Abstract
Autism is a heterogeneous disorder with multiple behavioral and biological phenotypes. Accelerated brain growth during early childhood is a well-established biological feature of autism. Onset pattern, i.e., early onset or regressive, is an intensely studied behavioral phenotype of autism. There is currently little known, however, about whether, or how, onset status maps onto the abnormal brain growth. We examined the relationship between total brain volume and onset status in a large sample of 2- to 4-y-old boys and girls with autism spectrum disorder (ASD) [n = 53, no regression (nREG); n = 61, regression (REG)] and a comparison group of age-matched typically developing controls (n = 66). We also examined retrospective head circumference measurements from birth through 18 mo of age. We found that abnormal brain enlargement was most commonly found in boys with regressive autism. Brain size in boys without regression did not differ from controls. Retrospective head circumference measurements indicate that head circumference in boys with regressive autism is normal at birth but diverges from the other groups around 4–6 mo of age. There were no differences in brain size in girls with autism (n = 22, ASD; n = 24, controls). These results suggest that there may be distinct neural phenotypes associated with different onsets of autism. For boys with regressive autism, divergence in brain size occurs well before loss of skills is commonly reported. Thus, rapid head growth may be a risk factor for regressive autism.
Keywords: MRI, neurodevelopment, trajectory, macrocephaly
Autism is a neurodevelopmental disorder with hallmark deficits in social interaction and communication, with restricted interests and repetitive behaviors (1). It is a behaviorally defined disorder that is typically diagnosed during early childhood. The prevalence of autism spectrum disorders (ASD) in the United States is estimated to be 1 in 110 children (2). Autism is diagnosed more frequently in males than females at a ratio of 4–1. Current research suggests that autism is a heterogeneous disorder (3, 4), with a broad range of severity and intellectual ability as well as a variety of comorbid conditions, such as epilepsy, anxiety, and gastrointestinal conditions (5, 6). The heterogeneity of this disorder is one of the major roadblocks to establishing etiologies that could then lead to more effective prevention and intervention. In the context of an ongoing, multidisciplinary effort to establish distinct autism phenotypes (the Autism Phenome Project, APP), we have examined the relationship between a behavioral feature of autism, onset status, and a commonly reported biological feature of autism, accelerated head growth and abnormal brain enlargement.
There is now ample evidence suggesting that brain growth in children with autism is accelerated, leading to an abnormally enlarged brain in early childhood (7). Studies using retrospective head circumference measurements as a proxy for brain size suggest that whereas children with autism are born with normal or slightly smaller brain size, the trajectory of growth accelerates during the first year of life (8–10). Several MRI studies of very young children with autism report a 5–10% abnormal enlargement in total brain volume that persists into early childhood (11–13).
An altered trajectory of brain growth is now widely cited as central to the neuropathology of autism (3). However, several additional questions remain to be explored. Little is known about how generalized the finding of brain enlargement is across all individuals with autism. Both microcephaly and macrocephaly have been reported in autism (14), and increased variability in head size is also observed (15). Does brain enlargement occur in the majority of individuals with autism or does it occur in just a subset of individuals? Are there any clinical correlates to brain enlargement? Also, studies of typical development have documented sex differences in the developing brain (16, 17). However, there is very little known about whether brain development in children with autism is sexually dimorphic as well.
To address these issues, we initiated a large-scale, multidisciplinary study, the APP, to explore potential biological and behavioral phenotypes in autism. The goal of the APP is to enroll a large sample of children and to carry out a comprehensive longitudinal analysis to begin to identify more homogeneous subgroups or phenotypes within the autism spectrum disorder population. Extensive behavioral and biological data are collected on all participants to correlate changes in brain growth with other biological and behavioral facets of autism. One example of a behavioral phenotype in autism is onset status. Whereas some children exhibit symptoms of autism in the first year of life, others experience a regression or loss of previously acquired skills in language and/or social domains. Although there are a number of complexities in determining onset status (18), children with autism can be characterized as regression (REG) or no regression (nREG), using parent report of early development. The neural underpinnings of onset status remain unclear because only one study has yet examined this question, in a small sample (19).
