Abstract
Striatal-enriched protein tyrosine phosphatase (STEP) is a brain-specific phosphatase that modulates key signaling molecules involved in synaptic plasticity and neuronal function. Targets include extracellular-regulated kinase 1 and 2 (ERK1/2), stress-activated protein kinase p38 (p38), the Src family tyrosine kinase Fyn, N-methyl-d-aspartate receptors (NMDARs), and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs). STEP-mediated dephosphorylation of ERK1/2, p38, and Fyn leads to inactivation of these enzymes, whereas STEP-mediated dephosphorylation of surface NMDARs and AMPARs promotes their endocytosis. Accordingly, the current model of STEP function posits that it opposes long-term potentiation and promotes long-term depression. Phosphorylation, cleavage, dimerization, ubiquitination, and local translation all converge to maintain an appropriate balance of STEP in the central nervous system. Accumulating evidence over the past decade indicates that STEP dysregulation contributes to the pathophysiology of several neuropsychiatric disorders, including Alzheimer's disease, schizophrenia, fragile X syndrome, epileptogenesis, alcohol-induced memory loss, Huntington's disease, drug abuse, stroke/ischemia, and inflammatory pain. This comprehensive review discusses STEP expression and regulation and highlights how disrupted STEP function contributes to the pathophysiology of diverse neuropsychiatric disorders.
I. Introduction
In the human genome, 107 genes encode the four families of protein tyrosine phosphatases (PTPs1) (Johnson and Hunter, 2005). Specifically, PTPs are either tyrosine-specific or dual-specificity phosphatases, and the tyrosine-specific PTPs are further subdivided into receptor-like or intracellular PTPs (Zhang, 2002). PTPs play an important role in both normal cellular function and the manifestation of disease. Consequently, PTPs are prime targets for drug discovery (Barr and Knapp, 2006; Barr, 2010). Initial interest in using PTP inhibitors in drug discovery was spawned in part by the fact that PTP1B knockout (KO) mice are resistant to an obesogenic diet and exhibit increased insulin sensitivity (Barr, 2010). These results suggest that PTP1B inhibition might be therapeutic in both type II diabetes and obesity. Accordingly, oligonucleotides antisense to PTP1B mRNA and small molecular inhibitors of PTP1B have since entered phase II clinical trials for the treatment of type II diabetes (http://clinicaltrials.gov/ct2/results?term=ISIS-113715).
Even so, the use of PTPs as therapeutic targets in the central nervous system (CNS) is still in its infancy. One PTP of particular promise for the treatment of neuropsychiatric disorders is striatal-enriched protein tyrosine phosphatase (STEP). STEP (encoded by the Ptpn5 gene) is a member of the family of intracellular tyrosine-specific phosphatases (Lombroso et al., 1991), and its closest relatives by sequence homology are HePTP and PTP-SL. Although STEP expression is restricted to the CNS, with the exception of the cerebellum (Lombroso et al., 1991; Lombroso et al., 1993), HePTP is found only in leukocytes (Adachi et al., 1992), and PTP-SL is expressed in the cerebellum and other brain regions (for review, see Hendriks et al., 2009). The current model of STEP function is that it opposes synaptic strengthening by dephosphorylating and inactivating key neuronal signaling molecules, including extracellular signal-regulated kinase 1 and 2 (ERK1/2), stress-activated protein kinase p38 (p38), the Src family tyrosine kinase (SFK) Fyn, N-methyl-d-aspartate receptors (NMDARs), and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) (Nguyen et al., 2002; Muñoz et al., 2003; Paul et al., 2003; Snyder et al., 2005; Braithwaite et al., 2006; Zhang et al., 2008; Kurup et al., 2010; Poddar et al., 2010).
STEP was discovered 20 years ago (Lombroso et al., 1991, 1993), and accumulating evidence implicates STEP dysregulation in the molecular basis of several neurological disorders. Up-regulation of STEP protein and/or activity contributes to the pathophysiology of diseases such as Alzheimer's disease (AD), schizophrenia (SZ), fragile X syndrome (FXS), epileptogenesis, and alcohol-induced memory loss (Snyder et al., 2005; Choi et al., 2007; Correa et al., 2009; Carty et al., 2010; Goebel-Goody et al., 2010; Kurup et al., 2010; Zhang et al., 2010; Briggs et al., 2011; Hicklin et al., 2011). On the other hand, too little STEP protein and/or activity can also negatively affect neuronal function and has been recently implicated in Huntington's disease (HD), drug abuse, stroke/ischemia, and inflammatory pain (Valjent et al., 2005; Braithwaite et al., 2008; Tashev et al., 2009; Xu et al., 2009; Saavedra et al., 2011; Yang et al., 2011). Proof-of-concept studies using peptides that manipulate STEP's ability to interact with its substrates, as well as characterization of STEP KO mice, establish that manipulating STEP function may be beneficial for the treatment of these disorders. Further enthusiasm for targeting STEP in drug discovery stems from the fact that the unique crystal structure of STEP has been resolved, and several structural characteristics distinguish STEP from other closely related PTPs (Eswaran et al., 2006). This review summarizes the current knowledge of STEP expression, regulation, and function, with particular emphasis on the dysfunction of STEP in neuropsychiatric disorders. Closing remarks outline efforts that may be useful for designing small-molecule inhibitors specific for STEP over other PTPs.
II. Basic Properties of Striatal-Enriched Protein Tyrosine Phosphatase: Localization, Developmental Expression, Splice Variants, Domain Structure and Function
A. Brain Region Specificity, Developmental Expression Patterns, and Cellular/Subcellular Localization
STEP exists as two major alternatively spliced isoforms, STEP61 and STEP46, and the baseline expression of these isoforms occurs in a brain region-specific manner (Boulanger et al., 1995; Bult et al., 1996). Both STEP61 and STEP46 are expressed in the striatum, central nucleus of the amygdala, and the optic nerve, whereas only STEP61 is expressed in the hippocampus, neocortex, spinal cord, and lateral amygdala (Boulanger et al., 1995; Lorber et al., 2004). Moreover, throughout rodent development, STEP61 and STEP46 are differentially expressed (Raghunathan et al., 1996). Whereas the levels of STEP61 are relatively abundant at birth and throughout adulthood, STEP46 does not appear until around postnatal day 6 (Raghunathan et al., 1996; Okamura et al., 1997). STEP46 progressively increases at postnatal day 14, reaches a plateau at 4 weeks of age, and remains constant throughout adulthood. The onset of STEP46 expression during the first few weeks of life may suggest a role in synaptogenesis. Consistently, the expression pattern of STEP and its colocalization with certain proteins changes throughout development (Kim et al., 2008b). For example, at postnatal day 8, STEP colocalizes with dopamine D2 receptors (D2Rs) in the substantia nigra compacta, where D2Rs act presynaptically, and does not colocalize much with the postsynaptic protein dopamine- and cAMP-regulated phosphoprotein of Mr 32,000 (DARPP-32) in the caudate putamen (Kim et al., 2008b). In contrast, in the adult brain, STEP extensively colocalizes with DARPP-32 in striatal medium spiny neurons (Lombroso et al., 1993; Raghunathan et al., 1996; Kim et al., 2008b). These findings suggest that STEP may interact with D2Rs presynaptically early in development and with DARPP-32 postsynaptically in the adult (Kim et al., 2008b).
At the cellular level, STEP is primarily restricted to neurons, but astroglia also show some expression, particularly after injury or ischemic insult (Hasegawa et al., 2000; Lorber et al., 2004). In all brain regions examined, STEP is found in both excitatory and inhibitory neurons, where it is poised to regulate the phosphorylation of its substrates. In excitatory neurons, STEP61 is targeted to postsynaptic densities (PSDs) (Oyama et al., 1995; Goebel-Goody et al., 2009) where one of its functions is to regulate NMDAR surface expression directly by dephosphorylating GluN2B Tyr1472 and indirectly by dephosphorylating and inactivating Fyn (Nguyen et al., 2002; Pelkey et al., 2002; Snyder et al., 2005; Braithwaite et al., 2006; Kurup et al., 2010). Even though inhibitory synaptic junctions do not present a prominent PSD, they still have complex protein matrices enriched in receptor proteins and signaling molecules, including STEP substrates (Kullmann and Lamsa, 2007; Keith and El-Husseini, 2008). Extensive colocalization of STEP with GAD65/67, a marker for GABAergic neurons, is found in hilar and stratum oriens interneurons of the hippocampus, where STEP modulates ERK1/2 activity (Choi et al., 2007). Moreover, loss of STEP reduces excitability of granule cell neurons and enhances excitability of hilar interneurons (Briggs et al., 2011). Taken together, these findings suggest that STEP contributes to the summation of excitatory and inhibitory input to control neuronal homeostasis.
At the subcellular level, STEP immunoreactivity staining demonstrates that it is normally expressed throughout striatal and central nucleus neurons in a punctate pattern, including the cell body, dendrites, and axonal processes (Boulanger et al., 1995). In contrast, STEP is expressed in neurites, but not cell bodies, in the globus pallidus and substantia nigra compacta, supporting the notion of a presynaptic role for STEP in these particular brain regions (Kim et al., 2008b). Biochemical characterizations further establish that STEP subcellular compartmentalization occurs with some degree of isoform specificity (Lombroso et al., 1993; Bult et al., 1996; Goebel-Goody et al., 2009). STEP46 is enriched in cytosolic fractions, whereas STEP61 is most enriched in light membrane fractions (which include endoplasmic reticulum, Golgi, and endosomes) (Lombroso et al., 1993; Bult et al., 1996). Electron microscopy studies demonstrate that STEP is targeted to the PSD (Oyama et al., 1995), and STEP61 is the primary isoform expressed there (N. Takagi, J. W. Gurd, and P. J. Lombroso, unpublished observations). In the monkey cerebral cortex, STEP61-specific antibodies label cell bodies of both pyramidal neurons and nonpyramidal interneurons, where the protein is localized in reticular endomembranes and nascent vesicles of the Golgi. In the neuropil, STEP61 predominates in dendrites, often as reactive rafts in association with the microtubules, and in dendritic spines, marking synaptic, perisynaptic, and extrasynaptic membranes. Figure 1 presents STEP61 localization patterns in dendritic spines of the prefrontal cortex, in relationship to incoming synapses to distinguish synaptic versus perisynaptic versus extrasynaptic STEP components. Quantitative assessments using high-resolution gold probes show that STEP61 is, for the most part, nonsynaptic in dendritic spines (C. D. Paspalas and P. J. Lombroso, unpublished observations), although we cannot exclude the possibility of the PSD hindering STEP immunodetection under the electron microscope. Corroborating these findings, STEP61 is found in both synaptic and extrasynaptic hippocampal fractions, and the concentration is greater extrasynaptically than synaptically (Goebel-Goody et al., 2009). Given that endocytosis-related proteins such as adaptor protein-2 and clathrin are also found in extrasynaptic regions (Blanpied et al., 2002; Rácz et al., 2004), we speculate that STEP61 activation at extrasynaptic membranes facilitates endocytosis of glutamate receptors in this part of the dendritic spine. Understanding and using STEP's regional, cellular, and subcellular localization might afford considerable flexibility in the therapeutic manipulation of its activities, especially given that function seems to differ depending on STEP location (see section IV.A).
Fig. 1.
STEP61 protein is captured in dendritic spine membranes in the macaque monkey prefrontal cortex, area 46. A1–B2, STEP-immunogold is found extrasynaptically (A1 and A2, arrowheads) and perisynaptically at a perforated synapse (B1 and B2, arrowheads); synaptic profiles are outlined in A2 and B2 to illustrate the synaptic, perisynaptic, and extrasynaptic membranes (color-coded). Double arrowheads point to the synapse active zone. C1–E2, when visualized with peroxidase, STEP61 marks perisynaptic and extrasynaptic membranes and is also found at the postsynaptic density of asymmetric, presumed glutamatergic, axospinous synapses. The raw data have been manipulated in the corresponding C2, D2, and E2 using image editing software to facilitate visualization of the immunoprecipitate against the postsynaptic density (between arrowheads). A nonreactive spine is shown in E1 and E2 (asterisk) for comparison. Arrowheads pointing to immunoreactive membranes are color-coded similarly to A2 and B2. For a technical account, please refer to Paspalas et al., 2009. The STEP antibody used was 23E5 (Boulanger et al., 1995). Scale bars, 200 nm.
B. Splice Variants, Domain Structure and Function
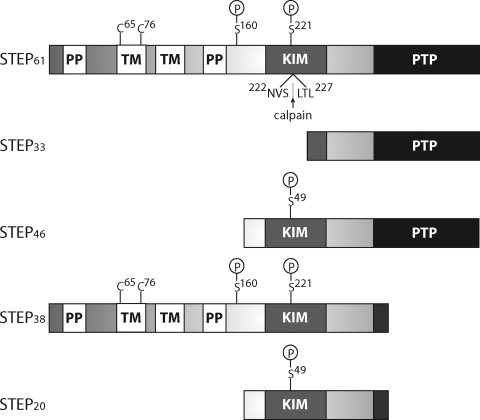
In addition to STEP61 and STEP46, two minor alternatively spliced variants of STEP include STEP38 and STEP20 (Fig. 2) (Lombroso et al., 1993; Sharma et al., 1995; Bult et al., 1996; Bult et al., 1997). STEP61 and STEP46 each contain a signature consensus PTP sequence, [I/V]HCxAGxxR[S/T]G, that is required for catalytic activity and a kinase-interacting motif (KIM) that is essential for substrate binding (Bult et al., 1996). STEP is one of only three PTPs known to contain a KIM domain; the two others are its closest relatives, HePTP and PTP-SL (Adachi et al., 1992; Hendriks et al., 2009). STEP38 and STEP20 do not contain the consensus PTP domain and are therefore catalytically inactive (Sharma et al., 1995; Bult et al., 1997). Although the function of STEP38 and STEP20 remains unknown, one possibility is that they act as dominant-negative variants that compete with active STEP variants for substrate binding. Both STEP38 and STEP20 contain a distinct 10-amino acid sequence at their carboxyl termini that is introduced during splicing and may play a role in their unknown function.
Fig. 2.
Schematic of STEP. There are four alternatively spliced variants of STEP (STEP61, STEP46, STEP38, and STEP20) and one calpain cleavage product (STEP33). STEP61 and STEP46 are the major isoforms expressed in the CNS. The KIM domain is required for substrate binding, and the consensus PTP sequence, [I/V]HCxAGxxR[S/T]G, is necessary for phosphatase activity. STEP38 and STEP20 do not contain the PTP sequence and are inactive variants of STEP with unknown function. It is possible that these two inactive isoforms function as dominant-negative variants that compete with active STEP variants for substrate binding, or they possess other functions yet to be discovered. STEP38 and STEP20 contain a unique 10-amino acid sequence at their carboxyl termini that is introduced during splicing. STEP33 is generated by calpain cleavage within the KIM domain between Ser224 and Leu225. Cleavage at this site disrupts the ability of STEP33 to interact with its substrates. STEP61 also contains an additional 172 amino acids in its amino-terminal region, which contains two transmembrane (TM) domains, two PP-rich regions, and an adjacent PEST sequence (not labeled). The TM regions target STEP61 to the endoplasmic reticulum, as well as the PSD. The KIM domain is required for binding to all substrates, whereas the PP regions impart substrate specificity. PKA phosphorylates STEP within the KIM domain (Ser221 and Ser49 on STEP61 and STEP46, respectively), as well as in the region adjacent to the PP regions (Ser160 on STEP61). Although the function of Ser160 phosphorylation on STEP61 remains unclear, current investigations are aimed at determining whether phosphorylation at this or other sites is a signal for calpain-mediated cleavage and/or ubiquitination. Finally, two cysteine residues Cys65 and Cys76 are found in the TM region that mediates STEP dimerization and reduces its phosphatase activity.
