Abstract
Two types of microvillar photoreceptors in the neural tube of amphioxus, an early chordate, sense light via melanopsin, the same photopigment as in “circadian” light detectors of higher vertebrates. Because in amphioxus melanopsin activates a Gq/phospholipase C cascade, like phototransduction in arthropods and mollusks, possible commonalities in the photoconductance were investigated. Unlike other microvillar photoreceptors, reversal of the photocurrent can only be attained upon replacement of extracellular Na+. In addition to Na+, Ca2+ is also permeant, as indicated by the fact that (a) in normal ionic conditions the photocurrent remains inward at Vm > ENa; (b) in Na-free solution a small residual inward photocurrent persists at Vm near resting level, provided that Ca is present; and (c) Vrev exhibits a modest shift with [Ca]o manipulations. The unusual reversal is accounted for by an uncommonly low permeability of the light-dependent channels to K+, as [K]o only marginally affects the photocurrent amplitude and its reversal. Lanthanum and ruthenium red (RuR), two TRP channel antagonists, reversibly suppress the response to photostimulation of moderate intensity; therefore, the melanopsin-initiated cascade may recruit ion channels of the same family as those of rhabdomeric photoreceptors. With brighter lights, blockage declines, so that both La3+ and RuR induce a right shift in the sensitivity curve without a reduction of its asymptote. Nonetheless, an effect on the transduction cascade, rather than the channels, was ruled out on the basis of the voltage dependency of the blockade and the lack of effects of intracellular application of the same substances. The mechanisms of action of these antagonists thus entail a state-dependent blockade, with a higher affinity for the channel in the closed conformation. Collectively, the results indicate a kinship of the light-sensitive channels of amphioxus with those of invertebrate rhabdomeric visual cells and support the representation of this lineage of photoreceptors among chordates.
INTRODUCTION
In the neural tube of amphioxus (Branchiostoma), two classes of microvilli-bearing cells express the photopigment melanopsin (Koyanagi et al., 2005) and have recently been shown to function as primary photoreceptors (Gomez et al., 2009). These cells, known as Joseph and Hesse cells, respectively, produce a depolarizing receptor potential caused by an increase in membrane conductance; the photoresponse is accompanied by a release of calcium from internal stores (Gomez et al., 2009) and is a result of the activation of the PLC signaling pathway (Angueyra et al., 2011); therefore, in many salient respects, they resemble rhabdomeric visual cells of arthropods and mollusks (Nasi et al., 2000).
Melanopsin, originally uncovered in dermal cells of amphibians (Provencio et al., 1998), was first implicated in light detection by the nervous system of higher vertebrates when it was found to underlie the photosensitivity of a subclass of ganglion cells in the retina (Peirson and Foster, 2006). These intrinsically photosensitive retinal ganglion cells (ipRGCs) subserve nonvisual functions like controlling the pupillary reflex and photoentraining circadian rhythms (Berson, 2003). The light response of ipRGCs also entails a depolarization of membrane voltage (Berson et al., 2002) and involves the phosphoinositide signaling pathway (Sekaran et al., 2007; Graham et al., 2008), prompting the suggestion that these cells descend from the same lineage as rhabdomeric photoreceptors (Plachetzki et al., 2005), notwithstanding the lack of discernible microvilli, which are the defining structural feature of canonical invertebrate receptors. Because molecular phylogeny has established that amphioxus is the most basal extant chordate (Putnam et al., 2008), and has remained remarkably close to its ancestral condition (Schubert et al., 2006), melanopsin-based light detection by Joseph and Hesse cells bridges the gap between classical microvillar photoreceptors of invertebrates and “circadian photoreceptors” of vertebrates (Koyanagi and Terakita, 2008).
Little is known about the functional properties of the light-dependent ion channels in melanopsin-using photosensitive cells. Moreover, their molecular identity remains to be elucidated; however, considering that the early steps of phototransduction initiated by melanopsin are akin to those of invertebrate microvillar receptors, it is plausible that the ion channels underlying the receptor potential may also be related to those of rhabdomeric visual cells. In this study, the light-dependent conductance of Joseph and Hesse cells was examined, with special emphasis on conduction and blockage. Although some surprising features were encountered, the data indicate a kinship to the superfamily of TRP channels and therefore support an evolutionary link between invertebrate vision and vertebrate circadian photoreception.
MATERIALS AND METHODS
Cell isolation
Amphioxus (Branchiostoma floridae) were obtained from Gulf Specimens Marine Laboratories and maintained in a seawater aquarium on a diet of marine phytoplankton and a 12/12-h light/dark cycle. Specimens were anesthetized by hypothermia, the rostral end was cut and pinned to a Sylgard-coated chamber, and the neural tube was excised. The tissue was then incubated with 750 U/ml pronase (50 min at 22°C; Boehringer Ingelheim) dissolved in artificial seawater (ASW: 480 mM NaCl, 10 KCl, 10 CaCl2, 49 MgCl2, 10 HEPES, and 5.4 glucose, pH 7.8), followed by extensive washing in ASW supplemented with 4% fetal calf serum and mechanical trituration with a fine-bore fire-polished Pasteur pipette. The resulting suspension was plated into a perfusion chamber mounted on the stage of an inverted microscope (Carl Zeiss). The coverslip bottom of the chamber was pretreated with concanavalin A (Sigma-Aldrich) to promote cell adhesion. Dissociated cells remain physiologically viable for several hours.
Electrophysiological recording
Patch pipettes for whole cell clamp were fabricated from thin-wall borosilicate capillaries (o.d., 1.5 mm; i.d., 1.1 mm) and fire-polished before use. The standard filling solution contained 100 mM KCl, 200 K-glutamate, 5 MgCl2, 5 Na2ATP, 12 NaCl, 1 EGTA, 300 sucrose, 10 HEPES, and 0.2 GTP, pH 7.3. Electrode resistance in ASW was 2–4 MΩ; series resistance was compensated electronically (maximum residual error, <2 mV). The soma of both Joseph and Hesse cells is compact and approximately spherical (typical diameter, 12–16 µm), and for whole cell clamp recording, cells lacking a visible axon stump were usually selected to optimize space clamp. Most cells remained viable with a stable photoresponse for recording periods ranging from 30 min to nearly 2 h. Extracellular solution changes were implemented by a system of manifolds that controlled the superfusion of the whole recording chamber. In those solutions designed to manipulate the concentration of external potassium (in the range of 2–50 mM), Na was reduced to 440 mM and a suitable amount of NMDG was used to maintain osmolarity. Perforated-patch recording was implemented with nystatin as the pore-forming agent, pre-diluting it in DMSO and subsequently in internal solution, and mixing by vortexing and sonication. The final concentration of nystatin was 0.3 mg/ml, whereas DMSO was 0.5%. All reagents were obtained from Sigma-Aldrich. Data were digitized with an analogue–digital interface (Data Translation), which also served to generate stimuli, under the control of software developed in-house; additional programs were used for offline analysis.
