In the 1934 Dixon lectures, Sir Henry Dale articulated that the chemical nature of each neuron is fixed and unchangeable (Dale, 1935). John Eccles elevated these observations to a principle in 1954 when he and colleagues postulated that “the same chemical transmitting substance is used at all junctions operated by a particular cell” (Eccles et al., 1954). Dale’s Principle had thus been interpreted to mean that each neuron used a single neurotransmitter, although Eccles later suggested that multiple substances may be released (Eccles, 1976). The pioneering work of Dale’s gave way to current models of multi-neurotransmitter modulation and co-release of neurotransmitters. Specifically in the striatum, Kocsis and Kitai (1977) demonstrated that stimulation of midbrain neurons produce a dual excitatory EPSP comprised of a fast and slow component, suggesting the involvement of glutamate and dopamine in this system. A recent study provided evidence of cholinergic and glutamatergic co-transmission from medial habenula (Ren et al., 2011). The medial habenula projects to the interpeduncular nucleus and is involved in aversive states such as pain or nicotine withdrawal (Salas et al., 2009). Photostimulation of habenular neurons expressing channelrhodopsin under control of the ChAT promoter produced both slow nicotinic currents and rapid glutamatergic currents (Ren et al., 2011). Other recent studies demonstrated glutamatergic co-release from dopaminergic and serotonergic cells (Chuhma et al., 2004; Varga et al., 2009; Hnasko et al., 2010), suggesting that co-release may be a general mechanism used in modulatory neurons to increase their repertoire of neurotransmitter actions.
The principal projection neuron in both the ventral and dorsal striatum is the GABAergic medium spiny neuron, the postsynaptic target of convergent input from distal glutamatergic and midbrain dopaminergic neurons. Midbrain dopamine neurons very densely innervate the striatum where the released dopamine, among other things, modulates excitatory glutamatergic transmission in this region. In this Journal Club article, I review the contribution of Stuber et al. (2010) to striatal circuitry. Here, they reported that midbrain dopaminergic neurons that project to the nucleus accumbens (NAc) shell co-release glutamate, but those that project to the dorsal striatum only release dopamine. I discuss how this may contribute to physiological and functional differences in these two regions. I propose that models of medium spiny neuron function must now consider how neuronal co-release of dopamine and glutamate influences synaptic transmission and plasticity in the NAc, and how this may influence behavior.
Key results: Conditional gene deletion and optogenetic control over neuromodulatory neurons
Accumulating evidence over the last 13 years indicates that dopaminergic neurons may also release glutamate. In single-cell cultures, dopaminergic neurons were shown to release glutamate (Sulzer et al., 1998), and in brain slices, stimulation of mesolimbic dopamine neurons elicited an excitatory postsynaptic potential in striatal neurons (Chuhma et al., 2004). Light and electron microscope studies provided evidence that the vesicular glutamate transporter VGLUT2, a regulator of extracellular glutamate levels and definitive marker of glutamatergic neurons, is expressed in the shell of the NAc (Hur et al., 2009). Using conditional genetic approaches to knock out VGLUT2 specifically in dopamine neurons, Hnasko et al. (2010) demonstrated that dopamine neurons could directly excite striatal neurons through glutamate release. Further, they demonstrated in human embryonic kidney cells that entry of glutamate via VGLUT2 acidifies vesicles and could in this non-neuronal system promote loading of dopamine into the same vesicles. Fig. 1 A represents the model of dopamine/glutamate co-transmission released from the same individual vesicles of ventral tegmental area (VTA) neurons. The work of Hnasko et al. (2010) was open to interpretation because they used electrical stimulation in the midbrain to elevate dopamine levels in the striatum. Because a population of VTA neurons expresses VGLUT2 but does not express tyrosine hydroxylase (TH), the rate-limiting enzyme that produces dopamine (Dobi et al., 2010), nonspecific excitation of all midbrain neurons by electrical stimulation precluded parsimonious interpretation.