STEP61 is targeted to endomembranes, such as the endoplasmic reticulum and the PSD, by the 172 amino acid sequence present at its amino terminus (Fig. 2) (Boulanger et al., 1995; Oyama et al., 1995; Bult et al., 1996). This sequence is not found on STEP46, which is restricted to the cytosol. Two polyproline-rich (PP) and PEST sequences are also present on the amino terminus of STEP61 (Bult et al., 1996). One function of the PP region is to impart substrate specificity for STEP isoforms (Nguyen et al., 2002). For example, the affinity of Fyn to associate with STEP61 is 10-fold greater than with STEP46, which does not contain the PP regions. A series of mutational analyses demonstrates that the first PP region (in addition to the KIM domain) is necessary for the interaction of STEP61 with Fyn (Nguyen et al., 2002). As such, it may be possible to use this first PP interface to selectively disrupt the protein-protein interaction between STEP and Fyn. The function of the second PP region remains unknown.
III. Striatal-Enriched Protein Tyrosine Phosphatase Regulation
STEP's expression and ability to bind to and dephosphorylate its substrates is regulated by several known mechanisms: phosphorylation, proteolytic cleavage, dimerization, ubiquitination, and local translation. Several of these mechanisms are disrupted in animal models of neuropsychiatric disorders and consequently lead to aberrant STEP activity (see section V). Here we review our current understanding of how STEP is regulated and the potential relevance for these mechanisms in specific neuropsychiatric disorders.
A. Phosphorylation
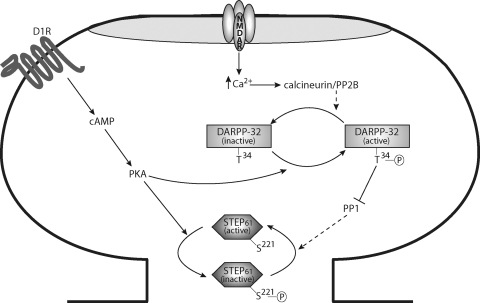
STEP phosphorylation at a serine residue within the KIM domain regulates the interactions of STEP with its substrates (Fig. 2) (Paul et al., 2000, 2003). In particular, dopamine D1R-induced PKA phosphorylation at residues Ser221 or Ser49 in STEP61 or STEP46, respectively, reduces the affinity of STEP for its substrates because of steric hindrance (Fig. 3). Although PKA also phosphorylates Ser160 in the unique amino-terminal portion of STEP61, the function of this residue remains unknown (Paul et al., 2000). Dephosphorylation of STEP at Ser221/Ser49 by NMDAR- or α7 nicotinic acetylcholine receptor (α7nAChR)-induced activation of protein phosphatase 2B (PP2B), otherwise known as calcineurin, and protein phosphatase 1 (PP1) enhances substrate affinity and consequently increases STEP dephosphorylation of its substrates (Fig. 3) (Paul et al., 2003; Snyder et al., 2005; Valjent et al., 2005). The ability of PP1 to dephosphorylate and activate STEP is also controlled by DARPP-32 (Valjent et al., 2005). When phosphorylated by cAMP-dependent protein kinase (PKA), DARPP-32 inhibits PP1 activity so that it no longer dephosphorylates or activates STEP (Fig. 3). In this way, two parallel pathways converge to regulate STEP phosphorylation and substrate binding: 1) direct phosphorylation of STEP by PKA and 2) indirect regulation of STEP phosphorylation via PKA-induced phosphorylation and activation of DARPP-32, which in turn inhibit PP1 activity. As discussed in more detail in section V, STEP phosphorylation is attenuated in AD (Snyder et al., 2005; Kurup et al., 2010) and enhanced in HD (Saavedra et al., 2011) and after amphetamine treatment (Valjent et al., 2005).
Fig. 3.
Regulation of STEP phosphorylation. D1R activation stimulates cAMP synthesis and activates PKA, which phosphorylates STEP61 at Ser221 in the KIM domain. Phosphorylation at Ser221 sterically inhibits the binding of STEP61 to its substrates. PKA also leads to phosphorylation and activation of DARPP-32 at Thr34. When phosphorylated at this site, DARPP-32 inhibits PP1 activity, which is the phosphatase that dephosphorylates Ser221 and promotes the interaction of STEP61 with its substrates. Conversely, NMDAR (or α7nAChR) stimulation initiates calcium influx and activation of calcineurin/PP2B to dephosphorylate and inactivate DARPP-32, thereby reducing DARPP-32-mediated inhibition of PP1 and increasing STEP61 activity by reducing phosphorylation of Ser221.
B. Calpain-Mediated Cleavage
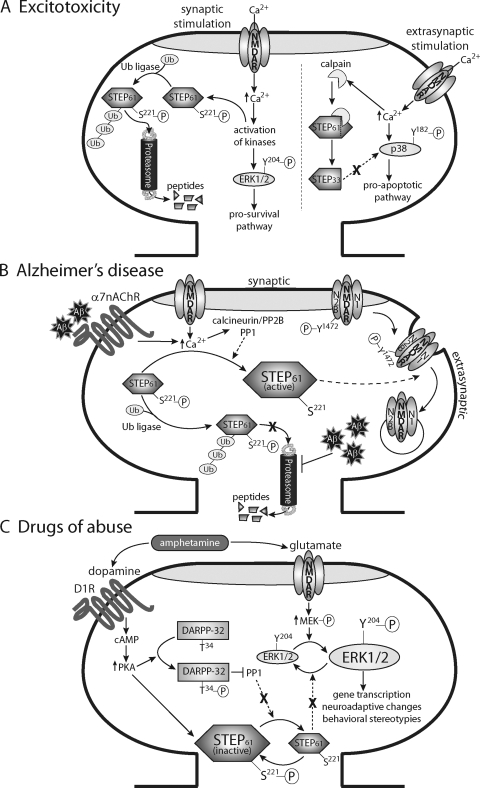
STEP61 is proteolytically cleaved by calpain between residues Ser224 and Leu225 in the KIM domain (Fig. 2) (Xu et al., 2009). Cleavage at this site produces a truncated STEP product, STEP33, that cannot associate with or dephosphorylate its substrates. In particular, proteolytic cleavage of STEP after extrasynaptic NMDAR stimulation results in the activation of one of STEP's substrates, p38, and initiates the cell death signaling cascade (Figs. 4 and 5A) (Xu et al., 2009). When STEP33 is produced after extrasynaptic NMDAR stimulation, p38 is no longer dephosphorylated and inactivated by STEP61. As a result, p38 phosphorylation is enhanced after activation of extrasynaptic NMDARs, and cell death signaling pathways are initiated (Fig. 5A). Because the GluN2B-specific antagonist ifenprodil attenuates the production of STEP33 after extrasynaptic NMDAR stimulation, extrasynaptic GluN2B-containing NMDARs seem to mediate cleavage of STEP61 (Xu et al., 2009; Poddar et al., 2010). A peptide that spans the STEP61 cleavage site blocks the proteolysis of STEP61 and is neuroprotective against glutamate excitotoxicity and oxygen-glucose deprivation (Xu et al., 2009). These findings demonstrate that treatments preventing the cleavage of STEP61 may be useful in stroke/ischemia (Gurd et al., 1999; Nguyen et al., 1999; Xu et al., 2009).
Fig. 4.
STEP substrates. When dephosphorylated at Ser221, STEP binds to and desphosphorylates ERK1/2, p38, Fyn, Pyk2, the NMDAR subunit GluN2B, and the AMPAR subunit GluA2. STEP dephosphorylates the regulatory active sites on ERK1/2 (Tyr204/187), p38 (Tyr182), Fyn (Tyr420) and Pyk2 (Tyr402) leading to their inactivation. STEP61 regulates the phosphorylation of GluN2B-containing NMDARs by two parallel mechanisms. First, when Fyn is inactivated by STEP61, Fyn-mediated phosphorylation of GluN2B Tyr1472 is reduced. Second, STEP61 dephosphorylates GluN2B Tyr1472 directly. Dephosphorylation of Tyr1472 promotes the interaction of GluN2B with clathrin adaptor proteins and leads to endocytosis of these receptors. It is noteworthy that in this model, dephosphorylation of GluN2B Tyr1472 is depicted to occur extrasynaptically, where clathrin adaptor proteins reside to mediate receptor internalization. This event must occur with a prior signal triggering movement of GluN2B-containing receptors from synaptic sites to extrasynaptic sites. Another possibility is that GluN2B Tyr1472 dephosphorylation occurs synaptically, and this event acts as the signal to trigger lateral movement of GluN2B-containing receptors to extrasynaptic sites. Further work is required to address these two possibilities. In addition, Pyk2 is upstream of Fyn-mediated phosphorylation and enhancement of GluN2B-containing NMDARs. STEP61 is also required for the internalization of GluA1/GluA2-containing AMPARs after mGluR stimulation. Although the molecular mechanisms underlying tyrosine-dependent internalization of AMPARs remains incompletely understood, STEP61 seems to promote the endocytosis of AMPARs in a manner similar to that in which it promotes NMDARs, by dephosphorylating a key tyrosine residue.
Fig. 5.
Disruptions in STEP associated with excitotoxicity, Alzheimer's disease, and drugs of abuse. A, extrasynaptic NMDAR stimulation invokes calpain-mediated proteolysis of STEP61 producing the truncated cleavage product STEP33. STEP33 is unable to bind to and dephosphorylate its substrates. The stress-activated MAPK p38 is preferentially activated by extrasynaptic NMDAR stimulation, and the cell death pathways are subsequently initiated. Cleavage of STEP61 is therefore likely to be a component of excitotoxic insults associated with stroke/ischemia and Huntington's disease. On the other hand, synaptic NMDAR stimulation leads to the activation of multiple kinases responsible for phosphorylating STEP61 and recruiting the ubiquitin proteasome system to dendritic spines. Preliminary evidence suggests that phosphorylation of STEP61 at Ser221 is required, but not sufficient, for ubiquitination of STEP61 (P. Kurup and P. J. Lombroso, unpublished observations). Synaptic NMDAR stimulation results in the degradation of STEP61, leads to an increase in ERK1/2 activation, and promotes neuronal survival. Ub, ubiquitin. B, in Alzheimer's disease, Aβ binding to α7nAChRs and synaptic NMDAR stimulation invoke activation of PP2B/calcineurin and PP1 to dephosphorylate STEP61 at Ser221, thereby increasing the affinity of STEP61 for its substrates. In a second pathway, Aβ inhibits the ubiquitin proteasome system and prevents degradation of STEP61. The net result is an accumulation of unphosphorylated and active STEP61 protein levels in AD, which leads to inappropriate dephosphorylation of GluN2B Tyr1472 and internalization of GluN2B-containing NMDARs. C, drugs of abuse, such as amphetamine, stimulate release of dopamine and glutamate and activate D1Rs and NMDARs, respectively. Stimulation of D1Rs initiates PKA-mediated phosphorylation of STEP61 and DARPP-32, which in turn inhibits PP1 activity. These two parallel pathways result in greater phosphorylation of STEP61 and less dephosphorylation of STEP61 substrates, including ERK1/2. A converging pathway is the NMDAR-mediated activation of MAPK kinase to phosphorylate and activate ERK1/2. Increased phosphorylation of ERK1/2 subsequently initiates gene transcription and promotes neuroadaptive changes that underlie behavioral abnormalities associated with drugs of abuse.
C. Dimerization
Within the hydrophobic region of the amino terminus of STEP61, two cysteine residues (Cys65 and Cys76) mediate STEP dimerization (Fig. 2) (Deb et al., 2011). Dimerization of STEP61, but not STEP46, occurs basally and decreases its phosphatase activity. Dimerization is achieved, at least in part, by the formation of intermolecular disulfide bonds between these two cysteine residues (Deb et al., 2011). Upon hydrogen peroxide-induced oxidative stress, further STEP61 dimerization occurs and results in even less phosphatase activity. Although STEP46 does not undergo basal dimerization, oxidative stress can induce the formation of STEP46 oligomers (Deb et al., 2011). Because oxidation of the active cysteine residue in the consensus tyrosine phosphatase sequence also inhibits tyrosine phosphatase activity (Lee et al., 1998), these findings may suggest a class of noncompetitive STEP inhibitors.
D. Ubiquitination
When multiple ubiquitin peptides are covalently attached to proteins, they are selectively targeted to the 26S proteasome for degradation. Apart from its role in normal cellular function, a growing body of literature shows impairment of the ubiquitin proteasome system (UPS) in CNS diseases, including AD, HD, schizophrenia, and Parkinson's disease (for review, see Hegde, 2010). STEP61 is rapidly ubiquitinated and degraded upon synaptic NMDAR activation (Fig. 5A) (Xu et al., 2009), presumably to diminish STEP activity and permit enhanced signaling of STEP substrates that promote synaptic plasticity. For example, ERK1/2 phosphorylation is positively correlated with ubiquitination and degradation of STEP61 after synaptic NMDAR activation, and this is followed by nuclear translocation of ERK1/2 and increased gene transcription (Fig. 5A). These events can have lasting effects on the formation and stabilization of dendritic spines and therefore contribute to long-term information storage. Although the molecular mechanisms underlying ubiquitination of STEP61 are still incompletely understood, the amino-terminal region of STEP61 contains two PEST sequences (Fig. 2) (Bult et al., 1996), and these are often found in proteins degraded by the UPS (Spencer et al., 2004). Current investigations are aimed at addressing whether phosphorylation of residues adjacent to the PEST sequences and/or other mechanisms are responsible for initiating STEP61 ubiquitination.
E. Local Translation
Local translation is the process by which certain messages are rapidly translated at distinct synaptic sites to induce or maintain synaptic plasticity. Accordingly, the expression of both long-term potentiation (LTP) and metabotropic glutamate receptor (mGluR) long-term depression (LTD) requires local dendritic translation (Huber et al., 2000; Sutton and Schuman, 2006; Bramham and Wells, 2007; Costa-Mattioli et al., 2009). It is now widely accepted that the mRNAs important for these plastic changes are transported along dendrites, where they reside in a suppressed state until the appropriate synaptic stimulus permits their translation (Wu et al., 1998; Li et al., 2001; Antar and Bassell, 2003; Shin et al., 2004; Bramham and Wells, 2007). Recent evidence suggests that STEP is a new member of the growing family of locally translated proteins. STEP mRNA and protein are found in puncta along dendrites and near PSDs in hippocampal cultures. Moreover, STEP translation is up-regulated within synaptoneurosomes after mGluR activation with (R,S)-3,5-dihydroxyphenylglycine (DHPG), suggesting that STEP is dendritically translated (Zhang et al., 2008). The increase in STEP protein occurs in a dose- and time-dependent manner and requires activation of the mitogen-activated protein kinase (MAPK) and phosphoinositide-3 kinase pathways, both of which are required for mGluR-stimulated translation and LTD. Moreover, the DHPG-mediated increase in STEP translation occurs primarily through mGluR5 activation, because the mGluR5 inhibitor, 2-methyl-6-(phenylethynyl)-pyridine, but not the mGluR1 inhibitor, (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385), blocks STEP translation. Taken together, these observations establish that STEP is rapidly translated in dendrites via mGluR5 receptor, MAPK, and phosphoinositide-3 kinase pathways (Zhang et al., 2008) and support findings demonstrating that a tyrosine phosphatase is necessary for the expression of mGluR-LTD (Moult et al., 2006).
Stimulation of β-adrenergic receptors also induces the translation of STEP (Hu et al., 2007). β-Adrenergic stimulation results in persistent LTP-like changes in the hippocampus and amygdala (Huang and Kandel, 1996; Watabe et al., 2000; Gelinas and Nguyen, 2005) and enhances memory formation in some rodent learning paradigms (Ferry and McGaugh, 1999; Gibbs and Summers, 2000; LaLumiere et al., 2003). Activation of β-adrenergic receptors with isoproterenol enhances STEP protein levels in a dose- and translation-dependent manner (Hu et al., 2007). Increased STEP levels after β-adrenergic activation may seem counterintuitive because one might expect STEP degradation to occur during memory consolidation. One possible explanation for this paradox is that newly synthesized STEP might limit the duration of ERK1/2 signaling initiated by β-adrenergic receptor stimulation in a negative feedback loop (Hu et al., 2007).