Light stimulation
Broadband light stimuli were generated by a tungsten-halogen light source (THQ; Oriel); infrared light (IR) was removed by a heat-absorbing filter (λ > 800 nm). A solenoid-driven shutter (Uniblitz), calibrated neutral density filters (Melles-Griot), and interference filters (Omega Optical) controlled the duration, intensity, and wavelength of stimulation. A pinhole restricted the illuminated region to a focused spot (≈150 µm). Alternatively, a blue LED (peak emission, 470 nm) was driven by a computer-controlled precision current pump and delivered a full-field light stimulus via a fiber optics bundle. Light was measured with a radiometer (UDT Sensors, Inc.) and converted to effective photon flux via an in vivo calibration, which made it possible to compare across stimulator arrangements. During experimental manipulations, the cells were illuminated at λ > 780 nm and viewed with the aid of an IR-sensitive CCD camera (Sony).
RESULTS
Ionic selectivity of the photoconductance
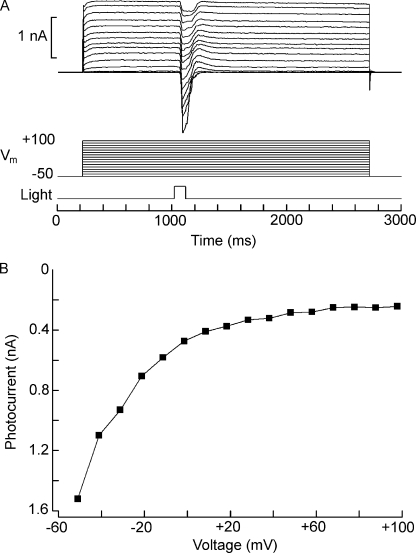
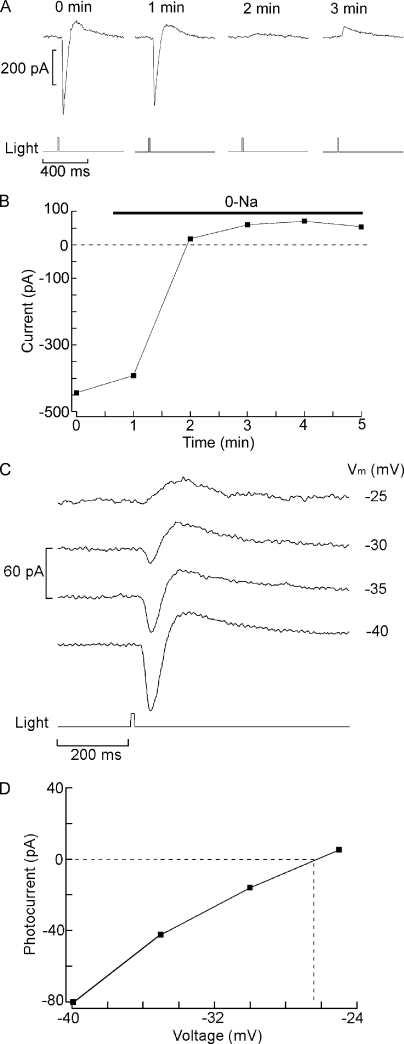
In a previous report, changes in extracellular Na and Ca were shown to influence the size of the light-evoked current of Hesse and Joseph cells (Gomez et al., 2009), but such manipulations were not combined with voltage stimulation to assess unambiguously the permeation properties of the photoconductance. We examined the dependency of the photocurrent on membrane voltage by maintaining voltage-clamped cells at a holding potential of −50 mV in the dark and applying once a minute a 2.5-s depolarizing step of increasing amplitude; 800 ms after the onset of the voltage stimulus, when the voltage-activated currents had largely stabilized, a flash of constant intensity was delivered, as illustrated in Fig. 1 A. Fig. 1 B shows the peak photocurrent amplitude plotted as a function of voltage. Surprisingly, although for both cell types the size of the photocurrent progressively decreased, it did not reverse polarity or even become null (Joseph cells, n = 5; Hesse cells, n = 6), in spite of the application of large depolarizations, in excess of +100 mV. This behavior contrasts sharply with all known depolarizing photoreceptors, as well as with vertebrate rods and cones, where reversal is attained at voltages in the range of 0 to +30 mV. Similar experiments were then conducted after replacing all extracellular sodium with Tris, but the light-evoked current remained inward in the same range of voltages, even though in Hesse cells its amplitude was reduced (n = 3). However, when the Na replacement was switched to the larger cation NMDG, reversal was observed. Fig. 2 A shows the response to a repetitive flash measured at 0 mV in a Joseph cell bathed initially in ASW and then superfused with Na-free (NMDG-substituted) solution: the photocurrent gradually decreased in amplitude and subsequently inverted polarity. An outward transient is visible after the primary inward photocurrent; this feature, which is especially pronounced with bright light stimuli and membrane depolarization, will be examined in a separate report. The graph in Fig. 2 B plots the peak photocurrent as a function of time. Hesse cells behaved in a comparable way. Fig. 2 C shows photocurrent traces in 0 Na ASW as membrane depolarization was progressively increased, whereas D illustrates the current-to-voltage relation. Reversal potential was similar between Hesse and Joseph cells, with a mean of −26.2 ± 1.84 mV (n = 5). Thus, Na carries a substantial fraction of the photocurrent, but Tris is also significantly permeant, like in the light-dependent channels of Limulus and Lima (Brown and Mote, 1974; Gomez and Nasi, 1996). Additional clues indicate that Ca may also be implicated: under physiological extracellular ionic conditions, reversal does not occur even when Vm is stepped to values well in excess of ENa (≈+73 mV), and after elimination of external Na, depolarization is needed to elicit an outward photocurrent. Complete Ca elimination proved deleterious, preventing a systematic examination of the photocurrent at different holding potentials: shortly after beginning the perfusion with Ca-free solution, the photocurrent transiently increased, but then it declined and unless control conditions were swiftly restored, responsiveness was irreversibly lost. We therefore tested the effect of lowering [Ca]o to 500 µM (a 20-fold reduction) in 0 Na (NMDG) solution: under these conditions the reversal potential was −29.3 ± 1.9 mV (n = 4), representing a negative shift of 3.1 mV with respect to standard [Ca]o. This result attests the permeation of Ca, complementing the observation that in Na-free solution a small residual inward current is measured at resting potential and can be eliminated by reducing extracellular calcium (not depicted).
Figure 1.
Failure to reverse the photocurrent in standard ionic conditions. (A) A Hesse cell was voltage clamped at −50 mV, and depolarizing steps of 2.5-s duration were applied every 60 s, increasing the amplitude at 10-mV increments. At 800 ms after the onset of the voltage stimulus, a 100-ms light flash of constant intensity was delivered (3.8 × 1012 photons/cm2). Photocurrent size decreased with depolarization but did not reverse polarity, even after stepping membrane voltage up to +100 mV. (B) Peak photocurrent amplitude plotted as a function of membrane voltage, revealing a pronounced inward rectification of the I-V relation.
Figure 2.
