Substratum compliance profoundly influenced HTM cell behaviors and modulated the response of HTM cells to Lat-B. The inclusion of substratum compliance that reflects healthy or glaucomatous HTM results in cell behaviors and responses to therapeutic agents in vitro that may more accurately reflect in vivo conditions.
Abstract
Purpose.
To determine the impact of substratum compliance and latrunculin-B (Lat-B), both alone and together, on fundamental human trabecular meshwork (HTM) cell behavior. Lat-B is a reversible actin cytoskeleton disruptor that decreases resistance to aqueous humor outflow and decreases intraocular pressure.
Methods.
HTM cells were cultured on polyacrylamide hydrogels possessing values for compliance that mimic those reported for normal and glaucomatous HTM, or tissue culture plastic (TCP). Cells were treated with 0.2 μM or 2.0 μM Lat-B in dimethyl sulfoxide (DMSO) or DMSO alone. The impact of substratum compliance and/or Lat-B treatment on cell attachment, proliferation, surface area, aspect ratio, and migration were investigated.
Results.
HTM cells had profoundly decreased attachment and proliferation rates when cultured on hydrogels possessing compliance values that mimic those found for healthy HTM. The effect of Lat-B treatment on HTM cell surface area was less for cells cultured on more compliant hydrogels compared with TCP. HTM cell migration was increased on stiffer hydrogels that mimic the compliance of glaucomatous HTM and on TCP in comparison with more compliant hydrogels. Lat-B treatment decreased cellular migration on all surfaces for at least 7 hours after treatment.
Conclusions.
Substratum compliance profoundly influenced HTM cell behaviors and modulated the response of HTM cells to Lat-B. The inclusion of substratum compliance that reflects healthy or glaucomatous HTM results in cell behaviors and responses to therapeutic agents in vitro that may more accurately reflect in vivo conditions.
Glaucoma is a common cause of blindness worldwide that is predicted to affect 79.6 million people by 2020.1 Although multiple factors have been identified that contribute to the onset of glaucoma, the primary risk factor is increased intraocular pressure (IOP).2 IOP is thought to be elevated because of a pathologic increase in resistance to aqueous outflow in the human trabecular meshwork (HTM).2–4 The HTM is a complex, three-dimensional structure comprised of interwoven beams of extracellular matrix and associated trabecular meshwork cells as well as a layer of cells adjacent to the inner wall of Schlemm's canal.5 It has been recently demonstrated that the compliance of the HTM is markedly decreased (becomes stiffer) in patients with glaucoma.4 Polyacrylamide hydrogels mimicking the recently published compliance of normal and glaucomatous HTM have been shown to markedly alter cytoskeletal dynamics, cell compliance, and the cellular response to latrunculin-B (Lat-B).3,4 In a variety of cell types, substratum compliance has been shown to modulate a variety of fundamental cell behaviors including cell morphology, migration, proliferation, and differentiation.6–10 Despite the expanding database demonstrating the fundamental importance of biophysical cues associated with the extracellular matrix, studies of HTM cell behavior are typically performed on flat, rigid substrates such as glass or plastic with elastic moduli in the giga-Pascal (GPa) range.11–14
The only clinically validated treatments for glaucoma are aimed at lowering IOP by decreasing aqueous humor production or decreasing the resistance to aqueous humor outflow.15 Lat-B, an actin cytoskeletal disruptor, decreases IOP by decreasing the resistance to aqueous humor outflow through the HTM and is currently in human clinical trials as a novel glaucoma treatment directed specifically at the trabecular meshwork.16–20 Recently, it has been demonstrated that HTM cells adhered to more rigid substrates had an altered response in actin fiber assembly after withdrawal of the drug suggesting that the effects of Lat-B treatment would be most pronounced in glaucomatous eyes with a stiffer HTM.3 However, the effect of substratum compliance on the fundamental behavior and morphology of HTM cells during and after Lat-B exposure has not been investigated. The purpose of this investigation was to determine the effect of Lat-B treatment in the context of substratum compliance on HTM cell morphology and fundamental behaviors including adhesion, proliferation, and migration. A better understanding of the effect that clinically relevant substratum compliance has on fundamental HTM cell behavior and their response to Lat-B treatment will inform the design of improved in vitro testing methodologies. Additionally, it will provide insight into how substratum compliance modulates HTM cell response with disease onset, progression, and treatment.
