microvascular pericytes express macrophage-like function, cell surface integrin alpha M, and macrophage marker ED-2. Microvasc Res. 1998;52:127–42. doi: 10.1006/mvre.1996.0049. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Barald KF, Lindberg KH, Hardiman K, Kavka AI, Lewis JE, Victor JC, Gardner CA, Poniatowski A. Immortalized cell lines from embryonic avian and murine otocysts: tools for molecular studies of the developing inner ear. Int J Dev Neurosci. 1997;15:523–40. doi: 10.1016/s0736-5748(96)00108-6. [DOI] [PubMed] [Google Scholar]
- Barber RD, Henderson RM. Inhibition by P1075 and pinacidil of a calcium-independent chloride conductance in conditionally-immortal renal glomerular mesangial cells. Br J Pharmacol. 1996;119:772–8. doi: 10.1111/j.1476-5381.1996.tb15739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, D’Aurizio F, Verardo R, Piazza S, Pignatelli A, Poz A, Baccarani U, Damiani D, Fanin R, Mariuzzi L, Finato N, Masolini P, Burelli S, Belluzzi O, Schneider C, Beltrami CA. Multipotent cells can be generated in vitro from several adult human organs (heart, liver and bone marrow) Blood. 2007;110:3438–46. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- Bernstein LR, Antoniades H, Zetter BR. Migration of cultured vascular cells in response to plasma and platelet-derived factors. J Cell Sci. 1982;56:71–82. doi: 10.1242/jcs.56.1.71. [DOI] [PubMed] [Google Scholar]
- Berrone E, Beltramo E, Buttiglieri S, Tarallo S, Rosso A, Hammes HP, Porta M. Establishment and characterization of a human retinal pericyte line: a novel tool for the study of diabetic retinopathy. Int J Mol Med. 2009;23:373–8. doi: 10.3892/ijmm_00000141. [DOI] [PubMed] [Google Scholar]
- Bowman PD, Betz AL, Goldstein GW. Primary culture of microvascular endothelial cells from bovine retina: selective growth using fibronectin coated substrate and plasma derived serum. In Vitro. 1982;18:626–32. doi: 10.1007/BF02796395. [DOI] [PubMed] [Google Scholar]
- Brighton CT, Lorich DG, Kupcha R, Reilly TM, Jones AR, Woodbury RA., 2nd The pericyte as a possible osteoblast progenitor cell. Clin Orthop Relat Res. 1992;275:287–99. [PubMed] [Google Scholar]
- Canfield AE, Sutton AB, Hoyland JA, Schor AM. Association of thrombospondin-1 with osteogenic differentiation of retinal pericytes in vitro. J Cell Sci. 1996;109:343–53. doi: 10.1242/jcs.109.2.343. [DOI] [PubMed] [Google Scholar]
- Chambers TJ, Owens JM, Hattersley G, Jat PS, Noble MD. Generation of osteoclast-inductive and osteoclastogenic cell lines from the H-2KbtsA58 transgenic mouse. Proc Natl Acad Sci USA. 1993;90:5578–5582. doi: 10.1073/pnas.90.12.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chunmeng S, Tianmin C. Skin: a promising reservoir for adult stem cell populations. Med Hypotheses. 2004;62:683–8. doi: 10.1016/j.mehy.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Collett GD, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96:930–8. doi: 10.1161/01.RES.0000163634.51301.0d. [DOI] [PubMed] [Google Scholar]
- Crisan M, Chen CW, Corselli M, Andriolo G, Lazzari L, Péault B. Perivascular multipotent progenitor cells in human organs. Ann N Y Acad Sci. 2009;1176:118–23. doi: 10.1111/j.1749-6632.2009.04967.x. [DOI] [PubMed] [Google Scholar]
- Crisan M, Zheng B, Zambidis ET, Yap S, Tavian M, Sun B, Giacobino JP, Casteilla L, Huard J, Péault B. Blood vessels as a source of progenitor cells in human embryonic and adult life. In: Bilko NM, Fehse B, Ostertag W, Stocking C, Zander AR, editors. Stem cells and their potential for clinical application, NATO Security through Science Series (A: Chemistry and Biology) Springer; Netherlands: 2007. pp. 137–147. [Google Scholar]
- Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF, Müller D. Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol. 2004;167:935–44. doi: 10.1083/jcb.200409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sachetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–67. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Dennis JE, Caplan AI. Analysis of the developmental potential of conditionally immortal marrow-derived mesenchymal progenitor cells isolated from the H-2Kb-tsA58 transgenic mouse. Connect Tissue Res. 1996a;35(1–4):93–9. doi: 10.3109/03008209609029179. [DOI] [PubMed] [Google Scholar]
