Abstract
Protease-activated receptor-1 (PAR1) is a guanine nucleotide-binding (G) protein-coupled receptor that elicits cellular responses to coagulant and anticoagulant proteases. Activation of PAR1 by the coagulant protease thrombin results in Ras homolog gene family member A (RhoA) activation, disassembly of adherens junctions, and disruption of the endothelial barrier. In contrast, activation of PAR1 with the anticoagulant protease activated protein C (APC) results in activation of Ras-related C3 botulinum toxin substrate 1 (Rac1) and endothelial barrier protection. We previously showed that APC cytoprotective signaling requires the compartmentalization of PAR1 in caveolar microdomains. However, the mechanism by which APC-activated PAR1 promotes cytoprotective signaling in human endothelial cells remains poorly understood. Here we show that APC-activated PAR1 cytoprotective signaling is mediated by β-arrestin recruitment and activation of the dishevelled-2 (Dvl-2) scaffold and not by G protein α inhibiting activity polypeptide 2 (Gαi) signaling. In human endothelial cells, PAR1 and β-arrestins form a preassembled complex and cosegregate in caveolin-1–enriched fractions. Remarkably, we found that depletion of β-arrestin expression by RNA interference resulted in the loss of APC-induced Rac1 activation but not of thrombin-stimulated RhoA signaling. APC also failed to protect against thrombin-induced endothelial barrier permeability in cells deficient in β-arrestin expression. We further demonstrate that APC activation of PAR1 results in β-arrestin–dependent recruitment of Dvl-2, which is critical for Rac1 signaling and endothelial barrier protection but not for thrombin-induced RhoA signaling. Our findings identify a role for β-arrestin and Dvl-2 scaffolds in APC-activated PAR1 cytoprotective signaling in human endothelial cells.
Keywords: biased agonism, endothelial dysfunction, inflammation, sepsis
Protease-activated receptor-1 (PAR1) is a seven-transmembrane guanine nucleotide-binding (G) protein-coupled receptor (GPCR) that transduces cellular responses to serine proteases generated by the coagulation and anticoagulation pathways. Thrombin is the key effector protease of the coagulation pathway and binds directly to and activates PAR1 on endothelial cells to promote proinflammatory responses resulting in disruption of the endothelial barrier and increased vascular permeability (1). In contrast, the anticoagulant protease activated protein C (APC) binds to a coreceptor, endothelial protein C receptor (EPCR), and exerts both anticoagulant and cytoprotective responses (2). Recombinant APC has been used as a drug for the treatment of severe sepsis (3), a pathological condition characterized by exacerbated inflammatory and coagulation responses. APC reduces coagulant activity by degrading upstream cofactors Va and VIIIa. In addition, APC bound to EPCR on endothelial cells promotes anti-inflammatory and cytoprotective signaling through the activation of PAR1 (4). APC cytoprotective signaling also requires the compartmentalization of PAR1 and its coreceptor EPCR in cholesterol-enriched caveolar microdomains (5, 6). A previous study further showed that the cytoprotective activity of a noncoagulant APC is critical for reducing mortality associated with sepsis in an experimental mouse model (7). However, the mechanism by which APC-activated PAR1 promotes cytoprotective cellular responses is poorly understood.
Activation of PAR1 occurs through proteolytic cleavage of its N terminus, revealing a tethered ligand sequence that binds intramolecularly to the receptor to trigger transmembrane signaling (8). Thus, it was assumed that different PAR1-activating proteases would trigger the same signaling cascades and exhibit linear efficacy. However, this is not the case. In cultured human endothelial cells, thrombin activation of PAR1 promotes coupling to G protein α subunits 12 and 13 (Gα12/13) and Gαq; activation of Ras homolog gene family, member A (RhoA) and other signaling effectors that cause disassembly of adherens junctions; reorganization of the actin cytoskeleton; and transient disruption of the endothelial barrier (9, 10). Conversely, APC cleaves and activates PAR1, albeit less efficiently than thrombin, and induces endothelial barrier stabilization through selective activation of Ras-related C3 botulinum toxin substrate 1 (Rac1) signaling (5, 11). Unlike thrombin, APC activation of PAR1 does not promote coupling to Gα12/13 or Gαq signaling but instead appears to require G protein α inhibiting activity polypeptide (Gαi) signaling for cytoprotection. However, inactivation of Gαi causes severe disruption of the endothelial barrier in both in vitro and in vivo experimental model systems (12, 13), indicating that Gαi signaling is critical for maintaining basal endothelial barrier integrity. Thus, it remains unclear whether Gαi is entirely responsible for APC-induced cytoprotective response.