Methods
HTM Cell Isolation and Culture
Primary HTM cells were isolated as previously described from corneal buttons deemed not suitable for transplant from donors with no prior history of ocular disease.21 All experiments complied with the tenets of the Declaration of Helsinki. Isolated cells were cultured in DME/F-12 medium containing 2.5 mM l-glutamine and 15 mM HEPES buffer (Hyclone, Fisher Scientific, Waltham, MA) that was supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrence, GA), and 1% penicillin-streptomycin with amphotericin b (Lonza, Walkerville, MD). All primary cultures were incubated for 4 days with 0.1 μM dexamethasone, and only cultures with increased myocilin expression were selected for use in this study. All studies were conducted using cells before the seventh passage.
Hydrogel Fabrication
Polyacrylamide (PA) hydrogels that mimic normal (homeomimetic, 4 kPa) and the range of glaucomatous (pathomimetic, 25, 50, 75, and 90 kPa) HTM compliances that have been previously documented4 were prepared by varying the cross-linker density as previously described.3,22 In brief, all hydrogels were sterilized with ultraviolet light and rinsed every 24 hours for at least 48 hours in PBS to fully hydrate the hydrogels and to ensure that no monomeric acrylamide remained. Free acrylamide has been previously shown to disrupt cytoskeletal structure.23 After hydration, the hydrogels were adhered to tissue culture plastic (TCP) via electrostatic and hydrophobic interactions, stored in HTM cell medium for 24 hours, and coated with a fibronectin/collagen (FNC) (AthenaES, Baltimore, MD) mixture for 15 minutes. TCP control samples were also treated with FNC before all experiments as well. Protein extraction of FNC coated hydrogels with 50 μL of mammalian protein extraction reagent (M-PER; ThermoScientific, Rockford, IL) followed by quantitation with a spectrophotometer (ND-1000; ThermoScientific) was performed. Protein extractions from five hydrogel substrates per compliance value as well as five TCP samples were analyzed. Atomic force microscopy was used to validate the compliance (Young's modulus) of the fully hydrated hydrogels.24
Latrunculin B Treatment
Lyophilized Lat-B (Cal Biochem, La Jolla, CA) was resuspended to a final concentration of 2.5 mM in dimethyl sulfoxide (DMSO) (Fisher, Pittsburg, PA). Fresh solutions of 0.2 or 2.0 μM Lat-B were prepared in serum free DME/F-12 with 2.5 mM l-glutamine or 15 mM HEPES buffer (Lonza) medium supplemented with 1% pennicillin/streptomycin with amphotericin b (Lonza, Walkerville, MD) for all experiments. Control samples were treated with an equivalent concentration of DMSO in serum free DME/F-12 or 15 mM HEPES buffer. After 30 minutes of exposure to Lat-B or DMSO, all samples were rinsed twice with serum-containing medium to neutralize the Lat-B25 and placed in 3 to 5 mL of DME/F-12 with 10% serum as above.
Microscopy
Phase contrast imaging was performed with an inverted microscope (Zeiss Axiovert 200M inverted microscope with a 10×/0.4 NA objective; Carl Zeiss Inc, New York, NY) and a camera (AxioCam HRm; Carl Zeiss Inc.) for studies related to cell attachment, area, proliferation, and migration. A migration tracking package (Axiovision ver. 4.8; Carl Zeiss Inc.) was used for migration analysis. For immunofluorescent imaging, HTM cells were labeled with an actin binding agent (phalloidin-568; Invitrogen, Carlsbad, CA) and nuclear label (DAPI; BioGenex, Fremont, CA) and fixed before, during, and after Lat-B treatment on homeomimetic and pathomimetic hydrogels as well as TCP. The cells were then imaged with an upright microscope (Zeiss Axio Scope.A1) and camera with a 10×/0.3 NA or 20×/0.5 NA objective (AxioCam HRc camera; Carl Zeiss Inc.).
Attachment
Cells from a total of 6 donors were used in these experiments. To measure cell attachment, 100,000 HTM cells were seeded onto 4, 25, 50, and 75 kPa hydrogels as well as tissue culture polystyrene (>1 GPa) substrates. Each hydrogel substrate was approximately 0.75 inch in diameter. Four fields from each substrate were obtained by phase contrast with a 10 × objective. The total number of cells was averaged for each substrate. From each of these donors, six replicates were performed for substrates of each compliance value (24 fields per donor and compliance value). Results from each donor were normalized to TCP within each experimental run to account for variation between donors. To determine the effects of Lat-B treatment, cells from three donors were seeded as describe above. HTM cells (100,000/substrate) were seeded onto 4 kPa and 25 kPa substrates and incubated at least 24 hours before Lat-B treatment. After Lat-B treatment for 30 minutes, the cells were rinsed and allowed to recover for 60 minutes before phase contrast imaging. HTM cell attachment after Lat-B treatment was then quantitated as described above.