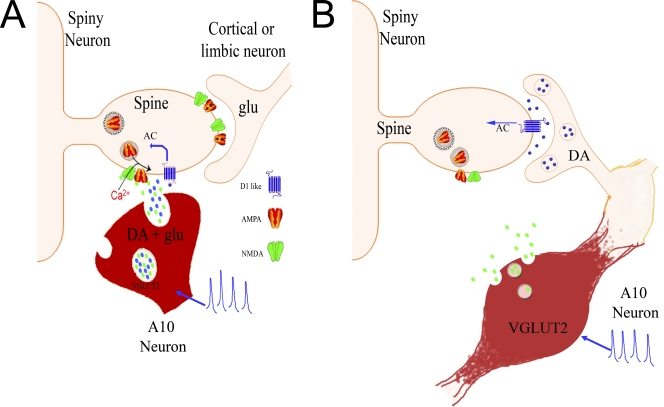
Figure 1.
Two models of dopamine/glutamate co-transmission. Glutamate is green, dopamine is blue, and VGLUT2 in A10 neurons is labeled red. Bursting activity of mesolimbic dopaminergic neurons has been shown to activate AMPA and NMDA receptors on medium spiny neurons (as shown in Tecuapetla et al., 2010), possibly promoting the insertion of AMPA receptors, whereas activation of D1-like receptors promotes intracellular postsynaptic changes through G-coupled activation of adenylyl cyclase (AC). (A) Co-transmission from the same presynaptic terminal. Evidence from Hnasko et al. (2010) indicates that VGLUT2 may promote loading of dopamine into vesicles. This is evidence that glutamate and dopamine are packaged and released from the same vesicle at all structures. (B) Co-transmission by the same neuron at separate structures. Although medial mesolimbic neurons express both TH and VGLUT2 mRNA, NAc sections show VGLUT2+ staining sequestered in varicosities sprouting from the axons of TH+ neurons (Kawano et al., 2006; Yamaguchi et al., 2011). This suggests that the same neuron releases different neurotransmitters at different structures. Either model may explain the physiological results now presented by three studies (Hnasko et al., 2010; Stuber et al., 2010; Tecuapetla et al., 2010).
Two articles from the summer of 2010 used optogenetics to specifically stimulate TH+ neurons, both producing glutamatergic postsynaptic responses that were restricted to the NAc. The first was by Tecuapetla et al. (2010), where they used an adeno-associated viral vector to deliver floxed channelrhodopsin DNA to allow for specific expression in neurons that have both TH+ and Cre. They used a fiber optic to stimulate the infected sliced tissue with light and recorded fast glutamatergic responses from medium spiny neurons. Further, they determined that burst activation at biologically relevant times produced temporal summation of NMDA currents in the NAc (Tecuapetla et al., 2010). The second was an elegant and key study that adds the final direct evidence that dopamine and glutamate are coming from the same VTA neurons. Stuber et al. (2010) used conditional gene disruption of VGLUT2 to determine whether glutamate is co-released from midbrain dopamine neurons of adult mice. To perform these experiments, mice carried one copy of cre recombinase driven by dopamine transporter elements and either one or two conditional VGLUT2 alleles. An adenovirus containing mcherry channelrhodopsin 2 (ChR2) was then injected into the VTA of these mice. Confocal microscopy confirmed that nearly all VTA neurons expressing mcherry (and therefore ChR2) also expressed TH. Coronal slices from the striatum were cut from control VGLUT2+/− mice or experimental VGLUT2−/− mice. Whole cell voltage-clamp recordings were then made from medium spiny neurons in either the NAc shell or dorsal striatum. A laser was placed 200 µm from the recording site to specifically stimulate terminals from mesolimbic dopaminergic neurons, and the extracellular postsynaptic currents were measured using whole cell voltage-clamped recordings. Optical stimulation of VGLUT2+/−, but not VGLUT2−/−, expressing dopamine terminals produced brief excitatory currents in medial spiny neurons that are blocked by glutamate receptor antagonists, unequivocally demonstrating that midbrain dopamine neurons projecting to the NAc shell can functionally co-release glutamate.