We have studied the relationship between brain size and autism onset status. We hypothesized that the classification of either regression or no regression may map onto particular trajectories of brain growth and brain size. Specifically, we evaluated total cerebral volume (TCV) using magnetic resonance imaging at age 3 y and retrospective head circumference measurements from birth through 18 mo of age. These data allowed us to produce brain growth trajectories in a large sample of children with autism relative to age-matched typically developing controls. Our sample size of 180 subjects allowed us to analyze sex differences in brain size and growth trajectories related to onset status as well.
Results
Participant characteristics are presented in Table 1. A total of 180 children, 2- to 4 y of age, participated in this study, 114 with ASD (101, autistic disorder; 11, pervasive developmental disorder-not otherwise specified) (PDD-NOS) and 66 age-matched typically developing (TD) controls. Of the children with ASD, 54% had ASD-REG and 46% ASD-nREG. There were no significant differences in age between any of the groups. There were also no sex differences in autism severity or developmental quotient (DQ). As expected, DQ was significantly higher in TD controls than children with ASD (P < 0.01). There was no significant difference between the ASD-REG and ASD-nREG groups on the Autism Diagnostic Observation Schedule-Generic (ADOS-G) severity score, which allows for comparison of autism severity across participants tested with different ADOS-G modules (P > 0.10). There was a marginally significant difference in DQ between the two groups of children with different onset status (P = 0.05). Groups were matched on gestational age, ethnicity, and socioeconomic status.
Table 1.
Participant characteristics
| TD | ASD-nREG | ASD-REG | |
| N (male/female) | 66 (42/24) | 53 (41/12) | 61 (51/10) |
| Age, mo | 36.3 (4.9) | 35.7 (5.4) | 37.7 (5.3) |
| Range, mo | 26.8–46.2 | 25.7–48.1 | 25.9–46.5 |
| ADOS severity | — | 7.4 (1.8) | 7.9 (1.7) |
| DQ | 104.8 (11) | 66.7 (22) | 59.1 (18) |
ASD-nREG, autism with no regression; ASD-REG, regressive autism; TD, typical development; ADOS, autism diagnostic observation schedule; DQ, development quotient. Age, ADOS severity score, and DQ: mean (SD).
Total Cerebral Volume.
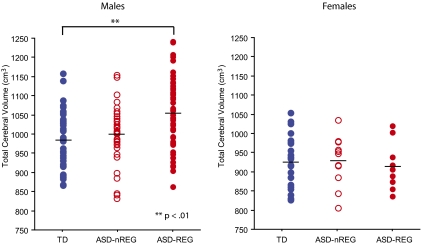
Table 2 provides results and effect sizes from our cross-sectional analysis of TCV. Fig. 1 depicts group differences in TCV for males and females separately. Volumetric differences between ASD-nREG, ASD-REG, and TD groups were analyzed using ANCOVA for males and females separately, as well as combined in a single model with sex as a covariate. The models assign separable variability to each covariate to avoid mistaking contributions from a secondary covariate (e.g., age) as arising from the covariate of interest (group), thus providing more accurate and precise estimates of true effects. Covariates analyzed include age, body mass index (BMI), DQ, age × group, and group × sex interactions (for the combined sex model). We report effect sizes equal to the t statistics that provide the P values, where each is equal to the magnitude of the effect divided by its SE from the model. Roughly, any effect size greater than 2 may be considered large, because its significance value is P = 0.05 or smaller. In males, TCV increased by 8.6 cm3 (0.8%) per month. In females, TCV increased by 6.1 cm3 (0.6%) per month. TCV was unrelated to BMI and DQ.
Table 2.
ANCOVA results for total cerebral volume
| Males |
Females |
|||||
| 984.7 (72.1) |
923.2 (73.5) |
|||||
| TD volume (cm3) | Difference (%) | Effect Size | P value | Difference (%) | Effect size | P value |
| ASD-nREG | 17.9 (1.8) | 1.06 | NS | 1.2 (0.1) | 0.05 | NS |
| ASD-REG | 63.4 (6.2) | 4.01 | 0.0001 | −2.4 (−0.2) | 0.09 | NS |
See Table 1 legend for definitions. TD volume, mean (SD). Difference is cm3 from TD mean. Covariates include age, body mass index, and DQ.
Fig. 1.