Proliferation
Approximately 100,000 HTM cells per well from three donors were cultured for five days on 4, 25, 50, and 75 kPa hydrogels as well as TCP. The same 10 × objective fields were imaged (four fields per surface) on Day 1 and again on Day 5. The percentage increase in cell number after five days of incubation was determined by dividing the sum of the cells in the four images per well on Day 5 by the sum from the same images taken on Day 1 (e.g., Day 5/Day 1). A total of six wells (24 fields) for each compliant substrate or TCP were measured from at least three donors (72 fields per compliant substrate or TCP) for percent increase. The percent increase measurements were then normalized to the TCP control samples for each donor to account for donor variation, and the mean percent increase was determined for each condition. Normalization was performed by defining the control TCP condition as a value of one and calculating all other values as ratios of this value. Three donors were analyzed to determine the effect of Lat-B treatment on proliferation using 4 kPa, 25 kPa, and TCP substrates. Images were taken 24 hours after Lat-B treatment and analyzed as described above. These results were then normalized to TCP within each experiment.
Area and Aspect Ratio
To measure cell area and aspect ratio, 100,000 HTM cells were seeded onto 4 and 25 kPa hydrogels, as well as TCP 24 hours before imaging. Phase contrast images were taken before Lat-B treatment, after 30 minutes of Lat-B treatment, 60 minutes after removal of Lat-B, and 7 hours after removal of Lat-B of the same cells on each plate. The perimeters of the cells from three donors per substrate and treatment were traced using software developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; ImageJ version1.42q; available at http://rsb.info.nih.gov/ij/index.html). At least 30 cells per donor for each substrate and treatment were traced and analyzed for total area and the aspect ratio (long axis/short axis) using the fitted ellipse model in the software. HTM cells from all donors were measured separately and to account for donor variation, cell area was normalized to the TCP control samples within each experiment.
Migration
Using a migration tracking module (Zeiss), individual cells were tracked at 20-minute intervals for 6 hours before Lat-B treatment, at 10-minute intervals during 30 minutes of Lat-B treatment, and at 20-minute intervals for 420 minutes after Lat-B removal. Only cells that remained in the field of view during the entire treatment course were analyzed. At least 29 cells for a given compliance value from each of three donors were tracked. The total distance traveled during each period was divided by the total time elapsed to determine the net migration rate.
Statistical Analysis
Data were analyzed using commercially available software (Sigma Plot 11; Systat Software, Chicago, IL). Analysis of variance (ANOVA) was used to determine significance within treatment groups with the Tukey post hoc test or Kruskal-Wallis ANOVA on rank tests used depending on the results of the normality test. Among the treatment groups with statistical significance, Mann-Whitney rank sum tests were used to determine the P value. All levels of significance are defined as ***P < 0.001. This stringent value was chosen to minimize the probability that the variability inherent in the use of primary cells from different donors would lead to erroneous conclusions.
Results
Adsorption of FNC to hydrogels
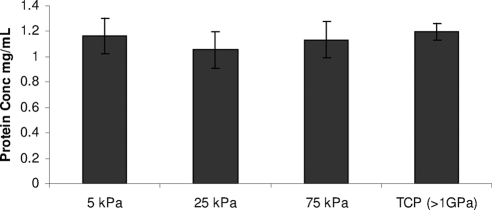
Protein extraction of FNC coated substrates yielded similar results on all substrates regardless of compliance value with no significant differences being detected in the amount of FNC adsorbed to any of the hydrogel or TCP substrates (Fig. 1).
Figure 1.
FNC evenly adsorbs to the surfaces of hydrogels and TCP. Protein extraction and quantification of FNC coated hydrogels and TCP demonstrated that similar amounts of FNC were present on all surfaces tested.
