Abstract
Disseminated cancer remains a largely fatal disease. While systemic therapy can have some initial success, it is rarely durable. Typically, populations of cancer cells resistant to therapy emerge quickly requiring progressively less effective 2nd, 3rd, and 4th line therapies until the patient succumbs. Cancer cells possess a large repertoire of heritable phenotypic strategies that can be used to confer resistance to one or more therapeutic drugs. In addition, environmental factors such as ischemia and hypoxia can reduce therapeutic effects by limiting drug delivery or toxicity. Here, we use a fitness generating function (G-function) approach to model tumor response with respect to evolutionary adaptation and micro-environmental conditions in response to various therapeutic strategies. We examine tumor cell death and evolution of resistance in single and two drug therapies as well as alternative “evolutionary” approaches. We demonstrate that even monotherapy would be highly successful in the absence of tumor evolution or environmentally-mediated resistance. However, environmental and evolutionary factors dramatically reduce the effectiveness of therapy. Two drug therapy in which adaptation requires two different phenotypic changes will maximally reduce tumor size and delay onset of resistance but actual eradication of the tumor population is rare. We demonstrate that multi-agent therapies in which the first drug both achieves tumor cell toxicity and drives phenotypic adaptation that renders the cell more vulnerable to a second therapy can be highly successful in maintaining durable tumor control. Examples of clinical trials that exploit these results are presented. We conclude that development of more lethal (cytotoxic) drugs is not likely to fundamentally change the outcome of therapy. Instead, new approaches that incorporate evolutionary strategies into target and drug selection are needed.
Keywords: Cancer therapy, tumor drug resistance, environmental resistance, phenotypic resistance, G-function, evolutionary adaptation, multi-drug therapy, double-bind therapy, predator facilitation
Introduction
With few exceptions, metastatic cancers remain incurable. A fundamental cause of treatment failures is the capacity of cancer cells to rapidly evolve therapeutic resistance. Many cancer therapies initially kill a large percentage of tumor cells resulting in tumor regression or stabilization. However, malignant cells have a significant evolutionary capacity and typically adapt to therapy through a large repertoire of available heritable phenotypic strategies. There are two general mechanisms of resistance: 1. Phenotypic adaptation in which cells evolve strategies, such as upregulation of p-glycoprotein, that allows survival in drug levels that would be lethal to the unadapted phenotype.1 2. De novo resistance in which pockets of cancer cells, although phenotypically sensitive, find protection by environmental factors such as ischemia and hypoxia that reduce the drug delivery and/or cytotoxicity.2 Repeated treatments yield subpopulations of resistant phenotypes that typically emerge resulting in cancer progression and patient death.
Typical cancer chemotherapy is administered in an evolutionarily static manner with drugs, doses, and schedules fixed according to rigid protocol. Therapy changes only in the event of unacceptable toxicity or unequivocal evidence of cancer progression. Yet, cancers are ecologically and evolutionarily dynamic systems that begin to adapt and change upon entry of the therapeutic agent. We have proposed that this mismatch between treatment and cancer dynamics contributes significantly to the rapid emergence of resistant subpopulations.
We propose that cancer therapy must become as adaptive and dynamic as the system it is treating. This requires application of Darwinian principles to understand the processes that lead to phenotypic adaptation and growth of tumor cells insensitive to therapy. Anticipating the evolutionary responses of cancers to treatment permits “evolutionarily enlightened” cancer therapy. We suggest conceptualizing the evolutionary dynamics of resistance in a manner analogous to the management and control of invasive pests.3 Attempts to eradicate disseminated invasive pests with chemical pesticides has been almost universally unsuccessful.4,5 However, control of pest populations with judicious use of pesticide or with biologic agents – predators, parasites, pathogens, and parasitoids – has often met with considerable success.6
A key factor in the evolutionary dynamics of adaptation to any therapy (whether pesticides or cytotoxic cancer drugs) is the phenotypic cost of resistance. In other words, successful adaptation to any toxic agent requires diversion of resources to support the phenotypic strategy. These resources are, therefore, not available for proliferation and, in the absence of the therapy, represent a decrease in fitness. For example, responses to pesticides typically require upregulation of xenobiotic metabolism. This is a low fitness cost adaptation but nevertheless is apparent in the common observation that resistance to a toxic agent declines with time after the agent is withdrawn. Interestingly, adaptations to a predator appear to involve a much larger change in phenotype that may compromise other vital activities. These types of adaptations may exact high fitness cost which is evident in the observations that pest control with biologic agents is generally far more successful and durable than with chemicals. Based on this, we propose cancer therapies that exact a high cost of adaptation will generally be more successful in controlling a population.7,8
An example of such strategies is the “evolutionary double-bind therapy” in which cellular adaptations to one treatment renders it increasingly vulnerable to a second therapeutic attack, and vice-versa. Thus, evolutionary approaches anticipate the cancer cell’s ability to evolve resistance and attempt to gain therapeutic benefit by either exploiting or blocking the expected adaptive strategies.
Here we examine the evolutionary dynamics of tumor cell adaptation to mono- or multi-drug chemotherapies using traditional treatment strategies. We demonstrate that the simulation outcomes are consistent with clinical observations. We then explore possible evolutionarily enlightened therapeutic strategies. Finally, we examine the consequences of the tumor microenvironment on all therapeutic treatments. We find that treatments that would ordinarily be effective in homogenous tumors fail when the cancer is non-homogeneous due to regions of hypoxia and ischemia.