Effects of replacement of extracellular sodium. (A) Joseph cell voltage clamped at 0 mV. After the first light stimulus, the solution was switched to 0 Na/NMDG, causing the photocurrent to become outward. (B) Plot of the photocurrent as a function of time for the full experiment, showing the stable outward light-evoked current in sodium-free solution. Light stimulus, 3.7 × 1013 photons/cm2. (C) Photostimulation (4.45 × 1013 photons/cm2) applied at different holding voltages to another Joseph cell, showing outwardly directed photocurrent above −30 mV. (D) l-V relation of the photocurrent in sodium-substituted extracellular solution in the same cell as C. The interpolated reversal potential was ≈−26.4 mV.
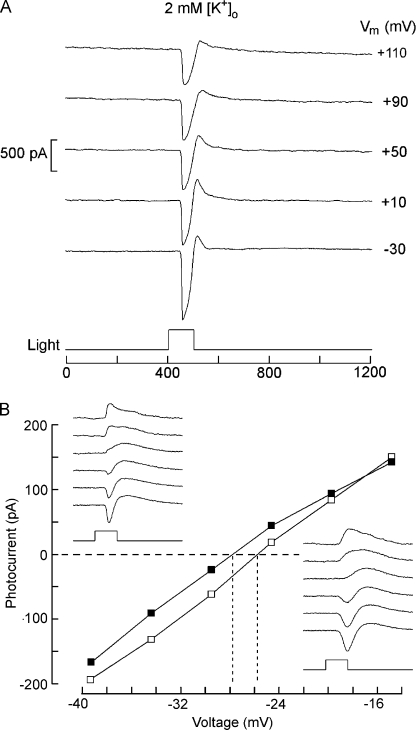
The light-sensitive conductance of most other photoreceptors discriminates poorly among cations, and a substantial participation of K ions accounts for the observed values of Vrev, hovering slightly positive of 0 mV. The unusual behavior of amphioxus photoreceptors suggests instead a marginal role of potassium in the light response. We directly assessed the effects of varying extracellular potassium in two ways. First, the photocurrent elicited by repetitive flashes (1/min) was measured at a fixed voltage (−50 mV) as the concentration of extracellular K+ was manipulated. Fig. 3 A shows traces obtained from a Hesse cell in physiological potassium (10 mM) as well as after iso-osmotic reduction of [K+]o to 2 mM, or elevation to 50 mM. Such manipulations produced only modest changes in the peak amplitude of the light response: the fivefold reduction, which increases the outwardly directed driving force by 213%, reduced the current on average by 5%, whereas the corresponding increase in potassium (lowering the driving force by 86%) augmented the size of the photocurrent by 7% (n = 3). Elevated potassium also induced the appearance of a prominent additional current component, the nature of which will be addressed in a separate study. The bar graph in Fig. 3 B summarizes the observations. Joseph cells behaved similarly. The second approach was to examine the effect of [K+]o changes on the reversal potential of the photocurrent. To the extent that potassium makes a significant contribution, increasing its gradient ought to induce a negative shift of Vrev, possibly allowing the photocurrent to invert polarity at voltages that can be applied with no adverse effects, without resorting to Na replacement. However, with 2 mM [K]o (EK ≈ −126 mV), depolarization up to +110 mV still failed to invert photocurrent polarity, as illustrated by Fig. 4 A. Therefore, we switched to low Na (NMDG) conditions. Fig. 4 B shows the I-V relation of the light-evoked current measured in a Joseph cell in normal extracellular potassium (10 mM) and upon reducing [K]o to 2 mM; the raw traces obtained in the two conditions are presented in the insets. The reversal potential of the photocurrent shifted only marginally to a more negative value. Pooling the measurements conducted in four Joseph cells, Vrev changed from −28 ± 2.4 mV (SD) to −30.7 ± 5.5 mV, a net average displacement of only 2.7 mV, whereas the corresponding change in EK is ≈40.5 mV. A similar outcome was obtained in Hesse cells. In contrast, when [K]o was instead increased to 50 mM, the apparent shift was more pronounced (average Vrev = +5.5 mV; n = 3); this could reflect either contamination by the late component that was greatly augmented in elevated [K]o (see large inward tail in Fig. 3 A) or the possibility that external K exerts some additional regulatory action on the properties of the light-dependent channels.
Figure 3.
Effects of [K]o manipulations on the amplitude of the light-evoked current. (A) A Hesse cell was voltage clamped and stimulated repetitively; the photocurrent size changed marginally as the extracellular potassium concentration was changed from 10 mM to either 2 or 50 mM. A fixed flash intensity was used throughout (2.8 × 1013 photons/cm2), and the holding potential was set at −50 mV. (B) Peak photocurrent amplitude normalized with respect to the value obtained at [K]o = 10 mM, averaged for three Hesse cells tested under the three ionic conditions. Error bars indicate SEM.
Figure 4.
Effects of extracellular potassium on the reversal of the photocurrent. (A) Increasing the potassium gradient by lowering [K]o to 2 mM in the presence of normal extracellular Na fails to bring about reversal of the light-evoked current when the membrane is depolarized up to +110 mV. Hesse cell: light stimulus, 6.1 × 1013 photons/cm2. (B) Effect of reducing [K]o on the reversal potential measured in 0 Na. Sodium in the bath solution was replaced by NMDG, and the light response was examined at either 10 mM [K]o (open symbols; traces of bottom right inset) or 2 mM [K]o (closed symbols; top left inset), as the membrane potential was progressively depolarized at 5-mV increments. The reversal potential of the photocurrent shifted by only 2 mV in the negative direction when external potassium was reduced fivefold.
Rectification
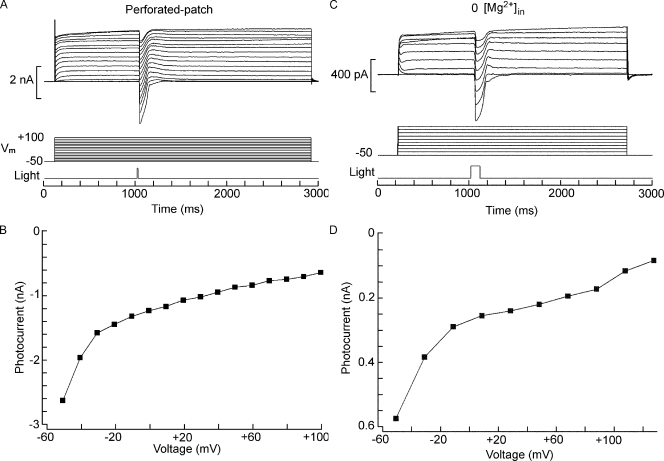
A striking feature of the I-V relation in ASW is a pronounced rectification in the inward direction, contrary to the characteristic outwardly rectifying photocurrents of other photoreceptors (Millecchia and Mauro, 1969; Bader et al., 1979; Gomez and Nasi, 1994). Inward rectification can result from voltage-dependent block of the permeation pathway by intracellular cations. We first examined whether such unusual nonlinearity may arise artifactually from the conditions of internal perfusion; the only nonphysiological constituent in the intracellular solution is HEPES, which has been previously reported to block certain conductances (Yamamoto and Suzuki, 1987; Hanrahan and Tabcharani, 1990) and can in fact contribute appreciably to the inward rectification of some IRK channels under whole cell clamp (Guo and Lu, 2000). However, when the procedure was repeated with perforated-patch recording to not alter the composition of the cytosol, the same strong inward rectification was observed (Fig. 5, A and B; n = 4); the effect is therefore genuine. Two classes of physiological molecules have been implicated in voltage-dependent block that results in nonlinear conduction, especially in some K-selective channels: magnesium (Vandenberg, 1987) and polyamines (Lopatin et al., 1994). Because of the extensive intracellular perfusion during whole cell clamp recording, soluble polyamines—if present in the native state—are expected to wash out; therefore, it is unlikely that they may account for the observed rectification. In contrast, the standard electrode-filling solution contains a significant concentration of free Mg2+, on the order of 0.63 mM (as calculated with the program MaxChelator). We tested the effect of omitting internal Mg altogether in two Hesse and a Joseph cell but observed no change in the I-V relation of the photocurrent; an example is illustrated in Fig. 5 (C and D). One can conclude that the rectification of the light-sensitive conductance of amphioxus photoreceptors cannot be explained as voltage-dependent blockage by the soluble intracellular factors that commonly operate in other systems; a plausible contributing factor could be a voltage dependency of the gating process.