Total cerebral volume (TCV) is enlarged in males with regressive autism (ASD-REG) relative to typical development (TD) (ANCOVA with group, age and Fisher post hoc corrections). TCV in the autism without regression group (ASD-nREG) did not differ from TD. There were no group differences for the females. Horizontal lines represent mean TCV for each group.
In males, relative to TD controls, the ASD-REG group had a significant increase in TCV of 63.4 cm3 (6.2%), whereas the ASD-nREG group did not differ from TD controls (Fig. 1). There were no age × group interactions. In females, there were no group differences nor any age × group interactions (Fig. 1). Results of the combined model confirmed those of sex-stratified models.
As a secondary analysis, we also examined the rate of megalencephaly in ASD-REG and ASD-nREG groups relative to the TD controls in males. We defined megalencephaly as TCV greater than 2 SDs above the TD mean (male TD control mean 984.7; SD 72). Out of 51 males in the ASD-REG group, 22% had megalencephaly. Out of 41 males in the ASD-nREG group, 5% had megalencephaly.
Finally, we also explored the relationship between TCV and autism severity. In children with ASD, there was no correlation between TCV and ADOS severity score (r = 0.05).
Head Circumference, Birth Through 18 Mo.
Cross-sectional analysis.
Retrospective head circumference measurements were available from a subset of 120 subjects (n = 42 TD, n = 34 ASD-nREG, n = 44 ASD-REG). A total of 717 measurements were obtained from birth through 18 mo of age. Head circumference measurements from TD participants fell within the interquartile ranges of the World Health Organization (WHO) Child Growth Standards at each time point. Thus, this TD sample is representative of the general population, and all ASD group comparisons were evaluated relative to the current TD sample.
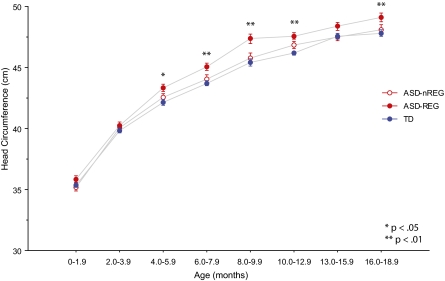
Fig. 2 depicts cross-sectional head circumference measurements at various age intervals from birth through 18 mo. Table 3 provides results and effect sizes from our cross-sectional ANCOVA analyses including age, sex, and group as covariates at 2- to 3-mo increments. As anticipated, sex had a strong effect on head circumference. Head circumference measurements in males were, on average, between 2.4–3.7% larger than female head circumference across this age span.
Fig. 2.
Cross-sectional analysis of head circumference measurements from birth through 18 mo of life. Groups do not differ from birth through 4 mo of age. The ASD-REG group diverges from the ASD-nREG and TD groups around 4–6 mo of age.
Table 3.
Cross-sectional differences in head circumference (cm) from typical development adjusted for age, group, and sex
| Age, mo | 0.0–1.9 | 2.0–3.9 | 4.0–5.9 | 6.0–7.9 | 8.0–9.9 | 10.0–12.9 | 13.0–15.9 | 16.0–18.9 |
| ASD-nREG cm | — | — | — | — | — | 0.72 | — | 2.02 |
| % | — | — | — | — | — | 1.55 | — | 4.32 |
| Effect size | — | — | — | — | — | 1.82 | — | 1.56 |
| P value* | NS** | NS | NS | NS | NS | 0.0363 | NS | 0.0611 |
| ASD-REG cm | — | — | 0.856 | 1.035 | 1.452 | 1.10 | 0.54 | 2.46 |
| % | — | — | 2.02 | 2.35 | 3.15 | 2.37 | 2.86 | 5.27 |
| Effect size | — | — | 2.35 | 3.03 | 2.96 | 3.58 | 1.45 | 2.80 |
| P value* | NS | NS | 0.023 | 0.003 | 0.005 | 0.0006 | 0.154 | 0.007 |
| Sample size TD | 87 | 34 | 31 | 30 | 14 | 32 | 25 | 14 |
| ASD-nREG | 64 | 21 | 19 | 17 | 18 | 19 | 20 | 18 |
| ASD-REG | 77 | 31 | 29 | 27 | 18 | 29 | 18 | 18 |
ASD-EO, early onset autism. For other definitions, see Table 1 legend. *Uncorrected, two-sided; **NS, P > 0.20.