Impact of Compliance and Lat-B Treatment on HTM Cell Attachment
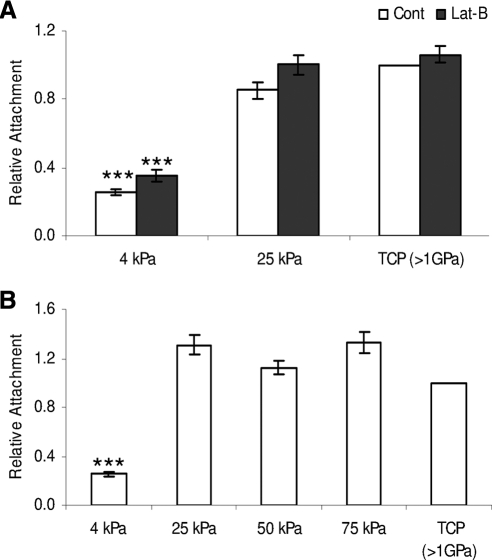
Similar numbers of HTM cells adhered to the 25, 50, and 75 kPa substrates and to TCP (Fig. 2A). In contrast, 65% to 75% fewer cells adhered to 4 kPa substrates (P < 0.001). Lat-B had no effect on cell attachment. With Lat-B treatment, HTM cells had not detached l hour after 2 μM Lat-B treatment on any substrate evaluated (Fig. 2B) with similar results obtained when employing 0.2 μM Lat-B (data not shown) (Fig. 2).
Figure 2.
Substratum compliance affected HTM cell attachment. Fewer HTM cells adhere to 4 kPa substrates compared with TCP. Treatment with 2 μM Lat-B did not result in HTM cell detachment (A). HTM cell attachment did not vary across pathomimetic, 25 to 75 kPa substrates (B). All data are reported as mean ± SEM (***P < 0.001).
Impact of Compliance and Lat-B on HTM Cell Proliferation
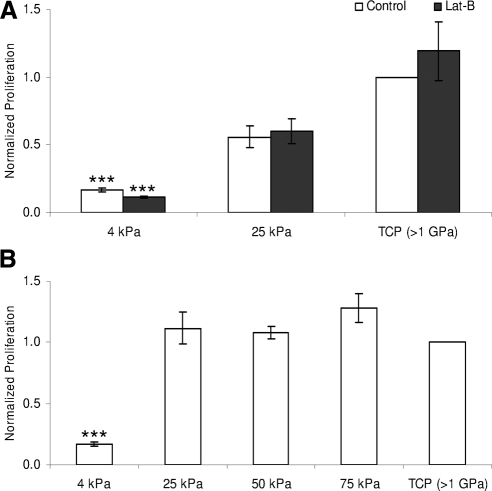
The number of cells on the 4 kPa hydrogels did increase during the 5-day incubation but the rate of proliferation was significantly lower (P < 0.001) than on the other substrates (Fig. 3). HTM cell proliferation did not vary significantly on 25, 50, or 75 kPa hydrogels compared with TCP (Fig. 3A). The most rapid proliferation rate of 13.6-fold was measured on TCP in cells from a 62-year-old donor while the slowest TCP proliferation rate was 3.2-fold and was measured in cells from a 69-year-old donor. Once normalized to their respective TCP controls, the data for all cells had similar trends and could be directly compared. HTM cell proliferation after recovery from Lat-B exposure was not affected regardless of concentration on any of the substrates examined (Fig. 3B).
Figure 3.
Substratum compliance affects HTM cell proliferation. HTM cell proliferation was reduced on 4 kPa substrates compared with 25 kPa and TCP substrates. Treatment with 2 μM Lat-B did not affect HTM cell proliferation on any of the tested substrates (A). HTM cell proliferation rates did not vary across pathomimetic, 25 to 75 kPa substrates (B). All data are reported as mean ± SEM (***P < 0.001).