Figure 5.
Rectification of the photocurrent. (A and B) The nonlinear conduction of the photocurrent is not an artifact of intracellular perfusion. A Hesse cell was whole cell clamped with the perforated-patch technique using nystatin. After access resistance attained a stable level, the protocol of light stimulation (7.2 × 1013 photons/cm2) and membrane depolarization was applied. The resulting light-evoked currents rectified in the inward direction like in control conditions. (C and D) Rectification is not a result of voltage-dependent block by intracellular magnesium ions. Similar experiments were performed with an intracellular solution in which Mg was omitted. The inward rectification was unaffected. Flash intensity: 3.8 × 1012 photons/cm2).
Blockers
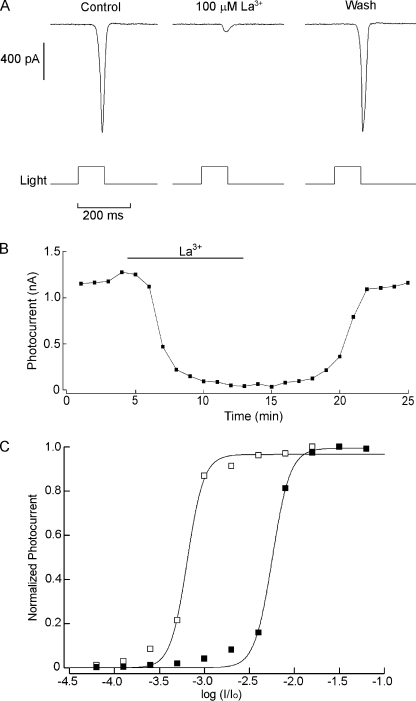
The light-sensitive conductance of the PLC-based phototransduction cascade of Drosophila melanogaster is comprised of the two founding members of the TRP superfamily of ion channels: TRP and TRPL. Channels belonging to the C subgroup of the TRP class are ubiquitous final effectors of phosphoinositide signaling cascades in a variety of systems (Clapham et al., 2005). Because the coupling of melanopsin stimulation to ionic fluxes in amphioxus also entails activation of the phosphoinositide pathway (Gomez et al., 2009; Angueyra et al., 2011), this family of channels constitutes plausible candidates to underlie the light-activated current in Hesse and Joseph cells. Several substances have been proven effective in blocking the current mediated by TRP-class channels, with varying degrees of effectiveness on different subtypes. Some trivalent cations are among them, such as gadolinium, which can block mammalian TRPC-1, TRPC-3, and TRPC-6 subtypes (Clapham et al., 2005). We tested the effects of extracellular application of 100 µM Gd3+ during periodic flash stimulation (1/min) of constant intensity but observed no significant depression in the amplitude of the photocurrent (not depicted; Joseph cells, n = 3; Hesse cells, n = 2). In contrast, when lanthanum, another standard antagonist that blocks the TRP-mediated light-evoked current in Drosophila photoreceptors (Hardie and Minke, 1992), was applied by bath superfusion at the same concentration (100 µM), it caused a profound, fully reversible suppression of the photocurrent, as shown in Fig. 6 A. Fig. 6 B illustrates the time course of the effect (much of the delay reflects the dead time for solution exchange in the perfusion chamber). The average inhibition was 79 ± 13% (SD) for Hesse cells (n = 4) and 82 ± 7% for Joseph cells (n = 5). In a few instances, a dual action of lanthanum on certain TRPs has been reported, with low concentrations producing a potentiation rather than inhibition (Strübing et al., 2001); in the present case, reduction of [La3+] to 20 µM simply decreased the blockage, but no hint of potentiation was observed (not depicted). Subsequently, full light intensity series were measured in control conditions and in the presence of 100 µM La3+. Surprisingly, as the stimuli were made brighter, the effectiveness of lanthanum progressively decreased, so that no significant change occurred in the asymptotic value of the stimulus–response relation, only a right shift of the curve. The average change in σ (the light intensity producing a response of half-maximal amplitude) was 1.36 ± 0.39 log (SEM; n = 4). In Fig. 6 C, the normalized intensity–response curve of the photocurrent is plotted for a cell tested in the two conditions. This apparent sensitivity shift fully accounts for the results obtained with fixed flashes of modest intensity (e.g., Fig. 6 A, where light intensity corresponds to an attenuation of −3.0 log in the scale of C).
Figure 6.
Blockade of the photocurrent by lanthanum. (A) Joseph cell stimulated every minute with light flashes of constant intensity (4.9 × 1012 photons × s−1cm−2). The panel shows photocurrent traces in control conditions at the peak of the inhibition during bath superfusion with 100 µM and after washing the blocker. (B) The photocurrent amplitude is plotted as a function of time to illustrate the time course of the blockage. (C) Normalized intensity–response curve determined in control conditions and in the presence of 100 µM La3+. Stimulating light was raised at 0.3 log increments. Lanthanum caused a substantial right shift of the curve by approximately one log unit.