After isolating sex differences to focus on group and age effects only, the model showed a pronounced increase in cross-sectional head circumference in the ASD-REG group relative to TD controls beginning at 4.0–5.9 mo of age that persisted through 18.9 mo of age. The ASD-REG group had an average head circumference enlargement of 2–3% above TD controls from 4 to 18.9 mo (except during 13–15.9 mo), reaching a difference of 5.3% between 16 and 18.9 mo. Head circumference in the ASD-nREG group did not differ from TD controls, except a modest 1.5% difference during the 10- to 14-mo cross-sectional interval.
Longitudinal analysis.
We examined individual longitudinal changes in head circumference between birth and 18 mo of age in a subset of children having at least three head circumference measurements (n = 95; 77 male, 18 female). Due to the paucity of repeated measurements in females, we restricted these analyses to males (ASD-nREG n = 26, ASD-REG n = 19, TD n = 32).
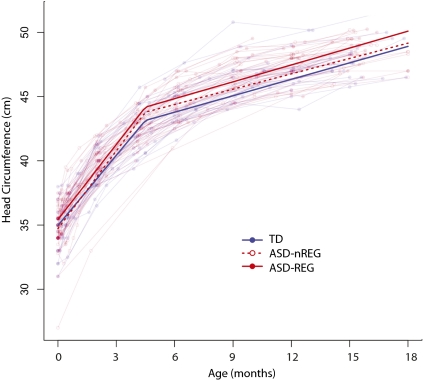
Fig. 3 depicts individual changes in head circumference over time (shaded lines) and results from a piecewise linear longitudinal model fit to each clinical group (bold lines). Table 4 provides the results of the comprehensive model including all groups. On the basis of preceding cross-sectional analyses suggesting that ASD-REG group divergence begins between 4 and 6 mo of age, we examined age- and group-dependent longitudinal head circumference growth before and after 4.5 mo of age.
Fig. 3.
Longitudinal head circumference growth in males. Lighter lines represent the growth trajectories of the brains of individuals in this study. Bold lines indicate the piecewise linear longitudinal model fit to each clinical group separately. Results from the longitudinal analysis confirm that the ASD-REG group is larger than the TD and ASD-nREG groups.
Table 4.
Results of longitudinal mixed-effects model for male head circumference
| TD (mean ± SD) cm |
||||
| At birth | At 4.0–5.0 mo | |||
| 34.6 ± 1.8 |
42.8 ± 1.4 |
|||
| Covariate | Effect | SE | P value | |
| ASD-nREG (cm) | 0.57 | 0.37 | 0.128 | |
| ASD-REG (cm) | 1.13 | 0.32 | 0.001 | |
| Age < 4.5 mo (cm/mo) | 1.80 | 0.05 | <0.001 | |
| Age ≥ 4.5 mo (cm/mo) | 0.44 | 0.02 | <0.001 | |
| ASD-nREG x age (<4.5 mo) | 0.21 | 0.07 | 0.003 | |
| ASD-REG x age (<4.5 mo) | 0.14 | 0.06 | 0.021 | |
See Table 1 legend for definitions.
As indicated in Table 4, age is a highly significant predictor of head circumference. Age acquires more predictive importance when group differences are taken into account. We found an average increase in head circumference of 1.80 cm per month in all subjects during the first 4.5 mo of life (P < 0.001), followed by more gradual increase of 0.44 cm per month thereafter (P < 0.001). Average head circumference in the ASD-REG group was 1.13 cm larger than that of the TD group (P < 0.001) across the entire age range. We found that the rates of growth relative to TD differed slightly between the ASD groups before 4.5 mo, with 0.21 cm/month in the ASD-nREG group (P = 0.003) and 0.14 cm per month in the ASD-REG group (P = 0.021). The age × group interaction was not significant after 4.5 mo of age.