Experimental Section
Tumor cell evolution to a population suppressing treatment
We model cancer treatments as “predators” on the growth rates of tumor cells. We start with a tumor population growing logistically, subjected to proliferation suppression from a treatment:
Where r is the cell’s growth rate in the absence of limitations, K is the carrying capacity, μ is cell mortality or suppression from the treatment, and x is the population density of tumor cells.
The proliferation suppression, μ, due to a chemotherapy regimen is constructed as a “predator” by considering the three aspects of ecological predation.9 The first of these is the encounter rate of predators with its prey. In ecology, this parameter increases with the abundance and activity of predators. This can easily be mapped to chemotherapy attributes such as dosage. The second aspect considers the lethality of the predator in the absence of vigilance of the prey. In ecology, this parameter increases with the ability of a prey to survive when directly confronted with a predator. For our model, this can be characterized as either the drug efficacy or the baseline resistance of a tumor cell to a chemotherapy. The third aspect considers the effectiveness of vigilance in reducing the lethality of the predator. In our model this translates to the effectiveness of resistance employed by a tumor cell.
Therefore, the term μ is given by:
where m is the dose of therapy administered, k is the baseline phenotypic resistance to a treatment in absence of any evolved resistance and may be high in some tumors indicating that the tumor is resistant to this form of therapy even prior to administration. The term be is also added as the amount of resistance that a tumor cell enjoys due to environmental factors such as hypoxia or ischemia.
The survival benefit to the cancer cell from evolving resistance in response to treatment is the term bp * v, where bp is the effectiveness of the resistance strategy in promoting safety, and v is the evolutionary strategy of the tumor cells in reaction to the treatment. The initial state of the tumor cell prior to evolving any level of resistance is represented by v = 0. Any intrinsic or a priori resistance is encompassed in the k term. With this expression, μ declines toward some minimum level as the cell’s strategy v increases away from 0 at a rate scaled by the effectiveness of the resistance strategy, bp. A small bp means that the resistance strategy is ineffective, a large bp means it is very effective at suppressing the treatment.
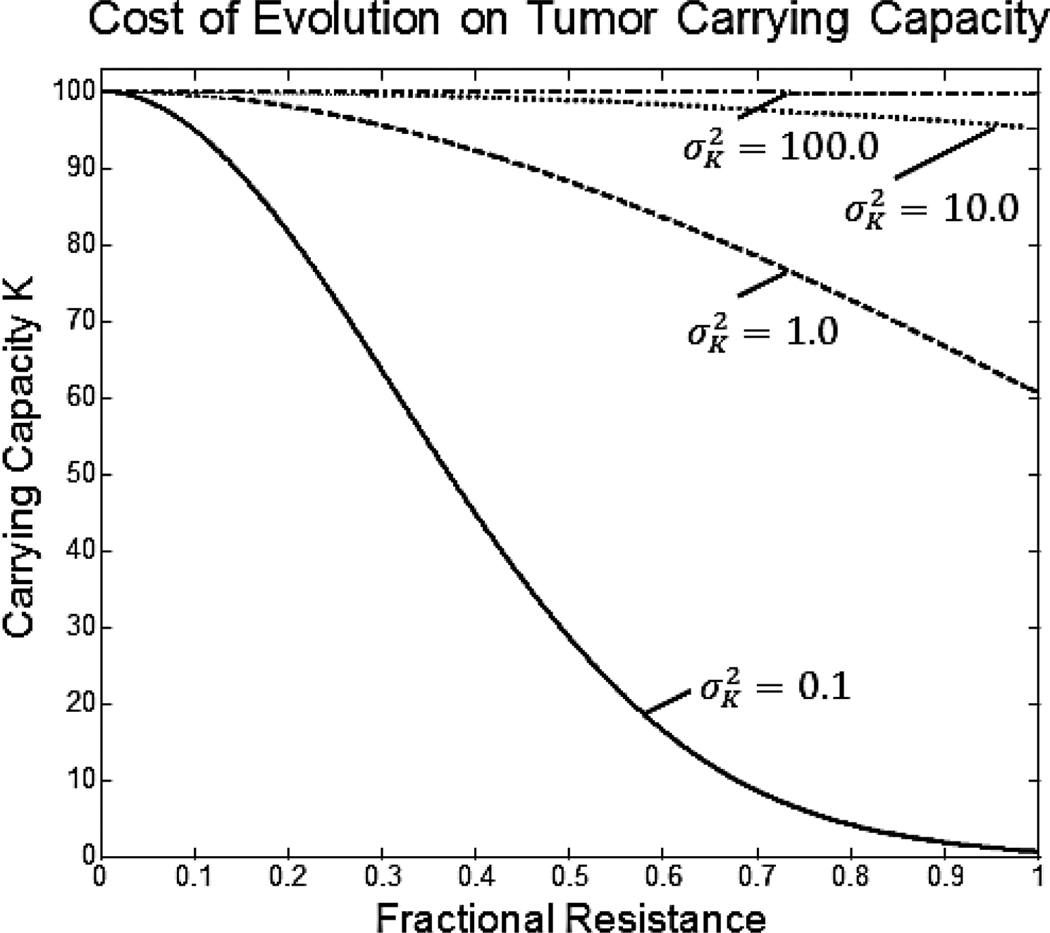
The cost of resistance in our model comes as a penalty to the tumor population’s carrying capacity, K. Resources cells use to defeat therapy toxicity are diverted from maintenance and proliferation, thus reducing carrying capacity:
Under this formulation, carrying capacity declines according to a Gaussian curve as a cell’s resistance strategy, v, deviates from 0. No resistance to the treatment, v = 0, maximizes carrying capacity at Kmax. The rate of decline in carrying capacity with the cell’s resistance strategy is determined by the variance term of the Gaussian curve, . For this model, does not represent the standard “niche breadth” as it would in ecological models. Rather, it represents the penalty to the tumor cell from evolving resistance. The value of will be a function of the actual resistance pathway used by the tumor cell. If evolving resistance requires simple upregulation of an already existing pathway, will be large resulting in a small penalty. On the other hand, if a significant phenotypic change is required, such as changes in metabolic processes, will be small resulting in a large penalty to the tumor cell when evolving treatment resistance. By anticipating the resistance pathway that tumor cells will utilize for a specific treatment, oncologists and drug researchers can predict treatment-specific costs of resistance to the cell.