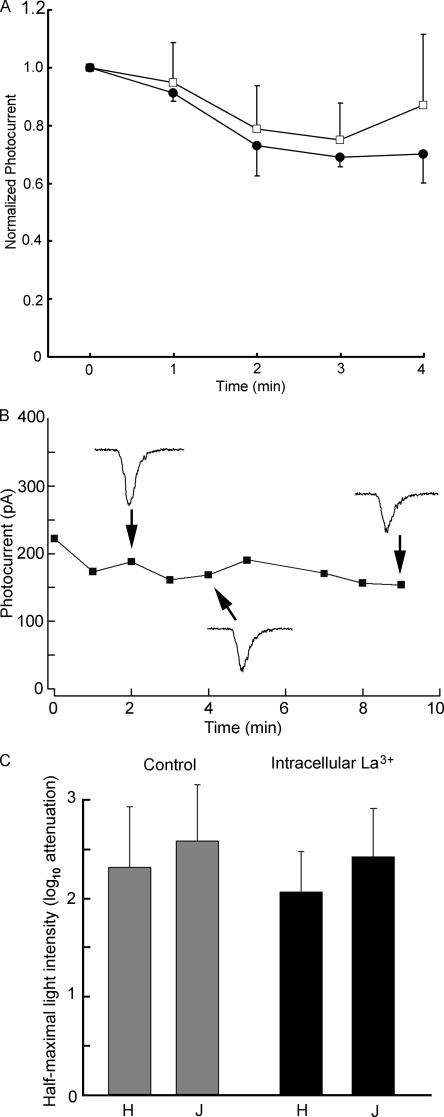
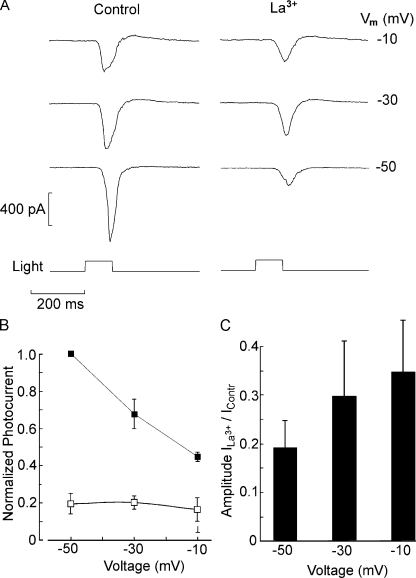
The reduction in sensitivity in the presence of lanthanum could indicate an effect on the phototransduction cascade, rather than blockage of ion channels. To clarify the site of action, lanthanum was applied directly to the intracellular compartment via the patch pipette. Periodic photostimulation (1/min) started immediately upon attaining the whole cell configuration; dim flashes were used for this purpose, a regimen that shows pronounced effects when La3+ is applied externally. After recording the currents evoked by fixed light stimuli for several minutes, intensity–response curves were also measured. Fig. 7 A shows the average peak photocurrent amplitude plotted as a function of time for Joseph cells dialyzed either with standard internal solution or with 100 µM La3+ added (n = 3 in each case): no significant differential decline in light response amplitude was observed. In Fig. 7 B, the time course of a similar experiment with a Hesse cell is displayed; the recording and dialysis time was extended, and the light-evoked current persisted virtually unabated for 9 min. The apparent sensitivity was also not appreciably affected in either type, as illustrated by the bar graph in Fig. 7 C, which shows the average value of σ, the light intensity that evokes a half-maximal response, in Hesse and Joseph cells under control conditions and with internal dialysis with 100 µM La3+. These results argue for an extracellular site of action of the blocker and likely implicate, as a target, the light-activated channels rather than the transduction machinery. Further support for this notion was sought by examining the effects of membrane voltage on the photocurrent suppression by lanthanum. To this end, the response to a fixed flash was measured at a holding potential of −50, −30, and −10 mV, both in control conditions and in the presence of 100 µM of extracellular La3+. Fig. 8 A shows the resulting photocurrent traces recorded in a Hesse cell; it is readily appreciated that the degree of response attenuation by La3+ is reduced with membrane depolarization. This is strengthened by the plot in Fig. 8 B, where for each cell the photocurrents were normalized with respect to the control response at −50 mV (n = 5). Because the two cell types behaved similarly, data from three Hesse and two Joseph cells were pooled for averaging. Finally, the bar graph in Fig. 8 C shows the mean response amplitude in the presence of La3+ relative to control, highlighting how suppression of the photocurrent decreased as the holding potential is made less negative.
Figure 7.
Effect of intracellular perfusion with La3+. Lanthanum was included in the electrode-filling solution at a concentration of 100 µM, and repetitive photostimulation (one flash/min) started immediately after accessing the cell interior. (A) Normalized average amplitude of the photocurrent in Joseph cells dialyzed with 100 µM lanthanum (closed symbols), compared with control solution (open symbols). No significant differences over time were observed between the two conditions (n = 3 in each group). (B) Similar experiment in a Hesse cell, extending the recording period to 9 min. Raw traces of the photoresponse are shown as insets. (C) Bar graph summarizing the effect of intracellular lanthanum on response sensitivity. The average light intensity required to elicit a response of half-maximal amplitude is plotted for Joseph and Hesse cells tested under the two conditions of intracellular perfusion.
Figure 8.
Voltage dependence of the block by lanthanum. (A) Photocurrent elicited by a light of approximately half-saturating intensity (2.6 × 1011 photons × s−1cm−2) in a Hesse cell voltage clamped sequentially at −50, −30, and −10 mV in ASW (left) and during exposure to 100 µM La3+ (right). The antagonistic effects of lanthanum diminished as the membrane was depolarized. (B) Pooled data (n = 5, three Hesse and two Joseph cells) showing the average relative amplitude of the photocurrent (normalized by the response in ASW at −50 mV) in control condition (closed squares) and in the presence of lanthanum (open squares). (C) Degree of blockage by lanthanum as a function of membrane potential. The bar graph plots the relative peak amplitude of the photocurrent during superfusion with La3+ versus in ASW.
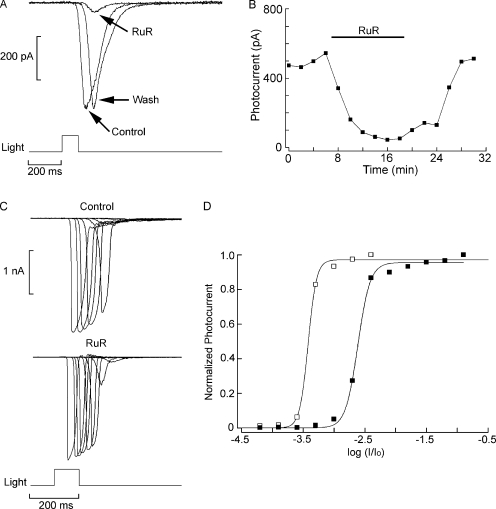
Ruthenium red (RuR) is another well-known antagonist of several TRP channels (Clapham et al., 2005), including those that mediate the photocurrent in Drosophila (Liu et al., 2007), and is typically effective at low micrometer concentrations. Extracellular administration of 5 µM RuR also depressed reversibly the photocurrent in both Joseph cells (mean inhibition, 85 ± 15% SD; n = 6) and Hesse cells (mean inhibition, 87 ± 13% SD; n = 5). The effect is illustrated in Fig. 9 A, whereas its time course is plotted in B. As observed with lanthanum, in the presence of RuR full-fledged photocurrents could still be elicited, provided that light intensity was suitably increased. In Fig. 9 C, superimposed traces of photocurrents evoked by flashes of increasing intensity are shown for a Hesse cell tested in ASW and during exposure to 5 µM RuR Finally, in Fig. 9 D, the normalized intensity–response curves are plotted in the two conditions; the average shift in σ was 1.24 ± 0.29 log (n = 5).
Figure 9.
Suppression of the light response by RuR. (A) A Joseph cell voltage clamped at −50 mV was stimulated with flashes of light (2.2 × 1011 photons × s−1cm−2) in ASW during superfusion with 5 µM RuR and after returning to control condition. The photocurrent was reversibly attenuated. (B) Time course of the effect of RuR. The peak amplitude of the light-evoked current is plotted as a function of time for the three phases of the experiment. (C) Intensity series measured in a different Joseph cell in control conditions and in the presence of 5 µM RuR. In the presence of the antagonist, large photocurrents could still be elicited when light intensity was increased. (D) Shift in sensitivity induced by RuR. The normalized peak amplitude of the photocurrent is plotted as a function of light attenuation in the two conditions. A substantial right shift of the stimulus–response curve was induced by RuR. Unattenuated light intensity: 5.1 × 1014 photons × s−1cm−2.