Discussion
The results presented in this report indicate that abnormal brain enlargement is not generalized across all individuals with autism. Accelerated head growth and brain enlargement was most consistently observed in the subset of children who had regressive autism. Specifically, total brain volume in 3-y-old males with regressive autism was ∼6% larger than that of age-matched typically developing controls. Indeed, 22% of boys with regressive autism had megalencephaly, whereas only 5% of boys without regression had megalencephaly. When did this abnormal enlargement occur? Analysis of head circumference data, which is a reliable proxy for total brain volume in young children (20), indicates that the divergence in head size began around 4–6 mo of age. Brain size and growth trajectory of children without regression did not differ from typically developing controls. Moreover, brain enlargement was not observed in girls with ASD, regardless of autism onset status. These findings provide suggestive evidence that the biological underpinnings of early onset and regressive forms of autism are different.
Behavioral Correlates of Abnormal Brain Enlargement.
Although there is ample evidence for an altered trajectory of brain growth during the first years of life (7), relatively little is known about the behavioral correlates of this altered trajectory. There is some evidence that increased head circumference is associated with increased autism severity and that macrocephaly may be associated with a delay in the onset of language (15), but clear and consistent associations have not been reported. MRI studies of brain enlargement in young children also have not shown any clear correlations with autism severity (12).
In the current study, we found an association between brain enlargement and regressive autism. The rate of regression in our sample of children with autism was 54%, which is similar to that of recent large-scale population-based studies (21, 22). Our head circumference findings are consistent with the notion that acceleration of head growth precedes onset of behavioral symptoms (10). We observed an increase in rate of head growth in children with regressive autism as early as 4–6 mo of age. By 18 mo of age, head size was 5% larger in children with regressive autism than typically developing controls. At age 3, total brain volume remained about 6% larger. Thus, accelerated growth appears to have taken place during the first 18 mo and thereafter the rate of further growth came in line with that of typically developing children. Importantly, these differences in brain size were observed even after controlling for age, sex, body mass index, and DQ.
To our knowledge, onset status has not been investigated in relationship to brain volume in previously published MRI studies of autism. One head circumference study of 28 males with autism (11 with regression) did not report an association between onset status and rate of head growth in the first year of life (19). It is likely that the substantially larger sample size and retrospective longitudinal data in the present study provided greater statistical power to detect differences in the pattern of head growth.
Brain Development in Girls with Autism.
Very little is known about the neuropathology of autism in females. There is some preliminary evidence suggesting that girls have similar (23) and possibly even more pronounced volumetric differences than boys (13, 24, 25). However, sample sizes in these studies are quite small, with fewer than 10 girls with autism in each study. The sample size of girls in the current study is the largest reported to date (n = 22 ASD, 24 TD). Interestingly, we found no differences in growth trajectory or brain size from birth to 3 y of age regardless of onset status. Clearly, additional studies with even larger sample sizes are needed to elucidate the neuropathology of autism in females. On the basis of available literature and results from the current study, it is likely that the pattern of pathology is different in females than in males.
Limitations.
One limitation of the present study is the sole reliance on parent report for establishing onset status. Recent papers have pointed out significant complexities in defining and measuring the onset of autism symptoms (18). Studies have demonstrated relatively low correspondence between parent report of onset and home videotape evidence of symptom trajectories (26), with one study showing that 45% of participants clearly demonstrated a regression on videotape that was not reported by parents (27). Similarly, a recent prospective study found that regression was evident in many infants who were developing autism but was reported by only a minority of parents (28). It may be, therefore, that some of the children characterized in the no-regression group in the current study may more appropriately be considered to be in the regressive group. Thus, we must be cautious in our interpretations of the current data, and replication, preferably using prospective direct observational methods of classifying onset, is needed. Nevertheless, parent report remains the most practical method of defining autism onset at the present time. Moreover, our strategy of using both the Autism Diagnostic Interview- Revised (ADI-R) and Early Development Questionnaire (EDQ) with additional parent interviewing to reconcile differences hopefully increased the reliability of parent report in the current study. The robust differences in brain growth trajectory of the different onset groups suggest there may well be an association between onset and neurobiological underpinnings that has emerged despite potential noise in the assays.
In addition, further exploration of head growth trajectories in the first 2 y of life is needed. The sample sizes for the head circumference analyses in the current study cover the first 2 mo of life predominantly. A more comprehensive study of longitudinal head circumference measurements would be informative.
Implications and Future Directions.