Impact of Compliance and Lat-B on HTM Cell Morphology
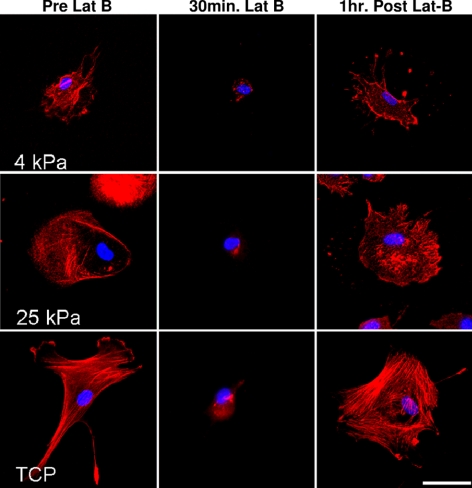
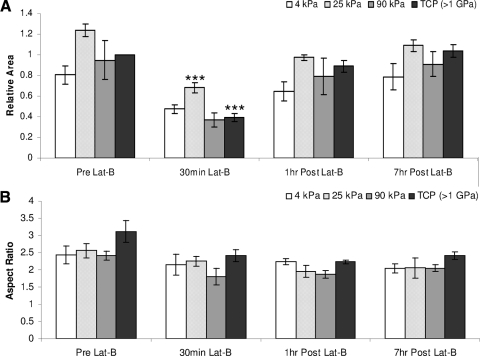
Substratum compliance dramatically impacted actin fiber formation and reassembly after Lat-B treatment. Stress fiber formation was observed before Lat-B treatment and after recovery only in HTM cells on TCP substrates. In contrast, HTM cells on softer substrates (4 to 25 kPa) did not exhibit actin stress fibers before or after Lat-B treatment (Fig. 4). Substratum compliance and Lat-B treatment dramatically altered HTM cell area and elongation on the softest surfaces (Fig. 5). Before Lat-B treatment, HTM cells on 4 kPa hydrogels had a smaller area than HTM cells on TCP (Fig. 5A). Lat-B treatment reduced the area of cells on TCP to the point that cells on 4 kPa hydrogels were slightly larger than cells on TCP. The variation between cell area on the 4 kPa after Lat-B removal hydrogels and the TCP returned to 20% and 40% at 1 hour and 7 hours, respectively. With the exception of 25 kPa surfaces, Lat-B treatment did not induce a statistically significant change in total cell area though cells trended toward being smaller during treatment and larger during recovery. In contrast, HTM cells on 25 kPa surfaces were significantly (P < 0.001) smaller after 30 minutes of Lat-B treatment compared with pretreatment levels. On TCP surfaces, HTM cells trended toward being more elongated before Lat-B treatment but this difference was not statistically significant (Fig. 5B).
Figure 4.
Representative images demonstrate the effect of substratum compliance and Lat-B treatment on HTM cell area. HTM cells on 4 kPa substrates were much smaller than cells on TCP both before and after Lat-B treatment. Note the dense packing of actin fibers 1 hour after Lat-B removal on TCP surfaces. This was consistent with an earlier report of abnormal fiber patterns at 90 minutes after Lat-B removal.3 Scale bar, 50 μm.
Figure 5.
Substratum compliance affects HTM cell area and aspect ratio before, during, and after Lat-B treatment. During the course of Lat-B treatment, HTM cells on 4 kPa and 90 kPa surfaces did not experience significant changes in total area. In contrast, HTM cells on 25 kPa were significantly smaller during Lat-B treatment compared with pretreatment and cell TCP surfaces were significantly smaller after 30 minutes of Lat-B treatment than at any other time point measured. One hour after Lat-B removal, HTM cells on TCP surfaces had returned to their pretreatment area compared with softer hydrogels (4 to 25 kPa) which took longer to recover (A). Data trends on all surfaces demonstrate that HTM cells are more elongated before Lat-B treatment. During the course of Lat-B treatment, all cells became less elongated (B). All data are reported as mean ± SEM (***P < 0.001).
Effect of Substratum Compliance and Lat-B on HTM Cell Migration
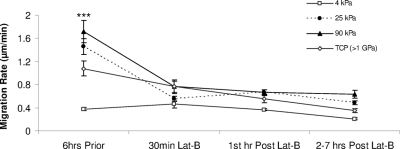
HTM cell migration on 25 and 90 kPa hydrogels as well as TCP (>1 GPa) surfaces was significantly faster than on 4 kPa surfaces (P < 0.001). Cell migration on 25 kPa, 90 kPa, and TCP surfaces averaged 1.45, 1.72, and 1.08 μm/minute, respectively, compared with 0.37 μm/minute on 4 kPa surfaces. In the second through seventh hours after recovery from Lat-B treatment, HTM cell migration decreased significantly (P < 0.001) to 0.20–0.63 μm/minute on all hydrogels and TCP (0.35 μm/minute). HTM cell migration rates did not approximate pre-Lat-B treatment values on any of the substrates examined during the course of our studies (at least 7 hours) (Fig. 6). Overall, HTM cell migration rate decreased 46% on 4 kPa, 66% on 25 kPa, and 63% on the 90 kPa hydrogels compared with a 68% decrease on TCP at 7 hours after Lat-B treatment.
Figure 6.
Substratum compliance and Lat-B treatment affects HTM cell migration. Before Lat-B treatment, HTM cells on 25 and 90 kPa surfaces migrated at an average rate of 1.45 and 1.72 μm/minute, respectively. While not statistically significant, both migration rates were faster than the 1.08 μm/minute measured on TCP surfaces. Migration rates on all three surfaces were significantly faster than the 0.37 μm/minute migration rate of HTM cells on 4 kPa surfaces before Lat-B treatment. HTM cell migration decreased significantly during Lat-B treatments and did not recover on any of the substrates tested including the 4 kPa surfaces. All data are reported as mean ± SEM (***P < 0.001).