DISCUSSION
Amphioxus is an organism of great importance for studies in evolutionary (Schubert et al., 2006; Gibson-Brown and Hartenstein, 2008) and developmental biology (Holland et al., 2004), owing to the unique position it occupies in the phylogenetic tree as the most basal extant chordate (Putnam et al., 2008). However, virtually no physiological investigations had been conducted on this animal. We recently characterized Joseph and Hesse cells as primary photoreceptors (Gomez et al., 2009). In the present study, we investigated the electrophysiological properties of the photocurrent, focusing on selectivity, rectification, and block.
These cells exhibit striking similarities, in terms of morphology and early stages of light transduction, to the lineage of rhabdomeric visual receptors, long thought to be a prerogative of arthropods and mollusks. Part of the thrust of this work was to ascertain whether such kinship extends to the ion channels recruited to serve as effectors of the light-signaling pathway. In Drosophila, genetic dissection of visual mechanisms initially led to the identification of two genes, trp (Cosens and Manning, 1969; Montell and Rubin, 1989) and trpl (Phillips et al., 1992), whose products mediate the photocurrent (Niemeyer et al., 1996; Reuss et al., 1997). In most other microvillar photoreceptors the light-operated ion channels have not yet been positively identified, but TRP-related proteins have been reported to express in rhabdomeric cells of squid (Monk et al., 1996) and Limulus (Bandyopadhyay and Payne, 2004). A plethora of TRP orthologues was subsequently found across virtually all eukaryotic organisms, including mammals, and comprises at least six subdivisions of a very diverse superfamily (Clapham et al., 2005). Their expression is widespread across tissues and cell types, but for many of them, the function remains poorly understood. Interestingly, a significant contingent of these channels has been implicated in various sensory tasks, so that the importance and ubiquity of the TRP family across sensory transduction modalities is gaining a growing recognition (Damann et al., 2008).
Although molecular and structural details on TRP channels are still sketchy, distinct functional and pharmacological commonalties have emerged, which can provide a basis for an initial characterization (Clapham et al., 2005; Owsianik et al., 2006). The susceptibility of the amphioxus photocurrent to lanthanum and RuR suggests a kinship to the light-sensitive conductance of rhabdomeric visual cells, like those of Drosophila (Hardie and Minke, 1992), as well as to the melanopsin-mediated light response of rat ipRGCs (Warren et al., 2006), where the participation of TRPC-6 channels has been proposed on the basis of immunological markers. A future goal will be the molecular identification of the light-dependent channels, and the recent sequencing of the amphioxus genome (Putnam et al., 2008) provides a powerful tool to aid such a quest. An initial genomic search of TRP channel orthologues yielded a large number of candidates (>80), but because the genome is not yet fully annotated, the obtained set likely includes putative genes of dubious relevance to a PLC-dependent phototransduction cascade (e.g., members of the TRP family that may be involved in mechanosensation or in temperature sensing). To restrict the pool of candidates, we resorted instead to a local tBLASTn with query sequences from other species that belong to the TRPC subcategory and either express in microvillar photoreceptors (e.g., Drosophila TRP and TRPL) or are suspected to be linked to the PLC signaling cascade (e.g., reported activation by Ca, DAG, etc.). A stringent search yielded an initial set of seven putative genes, and current efforts are geared toward conducting PCR amplifications using cDNA template from the neural tube of amphioxus. For those that will prove positive, assays of localization at the cellular level will ascertain possible expression in Hesse and Joseph cells, to be followed by functional manipulations.
The effectiveness of both lanthanum and RuR on the photocurrent of amphioxus displayed a distinct dependency on light intensity. Examination of the full intensity–response relation in control conditions versus during exposure to La3+ or RuR revealed that the asymptotic photocurrent amplitude is marginally altered, and the antagonistic effect stems from a right shift of the sensitivity curve. In fact, if supersaturating stimulation is used, one could erroneously dismiss the effectiveness of these antagonists (a call for caution in drawing conclusions about effects, or lack thereof, without a systematic exploration of stimulation parameters!). Nonetheless, such decreased sensitivity in the absence of diminished maximal amplitude of the photocurrent does not reflect interference with the light transduction cascade, as corroborated by the lack of inhibitory effects when La3+ was administered intracellularly. This result points to a site of action accessible extracellularly and is therefore more likely to implicate the ion channels themselves. This notion is also supported by the observed voltage dependency of the block by external La3+. Although from the mechanistic point of view such an effect admits more than one interpretation (i.e., occupancy of a site in the pore within the electrical field vs. voltage-dependent accessibility to the blocking site), both accounts point to the channel as the target. The observed dependency of blockade on light intensity is quite reminiscent of the interactions reported for a wide variety of channels and antagonists, in which the effectiveness of blockade is significantly altered depending of the probability of opening of the channel, which in turn depends on the conditions of stimulation. These phenomena are referred to as state-dependent blockade. For example, when tetracaine is administered to cyclic nucleotide–activated channels of rod photoreceptors, the curve relating current to cyclic nucleotide concentration is right-shifted, with no effect on the asymptote (Fodor et al., 1997). In other words, tetracaine effectively suppresses the current when low concentrations of cGMP are used, resulting in a modest channel activation, whereas at supersaturating cGMP concentrations, the same application of the blocker fails to exert a significant antagonism. The effect has been explained in terms of the antagonist having a higher affinity for the closed conformation of the channel, compared with the open state, and stabilizing it. According to the present results, La3+ and RuR behave as closed-state blockers of the photoconductance of amphioxus, and their effectiveness is decreased by channel opening.
In invertebrate microvillar photoreceptors, the permeability of the light-activated conductance is mixed cationic, with varying contributions by Na+ and Ca2+ in different species and a ubiquitous participation of K+. The reversal potential, therefore, is also somewhat species dependent but is typically confined to a relatively narrow range between 0 and +30 mV (Limulus: Millecchia and Mauro, 1969; Brown and Mote, 1974; Balanus: Brown et al., 1970; Drosophila: Hardie, 1991; Lima: Gomez and Nasi, 1996). In contrast, with Hesse and Joseph cells of amphioxus, it proved impossible to revert the photocurrent under standard ionic conditions, even at Vm > +100 mV. Manipulations of extracellular Na and Ca indicate that both ions permeate through the light-activated channels. The contribution of calcium is modest, gauging from the magnitude of the effect of altering [Ca2+]o; quantification of the relative selectivity of Na+/Ca2+ proved difficult, and a version of the Goldman-Hodgkin-Katz equation modified to include divalents (Fatt and Ginsborg, 1958) yielded variable permeability ratios (ranging from 0.34 to >1), depending on [Ca2+]o. Such inconsistency is not surprising, as there are several caveats regarding the applicability of such an analysis to the present case. In the first place, a margin of error is introduced by the fact that during the light response the concentration of cytosolic calcium undergoes substantial changes, the magnitude of which are not known. Second, the assumption of independence of ionic fluxes (implicit in the Goldman-Hodgkin-Katz equation) is most likely invalid, because as [Ca]o is reduced to micromolar levels, the current carried by Na increases (Gomez et al., 2009), which is indicative of ion interaction. Only for a few of TRP channel subtypes has a very positive reversal potential been reported, such as TRPV5/ECaC (Vrev > +50 mV; Vennekens et al., 2000), which display an especially high selectivity for Ca2+ (pCa/pNa > 100). In Joseph and Hesse cells, instead, the unusually positive reversal potential is accounted for by the very modest contribution of K, a feature that sets these channels apart from those of other microvillar photoreceptors, where the permeability of potassium and other monovalent cations like Cs compares favorably with that of Na (Hardie, 1991; Gomez and Nasi, 1996).