This study highlights the complexity and heterogeneity of autism. We have found that a commonly reported biological feature of autism, abnormal brain enlargement, is mainly present in a subset of male children with regressive autism. Investigations of how different genetic underpinnings or immunological profiles may map onto our findings are important next steps. In fact, the overarching goal of this research is to identify more homogeneous subgroups of individuals with autism to facilitate analysis of the etiology of each type. Ongoing imaging studies with this cohort of subjects that examine additional aspects of brain structure and function, such as white matter microstructure and connectivity, will very likely shed light on additional neural phenotypes that may relate to other behavioral or biological aspects of autism. Thus, whereas the children with early autism onset did not differ from typically developing controls with respect to brain growth trajectory and total brain volume at age 3, this does not imply that these children have normal brain development, but rather that changes are likely more subtle than what is detectable with these relatively gross volumetric measures. Similarly, females with autism did not exhibit abnormal brain enlargement or trajectory of growth, but another aspect of brain development or function must be altered.
The major finding of this study is that a subset of boys with regressive autism have normal head circumference at birth, which diverges from normality around 4–6 mo of age, well before any loss of skills were documented. Thus, rapid head growth beginning around 4–6 mo of age may be a risk factor for future loss of skills. Furthermore, whereas behavioral regression in autism usually occurs between 12 and 24 mo of age, we found that the brain changes that are associated with this form of autism begin as early as 4 mo of age. This calls into question the association of pediatric vaccinations, in particular the measles, mumps, and rubella (MMR) vaccine, administered close to the time of regression as a causal factor in the disorder. Clearly, additional studies need to be conducted to elucidate the precise neural underpinnings of this rapid head growth that precedes the behavioral onset of regressive autism.
Materials and Methods
Participants.
Subjects were enrolled in the Autism Phenome Project. At study entry, height, weight, and occipitofrontal circumference were measured by trained study personnel. Diagnostic instruments included the ADOS-G (29, 30) and the ADI-R (31). Diagnostic criteria for ASD were based on the Collaborative Programs of Excellence in Autism network. Participants met ADOS cutoff scores for either autism or ASD. In addition, the participants were over the ADI-R cutoff score for autism on either the social or communication subscale and within two points of this criterion on the other subscale. An ADOS severity score was calculated (32), which allows for comparison of autism severity across participants tested with different ADOS-G modules.
Developmental ability was obtained for all participants using the Mullen Scales of Early Development (MSEL) (33). A DQ was calculated as the average of the age equivalent scores on the visual reception, fine motor, receptive language, and expressive language scales, divided by chronological age, multiplied by 100.
For TD controls, inclusion criteria were developmental scores within two SDs on all scales of the MSEL. In addition, TD children were screened and excluded for autism using the Social Communication Questionnaire (SCQ-Lifetime Edition) (scores > 11).
All children were native English speakers, ambulatory, and had no suspected vision or hearing problems or known genetic disorders, and/or other neurological conditions. In the ASD group, one child with fragile X syndrome and five with a history of abnormal EEGs were excluded. Additional exclusionary criteria included physical contraindications to MRI. This study was approved by the University of California (UC) Davis Institutional Review Board, and informed consent was obtained by the parent or guardian of each participant.
Onset Status.
Onset classification was determined using the ADI-R (18). To be classified in the ASD-REG group, parents had to indicate that their child had acquired and then lost at least five words and/or demonstrated losses in social engagement, social responsiveness, and social interest. Parents of children classified as ASD-nREG, indicated that their child had not lost such language and social abilities. For validation purposes, the EDQ (34), a set of 70 questions that detail development and regression over the first 18 mo of life, was also administered. If parents gave inconsistent responses across the two measures, further parent interviewing was done to resolve the inconsistencies.
Imaging.
Scans were acquired during natural, nocturnal sleep (35) at the UC Davis Imaging Research Center on a 3T Total Imaging Matrix (TIM) Trio whole-body MRI system (Siemens Medical Solutions) using an eight-channel head coil. Success rate in acquiring images during natural sleep was 85%.