Discussion
In many glaucomatous patients, increased resistance to outflow through the trabecular meshwork is the suspected cause of increased IOP. A recent report documented that Young's moduli of trabecular meshwork from human glaucomatous donors were increased,3,4 which could increase the resistance to outflow. The results reported here demonstrate that changes in substratum compliance in the range found for both healthy and glaucomatous human meshwork profoundly influence fundamental behaviors and morphologies of HTM cells as well as their response to Lat-B treatment.
“Homeomimetic” substrates approximating values for compliance found for the normal meshwork (approximately 4 kPa) had significantly fewer HTM cells attach compared with “pathomimetic” stiffer substrates (25–75 kPa) that approximate the lower values for compliance (i.e., stiffer) found for the glaucomatous meshwork. Importantly, Lat-B treatment at the dose employed did not result in HTM cell detachment under 4 kPa homeomimetic, 25 kPa pathomimetic, or TCP conditions. This finding predicts that Lat-B should not alter the attachment of HTM cells in either healthy or glaucomatous trabecular meshwork.
Similar to HTM cell attachment, proliferation was significantly diminished (P < 0.001) on homeomimetic (4 kPa) hydrogels before Lat-B exposure. These results correlate with in vivo conditions where HTM cells have been shown to proliferate slowly.26 In contrast, cells under pathomimetic conditions (substrate compliance of 25 kPa or greater) proliferated at a similar rate to cells on TCP. These findings are interesting because in vivo the number of cells have been reported to be to decreased on the trabecular beams from glaucomatous donors.27,28 Previous reports suggest there is a stem cell population of HTM cells26 and perhaps these stem cells proliferate but cannot do so fast enough to prevent the net loss of HTM cells with glaucoma. In aggregate, previous reports in combination with these findings support the view that the proliferative capacity of HTM cells in vivo is determined by multiple cellular inputs including biophysical cues.
In addition to the absence of exogenous biophysical and biochemical cues, cell density may have impacted the observed proliferation results. Initial cell density is a function of the number of cells seeded, the surface area being seeded, and the attachment rate. We controlled for surface area and number of cells seeded and in our studies, the proliferation rate was strongly correlated with the cell attachment assay. Cell density has been shown to affect cell proliferation29 and it is possible that some of the decreased proliferation response to the 4 kPa hydrogels may be the result of the decreased initial cell density. Besides biophysical cues, increased cell to cell contact may also increase cell proliferation. It is also possible that some level of protein absorption was occurring during our surface treatments with FNC. If more FNC was absorbed deeper into the hydrogels then less surface FNC would be present. Further testing is needed in the future to address this possibility. Lat-B treatment had no effect on HTM cell proliferation on any substrate and these data support previous findings on the relative safety of Lat-B treatment.19,26
To better simulate many HTM cell behaviors observed in glaucomatous tissue in vivo, incorporation of multiple biophysical cues into in vitro experiments may be necessary. For example, increased IOP leads to increased stress on the HTM cells.28,30 Previous reports have shown that changes in the stress response elements classically observed in relation to cardiovascular endothelium also change in a similar fashion in HTM cells from glaucomatous patients.30–34
The collapse of the actin cytoskeleton that leads to changes in cell morphology as a result of Lat-B treatment is well documented.3,19,35,36 A recent report found substrate compliance to modulate the impact of Lat-B on HTM cytoskeletal dynamics.3 Data during treatment and after withdrawal of Lat-B in conjunction with previous findings demonstrates that substratum compliance affects HTM cell morphology as well as the response to Lat-B. The decrease in area as a result of Lat-B treatment was minimized in HTM cells on biologically relevant (homeomimetic and pathomimetic) substrates compared with TCP. This is consistent with the low density of actin stress fibers observed for HTM cells on the hydrogel surfaces compared with TCP and the dramatic loss of these fibers with Lat-B treatment. However, HTM cells on 25 kPa substrates were also significantly smaller during Lat-B treatment suggesting that there may not be a linear relationship between substratum compliance and HTM cell response to Lat-B. Further studies are needed to fully elucidate this relationship. Overall, these data reinforce that biomimetic substrates influence HTM cell response to Lat-B treatment and their use may provide results that are more predictive of the in vivo response of HTM cells to actin-disrupting agents than the response of HTM cells on TCP.