The specific design of growth rate, r, proliferation suppression, μ, and evolutionary cost included in K of our model describe but one of many possible formulations. For example, it is possible that tumor cells may not be growing logistically, but instead grow exponentially or according to some other density-dependent growth model. Modeling how a treatment suppresses tumor growth may also take alternative forms depending on the mode of action (kinematics), tumor type and stage, or the explicit number of cells present in the treated population.10 The cost to the cell of evolving resistance may not only include reductions of carrying capacity, but also other growth rate parameters. We feel the present model captures the essential features of treatment resistance, and provides a tool for investigating its consequences and manifestations.
Darwinian Dynamics
Darwinian dynamics couples population dynamics with strategy dynamics to model the evolutionary process. Consider that a tumor is a population of cells that, by virtue of inheritance and common ancestry, have the same set of evolutionarily feasible phenotypes. We can describe their fitness and population dynamics using a fitness generating function or G-function.11 The vector, u, gives all of the phenotypic strategies currently present in the tumor
The vector, x, indicates the current population sizes of each of these extant strategies (xi is the population size of those cells with strategy u1)
The G-function gives the per capita growth rate of some focal cell using strategy v within a population of tumor cells described by u and x. This yields an evolutionary game among the tumor cells as each cell’s fitness is determined by its strategy and the strategies and population sizes of other cells. Our model of tumor growth in response to therapy gives the following G-function:
The fitness function for any individual using strategy ui can be obtained by evaluating G at v = ui. By changing v to any strategy of the strategy set, one can see how the fitness of a focal individual is influenced by its strategy, v, the strategies employed by the other cells, u, and the density of the cells, x. In our present model, the strategy of the focal individual, v, and the density of cells, x, directly influences the fitness of a focal cell. The strategies of other cells, u, do not directly influence the focal cell, but do so indirectly via the effect of others’ strategies on the cell density and the tumor’s growth rate (see below).
Following Fisher’s Fundamental Theorem of Natural Selection, the population’s mean strategy value evolves in the direction of increasing fitness given by the fitness gradient ∂G/∂v:
Where s scales the speed of evolutionary change.12 The speed of evolutionary change will be large for tumor populations with high genetic variability, high mutation rates, and high cell population size. The population size of the tumor changes with respect to G evaluated at v = ui.
Together the population dynamic, , and the strategy dynamic, , represent the complete Darwinian dynamics of the system. The ecological (changes in x) and evolutionary (changes in u) dynamics generally converge on an “evolutionarily stable strategy” (ESS). An ESS is a strategy (or coexisting set of strategies) which cannot be invaded by cells with rare alternative strategies. At an ESS the system becomes both ecologically and evolutionarily stable .13
Results and Discussion
The G-function can be used to examine the evolution of resistance in a tumor population subjected to chemotherapy regimens. We first evaluated a monotherapy requiring a single adaptive strategy. While monotherapy is relatively uncommon in current clinical oncology, it provides a useful starting point. Most modern cancer therapies employ multidrug regimens where the mechanism(s) of phenotypic resistance to the multiple drugs may be similar or different. Therefore, we next examine a multidrug therapy where the mechanism of adaptation to each of the drugs is the same. For example, upregulation of xenobiotic metabolism such as p-glycoprotein can sometimes confer resistance to multiple drugs.14 We evaluate a third therapy where the cell’s resistance strategy is drug-specific. The cell must evolve different drug-specific strategies to gain resistance to the combination therapy.
We let each of these basic models begin with a population density x = 100 representing the maximum carrying capacity of Kmax = 100. For consistency across analyses we set the growth rate to r = 0.1, the cost of resistance to , and the effectiveness of the resistance strategy to bp = 5. Let the tumor populations start with minimal baseline phenotypic resistance (k = 0.1) and no de-novo environmental resistance (be = 0.0).
We examined the monotherapy with a drug dosage of m1 = 0.1. A multidrug therapy requiring the same evolutionary response can be modeled by increasing the dose of an existing treatment. To do this we simply added the dosage of a second treatment with an exposure of 0.12 onto the prior exposure for a combined m1+2 = 0.22.
Next, we examined a multidrug therapy where each drug required a distinct phenotypic adaptation. In this example, the two treatments act independently in suppressing tumor growth and the tumor cell has treatment-specific resistance responses. The fitness function now includes a separate μ for each treatment and there is an associated phenotypic resistance strategy v1 and v2 associated with each treatment, respectively.
The two resistance strategies of the cell combine to exact a cost on the cell’s carrying capacity. We have kept the same Gaussian form as the previous examples but now add the strategies together to determine how they impact carrying capacity. For this example we kept m1 = 0.1 and m2 = 0.12 to compare against two treatments requiring one response.