Stuber et al. (2010) further demonstrated that optogenetic stimulation elicited a strong dopamine signal in the dorsal striatum without a corresponding glutamatergic signal. This is consistent with the anatomical data from Kawano et al. (2006), showing only rare coexpression of VGLUT2 with TH+ neurons from the substantia nigra, the dopamine neurons that project to the dorsal striatum. Although this contradicts the physiological evidence from Kocsis and Kitai (1977), suggesting that glutamatergic and dopaminergic co-release in the dorsal striatum, the electrical stimulation in this early study may have directly or indirectly recruited a separate population of glutamatergic neurons.
The physiological evidence of glutamate/dopamine co-release in the NAc is seemingly contradicted by ultrastructural evidence derived from electron microscope studies that have shown that VGLUT2 is highly coexpressed in juvenile mesencephalic TH+ neurons projecting to the NAc (Dal Bo et al., 2008), yet expression regresses in the mature rat brain (Bérubé-Carrière et al., 2009). Further, Moss et al. (2011) demonstrated essentially no evidence of coexpression of VGLUT2 and TH in the adult NAc. However, the Yamaguchi et al. (2011) showed that four populations of mature mesencephalic neurons project to the dorsal striatum and the NAc: TH+ only, VGLUT2+ only, TH+/VGLUT2+ neurons, and a small subset of TH−/VGLUT2− neurons. Specifically, both the Hisano (Kawano et al., 2006) and Morales laboratories (Yamaguchi et al., 2011) have demonstrated that populations of neurons in the medial intrafascicular nucleus project to the NAc and either express TH only or TH/VGLUT2. The Hisano laboratory has also shown that VGLUT2 seems to be localized in varicosities along TH-stained axons, but very few release sites coexpress TH and VGLUT2 (Kawano et al., 2006). This evidence may explain why neurons with mRNA for VGLUT2 and TH fail to show ultrastructural evidence of coexpression at the synapse; VGLUT2/TH-expressing neurons may form separate asymmetric dopaminergic and symmetric glutamatergic processes, as has been demonstrated in cell culture (Sulzer et al., 1998). The model of neuronal, but not structure-specific, co-transmission in Fig. 1 B may reconcile the physiological results from optogenetic studies with the anatomical results, but in this model, one cannot assume that the biochemical content at any one axonal structure will be found at other structures.
Interpretation: How glutamate co-release might influence synaptic plasticity in the striatum
Coincident and convergent input often induces plasticity on a postsynaptic neuron. The NAc integrates processed information about the environment from basolateral amygdala, hippocampus, and prefrontal cortex (PFC), as well as projections from midbrain dopamine neurons. Previous studies have demonstrated how dopamine modulates this integrative process. For example, high frequency stimulation potentiates hippocampal inputs to the NAc while simultaneously depressing PFC synapses (Goto and Grace, 2005). The converse was also shown to be true; stimulation at PFC potentiates PFC–NAc synapses but depresses hippocampal–NAc synapses. In light of the new functional evidence of midbrain dopamine/glutamate co-transmission (references above), new experiments of NAc function will have to test whether midbrain glutamatergic inputs bias or filter either limbic or cortical inputs to guide goal-directed behavior.
Glutamate co-release from mesolimbic dopamine neurons may also influence these same neurons through presynaptic AMPA and NMDA receptors. Glutamate released may spill out from the synaptic cleft and bind to these receptors on dopamine neurons, modulating plasticity of dopaminergic terminals projecting to the NAc. Although the importance of glutamatergic receptors to physiological properties of the VTA is well documented (Zweifel et al., 2008), the specific influence of presynaptic glutamatergic neurotransmission on the axonal arbors of VTA neurons is relatively unknown, yet may be a fruitful line of inquiry for future studies.