Two proteins proposed to regulate dendritically translated mRNAs are cytoplasmic polyadenylation element (CPE) binding protein (CPEB) and fragile X mental retardation protein (FMRP) (Wu et al., 1998; Antar and Bassell, 2003). The 3′ untranslated region (UTR) of STEP contains two CPEs similar to those found in αCaMKII mRNA, a well characterized message regulated by CPEB (Wu et al., 1998; Rook et al., 2000; Huang et al., 2003), suggesting that the local translation of STEP may be regulated by CPEB. Support for this hypothesis comes from a recent microarray study that identified STEP as a potential CPEB-regulated mRNA (Piqué et al., 2008). Likewise, FMRP binds to mRNAs via a consensus G-quartet sequence in the 3′ UTR of the messages it represses (Darnell et al., 2001). The 3′ UTR of STEP contains a potential G-quartet sequence that is remarkably similar to a sequence proposed to bind to FMRP. Furthermore, high-throughput sequencing of RNA isolated by cross-linking immunoprecipitation to identify endogenous mRNA targets of FMRP in neurons reveals a highly significant association of STEP mRNA with FMRP across seven independent experiments (p = 9.97 ×10−5), including use of two different FMRP antibodies and multiple biological replicates (Darnell et al., 2011). In summary, these collective findings suggest that STEP mRNA associates with and may be repressed by CPEB and FMRP in dendrites until the appropriate stimuli, such as mGluR5 or β-adrenergic receptor stimulation, releases that suppression to permit local translation.
Although the mechanism behind transport of mRNAs and binding proteins to sites of dendritic translation remains unknown, recent findings demonstrate that major vault protein (MVP), a large ribonucleoprotein cargo particle, is localized to the nucleus-neurite axis alongside microtubules and intracellular organelles, such as free and attached ribosomes (Paspalas et al., 2009). Moreover, MVP associates with several mRNAs, including STEP, that are translated in response to synaptic activity, suggesting that MVP shuttles mRNA cargo to dendrites for local translation (Paspalas et al., 2009). It is likely that MVP also carries binding proteins such as CPEB and FMRP. Future work is required to rigorously address this hypothesis and solidify the role of MVP in transporting mRNAs to sites of dendritic translation.
IV. Striatal-Enriched Protein Tyrosine Phosphatase Substrates
A. Mitogen-Activated Protein Kinase Family
Two members of the MAPK family are known STEP substrates: ERK1/2 and p38 (Muñoz et al., 2003; Paul et al., 2003; Poddar et al., 2010). ERK1/2 regulates electrical membrane properties (via the Kv4 channel family and subsequent NMDAR activation), local dendritic protein synthesis, nuclear transcriptional regulation, neuronal survival, and the formation and stabilization of dendritic spines (for review, see Sweatt, 2004). Consequently, ERK1/2 is critical for the induction and maintenance of synaptic plasticity. ERK1/2 activation is achieved via phosphorylation of Thr202/185 and Tyr204/187 in its activation loop by a class of dual-specificity kinases, MAPK kinases (Robinson and Cobb, 1997). When phosphorylated, one function of ERK1/2 is to initiate transcription through activation of the transcription factors CREB and Elk1 in the nucleus (Davis et al., 2000). STEP dephosphorylates Tyr204/187 and consequently inactivates ERK1/2 (Fig. 4), thereby limiting the duration of ERK1/2 activity after NMDAR stimulation (Paul et al., 2003; Valjent et al., 2005). Recent findings by Paul and Connor (2010) establish a role specifically for GluN2B-containing receptors in mediating the dephosphorylation of both STEP and ERK1/2 after transient influx of calcium after glutamate stimulation.
The formation of fear memories and the expression of synaptic plasticity in the lateral amygdala require nuclear translocation of ERK1/2 and subsequent gene transcription (Schafe et al., 2000). As discussed, STEP normally opposes these processes by dephosphorylation and inactivation of ERK1/2 (Paul et al., 2007). Infusion of a substrate-trapping membrane-permeable fusion protein of STEP46 (TAT-STEPC-S) in the lateral amygdala of rats inhibits Pavlovian fear conditioning. This mutant STEP protein binds to its substrates, including ERK1/2, but cannot dephosphorylate or release them, thereby disrupting their downstream signaling. Moreover, the induction of LTP and nuclear translocation of ERK1/2 is blocked by bath application of TAT-STEPC-S to amygdala slices. Together, these findings confirm a role for STEP in opposing the formation of fear memories and in regulating synaptic plasticity (Paul et al., 2007).
STEP KO mice further establish that ERK1/2, among others, is a STEP substrate (Venkitaramani et al., 2009). These mice are viable, seem healthy, and exhibit normal fertility. STEP KO mice appear to have normal neuroanatomy, exhibiting no gross anatomical alterations in the striatum, hippocampus, or amygdala relative to wild-type (WT) littermates. As predicted, no perceptible levels of STEP protein are observed in total cerebral homogenates of STEP KO mice, whereas mice heterozygous for STEP express approximately 50% of STEP protein of that expressed by WT littermates (Venkitaramani et al., 2009).
ERK1/2 phosphorylation is significantly elevated in the striatum, hippocampus, and central/lateral amygdala of STEP KO mice compared with WT littermates (Venkitaramani et al., 2009, 2011), providing additional support for the regulation of ERK1/2 by STEP. DHPG normally enhances phosphorylation and activation of ERK1/2 (Kim et al., 2008a), and this is more pronounced in STEP KOs relative to WT, suggesting that STEP limits the activation of ERK1/2 after mGluR stimulation (Venkitaramani et al., 2009). Downstream of ERK1/2 activation, the transcription factors CREB and Elk1 are also hyperphosphorylated in STEP KOs compared with WT mice (Venkitaramani et al., 2011). Thus, alterations in STEP levels and/or activity can have a lasting impact on gene transcription via its regulation of ERK1/2.
Whereas ERK1/2 promotes learning and cell survival, p38 plays a role in excitotoxic cell death (Hardingham et al., 2002; Ivanov et al., 2006; Léveillé et al., 2008). Similar to ERK1/2, activation of p38 is achieved by dual phosphorylation at Thr180 and Tyr182. Upon phosphorylation, p38 initiates the cell death signaling cascades by phosphorylating Bcl-2 and regulating other proapoptotic proteins and transcription factors (for review, see Cuadrado and Nebreda, 2010). STEP dephosphorylates Tyr182 and consequently inactivates p38 (Fig. 4) (Muñoz et al., 2003; Xu et al., 2009; Poddar et al., 2010). Prolonged glutamate stimulation results in activation of extrasynaptic NMDARs and cleavage of STEP61, which promotes phosphorylation of p38 (Fig. 5A) (Xu et al., 2009). Conditions associated with excitotoxicity, such as HD and ischemic neuronal injury, are affiliated with hyperphosphorylation of p38, as well as decreased STEP61 levels and/or activity (Xu et al., 2009; Saavedra et al., 2011).
The ability of STEP to regulate two opposing MAPKs, ERK1/2 and p38, may seem puzzling; however, Xu et al. (2009) resolve this apparent paradox (Fig. 5A). As discussed above in section III, extrasynaptic NMDAR stimulation triggers calpain-mediated cleavage of STEP61 that selectively activates p38 and promotes cell death. In contrast, synaptic NMDAR stimulation leads to ubiquitination and degradation of STEP61 and subsequent ERK1/2 activation. These events seem to be mechanistically distinct because extrasynaptic NMDAR-mediated cleavage and inhibition of STEP61 fail to activate ERK1/2. Perhaps the answer to this conundrum lies in the subcellular localization of p38 and ERK1/2. Specifically, p38 is more concentrated in extrasynaptic membranes compared with synaptic membranes, so extrasynaptic stimulation could lead to the selective cleavage of the extracellular pool of STEP (Xu et al., 2009). Alternatively, it is possible that extrasynaptic NMDAR stimulation promotes dominant ERK-inactivating signals that supersede STEP cleavage (Hardingham and Bading, 2010). Nonetheless, STEP's role in regulating ERK1/2 and p38 after synaptic and extrasynaptic NMDAR activation, respectively, probably explains why extrasynaptic stimulation promotes cell death, whereas synaptic stimulation promotes cell survival (Xu et al., 2009).
B. Glutamate Receptors and Fyn
STEP regulates the phosphorylation and surface expression of both NMDARs and AMPARs (Fig. 4) (Snyder et al., 2005; Braithwaite et al., 2006; Zhang et al., 2008; Kurup et al., 2010; Zhang et al., 2010; Venkitaramani et al., 2011). In the case of NMDARs, STEP modulates the phosphorylation of the GluN2B subunit by two parallel pathways: by direct dephosphorylation of GluN2B Tyr1472 (Snyder et al., 2005; Kurup et al., 2010) and indirectly via dephosphorylation and inactivation of the SFK Fyn (Nguyen et al., 2002) (Fig. 4). Phosphorylation of Tyr1472 tightly and dynamically regulates the surface expression of GluN2B. Tyr1472 resides within a conserved tyrosine-dependent endocytic motif (YXXO: X = any amino acid, O = bulky hydrophobic amino acid) (Roche et al., 2001). When dephosphorylated, the tyrosine residue in this motif binds to clathrin adapter proteins via strong hydrophobic interactions and promotes endocytosis (for review, see Marsh and McMahon, 1999). In agreement, the phosphorylation of Tyr1472 and association of clathrin adapter proteins with GluN2B are inversely correlated (Nakazawa et al., 2006). Given that STEP directly interacts with NMDARs (Pelkey et al., 2002; Braithwaite et al., 2006) and dephosphorylates Tyr1472 (Snyder et al., 2005; Kurup et al., 2010), STEP probably promotes the interaction of GluN2B with clathrin adapter proteins to initiate endocytosis. Corroborating this hypothesis, the surface expression of GluN1/GluN2B receptor complexes is elevated in STEP KO mice (Zhang et al., 2010; Venkitaramani et al., 2011).
Full activation of Fyn is achieved by intermolecular autophosphorylation of a regulatory tyrosine residue, Tyr420, in its catalytic domain (Smart et al., 1981). When active, Fyn phosphorylates GluN2B at three sites, Tyr1252, Tyr1336, and Tyr1472 (Nakazawa et al., 2001), and all of these are phosphorylated in the brain (Goebel-Goody et al., 2009). STEP inactivates Fyn by dephosphorylating Tyr420 (Fig. 4) (Nguyen et al., 2002), thereby reducing Fyn-mediated phosphorylation of GluN2B. Given that Tyr1472 is the only site hyperphosphorylated in STEP KO mice (Zhang et al., 2010; Venkitaramani et al., 2011; T. R. Hicklin and M. D. Browning, unpublished observations), it seems that the other Fyn-mediated phosphorylation sites are not regulated by STEP. Taken together, endocytosis of GluN2B-containing NMDARs is regulated by STEP via two converging parallel pathways involving direct dephosphorylation of Tyr1472 and indirect regulation of Tyr1472 via Fyn.
STEP's regulation of surface NMDARs affects their function (Pelkey et al., 2002). In particular, STEP application decreases NMDAR excitatory postsynaptic currents (EPSCs) and prevents the induction of high-frequency stimulation (HFS) LTP. In contrast, inhibiting endogenous STEP with an anti-STEP antibody enhances NMDAR EPSCs and occludes HFS LTP. These effects are prevented by the noncompetitive NMDAR antagonist dizocilpine (MK801) and by a Src inhibitory peptide (Src40–58), suggesting that STEP acts as a “tonic brake” on synaptic transmission by opposing SFK-mediated enhancement of NMDARs (Pelkey et al., 2002). Congruent with these findings, θ-burst LTP is significantly increased in hippocampal slices from STEP KO mice relative to WT (Zhang et al., 2010). Therefore, the current model of STEP function proposes that STEP activity must be suppressed either by degradation of STEP or phosphorylation in the KIM domain in order for LTP to be observed. Ongoing efforts are directed at confirming the mechanism whereby STEP is repressed during LTP induction.
An additional level of complexity is that the focal adhesion kinase family kinase Pyk2/CAKβ also seems to be a substrate of STEP (Fig. 4) (Xu et al., 2010; Venkitaramani et al., 2011). Pyk2 is upstream of SFK-mediated phosphorylation and enhancement of NMDARs (Huang et al., 2001). When Pyk2 is activated by tyrosine phosphorylation at Tyr402, it phosphorylates and activates Fyn, which in turn phosphorylates GluN2B at Tyr1472. Thus, the consequence of Pyk2 activation is greater tyrosine phosphorylation and increased surface expression of NMDARs (Besshoh et al., 2005; Le et al., 2006). Likewise, Pyk2 activation results in enhanced ERK1/2 phosphorylation (Nicodemo et al., 2010). Inhibitors of Pyk2 block the induction of LTP (Huang et al., 2001), demonstrating an important role for Pyk2 in the expression of synaptic plasticity. On the other hand, STEP-mediated dephosphorylation of Pyk2 would oppose these processes. In line with this model, Pyk2 is hyperphosphorylated at Tyr402 in STEP KO mice (Venkitaramani et al., 2011).
STEP also seems to be the tyrosine phosphatase that mediates phosphorylation and internalization of AMPARs after mGluR stimulation (Zhang et al., 2008). Tyrosine phosphorylation of the GluA2 subunit is reduced after stimulation of mGluRs with DHPG (Moult et al., 2006; Gladding et al., 2009). The DHPG-induced dephosphorylation of GluA2 is diminished in the presence of TAT-STEPC-S, suggesting a requirement for STEP (Zhang et al., 2008). In conjunction with tyrosine dephosphorylation of GluA2, surface GluA1/GluA2 levels are reduced after DHPG treatment (Snyder et al., 2001; Moult et al., 2006; Zhang et al., 2008; Gladding et al., 2009). DHPG-mediated AMPAR endocytosis is abolished in STEP KO hippocampal slices and cultures and restored with the addition of a wild-type TAT-STEP fusion protein to STEP KO neurons (Zhang et al., 2008). These findings point to the following model: STEP is activated during mGluR stimulation to dephosphorylate GluA2-containing receptors and promote their endocytosis (Fig. 4). Consistent with this model, the surface expression of GluA1/GluA2-containing AMPARs is significantly elevated in STEP KO mice (Zhang et al., 2008; Venkitaramani et al., 2011). Although GluA2 does seem to be phosphorylated by SFKs (Hayashi and Huganir, 2004), it remains unknown whether the regulation of GluA2 tyrosine phosphorylation by STEP is direct or indirect via SFKs or whether both mechanisms are involved.
Scholz et al. (2010) recently shed some light on the molecular mechanisms governing AMPAR endocytosis after mGluR activation and report findings supporting the hypothesis that tyrosine dephosphorylation plays a role. They demonstrate that GluA2 directly interacts with the synaptic protein BRAG2, which is guanine-exchange factor for the GTPase Arf6. BRAG2 is a synaptically localized protein with a similar developmental profile and subcellular distribution as GluA2. When activated by BRAG2, Arf6 recruits adaptor protein-2 and clathrin to synaptic membranes (Krauss et al., 2003), thereby promoting endocytosis of GluA2 (Scholz et al., 2010). Arf6 activation requires dephosphorylation of GluA2 Tyr876 by an unknown tyrosine phosphatase. Linking STEP to the activation of Arf6 and endocytosis of GluA2 is the subject of current investigation.