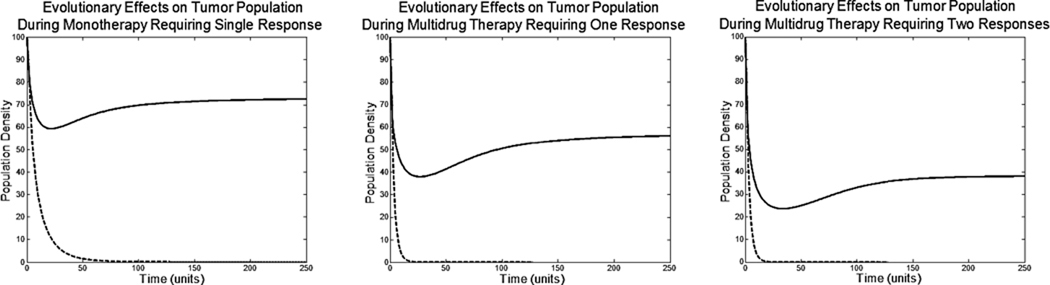
In the absence of any evolution, all three therapies successfully eliminated the tumor population. Unfortunately, with evolution, monotherapy resulted in only a 27% reduction in cell density at the ESS of u = 1.06. The multidrug therapy requiring a single strategy response resulted in a 43% reduction in cell density at an ESS of u = 1.45. This additional 16% drop is due to cells diverting more resources from proliferation to cope with the multidrug treatment. The multidrug therapy requiring two independent responses resulted in a 62% drop in cell density with an ESS of u1 = 0.93 and u2 = 1.02. Though resistance emerged more slowly when multiple adaptive strategies were required, the therapy eventually became ineffective.
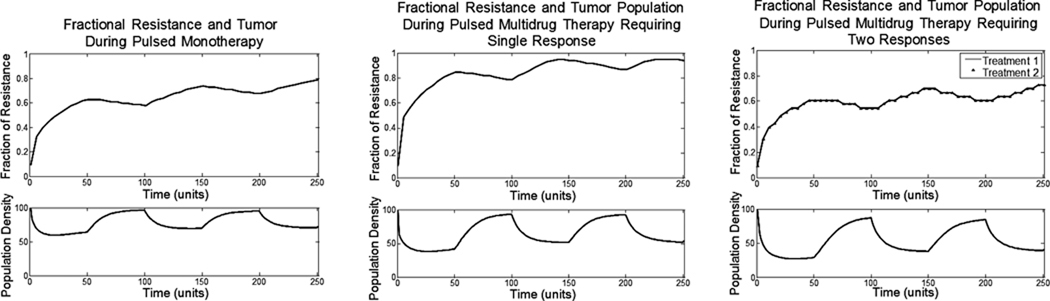
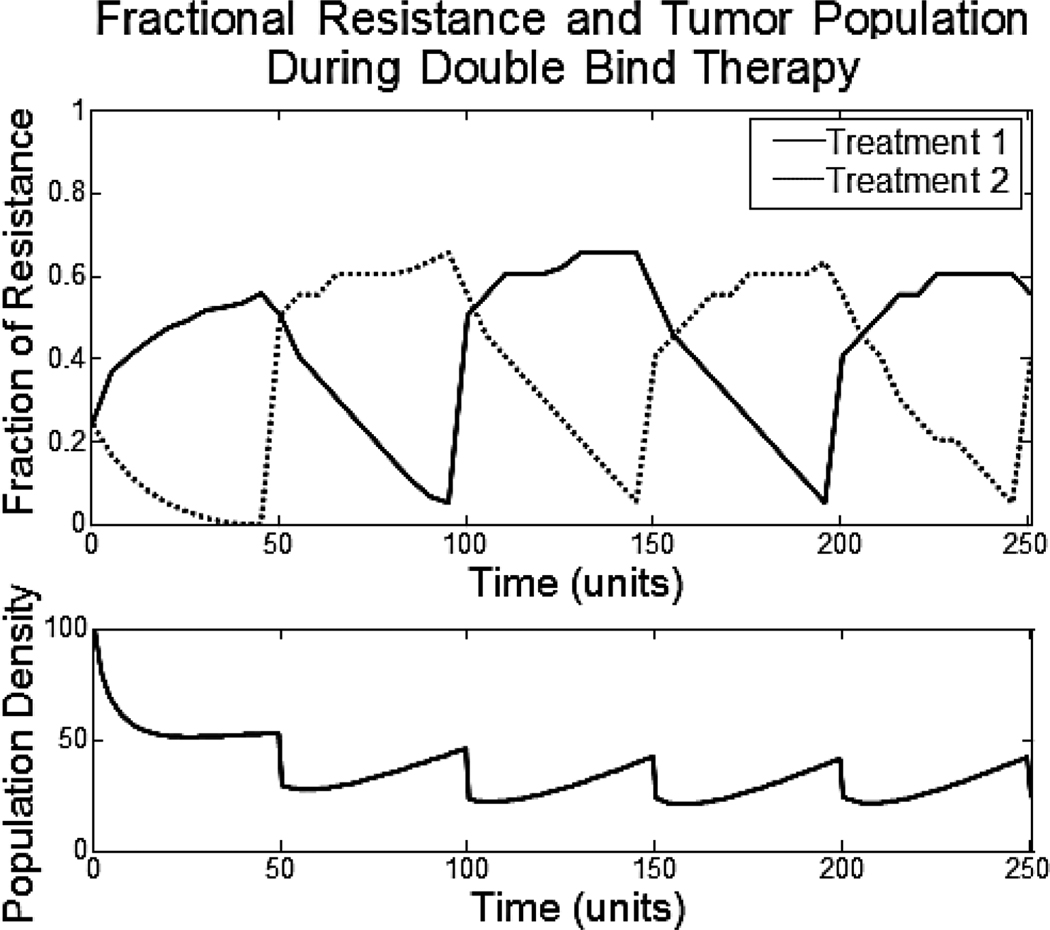
Commonly, chemotherapy involves an infusion of a drug into the patient. In between infusions, the drug clears the body and there are lengths of time with little to no drug present. We can model these sorts of pulsed treatments by alternating periods when the treatment is present (50 time units) with periods when it is absent (50 time units). For each of the three regimens we followed the ecological and evolutionary dynamics of the tumor cells (Fig. 3).