- Dennis JE, Caplan AI. Differentiation potential of conditionally immortalized mesenchymal progenitor cells from adult marrow of a H-2Kb-tsA58 transgenic mouse. J Cell Physiol. 1996b;167:523–38. doi: 10.1002/(SICI)1097-4652(199606)167:3<523::AID-JCP16>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Diaz-Flores L, Gutierrez R, Lopez-Alonso A, Gonzalez R, Varela H. Pericytes as a supplementary source of osteoblasts in periosteal osteogenesis. Clin Orthop Relat Res. 1992;275:280–6. [PubMed] [Google Scholar]
- Doherty MJ, Canfield AE. Gene expression during vascular pericyte differentiation. Crit Rev Eukaryot Gene Expr. 1999;9:1–17. [PubMed] [Google Scholar]
- Doherty MJ, Ashton BA, Walsh S, Beresford JN, Grant ME, Canfield AE. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res. 1998;13:828–38. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P. Isolation and characterization of cerebral microvascular pericytes. Methods Mol Med. 2003;89:375–82. doi: 10.1385/1-59259-419-0:375. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P. Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des. 2008;14:1581–93. doi: 10.2174/138161208784705469. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–24. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Owen C, Balabanov R, Murphy S, Beaumont T, Rafols J. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvascular Res. 2000;60:55–69. doi: 10.1006/mvre.2000.2244. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Wang X, Mehedi A, Kreipke CW, Rafols JA. Differential expression of capillary VEGF isoforms following traumatic brain injury. Neurol Res. 2007;29:395–403. doi: 10.1179/016164107X204729. [DOI] [PubMed] [Google Scholar]
- Duda DG, Cohen KS, di Tomaso E, Au P, Klein RJ, Scadden DT, Willett CG, Jain RK. Differential CD146 expression on circulating versus tissue endothelial cells in rectal cancer patients: implications for circulating endothelial and progenitor cells as biomarkers for antiangiogenic therapy. J Clin Oncol. 2006 Mar 20;24(9):1449–53. doi: 10.1200/JCO.2005.04.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman DA, Jiang Y, Tyburski J, Wilson RF, Steffes C. Pericytes and their role in microvasculature homeostasis. J Surg Res. 2006;135:305–11. doi: 10.1016/j.jss.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Ehler E, Jat PS, Noble MD, Citi S, Draeger A. Vascular smooth muscle cells of H-2Kb-tsA58 transgenic mice. Characterization of cell lines with distinct properties. Circulation. 1995;92:3289–96. doi: 10.1161/01.cir.92.11.3289. [DOI] [PubMed] [Google Scholar]
- Fanning E. Simian virus 40 large T antigen: the puzzle, the pieces, and the emerging picture. J Virol. 1992;66:1289–93. doi: 10.1128/jvi.66.3.1289-1293.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girós A, Morante J, Gil-Sanz C, Fairén A, Costell M. Perlecan controls neurogenesis in the developing telencephalon. BMC Dev Biol. 2007;5:29. doi: 10.1186/1471-213X-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin JD, D’Amore PA. Culture of retinal capillary cells using selective growth media. Microvascular Res. 1983;26:74–80. doi: 10.1016/0026-2862(83)90056-0. [DOI] [PubMed] [Google Scholar]
- Groves AK, Entwistle A, Jat PS, Noble M. The characterization of astrocyte cell lines that display properties of glial scar tissue. Dev Biol. 1993;159:87–104. doi: 10.1006/dbio.1993.1223. [DOI] [PubMed] [Google Scholar]
- Herrera MB, Bruno S, Buttiglieri S, Tetta C, Gatti S, Deregibus MC, Bussolati B, Camussi G. Isolation and characterization of a stem cell population from adult human liver. Stem Cells. 2006;24:2840–50. doi: 10.1634/stemcells.2006-0114. [DOI] [PubMed] [Google Scholar]