C. Behavioral Implications for the Regulation of Striatal-Enriched Protein Tyrosine Phosphatase Substrates
As discussed above, the loss of STEP results in hyperphosphorylation of STEP substrates (Venkitaramani et al., 2009, 2011; Zhang et al., 2010). In the case of NMDARs and AMPARs, increased tyrosine phosphorylation results in greater surface expression of these receptors in STEP KOs, presumably as a result of reduced clathrin-mediated endocytosis and/or greater stability of these receptors in membrane compartments. As for ERK1/2, Fyn, and Pyk2, hyperphosphorylation in STEP KO mice likely leads to exaggerated activation of these enzymes. The loss of STEP might therefore benefit cognition by increasing the activation of some STEP substrates concomitantly with enhancing the surface expression of NMDARs and AMPARs.
To test this hypothesis, STEP KO mice have been characterized in hippocampal- and amygdala-dependent behavioral paradigms (Venkitaramani et al., 2011; P. Olausson, J. Taylor, and P. J. Lombroso, unpublished observations). Although STEP KO mice trained in a hippocampus-dependent Morris water maze task do not differ from WT mice in spatial memory acquisition, STEP KO mice perform significantly better than WT during a reversal training task in the water maze (Venkitaramani et al., 2011). The fact that STEP KO mice outperform WT mice in a reversal task suggests that STEP KO mice exhibit a greater degree of cognitive flexibility. STEP KO mice also outperform WT mice in a water-escape motivated radial arm maze, which simultaneously tests spatial working and reference memory. During water-escape motivated radial arm maze task acquisition (the first 2 days of training), STEP KO mice commit fewer reference and working memory errors compared with WT mice (Venkitaramani et al., 2011).
To extend these findings, STEP KO and WT were tested in an amygdala-dependent behavioral paradigm (P. Olausson, J. Taylor, and P. J. Lombroso, unpublished observations). Specifically, mice were trained on an operant task requiring an instrumental response, followed by cued fear conditioning training. Mice were then tested for instrumental performance in the presence of the fear-associated stimulus. Compared with WT, STEP KO mice exhibit a greater degree of fear memory, as measured by suppression of the instrumental response. Taken together, the behavioral characterization of STEP KO mice indicates that deletion of STEP clearly benefits certain aspects of cognitive function. It is noteworthy that enhanced cognitive function in STEP KO mice relative to WT mice is not influenced by certain nonmnemonic factors. For example, STEP KO mice do not differ from WT mice in some behavioral paradigms testing anxiety, motor coordination, and motor learning (Venkitaramani et al., 2011).
The data showing that STEP KO mice outperform WT mice in several tasks are consistent with studies showing that transgenic mice with increased phosphorylation and/or activation of STEP substrates benefits cognition. For example, overexpression of GluN2B in the mouse forebrain yields enhanced spatial memory in a Morris water maze, fear memory, and object recognition memory compared with WT mice (Tang et al., 1999). Moreover, transgenic mice that overexpress forebrain type I adenylyl cyclase, which gives rise to enhanced ERK1/2 activation, exhibit enhanced object memory relative to WT mice (Wang et al., 2004). In addition, transgenic mice deficient in the immune receptor TRL3 exhibit increased hippocampal ERK1/2 and GluR1 signaling and show superior spatial working memory in a Morris water maze task compared with WT mice (Okun et al., 2010). Taken together, the fact that genetic elimination of STEP improves some aspects of learning and memory is probably due to increased activation of ERK1/2, Fyn, and Pyk2 as well as increased surface expression of NMDARs and AMPARs.
V. Dysregulation of Striatal-Enriched Protein Tyrosine Phosphatase in Neuropsychiatric Disorders
The first decade of STEP research elucidated the molecular mechanisms and post-translational modifications that regulate STEP's activity and translation. More recently, considerable effort has been dedicated to understanding how STEP dysregulation contributes to the pathophysiology of neuropsychiatric disorders. In some cases, STEP protein and/or activity is up-regulated (AD, SZ, FXS, epileptogenesis, and alcohol-induced memory loss) (Table 1). In others, STEP protein and/or activity is down-regulated (HD, drug abuse, stroke/ischemia, and inflammatory pain) (Table 1). Here we will review the abnormalities in STEP that have been documented in neuropsychiatric disorders over the past several years and discuss the potential therapeutic implications of these findings. It is important to emphasize that STEP is one of 400 proteins localized within dendritic spines, and disruptions in many of these proteins likely contribute to the pathophysiology of neuropsychiatric disorders. Even so, by increasing our understanding of STEP's involvement in these disorders, we hope to uncover converging properties that account for synaptopathological conditions in mental illness.
TABLE 1.
Neuropsychiatric disorders associated with changes in STEP
Phosphorylation of STEP at the regulatory serine site in the KIM domain reduces the affinity of STEP for its substrates leading to less dephosphorylation by STEP.
| Disorder | STEP Abnormalities Associated with the Disorder | Remarks | References |
|---|---|---|---|
| Alzheimer's disease | Phosphorylation ↓ Protein levels ↑ | Aβ binds to α7nAChRs and triggers activation of PP2B/calcineurin and PP1 to dephosphorylate STEP61; increased STEP61 protein levels are due to Aβ-induced inhibition of the ubiquitin proteasome system; genetic reduction of STEP attenuates cognitive, biochemical, and electrophysiological deficits in AD mice | Snyder et al., 2005; Kurup et al., 2010; Zhang et al., 2010 |
| Schizophrenia | Protein levels ↑ | STEP KOs are resistant to PCP-induced hyperlocomotion and cognitive deficits; neuroleptics lead to STEP61 phosphorylation and promote phosphorylation and activation of STEP substrates | Correa et al., 2009; Carty et al., 2010 |
| Fragile X syndrome | Protein levels ↑ | STEP mRNA associates with the RNA-binding protein FMRP; absence of FMRP increases translation of STEP61; STEP deletion reduces audiogenic seizures in Fmr1 KO mice | Goebel-Goody et al., 2010; Darnell et al., 2011 |
| Epileptogenesis | Protein expression in inhibitory hilar interneurons—High | Expression in inhibitory interneurons prevents ERK1/2 activation and promotes cell death; STEP KOs are more resistant to pilocarpine-induced SE | Choi et al., 2007; Briggs et al., 2011 |
| Alcohol-induced memory loss | Activity ↑ | STEP contributes to the ethanol-induced inhibition of NMDAR function, LTP, and fear conditioning | Alvestad et al., 2003; Wu et al., 2010; Hicklin et al., 2011 |
| Huntington's disease | mRNA ↓ Protein↓ Phosphorylation ↑ | STEP increases vulnerability of neurons to undergo cell death | Saavedra et al., 2011 |
| Drugs of abuse | Phosphorylation ↑ | Increased STEP61 phosphorylation is associated with greater ERK1/2 phosphorylation; infusion of TAT-STEPC-S into VLS prevents amphetamine-induced behavioral stereotypies | Valjent et al., 2005; Tashev et al., 2009 |
| Stroke/ischemia | mRNA in CA1/2 after global ischemia ↓ mRNA post-tMCAO ↓ mRNA in DG ↑ | STEP61 is cleaved into STEP33 after tMCAO; use of competitive peptide spanning cleavage site attenuates cell death after glutamate excitotoxicity or oxygen-deprivation models | Braithwaite et al., 2008; Xu et al., 2009 |
| Inflammatory pain | Interaction with Fyn ↓ | Reduced STEP61-Fyn association leads to phosphorylation and activation of Fyn as well as phosphorylation of GluN2B Tyr1472 | Yang et al., 2011 |
A. Alzheimer's Disease
AD is a debilitating neurodegenerative disorder associated with memory impairments, and it is estimated that 80 million people worldwide will have AD by 2040 (Forlenza et al., 2010). Characteristic features of AD include hyperphosphorylation of τ, accumulation of β-amyloid (Aβ), and the formation of amyloid plaques, all of which have been implicated in synaptic loss and cognitive decline (Hardy and Selkoe, 2002; Lacor et al., 2004). Excess Aβ attenuates NMDAR EPSCs, reduces LTP, and facilitates LTD (Kim et al., 2001; Selkoe, 2002; Snyder et al., 2005), suggesting that the effects of Aβ on synaptic plasticity are responsible, at least in part, for the cognitive decline observed in AD. Recent findings establish that Aβ modulates STEP via two parallel pathways that are not mutually exclusive (Fig. 5B): 1) dephosphorylation of STEP61 at Ser221 by Aβ-induced activation of PP2B/calcineurin and PP1 (Snyder et al., 2005), and 2) reduced degradation of STEP61 via Aβ-mediated inhibition of the UPS (Kurup et al., 2010). In both instances, elevated levels of Aβ result in up-regulation of STEP activity and consequently lead to decreased phosphorylation and surface expression of GluN2B-containing NMDARs, as well as reduced cognitive ability (Kurup et al., 2010; Zhang et al., 2010).
In the dephosphorylation pathway, Aβ binding to the α7 nicotinic receptor and NMDAR stimulation initiates calcium influx into neurons, which in turn activates PP2B/calcineurin and PP1 and triggers subsequent dephosphorylation of STEP61 at the regulatory Ser221 (Fig. 5B) (Dineley et al., 2001; Stevens et al., 2003; Snyder et al., 2005). As a result, the affinity of STEP61 for its substrates is thereby increased (Paul et al., 2003). At the same time, Aβ impairs the UPS, and STEP61 protein levels are elevated as a result of reduced degradation by the proteasome (Kurup et al., 2010). Consistent with these findings, increased STEP61 protein levels are observed in three mouse models of AD (Tg-2576, J20, 3xTg-AD) (Chin et al., 2005; Kurup et al., 2010; Zhang et al., 2010) and in the prefrontal cortex of patients with AD (Kurup et al., 2010). Regardless of which pathway is activated, the net result is the same: Aβ increases the level of active STEP61 in the brain and leads to inappropriate dephosphorylation of GluN2B Tyr1472 and internalization of GluN2B-containing NMDARs (Snyder et al., 2005; Kurup et al., 2010; Zhang et al., 2010).
Together, these studies suggest a critical role for STEP in mediating the aberrant endocytosis of NMDARs in AD and implicate STEP as a potential target for AD drug discovery. To show proof of concept for this hypothesis, Zhang et al. (2010) generated an AD mouse model (triple transgenic: 3xTg-AD) that is null for STEP. At 6 months old, 3xTg-AD mice normally show impairments in spatial reference memory, spatial working memory, and nonspatial hippocampus-dependent memory (Billings et al., 2005). Deletion of STEP reverses these cognitive deficits, given that double mutant mice (3xTg-AD/STEP KO) of similar age perform as well as WT in these tasks (Zhang et al., 2010). Moreover, the surface expression of GluN1/GluN2B receptors is restored to WT levels in double mutant mice, and hippocampal θ-burst LTP is enhanced in the double mutant mice compared with 3xTg-AD. Taken together, these findings demonstrate that genetic elimination of STEP reverses the cognitive, biochemical, and electrophysiological deficits associated with 3xTg-AD mice. An important discovery is that these changes occur without any alteration in Aβ expression or phosphorylated τ in the double mutant compared with 3xTg-AD mice (Zhang et al., 2010), suggesting that eliminating STEP is sufficient to improve cognitive function in the early stages of AD even in the presence of excess Aβ or phosphorylated τ. Although further studies are needed to determine whether the rescue persists over time (e.g., in aged AD mice null for STEP) or the optimal period for intervention (e.g., early versus late in AD progression), these results validate STEP as a potential target for drug discovery in AD.
Given that excess Aβ levels oftentimes precedes dementia, it is possible that changes in STEP and/or STEP substrates could be used as biomarkers for the early diagnosis of AD or to determine therapeutic efficacy (Forlenza et al., 2010). The most consistent biomarkers in current use for AD are the cerebrospinal fluid (CSF) concentrations of Aβ42, total τ, and phosphorylated τ. Low CSF concentrations of Aβ42, which are reflective of Aβ42 accumulating in the brain, and high CSF concentrations of total and phosphorylated τ are hallmark features of patients with AD, and these have been widely accepted to have good diagnostic accuracy for predicting dementia outcome (Forlenza et al., 2010). Nonetheless, given the heterogeneity of AD with persons with mild cognitive impairment and the invasive nature of obtaining CSF samples, the search for alternative biomarkers is essential. Perhaps STEP or STEP substrates could be assessed as biomarkers for early diagnosis or to determine the efficacy of a particular therapeutic strategy.
In support of this idea, the phosphorylation of ERK in lymphocytes from human peripheral blood has already been proposed as a biomarker to measure metabolic status for persons with FXS (Weng et al., 2008), so it is conceivable that ERK activation could be assessed in the CSF of patients with AD. Moreover, advancements in induced pluripotent stem cell technology may provide another assay to evaluate STEP function. Alternatively, it might be possible to use positron emission tomography neuroimaging technology with radioactively labeled compounds that target surface NMDARs. The prediction would be that surface labeled NMDARs would be reduced in patients with AD. An ongoing challenge would be to use the knowledge of STEP's involvement in the pathophysiology of AD to accurately discriminate between those with mild cognitive impairment who will ultimately progress to AD and healthy persons with nonprogressive cognitive impairment. A more practical possibility may be to use these techniques to assay real-time biochemical tracking of NMDAR surface expression and predict the likelihood of response to a STEP inhibitor.
B. Schizophrenia
SZ has a complex etiology, where genetics, the environment, and neuronal dysfunction all are contributing factors (Tsuang et al., 2001; for review, see Lewis and Levitt, 2002). Patients with SZ are plagued with positive symptoms (e.g., hallucinations, delusions), negative symptoms (e.g., flat affect, withdrawal), and cognitive deficits, and nearly 1% of the world's population older than 18 is afflicted with the disorder. One proposed mechanism implicated in the behavioral manifestation of SZ is NMDAR hypofunction (Goff and Coyle, 2001). Consistent with this hypothesis, postmortem studies of SZ brains show abnormalities in glutamate receptor density in the prefrontal cortex, thalamus, and temporal lobe (Gao et al., 2000; Ibrahim et al., 2000), and mice with reduced NMDAR expression have some SZ-like behaviors on cognitive tasks (Belforte et al., 2010). Moreover, SZ-like symptoms occur in persons who ingest the psychotomimetic drugs phencyclidine (PCP) or ketamine, which are both noncompetitive NMDAR antagonists (Rosenbaum et al., 1959; Javitt and Zukin, 1991; Krystal et al., 1994). Likewise, rodent models of SZ include injection of either PCP or the irreversible open channel NMDAR blocker MK801 (Moghaddam et al., 1997; Harris et al., 2003; Mouri et al., 2007; Bubenikova-Valesova et al., 2009).
The prevalent glutamate hypothesis of SZ posits that decreased neuronal surface expression of NMDARs contributes to the behavioral abnormalities (Gao et al., 2000; Ibrahim et al., 2000). Congruent with this hypothesis, genetic studies have implicated neuregulin (NRG1), a growth factor that promotes phosphorylation and retention of surface-bound NMDARs and its receptor ErbB4 (Bjarnadottir et al., 2007; Barros et al., 2009; but see also Pitcher et al., 2011) in the disease progression of SZ (Stefansson et al., 2002). One model proposes that NRG1/ErbB4 signaling normally activates Fyn and promotes phosphorylation of GluN2B Tyr1472 (Bjarnadottir et al., 2007), presumably leading to greater surface expression and function of NMDARs. Consistent with this hypothesis, NRG1/ErbB4 signaling promotes synaptic incorporation of NMDARs via NRG1/ErbB4-stimulated binding of PSD-95 to Erbin (Barros et al., 2009). Mutations in NRG1, as seen in patients with SZ (Stefansson et al., 2002), could therefore result in less Fyn-mediated phosphorylation of Tyr1472 and hypofunction of NMDARs. In agreement, Tyr1472 phosphorylation is reduced in mice heterozygous for NRG1, an effect that is rescued by the neuroleptic clozapine (Bjarnadottir et al., 2007).