Another striking feature of the light-evoked current is that the I-V relation of both Hesse and Joseph cells strongly rectifies inwardly. Although several TRPs exhibit a similar nonlinear conduction (Clapham et al., 2005), this attribute is unusual in photoreceptors, where the rectification is characteristically in the outward direction, not only for other microvilli-bearing visual cells (e.g., Millecchia and Mauro, 1969) but also for vertebrate rods (Bader et al., 1979) and ciliary invertebrate photoreceptors (Gomez and Nasi, 1994; Nasi and Gomez, 1999). The effect also obtains with perforated-patch recording, which minimally perturbs the intracellular milieu, thus ruling out artifacts attributable to the composition of the electrode-filling solution. Also, voltage-dependent block by intracellular magnesium, which accounts for the inward rectification in some K channels (Vandenberg, 1987), as well as TRP channels belonging to the V subgroup (Voets et al., 2003), seemed not to be involved; one can tentatively suggest that the nonlinear conduction may arise instead from an intrinsic voltage dependency of the gating machinery.
In summary, the ionic mechanisms underlying the receptor potential of melanopsin-expressing light-sensitive cells of amphioxus were examined together with the effects of pharmacological agents known to target TRP-class light-dependent channels in Drosophila. A significant parallelism could be established with respect to the photoconductance of rhabdomeric visual cells, but some distinct traits were also encountered, which will warrant further investigation. Nonetheless, it is worth stressing that even within the class of invertebrate photoreceptors, selectivity properties can vary widely: calcium permeability, for example, ranges from unmeasurably low in Limulus (Brown and Mote, 1974) to >50 times higher than sodium in Drosophila (Reuss et al., 1997). Although only molecular identification will provide definitive evidence, the present observations, in conjunction with our findings implicating inositol lipid signaling in phototransduction, reinforce the notion that this lineage of light sensors, emblematic of arthropod and molluscan eyes, is also represented among deuterostomia, and position Hesse and Joseph cells as possible intermediate links between invertebrate vision and ipRGC-mediated circadian photoreception in vertebrates.
Acknowledgments
This work was supported by National Science Foundation grant 0918930.
Edward N. Pugh Jr. served as editor.
Footnotes
Abbreviations used in this paper:
- ASW
- artificial seawater
- ipRGC
- intrinsically photosensitive retinal ganglion cell
- RuR
- ruthenium red
References
- Angueyra J.M., Pulido C., Malagón G., Nasi E., Gomez M.P. 2011. Melanopsin-expressing amphioxus photoreceptors transduce light via a phospholipase C signaling cascade. PLoS ONE. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C.R., Macleish P.R., Schwartz E.A. 1979. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J. Physiol. 296:1–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay B.C., Payne R. 2004. Variants of TRP ion channel mRNA present in horseshoe crab ventral eye and brain. J. Neurochem. 91:825–835 10.1111/j.1471-4159.2004.02773.x [DOI] [PubMed] [Google Scholar]
- Berson D.M. 2003. Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci. 26:314–320 10.1016/S0166-2236(03)00130-9 [DOI] [PubMed] [Google Scholar]
- Berson D.M., Dunn F.A., Takao M. 2002. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 295:1070–1073 10.1126/science.1067262 [DOI] [PubMed] [Google Scholar]
- Brown H.M., Hagiwara S., Koike H., Meech R.M. 1970. Membrane properties of a barnacle photoreceptor examined by the voltage clamp technique. J. Physiol. 208:385–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.E., Mote M.I. 1974. Ionic dependence of reversal voltage of the light response in Limulus ventral photoreceptors. J. Gen. Physiol. 63:337–350 10.1085/jgp.63.3.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D.E., Julius D., Montell C., Schultz G. 2005. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol. Rev. 57:427–450 10.1124/pr.57.4.6 [DOI] [PubMed] [Google Scholar]
- Cosens D.J., Manning A. 1969. Abnormal electroretinogram from a Drosophila mutant. Nature. 224:285–287 10.1038/224285a0 [DOI] [PubMed] [Google Scholar]
- Damann N., Voets T., Nilius B. 2008. TRPs in our senses. Curr. Biol. 18:R880–R889 10.1016/j.cub.2008.07.063 [DOI] [PubMed] [Google Scholar]
- Fatt P., Ginsborg B.L. 1958. The ionic requirements for the production of action potentials in crustacean muscle fibres. J. Physiol. 142:516–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor A.A., Gordon S.E., Zagotta W.N. 1997. Mechanism of tetracaine block of cyclic nucleotide–gated channels. J. Gen. Physiol. 109:3–14 10.1085/jgp.109.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson-Brown J.J., Hartenstein V. 2008. The amphioxus genome sequence illuminates the evolutionary origin of vertebrates. Dev. Genes Evol. 218:575–578 10.1007/s00427-008-0263-7 [DOI] [PubMed] [Google Scholar]
- Gomez M.P., Nasi E. 1994. The light-sensitive conductance of hyperpolarizing invertebrate photoreceptors: A patch-clamp study. J. Gen. Physiol. 103:939–956 10.1085/jgp.103.6.939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez M.P., Nasi E. 1996. Ion permeation through light-activated channels in rhabdomeric photoreceptors. Role of divalent cations. J. Gen. Physiol. 107:715–730 10.1085/jgp.107.6.715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez M.P., Angueyra J.M., Nasi E. 2009. Light-transduction in melanopsin-expressing photoreceptors of Amphioxus. Proc. Natl. Acad. Sci. USA. 106:9081–9086 10.1073/pnas.0900708106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D.M., Wong K.Y., Shapiro P., Frederick C., Pattabiraman K., Berson D.M. 2008. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J. Neurophysiol. 99:2522–2532 10.1152/jn.01066.2007 [DOI] [PubMed] [Google Scholar]
- Guo D., Lu Z. 2000. Pore block versus intrinsic gating in the mechanism of inward rectification in strongly rectifying IRK1 channels. J. Gen. Physiol. 116:561–568 10.1085/jgp.116.4.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan J.W., Tabcharani J.A. 1990. Inhibition of an outwardly rectifying anion channel by HEPES and related buffers. J. Membr. Biol. 116:65–77 10.1007/BF01871673 [DOI] [PubMed] [Google Scholar]