- Holley MC, Lawlor PW. Production of conditionally immortalised cell lines from a transgenic mouse. Audiol Neurootol. 1997;2:25–35. doi: 10.1159/000259227. [DOI] [PubMed] [Google Scholar]
- Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S, Price J, Modo M. Effect of inflammatory cytokines on major histocompatibility complex expression and differentiation of human neural stem/progenitor cells. Stem Cells. 2008;26:2444–54. doi: 10.1634/stemcells.2008-0116. [DOI] [PubMed] [Google Scholar]
- Jones AR, Clark CC, Brighton CT. Microvessel endothelial cells and pericytes increase proliferation and repress osteoblast phenotypic markers in rat calvarial bone cell cultures. J Orthop Res. 1995;13:553–61. doi: 10.1002/jor.1100130410. [DOI] [PubMed] [Google Scholar]
- Joó F, Karnushina I. A procedure for the isolation of capillaries from rat brain. Cytobios. 1973;8:41–8. [PubMed] [Google Scholar]
- Kachar B, Brownell WE, Altschuler R, Fex J. Electrokinetic shape changes of cochlear outer hair cells. Nature. 1986;322:365–8. doi: 10.1038/322365a0. [DOI] [PubMed] [Google Scholar]
- Kadoya K, Fukushi JI, Matsumoto Y, Yamaguchi Y, Stallcup WB. NG2 proteoglycan expression in mouse skin: altered postnatal skin development in the NG2 null mouse. J Histochem Cytochem. 2008;56:295–303. doi: 10.1369/jhc.7A7349.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda S, Landgren E, Ljungström M, Claesson-Welsh L. Fibroblast growth factor receptor 1-induced differentiation of endothelial cell line established from tsA58 large T transgenic mice. Cell Growth Differ. 1996;7:383–395. [PubMed] [Google Scholar]
- Katenkamp D, Stiller D. Cellular composition of the so-called dermatofibroma (histiocytoma cutis) Virchows Arch A Pathol Anat Histol. 1975;367:325–36. doi: 10.1007/BF01239339. [DOI] [PubMed] [Google Scholar]
- Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT, Mercier F. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–57. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- Kershaw TR, Rashid-Doubell F, Sinden JD. Immunocharacterization of H-2Kb-tsA58 transgenic mouse hippocampal neuroepithelial cells. Neuroreport. 1994;5:2197–2200. doi: 10.1097/00001756-199410270-00052. [DOI] [PubMed] [Google Scholar]
- Lardon J, Rooman I, Bouwens L. Nestin expression in pancreatic stellate cells and angiogenic endothelial cells. Histochem Cell Biol. 2002;117:535–540. doi: 10.1007/s00418-002-0412-4. [DOI] [PubMed] [Google Scholar]
- Li Q, Yu Y, Bischoff J, Mulliken JB, Olsen BR. Differential expression of CD146 in tissues and endothelial cells derived from infantile haemangioma and normal human skin. J Pathol. 2003;201:296–302. doi: 10.1002/path.1443. [DOI] [PubMed] [Google Scholar]
- Lovschall H, Mitsiadis TA, Poulsen K, Jensen KH, Kjeldsen AL. Coexpression of Notch3 and Rgs5 in the pericyte-vascular smooth muscle cell axis in response to pulp injury. Int J Dev Biol. 2007;51:715–21. doi: 10.1387/ijdb.072393hl. [DOI] [PubMed] [Google Scholar]
- Middleton J, Americh L, Gayon R, Julien D, Mansat M, Mansat P, Anract P, Cantagrel A, Cattan P, Reimund JM, Aguilar L, Amalric F, Girard JP. A comparative study of endothelial cell markers expressed in chronically inflamed human tissues: MECA-79, Duffy antigen receptor for chemokines, von Willebrand factor, CD31, CD34, CD105 and CD146. J Pathol. 2005;206:260–8. doi: 10.1002/path.1788. [DOI] [PubMed] [Google Scholar]
- Morgan JE, Beauchamp JR, Pagel CN, Peckham M, Ataliotis P, Jat PS, Noble MD, Farmer K, Partridge TA. Myogenic cell lines derived from transgenic mice carrying a thermolabile T antigen: a model system for the derivation of tissue-specific and mutation-specific cell lines. Dev Biol. 1994;162:486–98. doi: 10.1006/dbio.1994.1103. [DOI] [PubMed] [Google Scholar]
- Nadal JA, Scicli GM, Carbini LA, Nussbaum JJ, Scicli AG. Angiotensin II and retinal pericytes migration. Biochem Biophys Res Commun. 1999;266:382–5. doi: 10.1006/bbrc.1999.1834. [DOI] [PubMed] [Google Scholar]