Our findings demonstrate that HTM cell migration is extremely slow on homeomimetic hydrogels. Interestingly, the migration rate on pathomimetic substrates was increased compared with TCP. While not statistically different from TCP, these data trends suggest that a linear relationship between substratum compliance and migration rate may not exist. While increased HTM cell migration with the onset of glaucoma has not been studied in vivo, decreased cellularity (cells/unit area) has been documented.27,37 Furthermore, HTM cell migration has been previously shown to be increased when cells are stimulated by aqueous humor from glaucomatous donors.37 The significantly increased migration rate on stiffer hydrogels and TCP in combination with previous findings,37 suggests that the migration rate could be due to a combination of biophysical and biochemical cues. Lat-B treatment significantly diminished HTM cell migration on stiffer substrates. Importantly, HTM cell migration was significantly slower on all substrates 2 to 7 hours after Lat-B treatment demonstrating that Lat-B continues to have a profound impact on HTM cell behavior for several hours after HTM cell area and compliance are known to recover.3
Overall, substratum compliance profoundly influences HTM cell behaviors. We have previously reported that cell proliferation in four different types of vascular endothelial cells is dramatically affected by substratum compliance and that response was affected by the anatomic origin. In endothelial cells from aorta, saphenous vein, and umbilical vein, hydrogels of all tested compliances significantly downregulated proliferation compared with TCP. In contrast to other endothelial cell types and in similarity with the HTM results, microvascular-dermal endothelial cells were only downregulated on softer hydrogels compared with TCP.22 The inclusion of biophysical characteristics that reflect both healthy and glaucomatous HTM for in vitro assays results in cell behaviors and responses to therapeutic agents such as Lat-B that may more accurately reflect in vivo conditions. Inclusion of these biophysical cues, such as substratum compliance, with in vitro studies will likely lead to a greater understanding of HTM cell behaviors and their response to therapeutic agents. By more closely modeling in vivo HTM cell behavior, we can better understand the role of HTM cells in the onset and progression of glaucoma as well as test novel therapies for the treatment of the disease.
Acknowledgments
The authors thank Marissa L. Hughbanks for cell counting, Leslie M. Kon for migration tracking, and Wai F. Cheung, Yow-Ren Chang, and Irene Ly for hydrogel production.
Footnotes
Supported by National Institute of Health Grants R01 EY019475 and P30 EY12576, a grant from National Glaucoma Research, a program of the American Health Assistance Foundation, and an unrestricted grant from Research to Prevent Blindness.
Disclosure: J.A. Wood, None; C.T. McKee, None; S.M. Thomasy, None; M.E. Fischer, None; N.M. Shah, None; C.J. Murphy, None; P. Russell, None
References
- 1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rhee DJ, Haddadin RI, Kang MH, Oh D-J. Matricellular proteins in the trabecular meshwork. Exp Eye Res. 2009;88:694–703 [DOI] [PubMed] [Google Scholar]
- 3. McKee CT, Wood JA, Shah NM, et al. The effect of biophysical attributes of the ocular trabecular meshwork associated with glaucoma on the cell response to therapeutic agents. Biomaterials. 2011;32:2417–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Last JA, Pan T, Ding Y, et al. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Inves Ophthalmol Vis Sci. 2011;52:2147–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson M. What controls aqueous humour outflow resistance? Exp Eye Res. 2006;82:545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wood JA, Liliensiek SJ, Russell P, Nealey PF, Murphy CJ. Biophysical cueing and vascular endothelial cell behavior. Materials. 2010;3:1620–1639 [Google Scholar]
- 7. Pelham RJ, Wang Y-L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143 [DOI] [PubMed] [Google Scholar]
- 9. Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86:617–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689 [DOI] [PubMed] [Google Scholar]
- 11. Kahook MY, Ammar DA. In vitro effects of antivascular endothelial growth factors on cultured human trabecular meshwork cells. J Glaucoma. 2010;19:437–441 [DOI] [PubMed] [Google Scholar]
- 12. Zhou L, Zhang SR, Yue BY. Adhesion of human trabecular meshwork cells to extracellular matrix proteins. Roles and distribution of integrin receptors. Inves Ophthalmol Vis Sci. 1996;37:104–113 [PubMed] [Google Scholar]