Figure 3.
The top axis shows the fractional resistance, vi, to the one or two treatments given over time. The bottom axis shows the population density of the tumor. Periods of treatment (50 time units) alternate with periods of no treatment (50 time units).
In all three cases, when the drug is not present in the system (exposure is zero), the tumor population grows logistically. Between treatments, tumor growth is only limited by the decreased carrying capacity caused by the tumor cells’ resistance strategy. With each treatment cycle the population size and resistance strategy of the cancer cells fluctuate; but the overall evolutionary trajectory is towards increased resistance. The increase in resistance when the treatment is active is much larger than the decrease in resistance that occurs when the drug is absent. In these types of mono and multidrug therapies, the cost of resistance is not great enough to evolve back to having no resistance. With enough cycles, the tumor population becomes completely resistant and the fluctuations in tumor size become small.
Thus, consistent with clinical observations, multiple drugs using different mechanisms for cell destruction and requiring different adaptive strategies improve tumor response. Unfortunately, the tumor cells can still adapt so that complete tumor eradication will not be commonly observed – again consistent with clinical observations.1
Evolutionary Double-Bind Therapies: Adaptation to one treatment increases vulnerability to the other
We propose that cancer therapy needs to become more strategic by anticipating the evolutionary response of the tumor cells and then exploiting it. One such approach uses one therapy to both kill cancer cells and drive evolution toward a phenotype that can then be targeted by a second therapy. This is similar to predator facilitation in which one predator is able to exploit the prey’s adaptive response to another predator.15,16 Here we alter the model so that resistance to one treatment makes the cell more vulnerable to the other treatment. In the formulation below, evolving resistance to one treatment reduces the effectiveness of evolving resistance to a second treatment, and vice versa.
All parameters have been kept the same as with the previous models shown. The two therapies are given sequentially, switching every 50 time units. This shows how each treatment drives the tumor cells toward a treatment-specific adaptation that makes them more susceptible to other treatment.
The double bind therapy begins by administering only the first drug. This forces the tumor cells to increase phenotypic resistance to the first drug and decrease phenotypic resistance to the second drug. This dynamic makes the tumor cells susceptible to the second drug in two distinct ways. First, the cells actually de-evolve their resistance to the second drug so as to enhance their effectiveness in adapting to the first drug. This leaves the cells directly susceptible to the second drug once administered. Second, if the cells evolve resistance to both drugs, the benefit of one cancels out the benefit of the other and vice versa, leaving the tumor cells sensitive to both drugs while continuing to exhaust resources to maintain resistance. Keeping this in mind, any evolution of resistance to the second drug will be less beneficial to the tumor cells until the strategy conferring resistance to the first drug decreases.
Though the tumor population may appear relatively stable after some time during this double bind treatment, the tumor population is constantly exhausting resources as it switches between evolutionary responses.
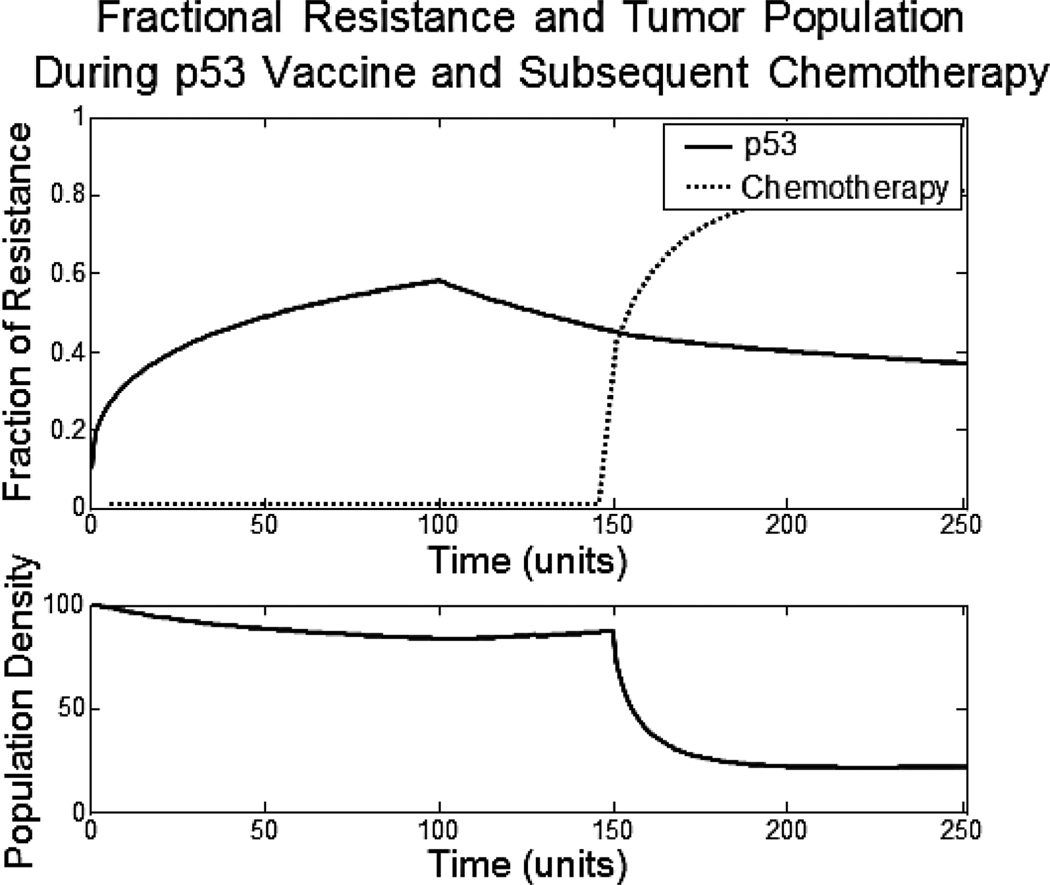
A recent study of the p53 cancer vaccine with chemotherapy may represent a double bind therapy.17 The study tested for the immunologic and clinical effects of the p53 cancer vaccine in patients with lung cancer. On its own, the p53 vaccine did not yield a significant clinical response. Yet, follow up chemotherapy achieved a response rate of 62%. Historically, the response rate to this second line chemotherapy is <8%. Furthermore, the best chemotherapy response was seen in those patients who had the best immune response (based on blood studies) to the p53 vaccine. It is likely that the tumor cells adapted to the immunotherapy by down-regulating p53, rendering them more vulnerable to the chemotherapy. Below we use the model to show this potential double bind (Fig. 5).