For each participant, a 3D T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) scan (TR 2170 ms; TE 4.86 ms; matrix 256 × 256; 192 sagittal slices, 1-mm isotropic voxels) was obtained. A T2-weighted scan was also obtained for clinical evaluation when possible (i.e., when the child remained asleep). All MPRAGE and available T2 scans were reviewed by a pediatric neuroradiologist and screened for significant, unexpected clinical findings. A calibration phantom (Magpham Alzheimer's Disease Neuroimaging Initiative; Phantom Laboratory) was scanned at the end of each MRI session using an MPRAGE pulse sequence matched to the study sequence. The phantom was used to derive a 3D image distortion map for each child's MRI scan. Distortion correction was then carried out on each participant's images (Image Owl). This distortion correction step ensures accuracy in volumetric measurements of TCV by removing any distortion associated with scanner hardware or head placement.
Total Cerebral Volume.
Image preprocessing included removing nonbrain tissue (36), and correcting inhomogeneity (37). TCV was measured using a template-based automated method. Each participant image was warped to a study-specific template. A mask, with the brainstem and cerebellum removed, was created on the template by carrying out a manual tracing of the cerebrum (38). The mask was applied to all participant images. Images were transformed back to native space and TCV was calculated. This automated protocol was validated by carrying out reliability checks with 10 manually defined volumes. Intraclass correlation coefficient was 0.98.
Head Circumference.
Retrospective head circumference measurements were obtained from pediatric medical records (i.e., well-baby visits) obtained through the APP. Birth head circumference measurements were obtained from labor and delivery records. Occipitofrontal measurements were abstracted from the medical records.
Biostatistical Analysis.
Cross-sectional analyses of TCV and head circumference were performed by using multiple linear regression (ANCOVA) that included age, sex, BMI, DQ, and group as covariates. Longitudinal time series of individual head circumference growth in males from birth through 18 mo were analyzed using a piecewise linear longitudinal random-effects model (39). Our model set a change point at 4.5 mo, consistent with the initial cross-sectional analysis and verified in the longitudinal data. The piecewise linear model yielded a better and more interpretable fit than did a quadratic age model of equal complexity (having an equal number of model parameters) or higher-order polynomials with change points. An additional advantage of the piecewise linear model was that a quadratic fit was not forced at age extremes (birth and 18 mo), thus avoiding potential bias at the endpoints. For all analyses, a uniform application of the Akaike information criterion (40) determined the inclusion and exclusion of covariates to yield the simplest, best-fitting models among all other choices.
Acknowledgments
The authors acknowledge the following individuals for their help in the logistics of family visits and data collection: L. Deprey, K. Harrington, J. Nguyen, K. Ross, S. Rumberg, P. Shoja, and A. Stark. We thank E. Fletcher for technical assistance and K. Camilleri, C. Green, C. McCormick, S. Subramanian, R. Scholz, S. Sepehri, M. Shen, and C. Rossi for invaluable assistance in acquiring MRI data; and we especially thank all of the families and children who participated in the Autism Phenome Project study. Funding for this study was provided by the National Institute of Mental Health (1R01MH089626, U24MH081810, and 1K99MH085099) and the University of California Davis Medical Investigation of Neurodevelopmental Disorders Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.American Psychiatric Association, ed . Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.Rice C. Prevalence of Autism Spectrum Disorders: National Center on Birth Defects and Developmental Disabilities. Atlanta: Centers for Disease Control; 2006. [Google Scholar]
- 3.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Geschwind DH, Levitt P. Autism spectrum disorders: Developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Hara H. Autism and epilepsy: A retrospective follow-up study. Brain Dev. 2007;29:486–490. doi: 10.1016/j.braindev.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Lecavalier L. Behavioral and emotional problems in young people with pervasive developmental disorders: Relative prevalence, effects of subject characteristics, and empirical classification. J Autism Dev Disord. 2006;36:1101–1114. doi: 10.1007/s10803-006-0147-5. [DOI] [PubMed] [Google Scholar]