- 13. Shearer TW, Crosson CE. Adenosine A1 receptor modulation of MMP-2 secretion by trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2002;43:3016–3020 [PubMed] [Google Scholar]
- 14. Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979;18:1043–1049 [PubMed] [Google Scholar]
- 15. Heijl A, Leske MC, Bengtsson B, Hyman L, Hussein M, the Early Manifest Glaucoma Trial Group Reduction of intraocular pressure and glaucoma progression. Arch Ophthalmol. 2002;1268–1279 [DOI] [PubMed] [Google Scholar]
- 16. Kaufman PL. Enhancing trabecular outflow by disrupting the actin cytoskeleton, increasing uveoscleral outflow with prostaglandins, and understanding the pathophysiology of presbyopia interrogating Mother Nature: asking why, asking how, recognizing the signs, following the trail. Exp Eye Res. 2008;86:3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rao PV, Deng P, Sasaki Y, Epstein DL. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp Eye Res. 2005;80:197–206 [DOI] [PubMed] [Google Scholar]
- 18. Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42:1029–1037 [PubMed] [Google Scholar]
- 19. Ethier CR, Read AT, Chan DW. Effects of latrunculin-B on outflow facility and trabecular meshwork structure in human eyes. Invest Ophthalmol Vis Sci. 2006;47:1991–1998 [DOI] [PubMed] [Google Scholar]
- 20. Okka M, Tian B, Kaufman PL. Effects of latrunculin B on outflow facility, intraocular pressure, corneal thickness, and miotic and accommodative responses to pilocarpine in monkeys. Trans Am Ophthalmol Soc. 2004;102:251–257 [PMC free article] [PubMed] [Google Scholar]
- 21. Rhee DJ, Tamm ER, Russell P. Donor corneoscleral buttons: a new source of trabecular meshwork for research. Exp Eye Res. 2003;77:749–756 [DOI] [PubMed] [Google Scholar]
- 22. Wood JA, Shah NM, McKee CT, et al. The role of substratum compliance of hydrogels on vascular endothelial cell behavior. Biomaterials. 2011;32:5056–5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arocena M. Effect of acrylamide on the cytoskeleton and apoptosis of bovine lens epithelial cells. Cell Biol Int. 2006;30:1007–1012 [DOI] [PubMed] [Google Scholar]
- 24. Radmacher M, Tillamnn R, Fritz M, Gaub H. From molecules to cells: imaging soft samples with the atomic force microscope. Science. 1992;257:1900–1905 [DOI] [PubMed] [Google Scholar]
- 25. Spector I, Shochet NR, Blasberger D, Kashman Y. Latrunculins–novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil Cytoskel. 1989;13:127–144 [DOI] [PubMed] [Google Scholar]
- 26. Kelley MJ, Rose AY, Keller KE, Hessle H, Samples JR, Acott TS. Stem cells in the trabecular meshwork: present and future promises. Exp Eye Res. 2008;88:747–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91:564–579 [DOI] [PubMed] [Google Scholar]
- 28. Gasiorowski JZ, Russell P. Biological properties of trabecular meshwork cells. Exp Eye Res. 2009;88:671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heng BC, Bezerra PP, Preiser PR, et al. Effect of cell-seeding density on the proliferation and gene expression profile of human umbilical vein endothelial cells within ex vivo culture. Cytotherapy. 2011;13:606–617 [DOI] [PubMed] [Google Scholar]
- 30. Gonzalez P, Epstein DL, Borras T. Genes upregulated in the human trabecular meshwork in response to elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2000;41:352–361 [PubMed] [Google Scholar]
- 31. Wang N, Chintala SK, Fini ME, Schuman JS. Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype. Nat Med. 2001;7:304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang N, Chintala SK, Fini ME, Schuman JS. Ultrasound activates the TM ELAM-1/IL-1/NF-kappaB response: a potential mechanism for intraocular pressure reduction after phacoemulsification. Invest Ophthalmol Vis Sci. 2003;44:1977–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adam MF, Belmouden A, Binisti P, et al. Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum Mol Genet. 1997;6:2091–2097 [DOI] [PubMed] [Google Scholar]
- 34. Triyoso DH, Good TA. Pulsatile shear stress leads to DNA fragmentation in human SH-SY5Y neuroblastoma cell line. J Physiol. 1999;515:355–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sabanay I, Tian B, Gabelt BAT, Geiger B, Kaufman PL. Latrunculin B effects on trabecular meshwork and corneal endothelial morphology in monkeys. Exp Eye Res. 2006;82:236–246 [DOI] [PubMed] [Google Scholar]
- 36. Peterson JA, Tian B, Geiger B, Kaufman PL. Effect of latrunculin-B on outflow facility in monkeys. Exp Eye Res. 2000;70:307–313 [DOI] [PubMed] [Google Scholar]
- 37. Hogg P, Calthorpe M, Batterbury M, Grierson I. Aqueous humor stimulates the migration of human trabecular meshwork cells in vitro. Invest Ophthalmol Vis Sci. 2000;41:1091–1098 [PubMed] [Google Scholar]