Figure 5.
The top axis shows the fractional resistance, vi, to the two treatments given over time. The bottom axis shows the population density of the tumor.
A treatment mimicking the p53 vaccine is given for the first 100 time units. This treatment is not cytotoxic. The only decrease in cancer cell population density comes from the decline in carry capacity, the cost of increasing resistance. In our model, the resistance to p53 increases to 0.6. During the next 50 time units, neither the p53 vaccine nor chemotherapy is administered. While there may be some loss of resistance to the p53 treatment during this time, it is not substantial. Lastly, the cytotoxic chemotherapy treatment, which is a double bind with the p53 vaccine, is administered for the last 100 time units. Due to the high resistance the cells have to the p53 vaccine, the chemotherapy has drastic and potentially decisive effects on the tumor population density.
Consequences of De-Novo Environmental Resistance
The tumor microenvironment can play a large role in conferring de novo resistance. The microenvironment of the tumor volume is primarily governed by vasculature structure and blood flow. A consistent, functioning blood supply reduces overall heterogeneity of the tumor by regularly supplying nutrients and removing metabolites. On the other hand, a dysfunctional vasculature creates chaotic blood flow and tumor sub regions of hypoxia and ischemia.18
Here we assume that environmental resistance results from these regions of hypoxia and ischemia that reduce local delivery of a drug. This is a simplification since other factors likely play a role such as hypoxia-induced reductions of the cytotoxicity of some drugs even in normal concentration because of the need for an oxygen radical intermediate to induce cell damage.19
To show the micro-environmental effects, we repeat the same three treatments discussed above but we give the tumor populations de-novo environmental resistance. All parameters are kept the same except for moderate environmental resistance. We change be = 0 (indicating no environmental resistance) to be = 1.
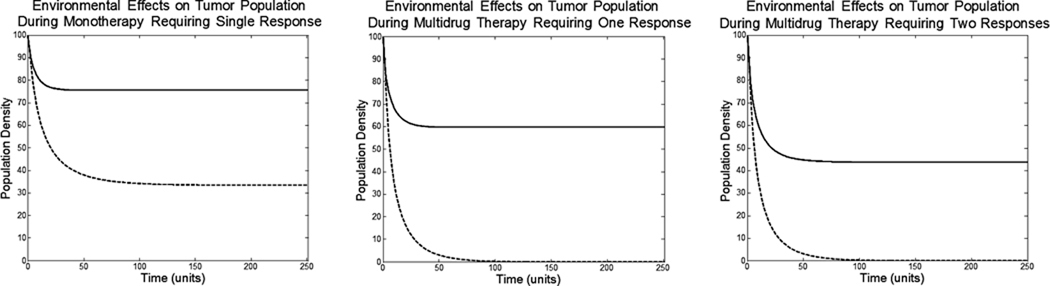
The de-novo environmental resistance renders monotherapy ineffective, even if the cells have no ability to evolve phenotypic resistance. Monotherapy causes only a 22% reduction in cell density at an ESS of u = 0.94. Multidrug therapy requiring just one strategy response causes a 40% reduction in cell density u = 1.33 and multidrug therapy requiring two responses causes a 52% decrease at u1 = 0.80 and u2 = 0.90.
The results generated from tumor populations enjoying no de-novo environmental resistance (figure 2) applies to cancers that have relatively uniform phenotypes and are well-perfused, such as testicular cancer and some lymphomas.20 However, the majority of epithelial cancers including lung, breast, colon, kidney, and pancreas are heterogeneous and give results more consistent with figure 6.21 Weak initial exposure to the treatment provides a refuge for tumor cells allowing a buildup of evolutionary resistance before irrecoverable losses in tumor cell population density.
Figure 2.
Without the ability of phenotypic evolution, shown as dotted lines, these treatments would be more than sufficient to eliminate the tumor. Unfortunately, with evolution of phenotypic resistance, treatments are unable to eradicate the tumor population.