It is important to mention that another model has been put forth to explain hypofunction of NMDARs in SZ. In this model, stimulation of NRG1 attenuates NMDAR activation by suppressing Src-mediated potentiation of NMDARs and phosphorylation of GluN2B (Pitcher et al., 2011), and overactivation of NRG1 in SZ therefore leads to NMDAR hypofunction (Hahn et al., 2006). One distinction to note is that the work of Pitcher et al. (2011) examines the effect of NRG1 on Src, whereas Bjarnadottir et al. (2007) identifies the effect of NRG1 on Fyn. Although these are both SFKs, it is possible that there is differential involvement of these kinases with NRG1 that mediates the effects on NMDARs. Although these two models are seemingly contradictory, reduced surface expression, phosphorylation, and/or function of NMDARs probably contribute to the hypofunction of glutamatergic synapses in SZ. Further research that rigorously addresses the molecular basis behind NRG1/ErbB4 signaling in SZ is required.
One possible explanation that may account for NMDAR hypofunction in SZ is up-regulation of STEP61 protein and/or activity. Accordingly, STEP61 protein levels are significantly increased in the anterior cingulate cortex of patients with SZ (Carty et al., 2010). Moreover, STEP KO mice show reduced PCP-induced locomotor activity and less PCP-induced cognitive deficits than WT (Correa et al., 2009). These findings suggest that genetic elimination of STEP is protective against the psychotomimetic effects of PCP.
Given that some patients with SZ show improvement upon treatment with antipsychotic medications, a logical question is whether STEP and/or STEP substrates are affected by these treatments (Carty et al., 2010). Injections of WT mice with three different neuroleptics (haloperidol, clozapine, and risperidone) for 21 days enhances the phosphorylation of STEP61 at Ser221, suggesting that these antipsychotics reduce the affinity of STEP for its substrates. Along those lines, the phosphorylation levels of GluN2B Tyr1472, Pyk2 Tyr402, and ERK1/2 Tyr204/187, as well as the surface expression of GluN2B, are all elevated after neuroleptic treatment. These results establish that neuroleptic medications exert their beneficial effects, at least in part, through the inactivation of STEP61 and promote the phosphorylation and activation of STEP substrates (Carty et al., 2010).
C. Fragile X Syndrome
FXS is the most common hereditary form of mental disability and is one of the most common known inherited forms of autism (Cornish et al., 2008). Cognitive disability, anxiety, and seizures are among the core symptoms of FXS, and approximately 25 to 30% of those with FXS meet the criteria for autism. Most patients with FXS have a CGG-expansion in the 5′ UTR of the Fmr1 gene that prevents transcription of the gene and subsequent translation of the protein it encodes, FMRP (for review, see Bassell and Warren, 2008). As mentioned earlier, the function of FMRP is to bind to and suppress translation of several mRNAs localized downstream of mGluR stimulation in dendrites (Li et al., 2001; Antar and Bassell, 2003; Bear et al., 2004). In FXS, FMRP is functionally absent, so the translation of some of those mRNAs is up-regulated (Huber et al., 2002; Zalfa et al., 2003; Lu et al., 2004; Hou et al., 2006; Gross et al., 2010).
A pivotal finding in the FXS field is work by Huber and colleagues (2002) establishing that mGluR-dependent LTD is up-regulated in the mouse model of FXS, the Fmr1 KO. This work led to the development of the mGluR hypothesis, which posits that stimulation of mGluRs leads to local translation of synaptic proteins that are responsible for mediating mGluR-LTD and proposes that many FXS-related phenotypes originate in exaggerated signaling through mGluRs (Bear et al., 2004). In section III.E, we reviewed evidence that the mGluR agonist DHPG leads to a rapid, dose-dependent increase in the translation of STEP (Zhang et al., 2008). Given its role in mediating endocytosis of NMDARs and AMPARs (Snyder et al., 2005; Braithwaite et al., 2006; Zhang et al., 2008; Kurup et al., 2010), STEP has been regarded as an “LTD protein” (Lüscher and Huber, 2010). In addition, STEP mRNA associates with FMRP (Darnell et al., 2011), and STEP translation is aberrantly up-regulated in Fmr1 KOs (Goebel-Goody et al., 2010). Thus, an updated model of the FXS mGluR theory emerges whereby exaggerated signaling through mGluRs causes dysregulation of STEP translation and subsequently increases the endocytosis rate of glutamate receptors. These events might explain the enhanced mGluR-LTD and behavioral deficits observed in Fmr1 KOs.
To determine whether STEP may be a promising therapeutic target in FXS, mice were generated that are null for both STEP and Fmr1 (Goebel-Goody et al., 2010). Expanding upon the observation that STEP KO mice are more resistant to pilocarpine-induced seizures (Briggs et al., 2011), STEP/Fmr1 double KO mice exhibit fewer audiogenic seizures and fewer seizure-induced c-Fos–positive neurons in the periaqueductal gray relative to Fmr1 KOs (Goebel-Goody et al., 2010). Although a major thrust of drug discovery in FXS has focused on inhibitors of mGluRs, our findings implicate STEP as a potential target downstream of mGluR stimulation.
D. Epileptogenesis
Epilepsy is a multifactorial, multifaceted brain disorder caused by repetitive, spontaneous seizures (for review, see Jensen, 2011). The onset of seizures is caused by aberrant neuronal activity restricted within one brain region (partial/focal seizures) or spread across multiple areas of the brain (absence/petit mal seizures or generalized tonic-clonic/grand mal seizures). The imbalance of synaptic excitation and inhibition is thought to lead to increased neuronal activity in epilepsy, so many medications have targeted these aspects of the disease. Even so, despite successful seizure management, more than 50% of patients with epilepsy have lasting cognitive and psychiatric impairments, indicating a complex interplay between perturbations of neuronal excitability and neuropsychiatric disorders (Jensen, 2011).
To study epilepsy in rodents, pilocarpine, a muscarinic agonist, is administered, normally induces status epilepticus (SE), and leads to the development of temporal lobe epilepsy and spontaneous seizures (ictogenesis) (Turski et al., 1983). The emergence of spontaneous seizures is believed to be due to pilocarpine-induced degradation of inhibitory interneurons in the dentate gyrus and subsequent disinhibition of granule cells (Sloviter, 1987). STEP61 is highly expressed in somatostatin-positive inhibitory interneurons of the hilus, which are extremely sensitive to pilocarpine-induced excitotoxicity (Choi et al., 2007). This subset of neurons acts as an important inhibitory feedback mechanism to the dentate gyrus. Specifically, hilar interneurons receive excitatory input from granule cell neurons, and they also synapse upon and provide inhibitory tone back onto these granule cell neurons. At baseline and after pilocarpine-induced SE, high levels of STEP61 suppress ERK1/2 activation in hilar interneurons, thereby preventing ERK1/2-mediated neuroprotection and promoting cell death (Choi et al., 2007). As a consequence, a reduction in the number of hilar interneurons decreases the inhibitory tone of granule cell neurons and thus increases their activity. Administration of either TAT-STEPC-S or pharmacological inhibitors that block STEP dephosphorylation are neuroprotective and rescue hilar interneurons from cell death (Choi et al., 2007). These findings lend support to the hypothesis that reducing STEP activity, specifically in hilar interneurons, may attenuate SE-induced excitotoxic cell death and restore the balance of excitation-inhibition onto granule cell neurons.
Genetic ablation of STEP consistently renders mice more resistant to pilocarpine-induced SE than WT or heterozygous mice (Briggs et al., 2011). Using calcium imaging and cell-attached recordings, Briggs et al. (2011) discovered that the basis for these findings is fold. During repetitive ictal-like stimulation, STEP KO granule cell neurons have reduced excitability, and inhibitory hilar interneurons show increased excitability. Together, these results suggest that the inhibitory input onto granule cell neurons is stronger in STEP KOs than WT mice and clarifies why STEP KOs have larger pilocarpine-induced seizure thresholds. Further studies are needed to determine whether the enhanced inhibitory tone in STEP KOs also explains the mechanism behind the reduced audiogenic seizures that are present in STEP/Fmr1 double KO mice (see section V.C). In summary, these collective findings demonstrate that the expression of STEP61 in hilar interneurons enhances the vulnerability of these neurons to cell death and increases the likelihood of pilocarpine-induced SE. Given that excitotoxic cell death of hippocampal interneurons may also give rise to cognitive deficits (Jensen, 2011), reducing STEP activity in these particular neurons may also be able to reverse some of these impairments.
E. Alcohol-Induced Memory Loss
Alcohol intoxication interferes with the ability to form new memories but does not affect previously established long-term memories (McIntosh and Chick, 2004). Considerable evidence suggests that modifications in synaptic circuitry are responsible for this alcohol-induced inhibition of learning and memory. For example, short-term ethanol treatment inhibits NMDAR function and blocks the induction of LTP (Lovinger et al., 1989; Blitzer et al., 1990; Morrisett and Swartzwelder, 1993; Schummers et al., 1997). Ethanol also induces dephosphorylation of GluN2B Tyr1472 (Alvestad et al., 2003). Inhibition of tyrosine phosphatases with a broad-spectrum inhibitor prevents ethanol-induced inhibition of NMDARs, suggesting that ethanol activates a tyrosine phosphatase (Alvestad et al., 2003).
A recent study demonstrates that STEP is necessary for ethanol inhibition of NMDAR function, LTP, and fear learning (Hicklin et al., 2011). In particular, ethanol fails to depress NMDAR currents in the presence of Tat-STEPC-S or in STEP KO mice. Moreover, the ethanol-induced inhibition of LTP induction is also blocked in STEP KO mice. These findings demonstrate that activation of STEP may be required for ethanol to exert its inhibitory effects on NMDAR-dependent synaptic plasticity. The mechanism by which ethanol fails to inhibit NMDARs in the absence of STEP is due, at least in part, to STEP's regulation of GluN2B phosphorylation. Whereas ethanol reduces Tyr1472 phosphorylation in WT slices, Tyr1472 phosphorylation remains unchanged in the presence of ethanol in slices from STEP KOs. STEP KO mice are also resistant to ethanol-induced inhibition of fear conditioned learning, suggesting that STEP plays a key role in mediating ethanol's inhibitory effects on NMDARs, LTP, and learning (Hicklin et al., 2011).
These studies implicate STEP in short-term ethanol-induced memory loss but do not address whether STEP is involved in the synaptic changes observed during long-term ethanol consumption or withdrawal. Given that long-term ethanol consumption impairs spatial cognitive performance (for review, see Matthews and Morrow, 2000) and that NMDAR surface expression is altered during long-term ethanol exposure and ethanol withdrawal (Clapp et al., 2010), it is conceivable that STEP plays a role in these neuroadaptive changes. Indeed, recent findings establish that long-term consumption of a liquid-ethanol diet modifies NMDARs such that they are no longer inhibited by a challenge (short-term) dose of ethanol (Wu et al., 2010). These findings are correlated with an increase in the protein levels of STEP33 and phosphorylation of p38, suggesting that long-term ethanol consumption may lead to calpain-mediated cleavage of STEP61 and subsequent activation of p38 (Wu et al., 2010). Additional studies are needed to confirm the involvement of STEP in long-term ethanol consumption and withdrawal.
An important consideration is that ethanol also enhances inhibitory GABAergic synaptic transmission (for review, see Kumar et al., 2009). Similar to WT, GABAA receptor (GABAAR) function continues to be enhanced by ethanol in STEP KOs (Hicklin et al., 2011). These findings establish that ethanol-induced enhancement of GABAARs does not seem to be regulated by STEP. Even so, it is possible that individual GABAAR subunits or other inhibitory receptors, such as GABAB receptors (GABABRs), are regulated by STEP. Tyrosine kinase inhibitors reduce the activity of GABAARs (Moss et al., 1995; Valenzuela et al., 1995; Wan et al., 1997); however, there are currently no reports of similar effects on GABABRs. It is noteworthy that very limited information has been collected regarding the PTPs that regulate dephosphorylation of GABARs. Future studies examining the role of STEP and its opposing SFKs in regulating GABARs will lead to a better understanding of ethanol-induced enhancement of inhibitory function.
F. Huntington's Disease
Up to this point, we have described neuropsychiatric disorders in which STEP up-regulation contributes to the manifestation of disease symptoms. Too little STEP protein can also have a negative impact on neuronal function and contribute to mental illness. One pertinent example is HD (Saavedra et al., 2011), which is a progressive, fatal, neurodegenerative disorder characterized by poor muscle coordination, mood disorders, and dementia (for review, see Ross and Tabrizi, 2011). The cause of HD is an abnormal CAG expansion in exon-1 of the huntingtin (htt) gene (Huntington's Disease Collaborative Research Group, 1993), resulting in a neurodegenerative disorder that specifically targets striatal projection neurons (Reiner et al., 1988). In contrast to AD, where STEP protein levels are elevated compared with controls (Chin et al., 2005; Kurup et al., 2010; Zhang et al., 2010), both STEP message and protein levels are significantly decreased with age in the striatum of an HD mouse model (R6/1) (Saavedra et al., 2011). At the same time, the phosphorylation of STEP significantly increases over time in these mice, which is correlated with enhanced PKA activity and reduced calcineurin activity. Consistent with decreased STEP expression and activity, the phosphorylation of both ERK1/2 and p38 are elevated in R6/1 mice later in life (20–30 weeks). Decreased STEP protein levels and hyperphosphorylation of STEP are also observed in several other mouse models of HD (R6/2, Tet/HD94, and HdhQ7/Q111), confirming the findings from R6/1 mice (Saavedra et al., 2011). Intrastriatal injections of the NMDAR agonist quinpirole induce excitotoxic cell death and result in lesion formation, which is dependent on calcineurin activation (Xifró et al., 2009). It is noteworthy that R6/1 mice show increased resistance to quinpirole-induced excitotoxicity (Hansson et al., 2001). Concurrent injections of quinpirole with WT TAT-STEP exacerbate the striatal lesions in both WT and R6/1 mice, suggesting that STEP acts downstream of calcineurin to mediate cell death after this excitotoxic insult (Saavedra et al., 2011). Together, these findings demonstrate that STEP increases the vulnerability of striatal neurons to undergo cell death after an excitotoxic insult and establish that the down-regulation of STEP in HD mouse models may be responsible for their increased resistance to excitoxicity (Saavedra et al., 2011). Future investigations should identify the mechanism behind reduced STEP mRNA and protein expression in HD.
G. Drugs of Abuse
Psychostimulant drugs such as cocaine and amphetamine exert their addictive and rewarding effects by altering the circuitry of glutamatergic projections from the prefrontal cortex to dopaminergic neurons in the ventral tegmental area (Nestler et al., 1996). Elevated levels of ERK1/2 phosphorylation are observed in corticolimbic areas after cocaine administration (Valjent et al., 2000). Moreover, short-term cocaine potentiates synaptic responses in ventral tegmental area dopamine neurons by promoting the rapid insertion of surface glutamate receptors (Borgland et al., 2004; Sun et al., 2008; Ferrario et al., 2011). ERK1/2 activation is also observed after amphetamine injections (Choe et al., 2002), and this requires both D1Rs and NMDARs (Valjent et al., 2005). The amphetamine-induced phosphorylation of ERK1/2 requires PKA-mediated phosphorylation of DARPP-32 and its subsequent inhibition of PP1 (Fig. 5C). Thus, inhibition of PP1 by DARPP-32 and activation of PKA converge to enhance phosphorylation of STEP and reduce the affinity of STEP for its substrates (Valjent et al., 2005). As a result, greater phosphorylation of ERK1/2 may contribute to the development of addiction, the onset of behavioral stereotypies, and reward-controlled learning by facilitating long-lasting changes in corticostriatal circuitry by inducing gene transcription (Fig. 5C).