Another key question that needs to be explored is how specific synapses in the NAc are modulated by burst firing of action potentials from mesolimbic dopamine neurons. Upon the presentation of unexpected rewards, midbrain dopaminergic neurons transition from slow tonic firing to burst firing (Schultz, 1998). Burst firing of midbrain dopaminergic neurons elevate striatal dopamine levels, and this is exploited by addictive drugs such as nicotine (Zhang et al., 2009). The burst firing of mesolimbic dopamine neurons may serve to potentiate the NAc medium spiny neurons through the ionotropic release of glutamate, whereas dopamine-triggered intracellular cascades could influence long-term plasticity. Tecuapetla et al. (2010) applied light pulses to channelrhodopsin-bearing TH+ neurons at burst frequencies and found that glutamatergic currents onto medium spiny neurons were depressed at the end of the burst, but that NMDA currents exhibited temporal summation. This NMDA current may potentiate plastic changes in the striatum. Using the conditional gene disruption and channelrhodopsin techniques highlighted in Stuber et al. (2010), one can specifically test how burst firing of midbrain dopamine neurons with or without VGLUT2 modifies striatal plasticity by measuring relative changes in the AMPA and NMDA currents using a tonic stimulation after a burst.
Concluding remarks
The neurophysiological evidence of co-transmission of glutamate and dopamine at the NAc is significant because glutamatergic currents may transmit transient reward-related activity and, coincident with increases in extracellular dopamine, may potentiate this circuit. Further, these results imply that Eccles’s version of Dale’s Principle of one neurotransmitter used by each neuron still holds true in some, but not all, neuromodulatory projections. Several exceptions to Eccles’s version of Dale’s Principle are currently known; it is currently no longer controversial to consider this formulation obsolete (Nicoll and Malenka, 1998). However, the recent anatomical evidence indicating that VGLUT2 (and therefore glutamate) is found on separate structures sprouting from DA neurons (Kawano et al., 2006; Yamaguchi et al., 2011) suggests a break from the original formulation of Dale’s Principle. That is, knowing the biochemical contents of any single axonal structure is not an absolute indicator for all structures in that axon. As more precise neurotransmitter markers (such as VGLUT2) and more sophisticated genetic approaches become more available, Dale’s Principle will likely yield to a more diverse and heterogeneous taxonomy of neurotransmitter release.
Please participate in a discussion of this Journal Club article on the JGP Facebook page (www.facebook.com/JGenPhysiol).
Acknowledgments
I would like to thank John Dani for his critical reading and thoughtful suggestions regarding this manuscript.
J.I. Broussard acknowledges the joint participation by Diana Helis Henry Medical Research Foundation through its direct engagement in the continuous active conduct of medical research in conjunction with Baylor College of Medicine and the “Dopamine Signaling Dysfunction Precedes and Predicts Neuron Loss in an Animal Model of PD Program.”
John Dani served as faculty advisor.
Angus C. Nairn served as editor.
Footnotes
Abbreviations used in this paper:
- NAc
- nucleus accumbens
- PFC
- prefrontal cortex
- TH
- tyrosine hydroxylase
- VTA
- ventral tegmental area
References
- Bérubé-Carrière N., Riad M., Dal Bo G., Lévesque D., Trudeau L.E., Descarries L. 2009. The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J. Comp. Neurol. 517:873–891 10.1002/cne.22194 [DOI] [PubMed] [Google Scholar]
- Chuhma N., Zhang H., Masson J., Zhuang X., Sulzer D., Hen R., Rayport S. 2004. Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. J. Neurosci. 24:972–981 10.1523/JNEUROSCI.4317-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Bo G., Bérubé-Carrière N., Mendez J.A., Leo D., Riad M., Descarries L., Lévesque D., Trudeau L.E. 2008. Enhanced glutamatergic phenotype of mesencephalic dopamine neurons after neonatal 6-hydroxydopamine lesion. Neuroscience. 156:59–70 10.1016/j.neuroscience.2008.07.032 [DOI] [PubMed] [Google Scholar]
- Dale H. 1935. Pharmacology and nerve-endings (Walter Ernest Dixon Memorial Lecture): (Section of Therapeutics and Pharmacology). Proc. R. Soc. Med. 28:319–332 [PMC free article] [PubMed] [Google Scholar]