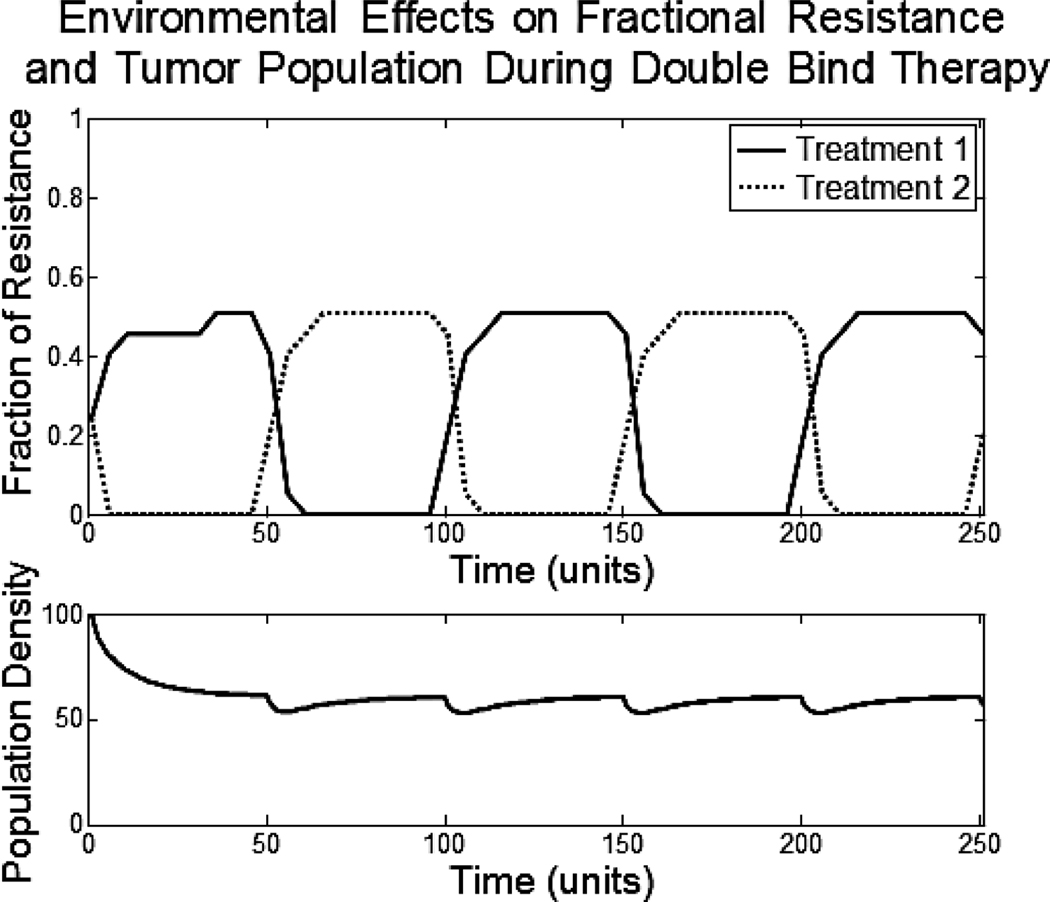
Figure 6.
The consequences of de-novo environmental resistance can be seen immediately. Even without the ability of phenotypic evolution, shown as the dotted lines, the monotherapy cannot eliminate the tumor population. The de-novo environmental resistance allows faster evolution of phenotypic resistance and less overall effect on tumor population.
We examine the double bind therapy under environmental resistance. All parameters have been kept the same as with the previous examples given.
The population density drops 44% on average. This is a 24% increase above the double bind therapy given with no environmental resistance. Fortunately, the double bind therapy is still forcing the tumor cells to constantly exhaust resources as they evolutionarily switch between the two resistance strategies. These forced evolutionary dynamics will provide long term control of the tumor population and prevent repopulation, as would otherwise be seen with monotherapy and multidrug therapies.
Conclusion
We developed an evolutionary model of tumor response and adaptation to various therapeutic strategies. In general we demonstrated that the evolutionary capacity of cancer cells severely limit the probability for robust tumor control using systemic, cytotoxic drugs. In fact, tumor populations typically begin to show emergence of a resistant population after a single treatment even when the cost of resistance is high.
Consistent with clinical observations, we demonstrate that increasing the dosage of a single treatment or administering two treatments that require one or two independent evolutionary responses is more effective in reducing the tumor population, but the tumor will typically survive and recur with resistant cells.
We explore an alternative strategy which we have termed “evolutionary double bind therapy”. In this approach, the mechanisms of adaptation to two treatments are explicitly considered in designing the administration of the treatments. The goal is to use the tumor cells’ adaptation to one treatment to increase the effectiveness of the second treatment. Model simulations demonstrate that such a strategy will not result in complete tumor eradication but may yield prolonged control of the tumor – far in excess of the results obtained from conventional therapeutic strategies.
Finally, our models demonstrate the highly significant role of environmental factors in the evolution of therapy resistance in cancer. We consistently find that drug therapies that would ordinarily be effective in a homogenous tumor fail when tumor characteristics are heterogeneous. These characteristics, such as regions of hypoxia and ischemia, inhibit the intended dosage of the drug and allow subsets of tumor cells to rapidly evolve resistance. Models of the double bind strategy demonstrate that tumor control can be more consistently achieved even in heterogeneous tumor environments. However, our models clearly indicate that greater focus on controlling the tumor microenvironment as part of cancer therapy is imperative to improve clinical outcomes.
In conclusion, development of more lethal drugs, including targeted therapies, may achieve some tumor response but durable control will be unlikely because the evolutionary capacity of cancer cells. We particularly highlight the environmental contribution to the evolutionary dynamics of resistance that results from vascular heterogeneity commonly observed in human cancer. In fact, we find that evolution of resistance, even in phenotypically sensitive cells, is a virtual certainty in poorly perfused regions of a tumor. Thus, we propose that durable control of clinical cancer will require not new drugs, but fundamental changes in therapeutic strategies to overcome or exploit these sources of resistance.