This model is congruent with a study by Tashev et al. (2009), which shows that infusion of the substrate-trapping isoform of STEP (TAT-STEPC-S) into the ventrolateral striatum prevents amphetamine-induced behavioral stereotypies. As discussed earlier, TAT-STEPC-S binds to ERK1/2, does not dephosphorylate it, and in turn blocks nuclear translocation of ERK1/2. Thus, ERK1/2-mediated gene transcription must be necessary for the manifestation of amphetamine-induced behavioral stereotypies. Alternatively, given that ERK1/2 activation after amphetamine exposure requires D1R signaling (Valjent et al., 2005), it is possible that dopamine/D1R-induced activation of PKA by amphetamine leads to phosphorylation and inactivation of STEP (Paul et al., 2000, 2003). Tat-STEPC-S also blocks the expression of corticostriatal HFS-LTP and facilitates a low-frequency stimulation-dependent form of LTD (Tashev et al., 2009), supporting the notion that STEP opposes synaptic strengthening and promotes synaptic depression. Therefore, STEP inhibition and enhanced phosphorylation of STEP substrates probably contribute to the neuroadaptive changes in synaptic efficacy that are acquired during drug sensitization and drug-seeking behaviors.
H. Stroke/Ischemia
Eighty percent of all stroke cases are ischemic insults caused by blocked blood flow and oxygen deprivation to a focal brain area, often because of a blood clot (Durukan et al., 2008). Ischemia leads to massive cell death in an irreversibly damaged infarct core with a surrounding, potentially salvageable area called the penumbra (Feuerstein and Wang, 2000). If the blockage clears, reperfusion of oxygenated blood triggers glutamate release, calcium entry into the cell, and production of oxygen radicals (Hossmann, 1998). Drug development strategies have focused on ways to reduce the period of blockade, increase penumbral salvage, or decrease reperfusion damage (Durukan et al., 2008). Even so, this field represents a large unmet clinical need. Thrombolytic treatment with recombinant tissue plasminogen activator is the only currently approved treatment but is effective only within 3 to 4 h of clinical symptom onset, limiting its practical use (Amaro and Chamorro, 2011).
Emerging work suggests that ischemic insult profoundly modifies both the expression and activity of STEP (Braithwaite et al., 2008). In an adult rat model, global ischemia followed by reperfusion leads to a significant reduction in STEP message in areas cornu ammonis 1 and 2 of the hippocampus, both of which are particularly susceptible to ischemic damage. Concomitantly, STEP mRNA is up-regulated in the ischemia-resistant dentate gyrus, which is protected from damage, after global ischemia. STEP message is also decreased after transient medial cerebral artery occlusion (tMCAO), first in the ischemic core and then in the penumbra in a pattern that precedes cell death. In addition to changes at the transcriptional level, tMCAO induces post-transcriptional alterations and leads to a redistribution of STEP from membrane-bound to soluble compartments (Braithwaite et al., 2008). Within 45 min of tMCAO, STEP61 is cleaved by calpain into STEP33 (Braithwaite et al., 2008) and initiates cell death pathways by activating p38 (Xu et al., 2009). Given that NMDAR tyrosine phosphorylation and surface expression is increased after cerebral hypoxia/ischemia (Gurd et al., 1999; Besshoh et al., 2005), it is likely that calpain-mediated cleavage, and STEP inactivation is responsible. A competitive peptide that spans the cleavage site on STEP significantly attenuates cell death after either glutamate excitotoxicity or oxygen-glucose deprivation models (Xu et al., 2009), suggesting that therapeutic strategies preventing STEP cleavage may prove beneficial for treating patients who have had strokes.
I. Inflammatory Pain
Hyperfunction of GluN2B-containing NMDARs is thought to contribute to pain sensitivity and hyperexcitability of nociceptive sensory neurons in the spinal dorsal horn (Gogas, 2006). Accordingly, selective GluN2B receptor antagonists effectively alleviate inflammatory and neuropathic pain. The underlying mechanism seems to involve PKA, Fyn, and STEP (Yang et al., 2011). Intrathecal injections of the PKA agonist forskolin or complete Freund's adjuvant (CFA), an inducer of chronic pain, drive GluN2B-containing NMDARs into PSD fractions and increase Tyr1472 phosphorylation. Both treatments also decrease paw withdrawal thresholds to Von Frey stimuli, suggesting increased sensitivity to pain. The SFK inhibitor PP2 blocks the forskolin- or CFA-induced increase in PSD-associated GluN2B, as well as Tyr1472 phosphorylation, demonstrating that SFKs act downstream of PKA activation. It is noteworthy that the association of Fyn with STEP is decreased in mice after CFA injections, and this disruption leads to increased phosphorylation of Fyn at Tyr420 (Yang et al., 2011). Given that PKA phosphorylates STEP and reduces STEP's affinity for its substrates (Paul et al., 2000, 2003), it is certainly possible that the effects of forskolin and CFA on GluN2B phosphorylation and surface expression (Yang et al., 2011) are due to reduced STEP activity. Additional work is needed to determine the therapeutic relevance that increasing STEP activity might have on inflammatory and neuropathic pain.
VI. Closing Remarks
A. The Outlook of Drug Discovery for Striatal-Enriched Protein Tyrosine Phosphatase Inhibitors
As described above, some, but not all, neuropsychiatric disorders might benefit from the development of STEP inhibitors. The active site pocket in STEP, as well as other PTPs, is particularly deep and positively charged (Barr, 2010). The pocket's depth confers specificity for phosphotyrosines over phosphoserines or phosphothreonines, because the latter groups are not long enough to extend all the way to the bottom of the pocket. The positive charge in the pocket attracts and complements the hard negative charge of the phosphate-group substrate. Even though highly charged phosphomimetics bind well to the active site, they exhibit low cell permeability and thus identification of small molecule inhibitors for PTPs has proven challenging. Five-membered ring phosphonate mimetics (isothiazolidinone and thiadiazolidone) have recently been developed with markedly improved cell permeability and good pocket binding. Another strategy that could be used to address the permeability issue would be to link phosphomimetics with cell-permeable peptides or lipophilic fatty acids (Barr, 2010).
A flexible Trp-Pro-Asp loop (WPD sequence) flanks the active site pocket in PTPs (Barr, 2010). This loop is important in enzymatic function, as it often undergoes important conformational changes upon substrate binding. STEP belongs to a class of PTPs (those that bind to MAPKs) that have a WPD sequence quite different from most other PTPs (WPDXGXP) (Barr and Knapp, 2006). In most other PTPs, the relevant sequence is His-Gly-Val-Pro, whereas STEP's sequence is Gln-Lys-Thr-Pro. Other varieties exist, including His-Lys-Thr-Pro in STEP-like PTPs and His-Gln-Thr-Pro in HePTP. These sequence-specific differences may impart substrate-specificity and allow the design of more selective drugs for MAPK PTPs over other PTPs. Moreover, the unusual conformation of the WPD loop in STEP separates it from the rest of the MAPK PTPs and could be used to maximize selectivity for STEP over its close relatives. Unlike most PTPs, the WPD in STEP seems to be stable (uninfluenced by substrate binding) (Barr and Knapp, 2006). In addition, a chemical linking strategy where several small molecules are combined to span the active site and different adjoining pockets might further increase the selectivity of compounds for STEP. Accordingly, this method has been successful in increasing the selectivity of inhibitors toward PTP1B over a close relative (Barr, 2010).
B. Summary and Future Directions
Since its discovery more than 2 decades ago, a considerable body of research has extended our understanding of the role STEP plays in synaptic plasticity and neuropsychiatric disorders. STEP dephosphorylates and inactivates signaling enzymes, including ERK1/2, p38, and Fyn. Moreover, dephosphorylation of NMDARs and AMPARs by STEP promotes their endocytosis by facilitating interactions with clathrin adapter proteins. For these reasons, STEP opposes LTP and facilitates LTD. The molecular mechanisms governing STEP's expression and activity are proving to be a complex affair, where phosphorylation, cleavage, dimerization, ubiquitination, and local translation all converge to maintain an appropriate balance STEP function in the CNS. It is evident from recent studies that disrupting any of these mechanisms can flip the balance of STEP activity to make it more or less active. Either of these results could contribute to the manifestation of symptoms associated with neuropsychiatric diseases. One of the primary conundrums of STEP research is that it is up-regulated in some neuropsychiatric disorders and down-regulated in others. How can too much or too little STEP promote diseases of such heterogeneous nature? One explanation might be that each disorder involves a distinct pathway that alters STEP function, and the interplay of STEP with other affected partners negatively influences synaptic function.
An open question to the development of therapeutics targeting STEP is how to impart substrate and/or brain region specificity without generating unwanted side effects. Given that STEP KO mice seem healthy with no apparent gross anatomical abnormalities (Venkitaramani et al., 2009), inhibitors of STEP function will probably be well tolerated and improve some neuropsychiatric disorders. Indeed, memory impairment is improved in AD mice that are null for STEP (Zhang et al., 2010). Even so, we cannot exclude the possibility that other phosphatases that regulate STEP substrates might compensate for the loss of STEP in STEP KOs or decreased STEP function in the presence of a STEP compound inhibitor. For example, perhaps the dual specificity phosphatase MAPK phosphatase is up-regulated in STEP KOs to prevent exaggerated activation of ERK1/2 and its downstream nuclear targets. Future work is required to rigorously address this issue.
Some of the most effective PTP inhibitors target the conserved catalytic phosphatase site (Popov, 2011); however, these are often too general and lack selective inhibition. To enhance inhibitor specificity, it is attractive to propose the development of compounds that interfere with STEP substrate binding. As discussed in section II.B, the interaction of Fyn with STEP61 requires both the first PP and KIM domains on STEP61. In contrast, the KIM domain is the only known region necessary for ERK1/2 binding. It is plausible to suggest that peptides that block the interaction of Fyn with STEP61 at the PP region might not interfere with the interaction of ERK1/2 to STEP61. By increasing our knowledge of how each of the substrates interacts with STEP, we may be able to develop substrate-interfering peptides. This strategy is currently being employed to develop cell penetrating c-Jun N-terminal kinase inhibitors to block the interaction of c-Jun N-terminal kinase with its scaffolding partners in the treatment of neurodegenerative diseases (Antoniou et al., 2011). Alternatively, cell-permeable peptides that mimic endogenous inhibitors of STEP may provide a degree of isoform specificity. There is precedence for this technology; tatCN21, a potent and selective inhibitor of CaMKII derived from the natural CaMKII inhibitor CaM-KIIN, has no effect on the activity of CaMKI, CaMKIV, or CaMKK (Coultrap et al., 2011). Large-scale screenings to identify and characterize potential endogenous proteins that interact with and modulate STEP activity will be necessary to move this possibility forward.
Perhaps a greater challenge is the prospect of potentiating STEP activity in neuropsychiatric disorders in which STEP is down-regulated. Given that the interaction of STEP with its substrates is decreased by PKA phosphorylation in the KIM domain, one logical solution might be to use PKA inhibitors to enhance STEP function; however, these inhibitors have their own set of off-target shortcomings (Murray, 2008). Another approach might be to inhibit endogenous suppressors of STEP translation (e.g., CPEB, FMRP) or transcription to increase the expression of STEP. These options prompt the question of how to effectively potentiate STEP activity without increasing the propensity to develop neuropsychiatric disorders where STEP is up-regulated. Will increasing STEP expression improve HD without causing AD? Will subtle increases in STEP function relieve inflammatory pain without causing SZ? If we are fortunate, accurate drug dosing may solve this drawback. Another possible outcome is that modulators of STEP activity can be targeted to particular brain regions that are affected in various neuropsychiatric disorders. Given that STEP expression varies at regional, cellular, and subcellular levels, distinct pools of STEP may contribute to the manifestation of particular neuropsychiatric disorders. It is therefore exciting to consider the possibility that therapeutic interventions targeting specific pools of STEP could be developed. Further research aimed at determining the role of STEP in other synaptopathological conditions may help uncover mechanisms governing STEP regulation in the CNS and lead to the development of effective therapeutic interventions.
Acknowledgments
This work was supported by the National Institutes of Health National Institute of Mental Health [Grants MH091037, MH052711] (to P.J.L.); FRAXA (to S.M.G.G. and P.J.L.); and Institute for the Study of Aging (to P.J.L.). We thank Dr. Marilee Ogren for critically reading and editing the manuscript. We also thank all members of the Lombroso laboratory for helpful discussions on the models and suggestions on how to improve the manuscript.
Authorship Contributions
Conducted experiments: Paspalas.
Wrote or contributed to the writing of the manuscript: Goebel-Goody, Baum, Paspalas, Fernandez, Carty, Kurup, and Lombroso.
This article is available online at http://pharmrev.aspetjournals.org.
- α7nAChR
- α7 nicotinic acetylcholine receptor
- Aβ
- β-amyloid
- AD
- Alzheimer's disease
- AMPAR
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- CaMK
- Ca2+/calmodulin-dependent protein kinase
- CFA
- complete Freund's adjuvant
- CNS
- central nervous system
- CPE
- cytoplasmic polyadenylation element
- CPEB
- cytoplasmic polyadenylation element binding protein
- CSF
- cerebrospinal fluid
- D1R
- dopamine D1 receptor
- D2R
- dopamine D2 receptor
- DARPP-32
- dopamine- and cAMP-regulated phosphoprotein of Mr 32,000
- DHPG
- (R,S)-3,5-dihydroxyphenylglycine
- EPSC
- excitatory postsynaptic current
- ERK
- extracellular signal-regulated kinase
- FMRP
- fragile X mental retardation protein
- FXS
- fragile X syndrome
- GABAAR
- GABAA receptor
- GABABR
- GABAB receptor
- HD
- Huntington's disease
- HePTP
- hematopoietic tyrosine phosphatase
- HFS
- high-frequency stimulation
- KIM
- kinase-interacting motif
- KO
- knockout
- LTD
- long-term depression
- LTP
- long-term potentiation
- LY367385
- (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid
- MAPK
- mitogen-activated protein kinase
- mGluR
- metabotropic glutamate receptor
- MK801
- dizocilpine
- MVP
- major vault protein
- NMDAR
- N-methyl-d-aspartate receptor
- NRG1
- neuregulin
- p38
- stress-activated protein kinase p38
- PCP
- phencyclidine; PEST
- PKA
- cAMP-dependent protein kinase
- PP
- polyproline
- PP1
- protein phosphatase 1
- PP2B
- protein phosphatase 2B (calcineurin)
- PSD
- postsynaptic density
- PTP
- protein tyrosine phosphatase
- SE
- status epilepticus
- SFK
- Src family tyrosine kinase
- STEP
- striatal-enriched protein tyrosine phosphatase
- SZ
- schizophrenia
- TAT-STEPC-S
- substrate-trapping membrane-permeable fusion protein of STEP46
- tMCAO
- transient medial cerebral artery occlusion
- UPS
- ubiquitin proteasome system
- UTR
- untranslated region
- WT
- wild type.