- Hardie R.C. 1991. Whole-cell recordings of the light induced current in dissociated Drosophila photoreceptors: evidence for feedback by calcium permeating the light-sensitive channels. Proc. Biol. Sci. 245:203–210 10.1098/rspb.1991.0110 [DOI] [Google Scholar]
- Hardie R.C., Minke B. 1992. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 8:643–651 10.1016/0896-6273(92)90086-S [DOI] [PubMed] [Google Scholar]
- Holland L.Z., Laudet V., Schubert M. 2004. The chordate amphioxus: an emerging model organism for developmental biology. Cell. Mol. Life Sci. 61:2290–2308 10.1007/s00018-004-4075-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M., Terakita A. 2008. Gq-coupled rhodopsin subfamily composed of invertebrate visual pigment and melanopsin. Photochem. Photobiol. 84:1024–1030 10.1111/j.1751-1097.2008.00369.x [DOI] [PubMed] [Google Scholar]
- Koyanagi M., Kubokawa K., Tsukamoto H., Shichida Y., Terakita A. 2005. Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr. Biol. 15:1065–1069 10.1016/j.cub.2005.04.063 [DOI] [PubMed] [Google Scholar]
- Liu C.H., Wang T., Postma M., Obukhov A.G., Montell C., Hardie R.C. 2007. In vivo identification and manipulation of the Ca2+ selectivity filter in the Drosophila transient receptor potential channel. J. Neurosci. 27:604–615 10.1523/JNEUROSCI.4099-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin A.N., Makhina E.N., Nichols C.G. 1994. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 372:366–369 10.1038/372366a0 [DOI] [PubMed] [Google Scholar]
- Millecchia R., Mauro A. 1969. The ventral photoreceptor cells of Limulus. III. A voltage-clamp study. J. Gen. Physiol. 54:331–351 10.1085/jgp.54.3.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk P.D., Carne A., Liu S.H., Ford J.W., Keen J.N., Findlay J.B. 1996. Isolation, cloning, and characterisation of a trp homologue from squid (Loligo forbesi) photoreceptor membranes. J. Neurochem. 67:2227–2235 10.1046/j.1471-4159.1996.67062227.x [DOI] [PubMed] [Google Scholar]
- Montell C., Rubin G.M. 1989. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 2:1313–1323 10.1016/0896-6273(89)90069-X [DOI] [PubMed] [Google Scholar]
- Nasi E., Gomez M.P. 1999. Divalent cation interactions with light-dependent K channels. Kinetics of voltage-dependent block and requirement for an open pore. J. Gen. Physiol. 114:653–672 10.1085/jgp.114.5.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasi E., Gomez M., Payne R. 2000. Phototransduction mechanisms in microvillar and ciliary photoreceptors of invertebrates. Molecular Mechanisms in Visual Transduction. Handbook of Biological Physics, Volume 3. Hoff A.J., Stavenga D.G., de Grip W.J., Pugh E.N., Elsevier Science, Amsterdam: 389–448 [Google Scholar]
- Niemeyer B.A., Suzuki E., Scott K., Jalink K., Zuker C.S. 1996. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 85:651–659 10.1016/S0092-8674(00)81232-5 [DOI] [PubMed] [Google Scholar]
- Owsianik G., Talavera K., Voets T., Nilius B. 2006. Permeation and selectivity of TRP channels. Annu. Rev. Physiol. 68:685–717 10.1146/annurev.physiol.68.040204.101406 [DOI] [PubMed] [Google Scholar]
- Peirson S., Foster R.G. 2006. Melanopsin: another way of signaling light. Neuron. 49:331–339 10.1016/j.neuron.2006.01.006 [DOI] [PubMed] [Google Scholar]
- Phillips A.M., Bull A., Kelly L.E. 1992. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 8:631–642 10.1016/0896-6273(92)90085-R [DOI] [PubMed] [Google Scholar]
- Plachetzki D.C., Serb J.M., Oakley T.H. 2005. New insights into the evolutionary history of photoreceptor cells. Trends Ecol. Evol. (Amst.). 20:465–467 10.1016/j.tree.2005.07.001 [DOI] [PubMed] [Google Scholar]
- Provencio I., Jiang G., De Grip W.J., Hayes W.P., Rollag M.D. 1998. Melanopsin: An opsin in melanophores, brain, and eye. Proc. Natl. Acad. Sci. USA. 95:340–345 10.1073/pnas.95.1.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam N.H., Butts T., Ferrier D.E., Furlong R.F., Hellsten U., Kawashima T., Robinson-Rechavi M., Shoguchi E., Terry A., Yu J.K., et al. 2008. The amphioxus genome and the evolution of the chordate karyotype. Nature. 453:1064–1071 10.1038/nature06967 [DOI] [PubMed] [Google Scholar]
- Reuss H., Mojet M.H., Chyb S., Hardie R.C. 1997. In vivo analysis of the drosophila light-sensitive channels, TRP and TRPL. Neuron. 19:1249–1259 10.1016/S0896-6273(00)80416-X [DOI] [PubMed] [Google Scholar]
- Schubert M., Escriva H., Xavier-Neto J., Laudet V. 2006. Amphioxus and tunicates as evolutionary model systems. Trends Ecol. Evol. (Amst.). 21:269–277 10.1016/j.tree.2006.01.009 [DOI] [PubMed] [Google Scholar]
- Sekaran S., Lall G.S., Ralphs K.L., Wolstenholme A.J., Lucas R.J., Foster R.G., Hankins M.W. 2007. 2-Aminoethoxydiphenylborane is an acute inhibitor of directly photosensitive retinal ganglion cell activity in vitro and in vivo. J. Neurosci. 27:3981–3986 10.1523/JNEUROSCI.4716-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strübing C., Krapivinsky G., Krapivinsky L., Clapham D.E. 2001. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 29:645–655 10.1016/S0896-6273(01)00240-9 [DOI] [PubMed] [Google Scholar]
- Vandenberg C.A. 1987. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc. Natl. Acad. Sci. USA. 84:2560–2564 10.1073/pnas.84.8.2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennekens R., Hoenderop J.G.J., Prenen J., Stuiver M., Willems P.H.G.M., Droogmans G., Nilius B., Bindels R.J.M. 2000. Permeation and gating properties of the novel epithelial Ca2+ channel. J. Biol. Chem. 275:3963–3969 10.1074/jbc.275.6.3963 [DOI] [PubMed] [Google Scholar]
- Voets T., Janssens A., Prenen J., Droogmans G., Nilius B. 2003. Mg2+-dependent gating and strong inward rectification of the cation channel TRPV6. J. Gen. Physiol. 121:245–260 10.1085/jgp.20028752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren E.J., Allen C.N., Brown R.L., Robinson D.W. 2006. The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells. Eur. J. Neurosci. 23:2477–2487 10.1111/j.1460-9568.2006.04777.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D., Suzuki N. 1987. Blockage of chloride channels by HEPES buffer. Proc. R. Soc. Lond. B Biol. Sci. 230:93–100 10.1098/rspb.1987.0011 [DOI] [PubMed] [Google Scholar]