Figure 1.
Cost of resistance to therapy. As the tumor population evolves phenotypic resistance, the cost of that resistance is reflected in a decreasing carrying capacity. The magnitude of this cost is reflected by the parameter .
Figure 4.
The top axis shows the fractional resistance, vi, to the two treatments given over time. The bottom axis shows the population density of the tumor.
Figure 7.
The top axis shows the fractional resistance, vi, to the two treatments given over time. The bottom axis shows the population density of the tumor.
Acknowledgment
We are indebted to Thomas Vincent, our deceased friend and colleague who provided good ideas and inspiration for the approach of this paper. We are also grateful to Paul Orlando for his contributions and support and to Donna Cunningham for her editing expertise.
Financial support came from Grant 1U54CA143970-01 from the Moffitt Cancer Center PSOC, NIH/NCI and a grant from the McDonnell Foundation.
Abbreviations
- ESS
Evolutionarily Stable Strategy
References
- 1.Fodale V, Pierobon M, Liotta L, Petricoin E. Mechanism of cell adaptation: when and how do cancer cells develop chemoresistance? Cancer Journal. 2011;17(2):89–95. doi: 10.1097/PPO.0b013e318212dd3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milane L, Duan Z, Amiji M. Role of hypoxia and glycolysis in the development of multi-drug resistance in human tumor cells and the establishment of an orthotopic multi-drug resistant tumor model in nude mice using hypoxic pre-conditioning. Cancer Cell Int. 2011;11:3. doi: 10.1186/1475-2867-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gatenby RA. A change of strategy in the war on cancer. Nature. 2009;459:508–509. doi: 10.1038/459508a. [DOI] [PubMed] [Google Scholar]
- 4.Denholm I, Rowland MW. Tactics for managing pesticide resistance in arthropods: theory and practice. Ann Rev of Entomology. 1992;37:91–112. doi: 10.1146/annurev.en.37.010192.000515. [DOI] [PubMed] [Google Scholar]
- 5.Dlugosch KM, Parker IM. Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol. 2008;17:431–449. doi: 10.1111/j.1365-294X.2007.03538.x. [DOI] [PubMed] [Google Scholar]
- 6.Holt RD, Hochberg ME. When is biological control evolutionarily stable (or is it)? Ecology. 1997;78:1673–1683. [Google Scholar]
- 7.Holt RD, Hochberg ME, Barfield M. Population dynamics and the evolutionary stability of biological control. In: Hawkins B, Cornell H, editors. Theoretical Approaches to Biological Control. 1st ed. Cambridge, UK: Cambridge University Press; 1999. pp. 219–230. [Google Scholar]
- 8.Gatenby RA, Brown JS, Vincent T. Lessons from Applied Ecology: Cancer Control Using an Evolutionary Double Bind. Cancer Research. 2009;69:7499–7502. doi: 10.1158/0008-5472.CAN-09-1354. [DOI] [PubMed] [Google Scholar]
- 9.Brown JS. Vigilance, patch use and habitat selection: Foraging under predation risk. Evolutionary Ecology Research. 1999;1:49–71. [Google Scholar]
- 10.Ale SB, Brown JS. The contingencies of group size and vigilance. Evolutionary Ecology Research. 2007;9:1263–1276. [Google Scholar]
- 11.Vincent TL, Brown JS. Evolutionary Game Theory, Natural Selection, and Darwinian Dynamic. 1st ed. New York: Cambridge University Press; 2005. [Google Scholar]
- 12.Vincent TL, Cohen Y, Brown JS. Evolution via strategy dynamics. Theoretical Population Biology. 1993;44:149–176. [Google Scholar]
- 13.Cohen Y, Vincent TL, Brown JS. A G-function approach to fitness minima, fitness maxima, evolutionarily stable strategies and adaptive landscapes. Evolutionary Ecology Research. 1999;1:923–942. [Google Scholar]
- 14.Baguley BC. Multiple drug resistance mechanisms in cancer. Mol Biotechnol. 2010;46(3):308–316. doi: 10.1007/s12033-010-9321-2. [DOI] [PubMed] [Google Scholar]
- 15.Charnov EL, Orians GH, Hyatt K. Ecological Implications of Resource Depression. The American Naturalist. 1976;110(972):247–259. [Google Scholar]
- 16.Kotler BP, Blaustein L, Brown JS. Predator facilitation: the combined effect of snakes and owls on the foraging behavior of gerbils. Ann Zool Fenn. 1992;29:199–206. [Google Scholar]
- 17.Antonia SJ, et al. Combination of p53 Cancer Vaccine with Chemotherapy in Patients with Extensive Stage Small Cell Lung Cancer. Clin Cancer Res. 2006;12(3):878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 18.Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Natural Medicine. 1997;3(2):177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 19.Sinha BK, Katki AG, Batist G, Cowan KH, Myers CE. Differential formation of hydroxyl radicals by adriamycin in sensitive and resistant MCF-7 breast tumor cell lines: implications for the mechanism of action. Biochemistry. 1987;26:3776–3781. doi: 10.1021/bi00387a006. [DOI] [PubMed] [Google Scholar]
- 20.Holland JF, Frei E. Cancer Medicine. 7th ed. Hamilton, Ontario, Canada: BC Decker Inc.; 2006. pp. 1470–1471.pp. 1826–1827. [Google Scholar]
- 21.Trédan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99(19):1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]