References
- Adachi M, Sekiya M, Arimura Y, Takekawa M, Itoh F, Hinoda Y, Imai K, Yachi A. (1992) Protein-tyrosine phosphatase expression in pre-B cell NALM-6. Cancer Res 52:737–740 [PubMed] [Google Scholar]
- Alvestad RM, Grosshans DR, Coultrap SJ, Nakazawa T, Yamamoto T, Browning MD. (2003) Tyrosine dephosphorylation and ethanol inhibition of N-methyl-d-aspartate receptor function. J Biol Chem 278:11020–11025 [DOI] [PubMed] [Google Scholar]
- Amaro S, Chamorro Á. (2011) Translational stroke research of the combination of thrombolysis and antioxidant therapy. Stroke 42:1495–1499 [DOI] [PubMed] [Google Scholar]
- Antar LN, Bassell GJ. (2003) Sunrise at the synapse: the FMRP mRNP shaping the synaptic interface. Neuron 37:555–558 [DOI] [PubMed] [Google Scholar]
- Antoniou X, Falconi M, Di Marino D, Borsello T. (2011) JNK3 as a therapeutic target for neurodegenerative diseases. J Alzheimers Dis 24:633–642 [DOI] [PubMed] [Google Scholar]
- Barr AJ. (2010) Protein tyrosine phosphatases as drug targets: strategies and challenges of inhibitor development. Future Med Chem 2:1563–1576 [DOI] [PubMed] [Google Scholar]
- Barr AJ, Knapp S. (2006) MAPK-specific tyrosine phosphatases: new targets for drug discovery? Trends Pharmacol Sci 27:525–530 [DOI] [PubMed] [Google Scholar]
- Barros CS, Calabrese B, Chamero P, Roberts AJ, Korzus E, Lloyd K, Stowers L, Mayford M, Halpain S, Müller U. (2009) Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proc Natl Acad Sci USA 106:4507–4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. (2008) Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron 60:201–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. (2004) The mGluR theory of fragile X mental retardation. Trends Neurosci 27:370–377 [DOI] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. (2010) Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci 13:76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besshoh S, Bawa D, Teves L, Wallace MC, Gurd JW. (2005) Increased phosphorylation and redistribution of NMDA receptors between synaptic lipid rafts and post-synaptic densities following transient global ischemia in the rat brain. J Neurochem 93:186–194 [DOI] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. (2005) Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron 45:675–688 [DOI] [PubMed] [Google Scholar]
- Bjarnadottir M, Misner DL, Haverfield-Gross S, Bruun S, Helgason VG, Stefansson H, Sigmundsson A, Firth DR, Nielsen B, Stefansdottir R, et al. (2007) Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1+/- knock-outs compared with wild-type mice. J Neurosci 27:4519–4529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD. (2002) Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron 36:435–449 [DOI] [PubMed] [Google Scholar]
- Blitzer RD, Gil O, Landau EM. (1990) Long-term potentiation in rat hippocampus is inhibited by low concentrations of ethanol. Brain Res 537:203–208 [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. (2004) Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci 24:7482–7490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger LM, Lombroso PJ, Raghunathan A, During MJ, Wahle P, Naegele JR. (1995) Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci 15:1532–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite SP, Adkisson M, Leung J, Nava A, Masterson B, Urfer R, Oksenberg D, Nikolich K. (2006) Regulation of NMDA receptor trafficking and function by striatal-enriched tyrosine phosphatase (STEP). Eur J Neurosci 23:2847–2856 [DOI] [PubMed] [Google Scholar]
- Braithwaite SP, Xu J, Leung J, Urfer R, Nikolich K, Oksenberg D, Lombroso PJ, Shamloo M. (2008) Expression and function of striatal enriched protein tyrosine phosphatase is profoundly altered in cerebral ischemia. Eur J Neurosci 27:2444–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Wells DG. (2007) Dendritic mRNA: transport, translation and function. Nat Rev Neurosci 8:776–789 [DOI] [PubMed] [Google Scholar]
- Briggs SW, Walker J, Asik K, Lombroso P, Naegele J, Aaron G. (2011) STEP regulation of seizure thresholds in the hippocampus. Epilepsia 52:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubenikova-Valesova V, Svoboda J, Horacek J, Vales K. (2009) The effect of a full agonist/antagonist of the D1 receptor on locomotor activity, sensorimotor gating and cognitive function in dizocilpine-treated rats. Int J Neuropsychopharmacol 12:873–883 [DOI] [PubMed] [Google Scholar]
- Bult A, Zhao F, Dirkx R, Jr, Raghunathan A, Solimena M, Lombroso PJ. (1997) STEP: a family of brain-enriched PTPs. Alternative splicing produces transmembrane, cytosolic and truncated isoforms. Eur J Cell Biol 72:337–344 [PubMed] [Google Scholar]
- Bult A, Zhao F, Dirkx R, Jr, Sharma E, Lukacsi E, Solimena M, Naegele JR, Lombroso PJ. (1996) STEP61: a member of a family of brain-enriched PTPs is localized to the endoplasmic reticulum. J Neurosci 16:7821–7831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty NC, Kurup P, Xu J, Austin DR, Chen G, Lombroso PJ. (2010) Striatal enriched protein tyrosine phosphatase (STEP) mediates the beneficial effects of antipsychotic drugs. Soc Neurosci Abstr 36:452.2 [Google Scholar]
- Chin J, Palop JJ, Puoliväli J, Massaro C, Bien-Ly N, Gerstein H, Scearce-Levie K, Masliah E, Mucke L. (2005) Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer's disease. J Neurosci 25:9694–9703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe ES, Chung KT, Mao L, Wang JQ. (2002) Amphetamine increases phosphorylation of extracellular signal-regulated kinase and transcription factors in the rat striatum via group I metabotropic glutamate receptors. Neuropsychopharmacology 27:565–575 [DOI] [PubMed] [Google Scholar]
- Choi YS, Lin SL, Lee B, Kurup P, Cho HY, Naegele JR, Lombroso PJ, Obrietan K. (2007) Status epilepticus-induced somatostatinergic hilar interneuron degeneration is regulated by striatal enriched protein tyrosine phosphatase. J Neurosci 27:2999–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp P, Gibson ES, Dell'acqua ML, Hoffman PL. (2010) Phosphorylation regulates removal of synaptic N-methyl-D-aspartate receptors after withdrawal from chronic ethanol exposure. J Pharmacol Exp Ther 332:720–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K, Turk J, Hagerman R. (2008) The fragile X continuum: new advances and perspectives. J Intellect Disabil Res 52:469–482 [DOI] [PubMed] [Google Scholar]
- Correa PR, Fernandez SM, Park JI, Lombroso PJ. (2009) STriatal-Enriched protein tyrosine Phosphatase (STEP) knock-out mice are resistant to the effects of phencyclidine on behavioral and biochemical measures. Soc Neurosci Abstr 35:135.16 [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. (2009) Translational control of long-lasting synaptic plasticity and memory. Neuron 61:10–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap SJ, Vest RS, Ashpole NM, Hudmon A, Bayer KU. (2011) CaMKII in cerebral ischemia. Acta Pharmacol Sin 32:861–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado A, Nebreda AR. (2010) Mechanisms and functions of p38 MAPK signalling. Biochem J 429:403–417 [DOI] [PubMed] [Google Scholar]
- Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. (2001) Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell 107:489–499 [DOI] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, et al. (2011) FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146:247–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. (2000) The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci 20:4563–4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb I, Poddar R, Paul S. (2011) Oxidative stress-induced oligomerization inhibits the activity of the non-receptor tyrosine phosphatase STEP61. J Neurochem 116:1097–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley KT, Westerman M, Bui D, Bell K, Ashe KH, Sweatt JD. (2001) Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer's disease. J Neurosci 21:4125–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durukan A, Strbian D, Tatlisumak T. (2008) Rodent models of ischemic stroke: a useful tool for stroke drug development. Curr Pharm Des 14:359–370 [DOI] [PubMed] [Google Scholar]
- Eswaran J, von Kries JP, Marsden B, Longman E, Debreczeni JE, Ugochukwu E, Turnbull A, Lee WH, Knapp S, Barr AJ. (2006) Crystal structures and inhibitor identification for PTPN5, PTPRR and PTPN7: a family of human MAPK-specific protein tyrosine phosphatases. Biochem J 395:483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Li X, Wolf ME. (2011) Effects of acute cocaine or dopamine receptor agonists on AMPA receptor distribution in the rat nucleus accumbens. Synapse 65:54–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. (1999) Clenbuterol administration into the basolateral amygdala post-training enhances retention in an inhibitory avoidance task. Neurobiol Learn Mem 72:8–12 [DOI] [PubMed] [Google Scholar]
- Feuerstein GZ, Wang X. (2000) Animal models of stroke. Mol Med Today 6:133–135 [DOI] [PubMed] [Google Scholar]
- Forlenza OV, Diniz BS, Gattaz WF. (2010) Diagnosis and biomarkers of predementia in Alzheimer's disease. BMC Med 8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XM, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. (2000) Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry 157:1141–1149 [DOI] [PubMed] [Google Scholar]
- Gelinas JN, Nguyen PV. (2005) Beta-adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J Neurosci 25:3294–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs ME, Summers RJ. (2000) Separate roles for beta2- and beta3-adrenoceptors in memory consolidation. Neuroscience 95:913–922 [DOI] [PubMed] [Google Scholar]
- Gladding CM, Collett VJ, Jia Z, Bashir ZI, Collingridge GL, Molnár E. (2009) Tyrosine dephosphorylation regulates AMPAR internalisation in mGluR-LTD. Mol Cell Neurosci 40:267–279 [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. (2009) Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience 158:1446–1459 [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Wallis ED, Li L, Hall DV, Royston S, Baum M, Naegele JR, Lombroso PJ. (2010) Loss of striatal-enriched protein tyrosine phosphatase (STEP) reverses deficits in a fragile X syndrome mouse model. Soc Neurosci Abstr 36:452.4 [Google Scholar]
- Goff DC, Coyle JT. (2001) The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry 158:1367–1377 [DOI] [PubMed] [Google Scholar]
- Gogas KR. (2006) Glutamate-based therapeutic approaches: NR2B receptor antagonists. Curr Opin Pharmacol 6:68–74 [DOI] [PubMed] [Google Scholar]
- Gross C, Nakamoto M, Yao X, Chan CB, Yim SY, Ye K, Warren ST, Bassell GJ. (2010) Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J Neurosci 30:10624–10638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurd JW, Bissoon N, Nguyen TH, Lombroso PJ, Rider CC, Beesley PW, Vannucci SJ. (1999) Hypoxia-ischemia in perinatal rat brain induces the formation of a low molecular weight isoform of striatal enriched tyrosine phosphatase (STEP). J Neurochem 73:1990–1994 [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, et al. (2006) Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med 12:824–828 [DOI] [PubMed] [Google Scholar]
- Hansson O, Guatteo E, Mercuri NB, Bernardi G, Li XJ, Castilho RF, Brundin P. (2001) Resistance to NMDA toxicity correlates with appearance of nuclear inclusions, behavioural deficits and changes in calcium homeostasis in mice transgenic for exon 1 of the huntington gene. Eur J Neurosci 14:1492–1504 [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. (2010) Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci 11:682–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. (2002) Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci 5:405–414 [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297:353–356 [DOI] [PubMed] [Google Scholar]
- Harris LW, Sharp T, Gartlon J, Jones DN, Harrison PJ. (2003) Long-term behavioural, molecular and morphological effects of neonatal NMDA receptor antagonism. Eur J Neurosci 18:1706–1710 [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Morioka M, Goto S, Korematsu K, Okamura A, Yano S, Kai Y, Hamada JI, Ushio Y. (2000) Expression of neuron specific phosphatase, striatal enriched phosphatase (STEP) in reactive astrocytes after transient forebrain ischemia. Glia 29:316–329 [PubMed] [Google Scholar]
- Hayashi T, Huganir RL. (2004) Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. J Neurosci 24:6152–6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde AN. (2010) The ubiquitin-proteasome pathway and synaptic plasticity. Learn Mem 17:314–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks WJ, Dilaver G, Noordman YE, Kremer B, Fransen JA. (2009) PTPRR protein tyrosine phosphatase isoforms and locomotion of vesicles and mice. Cerebellum 8:80–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicklin TR, Wu PH, Radcliffe RA, Freund RK, Goebel-Goody SM, Correa PR, Proctor WR, Lombroso PJ, Browning MD. (2011) Alcohol inhibition of the NMDA receptor function, long-term potentiation, and fear learning requires striatal-enriched protein tyrosine phosphatase. Proc Natl Acad Sci USA 108:6650–6655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossmann KA. (1998) Experimental models for the investigation of brain ischemia. Cardiovasc Res 39:106–120 [DOI] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. (2006) Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron 51:441–454 [DOI] [PubMed] [Google Scholar]
- Hu Y, Zhang Y, Venkitaramani DV, Lombroso PJ. (2007) Translation of striatal-enriched protein tyrosine phosphatase (STEP) after beta1-adrenergic receptor stimulation. J Neurochem 103:531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Lu W, Ali DW, Pelkey KA, Pitcher GM, Lu YM, Aoto H, Roder JC, Sasaki T, Salter MW, et al. (2001) CAKbeta/Pyk2 kinase is a signaling link for induction of long-term potentiation in CA1 hippocampus. Neuron 29:485–496 [DOI] [PubMed] [Google Scholar]
- Huang YS, Carson JH, Barbarese E, Richter JD. (2003) Facilitation of dendritic mRNA transport by CPEB. Genes Dev 17:638–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. (1996) Modulation of both the early and the late phase of mossy fiber LTP by the activation of beta-adrenergic receptors. Neuron 16:611–617 [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. (2002) Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA 99:7746–7750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. (2000) Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288:1254–1257 [DOI] [PubMed] [Google Scholar]
- Huntington's Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72:971–983 [DOI] [PubMed] [Google Scholar]
- Ibrahim HM, Hogg AJ, Jr, Healy DJ, Haroutunian V, Davis KL, Meador-Woodruff JH. (2000) Ionotropic glutamate receptor binding and subunit mRNA expression in thalamic nuclei in schizophrenia. Am J Psychiatry 157:1811–1823 [DOI] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. (2006) Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol 572:789–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. (1991) Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148:1301–1308 [DOI] [PubMed] [Google Scholar]
- Jensen FE. (2011) Epilepsy as a spectrum disorder: implications from novel clinical and basic neuroscience. Epilepsia 52 (Suppl 1):1–6 [DOI] [PubMed] [Google Scholar]
- Johnson SA, Hunter T. (2005) Kinomics: methods for deciphering the kinome. Nat Methods 2:17–25 [DOI] [PubMed] [Google Scholar]
- Keith D, El-Husseini A. (2008) A Excitation Control: Balancing PSD-95 Function at the Synapse. Front Mol Neurosci 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Anwyl R, Suh YH, Djamgoz MB, Rowan MJ. (2001) Use-dependent effects of amyloidogenic fragments of (beta)-amyloid precursor protein on synaptic plasticity in rat hippocampus in vivo. J Neurosci 21:1327–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Markham JA, Weiler IJ, Greenough WT. (2008a) Aberrant early-phase ERK inactivation impedes neuronal function in fragile X syndrome. Proc Natl Acad Sci USA 105:4429–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Lee HJ, Kim YN, Yoon S, Lee JE, Sun W, Choi EJ, Baik JH. (2008b) Striatal-enriched protein tyrosine phosphatase regulates dopaminergic neuronal development via extracellular signal-regulated kinase signaling. Exp Neurol 214:69–77 [DOI] [PubMed] [Google Scholar]
- Krauss M, Kinuta M, Wenk MR, De Camilli P, Takei K, Haucke V. (2003) ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Igamma. J Cell Biol 162:113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214 [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Lamsa KP. (2007) Long-term synaptic plasticity in hippocampal interneurons. Nat Rev Neurosci 8:687–699 [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. (2009) The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 205:529–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup P, Zhang Y, Xu J, Venkitaramani DV, Haroutunian V, Greengard P, Nairn AC, Lombroso PJ. (2010) Abeta-mediated NMDA receptor endocytosis in Alzheimer's disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci 30:5948–5957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, et al. (2004) Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J Neurosci 24:10191–10200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Buen TV, McGaugh JL. (2003) Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. J Neurosci 23:6754–6758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le HT, Maksumova L, Wang J, Pallen CJ. (2006) Reduced NMDA receptor tyrosine phosphorylation in PTPalpha-deficient mouse synaptosomes is accompanied by inhibition of four src family kinases and Pyk2: an upstream role for PTPalpha in NMDA receptor regulation. J Neurochem 98:1798–1809 [DOI] [PubMed] [Google Scholar]