- Noble M, Groves AK, Ataliotis P, Jat PS. From chance to choice in the generation of neural cell lines. Brain Pathology. 1992;2:39–46. [PubMed] [Google Scholar]
- Ozerdem V, Monosov E, Stallcup WB. NG2 proteoglycan expression by pericytes in pathological microvasculature. Microvascular Res. 2002;63:129–34. doi: 10.1006/mvre.2001.2376. [DOI] [PubMed] [Google Scholar]
- Ozerdem V, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–27. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Paquet-Fifield S, Schlüter H, Li A, Aitken T, Gangatirkar P, Blashki D, Koelmeyer R, Pouliot N, Palatsides M, Ellis S, Brouard N, Zannettino A, Saunders N, Thompson N, Li J, Kaur P. A role for pericytes as microenvironmental regulators of human skin tissue regeneration. J Clin Invest. 2009;119:2795–806. doi: 10.1172/JCI38535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis K, Le ON, Russo P, St-Cyr M, Fournier H, Bu D. Characterization and response to interleukin 1 and tumor necrosis factor of immortalized murine biliary epithelial cells. Gastroenterology. 1995;109:1308–15. doi: 10.1016/0016-5085(95)90593-6. [DOI] [PubMed] [Google Scholar]
- Péault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T, Gussoni E, Kunkel LM, Huard J. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15:867–77. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B, Bartholomew A. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745–55. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar VS, Howell K, Csiszar K, Denton CP, Black CM, Abraham DJ. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res Ther. 2005;7:R1113–23. doi: 10.1186/ar1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Oka K, Matsumoto K, Nakamura T. p27 Nuclear localization and growth arrest caused by perlecan knockdown in human endothelial cells. Biochem Biophys Res Commun. 2010;12:403–8. doi: 10.1016/j.bbrc.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Schor AM, Canfield AE, Sutton AB, Arciniegas E, Allen TD. Pericyte differentiation. Clin Orthop Relat Res. 1995;313:81–91. [PubMed] [Google Scholar]
- Schor AM, Canfield AE, Sutton AB, Allen TD, Sloan P, Schor SL. The behaviour of pericytes in vitro: relevance to angiogenesis and differentiation. EXS. 1992;61:167–78. doi: 10.1007/978-3-0348-7001-6_26. [DOI] [PubMed] [Google Scholar]
- Shepro D, Morel NM. Pericyte physiology. Faseb J. 1993;7:1031–38. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- Suematsu M, Aiso S. Professor Toshio Ito: a clairvoyant in pericyte biology. Keio J Med. 2001;50:66–71. doi: 10.2302/kjm.50.66. [DOI] [PubMed] [Google Scholar]
- Takacs-Jarrett M, Sweeney WE, Avner ED, Cotton CU. Generation and phenotype of cell lines derived from CF and non-CF mice that carry the H-2K(b)-tsA58 transgene. Am J Physiol Cell Physiol. 2001;280:C228–36. doi: 10.1152/ajpcell.2001.280.1.C228. [DOI] [PubMed] [Google Scholar]
- Takacs-Jarrett M, Sweeney WE, Avner ED, Cotton CU. Morphological and functional characterization of a conditionally immortalized collecting tubule cell line. Am J Physiol. 1998;275:F802–11. doi: 10.1152/ajprenal.1998.275.5.F802. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp Hematol. 2008;36:350–9. doi: 10.1016/j.exphem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–9. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Walther N, Jansen M, Ergün S, Kascheike B, Ivell R. Sertoli cell lines established from H-2Kb-tsA58 transgenic mice differentially regulate the expression of cell-specific genes. Exp Cell Res. 1996;225:411–21. doi: 10.1006/excr.1996.0192. [DOI] [PubMed] [Google Scholar]
- Whitehead RH, Joseph JL. Derivation of conditionally immortalized cell lines containing the Min mutation from the normal colonic mucosa and other tissues of an ‘Immortomouse’/Min hybrid. Epithelial Cell Biol. 1994;3:119–25. [PubMed] [Google Scholar]
- Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci USA. 1993;90:587–591. doi: 10.1073/pnas.90.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]