- 7.Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol Psychiatry. 2005;58:1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 8.Lainhart JE, et al. Macrocephaly in children and adults with autism. J Am Acad Child Adolesc Psychiatry. 1997;36:282–290. doi: 10.1097/00004583-199702000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 10.Dawson G, et al. Rate of head growth decelerates and symptoms worsen in the second year of life in autism. Biol Psychiatry. 2007;61:458–464. doi: 10.1016/j.biopsych.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courchesne E, et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 12.Hazlett HC, et al. Magnetic resonance imaging and head circumference study of brain size in autism: Birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- 13.Schumann CM, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30:4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fombonne E, Rogé B, Claverie J, Courty S, Frémolle J. Microcephaly and macrocephaly in autism. J Autism Dev Disord. 1999;29:113–119. doi: 10.1023/a:1023036509476. [DOI] [PubMed] [Google Scholar]
- 15.Lainhart JE, et al. Head circumference and height in autism: A study by the Collaborative Program of Excellence in Autism. Am J Med Genet A. 2006;140:2257–2274. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giedd JN, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: Ages 4-18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy DN, et al. Gyri of the human neocortex: An MRI-based analysis of volume and variance. Cereb Cortex. 1998;8:372–384. doi: 10.1093/cercor/8.4.372. [DOI] [PubMed] [Google Scholar]
- 18.Ozonoff S, Heung K, Byrd R, Hansen R, Hertz-Picciotto I. The onset of autism: Patterns of symptom emergence in the first years of life. Autism Res. 2008;1:320–328. doi: 10.1002/aur.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webb SJ, et al. Rate of head circumference growth as a function of autism diagnosis and history of autistic regression. J Child Neurol. 2007;22:1182–1190. doi: 10.1177/0883073807306263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartholomeusz HH, Courchesne E, Karns CM. Relationship between head circumference and brain volume in healthy normal toddlers, children, and adults. Neuropediatrics. 2002;33:239–241. doi: 10.1055/s-2002-36735. [DOI] [PubMed] [Google Scholar]
- 21.Hansen RL, et al. Regression in autism: Prevalence and associated factors in the CHARGE study. Ambul Pediatr. 2008;8:25–31. doi: 10.1016/j.ambp.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Shumway S, et al. Brief report: Symptom onset patterns and functional outcomes in young children with autism spectrum disorders. J Autism Dev Disord. 2011 doi: 10.1007/s10803-011-1203-3. 10.1007/s10803-011-1203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sparks BF, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 24.Bloss CS, Courchesne E. MRI neuroanatomy in young girls with autism: A preliminary study. J Am Acad Child Adolesc Psychiatry. 2007;46:515–523. doi: 10.1097/chi.0b013e318030e28b. [DOI] [PubMed] [Google Scholar]
- 25.Schumann CM, Barnes CC, Lord C, Courchesne E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol Psychiatry. 2009;66:942–949. doi: 10.1016/j.biopsych.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldberg WA, Thorsen KL, Osann K, Spence MA. Use of home videotapes to confirm parental reports of regression in autism. J Autism Dev Disord. 2008;38:1136–1146. doi: 10.1007/s10803-007-0498-6. [DOI] [PubMed] [Google Scholar]
- 27.Ozonoff S, et al. Onset patterns in autism: Correspondence between home video and parent report. J Am Acad Child Adolesc Psychiatry. 2011;50:796–806, e1. doi: 10.1016/j.jaac.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozonoff S, et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010;49(3):256–266. e251–252. [PMC free article] [PubMed] [Google Scholar]
- 29.DiLavore PC, Lord C, Rutter M. The pre-linguistic autism diagnostic observation schedule. J Autism Dev Disord. 1995;25:355–379. doi: 10.1007/BF02179373. [DOI] [PubMed] [Google Scholar]
- 30.Lord C, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 31.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 32.Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39:693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- 34.Ozonoff S, Williams BJ, Landa R. Parental report of the early development of children with regressive autism: The delays-plus-regression phenotype. Autism. 2005;9:461–486. doi: 10.1177/1362361305057880. [DOI] [PubMed] [Google Scholar]
- 35.Nordahl CW, et al. Brief report: Methods for acquiring structural MRI data in very young children with autism without the use of sedation. J Autism Dev Disord. 2008;38:1581–1590. doi: 10.1007/s10803-007-0514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 38.Schumann CM, et al. The amygdala is enlarged in children but not adolescents with autism; The hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 40.Akaike H. A new look at the statistical model identification. IEEE Trans Autom Contr. 1974;19:716–723. [Google Scholar]