- Lee SR, Kwon KS, Kim SR, Rhee SG. (1998) Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem 273:15366–15372 [DOI] [PubMed] [Google Scholar]
- Léveillé F, El Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O, Buisson A. (2008) Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. FASEB J 22:4258–4271 [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. (2002) Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci 25:409–432 [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Ku L, Wilkinson KD, Warren ST, Feng Y. (2001) The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res 29:2276–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombroso PJ, Murdoch G, Lerner M. (1991) Molecular characterization of a protein-tyrosine-phosphatase enriched in striatum. Proc Natl Acad Sci USA 88:7242–7246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombroso PJ, Naegele JR, Sharma E, Lerner M. (1993) A protein tyrosine phosphatase expressed within dopaminoceptive neurons of the basal ganglia and related structures. J Neurosci 13:3064–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber B, Berry M, Hendriks W, den Hertog J, Pulido R, Logan A. (2004) Stimulated regeneration of the crushed adult rat optic nerve correlates with attenuated expression of the protein tyrosine phosphatases RPTPalpha, STEP, and LAR. Mol Cell Neurosci 27:404–416 [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. (1989) Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243:1721–1724 [DOI] [PubMed] [Google Scholar]
- Lu R, Wang H, Liang Z, Ku L, O'donnell WT, Li W, Warren ST, Feng Y. (2004) The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci USA 101:15201–15206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Huber KM. (2010) Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron 65:445–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M, McMahon HT. (1999) The structural era of endocytosis. Science 285:215–220 [DOI] [PubMed] [Google Scholar]
- Matthews DB, Morrow AL. (2000) Effects of acute and chronic ethanol exposure on spatial cognitive processing and hippocampal function in the rat. Hippocampus 10:122–130 [DOI] [PubMed] [Google Scholar]
- McIntosh C, Chick J. (2004) Alcohol and the nervous system. J Neurol Neurosurg Psychiatry 75 (Suppl 3):iii16-iii21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17:2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisett RA, Swartzwelder HS. (1993) Attenuation of hippocampal long-term potentiation by ethanol: a patch-clamp analysis of glutamatergic and GABAergic mechanisms. J Neurosci 13:2264–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SJ, Gorrie GH, Amato A, Smart TG. (1995) Modulation of GABAA receptors by tyrosine phosphorylation. Nature 377:344–348 [DOI] [PubMed] [Google Scholar]
- Moult PR, Gladding CM, Sanderson TM, Fitzjohn SM, Bashir ZI, Molnar E, Collingridge GL. (2006) Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptor-mediated long-term depression. J Neurosci 26:2544–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouri A, Noda Y, Enomoto T, Nabeshima T. (2007) Phencyclidine animal models of schizophrenia: approaches from abnormality of glutamatergic neurotransmission and neurodevelopment. Neurochem Int 51:173–184 [DOI] [PubMed] [Google Scholar]
- Muñoz JJ, Tárrega C, Blanco-Aparicio C, Pulido R. (2003) Differential interaction of the tyrosine phosphatases PTP-SL, STEP and HePTP with the mitogen-activated protein kinases ERK1/2 and p38alpha is determined by a kinase specificity sequence and influenced by reducing agents. Biochem J 372:193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AJ. (2008) Pharmacological PKA inhibition: all may not be what it seems. Sci Signal 1:re4. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. (2001) Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem 276:693–699 [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Watabe AM, Kiyama Y, Fukaya M, Arima-Yoshida F, Horai R, Sudo K, Ebine K, Delawary M, et al. (2006) NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity. EMBO J 25:2867–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Berhow MT, Brodkin ES. (1996) Molecular mechanisms of drug addiction: adaptations in signal transduction pathways. Mol Psychiatry 1:190–199 [PubMed] [Google Scholar]
- Nguyen TH, Liu J, Lombroso PJ. (2002) Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem 277:24274–24279 [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Paul S, Xu Y, Gurd JW, Lombroso PJ. (1999) Calcium-dependent cleavage of striatal enriched tyrosine phosphatase (STEP). J Neurochem 73:1995–2001 [PubMed] [Google Scholar]
- Nicodemo AA, Pampillo M, Ferreira LT, Dale LB, Cregan T, Ribeiro FM, Ferguson SS. (2010) Pyk2 uncouples metabotropic glutamate receptor G protein signaling but facilitates ERK1/2 activation. Mol Brain 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura A, Goto S, Nishi T, Yamada K, Yoshikawa M, Ushio Y. (1997) Postnatal ontogeny of striatal-enriched protein tyrosine phosphatase (STEP) in rat striatum. Exp Neurol 145:228–234 [DOI] [PubMed] [Google Scholar]
- Okun E, Griffioen K, Barak B, Roberts NJ, Castro K, Pita MA, Cheng A, Mughal MR, Wan R, Ashery U, et al. (2010) Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis. Proc Natl Acad Sci USA 107:15625–15630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama T, Goto S, Nishi T, Sato K, Yamada K, Yoshikawa M, Ushio Y. (1995) Immunocytochemical localization of the striatal enriched protein tyrosine phosphatase in the rat striatum: a light and electron microscopic study with a complementary DNA-generated polyclonal antibody. Neuroscience 69:869–880 [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Perley CC, Venkitaramani DV, Goebel-Goody SM, Zhang Y, Kurup P, Mattis JH, Lombroso PJ. (2009) Major vault protein is expressed along the nucleus-neurite axis and associates with mRNAs in cortical neurons. Cereb Cortex 19:1666–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Connor JA. (2010) NR2B-NMDA receptor-mediated increases in intracellular Ca2+ concentration regulate the tyrosine phosphatase, STEP, and ERK MAP kinase signaling. J Neurochem 114:1107–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. (2003) NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci 6:34–42 [DOI] [PubMed] [Google Scholar]
- Paul S, Olausson P, Venkitaramani DV, Ruchkina I, Moran TD, Tronson N, Mills E, Hakim S, Salter MW, Taylor JR, et al. (2007) The striatal-enriched protein tyrosine phosphatase gates long-term potentiation and fear memory in the lateral amygdala. Biol Psychiatry 61:1049–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Snyder GL, Yokakura H, Picciotto MR, Nairn AC, Lombroso PJ. (2000) The Dopamine/D1 receptor mediates the phosphorylation and inactivation of the protein tyrosine phosphatase STEP via a PKA-dependent pathway. J Neurosci 20:5630–5638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Askalan R, Paul S, Kalia LV, Nguyen TH, Pitcher GM, Salter MW, Lombroso PJ. (2002) Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron 34:127–138 [DOI] [PubMed] [Google Scholar]
- Piqué M, López JM, Foissac S, Guigó R, Méndez R. (2008) A combinatorial code for CPE-mediated translational control. Cell 132:434–448 [DOI] [PubMed] [Google Scholar]
- Pitcher GM, Kalia LV, Ng D, Goodfellow NM, Yee KT, Lambe EK, Salter MW. (2011) Schizophrenia susceptibility pathway neuregulin 1-ErbB4 suppresses Src upregulation of NMDA receptors. Nat Med 17:470–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poddar R, Deb I, Mukherjee S, Paul S. (2010) NR2B-NMDA receptor mediated modulation of the tyrosine phosphatase STEP regulates glutamate induced neuronal cell death. J Neurochem 115:1350–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov D. (2011) Novel protein tyrosine phosphatase 1B inhibitors: interaction requirements for improved intracellular efficacy in type 2 diabetes mellitus and obesity control. Biochem Biophys Res Commun 410:377–381 [DOI] [PubMed] [Google Scholar]
- Rácz B, Blanpied TA, Ehlers MD, Weinberg RJ. (2004) Lateral organization of endocytic machinery in dendritic spines. Nat Neurosci 7:917–918 [DOI] [PubMed] [Google Scholar]
- Raghunathan A, Matthews GA, Lombroso PJ, Naegele JR. (1996) Transient compartmental expression of a family of protein tyrosine phosphatases in the developing striatum. Brain Res Dev Brain Res 91:190–199 [DOI] [PubMed] [Google Scholar]
- Reiner A, Albin RL, Anderson KD, D'Amato CJ, Penney JB, Young AB. (1988) Differential loss of striatal projection neurons in Huntington disease. Proc Natl Acad Sci USA 85:5733–5737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJ, Cobb MH. (1997) Mitogen-activated protein kinase pathways. Curr Opin Cell Biol 9:180–186 [DOI] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. (2001) Molecular determinants of NMDA receptor internalization. Nat Neurosci 4:794–802 [DOI] [PubMed] [Google Scholar]
- Rook MS, Lu M, Kosik KS. (2000) CaMKIIalpha 3′ untranslated region-directed mRNA translocation in living neurons: visualization by GFP linkage. J Neurosci 20:6385–6393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum G, Cohen BD, Luby ED, Gottlieb JS, Yelen D. (1959) Comparison of sernyl with other drugs: simulation of schizophrenic performance with sernyl, LSD-25, and amobarbital (amytal) sodium; I. Attention, motor function, and proprioception. Arch Gen Psychiatry 1:651–656 [DOI] [PubMed] [Google Scholar]
- Ross CA, Tabrizi SJ. (2011) Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol 10:83–98 [DOI] [PubMed] [Google Scholar]
- Saavedra A, Giralt A, Rué L, Xifró X, Xu J, Ortega Z, Lucas JJ, Lombroso PJ, Alberch J, Pérez-Navarro E. (2011) Striatal-enriched protein tyrosine phosphatase expression and activity in Huntington's disease: a STEP in the resistance to excitotoxity. J Neurosci 31:8150–8162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. (2000) Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci 20:8177–8187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz R, Berberich S, Rathgeber L, Kolleker A, Köhr G, Kornau HC. (2010) AMPA receptor signaling through BRAG2 and Arf6 critical for long-term synaptic depression. Neuron 66:768–780 [DOI] [PubMed] [Google Scholar]
- Schummers J, Bentz S, Browning MD. (1997) Ethanol's inhibition of LTP may not be mediated solely via direct effects on the NMDA receptor. Alcohol Clin Exp Res 21:404–408 [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. (2002) Alzheimer's disease is a synaptic failure. Science 298:789–791 [DOI] [PubMed] [Google Scholar]
- Sharma E, Zhao F, Bult A, Lombroso PJ. (1995) Identification of two alternatively spliced transcripts of STEP: a subfamily of brain-enriched protein tyrosine phosphatases. Brain Res Mol Brain Res 32:87–93 [DOI] [PubMed] [Google Scholar]
- Shin CY, Kundel M, Wells DG. (2004) Rapid, activity-induced increase in tissue plasminogen activator is mediated by metabotropic glutamate receptor-dependent mRNA translation. J Neurosci 24:9425–9433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS. (1987) Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science 235:73–76 [DOI] [PubMed] [Google Scholar]
- Smart JE, Oppermann H, Czernilofsky AP, Purchio AF, Erikson RL, Bishop JM. (1981) Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src). Proc Natl Acad Sci USA 78:6013–6017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, et al. (2005) Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci 8:1051–1058 [DOI] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. (2001) Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci 4:1079–1085 [DOI] [PubMed] [Google Scholar]
- Spencer ML, Theodosiou M, Noonan DJ. (2004) NPDC-1, a novel regulator of neuronal proliferation, is degraded by the ubiquitin/proteasome system through a PEST degradation motif. J Biol Chem 279:37069–37078 [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, et al. (2002) Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet 71:877–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens TR, Krueger SR, Fitzsimonds RM, Picciotto MR. (2003) Neuroprotection by nicotine in mouse primary cortical cultures involves activation of calcineurin and L-type calcium channel inactivation. J Neurosci 23:10093–10099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. (2008) Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci 28:4216–4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. (2006) Dendritic protein synthesis, synaptic plasticity, and memory. Cell 127:49–58 [DOI] [PubMed] [Google Scholar]
- Sweatt JD. (2004) Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol 14:311–317 [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. (1999) Genetic enhancement of learning and memory in mice. Nature 401:63–69 [DOI] [PubMed] [Google Scholar]
- Tashev R, Moura PJ, Venkitaramani DV, Prosperetti C, Centonze D, Paul S, Lombroso PJ. (2009) A substrate trapping mutant form of striatal-enriched protein tyrosine phosphatase prevents amphetamine-induced stereotypies and long-term potentiation in the striatum. Biol Psychiatry 65:637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Stone WS, Faraone SV. (2001) Genes, environment and schizophrenia. Br J Psychiatry Suppl 40:s18–24 [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. (1983) Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res 9:315–335 [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Machu TK, McKernan RM, Whiting P, VanRenterghem BB, McManaman JL, Brozowski SJ, Smith GB, Olsen RW, Harris RA. (1995) Tyrosine kinase phosphorylation of GABAA receptors. Brain Res Mol Brain Res 31:165–172 [DOI] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. (2000) Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci 20:8701–8709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, et al. (2005) Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA 102:491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaramani DV, Moura PJ, Picciotto MR, Lombroso PJ. (2011) Striatal-enriched protein tyrosine phosphatase (STEP) knockout mice have enhanced hippocampal memory. Eur J Neurosci 33:2288–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaramani DV, Paul S, Zhang Y, Kurup P, Ding L, Tressler L, Allen M, Sacca R, Picciotto MR, Lombroso PJ. (2009) Knockout of striatal enriched protein tyrosine phosphatase in mice results in increased ERK1/2 phosphorylation. Synapse 63:69–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Q, Man HY, Braunton J, Wang W, Salter MW, Becker L, Wang YT. (1997) Modulation of GABAA receptor function by tyrosine phosphorylation of beta subunits. J Neurosci 17:5062–5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ferguson GD, Pineda VV, Cundiff PE, Storm DR. (2004) Overexpression of type-1 adenylyl cyclase in mouse forebrain enhances recognition memory and LTP. Nat Neurosci 7:635–642 [DOI] [PubMed] [Google Scholar]
- Watabe AM, Zaki PA, O'Dell TJ. (2000) Coactivation of beta-adrenergic and cholinergic receptors enhances the induction of long-term potentiation and synergistically activates mitogen-activated protein kinase in the hippocampal CA1 region. J Neurosci 20:5924–5931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng N, Weiler IJ, Sumis A, Berry-Kravis E, Greenough WT. (2008) Early-phase ERK activation as a biomarker for metabolic status in fragile X syndrome. Am J Med Genet B Neuropsychiatr Genet 147B:1253–1257 [DOI] [PubMed] [Google Scholar]
- Wu L, Wells D, Tay J, Mendis D, Abbott MA, Barnitt A, Quinlan E, Heynen A, Fallon JR, Richter JD. (1998) CPEB-mediated cytoplasmic polyadenylation and the regulation of experience-dependent translation of alpha-CaMKII mRNA at synapses. Neuron 21:1129–1139 [DOI] [PubMed] [Google Scholar]
- Wu PH, Coultrap S, Browning MD, Proctor WR. (2010) Correlated changes in NMDA receptor phosphorylation, functional activity, and sedation by chronic ethanol consumption. J Neurochem 115:1112–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xifró X, Giralt A, Saavedra A, García-Martínez JM, Díaz-Hernández M, Lucas JJ, Alberch J, Pérez-Navarro E. (2009) Reduced calcineurin protein levels and activity in exon-1 mouse models of Huntington's disease: role in excitotoxicity. Neurobiol Dis 36:461–469 [DOI] [PubMed] [Google Scholar]
- Xu J, Kurup P, Bartos JA, Hell JW, Lombroso PJ. (2010) Inhibition of pyk2 signaling by striatal-enriched tyrosine phosphatase (STEP). Soc Neurosci Abstr 36:452.13 [Google Scholar]
- Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, Baum ML, Bibb JA, Lombroso PJ. (2009) Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci 29:9330–9343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HB, Yang X, Cao J, Li S, Liu YN, Suo ZW, Cui HB, Guo Z, Hu XD. (2011) cAMP-dependent protein kinase activated Fyn in spinal dorsal horn to regulate NMDA receptor function during inflammatory pain. J Neurochem 116:93–104 [DOI] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. (2003) The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell 112:317–327 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kurup P, Xu J, Carty N, Fernandez SM, Nygaard HB, Pittenger C, Greengard P, Strittmatter SM, Nairn AC, et al. (2010) Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer's disease mouse model. Proc Natl Acad Sci USA 107:19014–19019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Venkitaramani DV, Gladding CM, Zhang Y, Kurup P, Molnar E, Collingridge GL, Lombroso PJ. (2008) The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci 28:10561–10566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY. (2002) Protein tyrosine phosphatases: structure and function, substrate specificity, and inhibitor development. Annu Rev Pharmacol Toxicol 42:209–234 [DOI] [PubMed] [Google Scholar]