- Dobi A., Margolis E.B., Wang H.L., Harvey B.K., Morales M. 2010. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J. Neurosci. 30:218–229 10.1523/JNEUROSCI.3884-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. 1976. From electrical to chemical transmission in the central nervous system. Notes Rec. R. Soc. Lond. 30:219–230 10.1098/rsnr.1976.0015 [DOI] [PubMed] [Google Scholar]
- Eccles J.C., Fatt P., Koketsu K. 1954. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J. Physiol. 126:524–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Grace A.A. 2005. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat. Neurosci. 8:805–812 10.1038/nn1471 [DOI] [PubMed] [Google Scholar]
- Hnasko T.S., Chuhma N., Zhang H., Goh G.Y., Sulzer D., Palmiter R.D., Rayport S., Edwards R.H. 2010. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 65:643–656 10.1016/j.neuron.2010.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur E.E., Edwards R.H., Rommer E., Zaborszky L. 2009. Vesicular glutamate transporter 1 and vesicular glutamate transporter 2 synapses on cholinergic neurons in the sublenticular gray of the rat basal forebrain: a double-label electron microscopic study. Neuroscience. 164:1721–1731 10.1016/j.neuroscience.2009.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano M., Kawasaki A., Sakata-Haga H., Fukui Y., Kawano H., Nogami H., Hisano S. 2006. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J. Comp. Neurol. 498:581–592 10.1002/cne.21054 [DOI] [PubMed] [Google Scholar]
- Kocsis J.D., Kitai S.T. 1977. Dual excitatory inputs to caudate spiny neurons from substantia nigra stimulation. Brain Res. 138:271–283 10.1016/0006-8993(77)90745-4 [DOI] [PubMed] [Google Scholar]
- Moss J., Ungless M.A., Bolam J.P. 2011. Dopaminergic axons in different divisions of the adult rat striatal complex do not express vesicular glutamate transporters. Eur. J. Neurosci. 33:1205–1211 10.1111/j.1460-9568.2011.07594.x [DOI] [PubMed] [Google Scholar]
- Nicoll R.A., Malenka R.C. 1998. A tale of two transmitters. Science. 281:360–361 10.1126/science.281.5375.360 [DOI] [PubMed] [Google Scholar]
- Ren J., Qin C., Hu F., Tan J., Qiu L., Zhao S., Feng G., Luo M. 2011. Habenula “cholinergic” neurons co-release glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron. 69:445–452 10.1016/j.neuron.2010.12.038 [DOI] [PubMed] [Google Scholar]
- Salas R., Sturm R., Boulter J., De Biasi M. 2009. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J. Neurosci. 29:3014–3018 10.1523/JNEUROSCI.4934-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. 1998. Predictive reward signal of dopamine neurons. J. Neurophysiol. 80:1–27 [DOI] [PubMed] [Google Scholar]
- Stuber G.D., Hnasko T.S., Britt J.P., Edwards R.H., Bonci A. 2010. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J. Neurosci. 30:8229–8233 10.1523/JNEUROSCI.1754-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D., Joyce M.P., Lin L., Geldwert D., Haber S.N., Hattori T., Rayport S. 1998. Dopamine neurons make glutamatergic synapses in vitro. J. Neurosci. 18:4588–4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F., Patel J.C., Xenias H., English D., Tadros I., Shah F., Berlin J., Deisseroth K., Rice M.E., Tepper J.M., Koos T. 2010. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J. Neurosci. 30:7105–7110 10.1523/JNEUROSCI.0265-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga V., Losonczy A., Zemelman B.V., Borhegyi Z., Nyiri G., Domonkos A., Hangya B., Holderith N., Magee J.C., Freund T.F. 2009. Fast synaptic subcortical control of hippocampal circuits. Science. 326:449–453 10.1126/science.1178307 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Wang H.L., Li X., Ng T.H., Morales M. 2011. Mesocorticolimbic glutamatergic pathway. J. Neurosci. 31:8476–8490 10.1523/JNEUROSCI.1598-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Zhang L., Liang Y., Siapas A.G., Zhou F.M., Dani J.A. 2009. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J. Neurosci. 29:4035–4043 10.1523/JNEUROSCI.0261-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel L.S., Argilli E., Bonci A., Palmiter R.D. 2008. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron. 59:486–496 10.1016/j.neuron.2008.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]