The ubiquitously expressed β-arrestin-1 and -2 isoforms are multifunctional adaptor proteins that bind to activated and phosphorylated GPCRs to promote desensitization and internalization. β-Arrestins also function as scaffolds that mediate non-heterotrimeric G protein signaling responses by seven-transmembrane receptors (14). The capacity of β-arrestins to initiate distinct GPCR signaling responses independent of heterotrimeric G proteins is commonly observed with “biased” agonists (15). Biased agonists stabilize unique GPCR active conformations that allow the receptor to couple selectively to specific signaling pathways (16). It is not known if activation of PAR1 by different proteases results in β-arrestin–initiated biased signaling. We previously showed that β-arrestins mediate thrombin-activated PAR1 desensitization but not receptor internalization, which occurs through a clathrin- and dynamin-dependent pathway (17, 18). β-Arrestins also contribute to thrombin activation of c-Src and phosphoinositide 3-kinase/Akt signaling in fibroblasts (19), suggesting that β-arrestins have diverse functions in PAR1 signal regulation. In addition to classic GPCRs, β-arrestins have been shown to function in other signaling cascades including regulation of the Wnt canonical and noncanonical signaling pathways (20). Noncanonical Wnt signaling promotes RhoA and Rac1 activation and modulation of the actin cytoskeleton. Wnt ligands bind to and activate Frizzled (Fz) seven-transmembrane receptors that result in recruitment of the cytosolic dishevelled (Dvl) scaffold protein and effector signaling. Previous studies showed that Wnt5A-induced Fz4 internalization occurs through Dvl-2 recruitment of β-arrestins, which also promotes Rac1 signaling in noncanonical Wnt pathways (21, 22). However, whether β-arrestins and Dvl function similarly in signal transduction pathways initiated by classic GPCRs is not known.
APC bound to its coreceptor EPCR cleaves and activates a subpopulation of PAR1 residing in caveolar microdomains and promotes cytoprotective signaling in human endothelial cells. However, it is not known how APC-activated PAR1 mediates cytoprotective signaling and whether β-arrestins function in this pathway. Here we report that β-arrestins, and not Gαi, function as critical mediators of APC-induced PAR1 cytoprotective signaling. We also demonstrate that APC cytoprotective signaling requires β-arrestin–dependent recruitment of Dvl-2. These findings reveal a unique function for β-arrestin and Dvl-2 scaffolds in APC-promoted PAR1 cytoprotective signaling in cultured human endothelial cells and provide further support for β-arrestins as negative regulators of sepsis-induced inflammatory responses observed in vivo (23, 24).
Results
PAR1, Gαi, and β-Arrestins Localize to Caveolin-Enriched Microdomains.
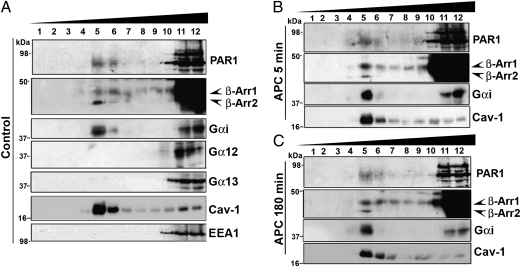
The compartmentalization of PAR1 in caveolar microdomains is critical for APC-promoted Rac1 activation and endothelial barrier protection (5, 6). To define the mediators of APC-activated PAR1 signaling, we examined whether Gαi and/or β-arrestins localize to caveolar microdomains together with PAR1 in human umbilical vein endothelial cell-derived EA.hy926 cells using sucrose density-gradient fractionation. In untreated control cells, endogenous PAR1 localized to both caveolin-1–enriched and heavy fractions containing the early endosome antigen-1 (EEA1) peripheral membrane protein (Fig. 1A). These findings confirm that PAR1 is present in caveolar microdomains (5, 6). In addition, both β-arrestin-1 and -2 isoforms were detected in caveolin-1–enriched fractions in control cells (Fig. 1A), although the majority of β-arrestins segregated to heavy fractions. Like PAR1 and β-arrestins, the Gαi protein cosegregated in caveolin-1–enriched and EEA1-containing fractions (Fig. 1A). However, PAR1, β-arrestins, and Gαi distribution was not altered significantly following acute or prolonged exposure to APC (Fig. 1 B and 1C). In contrast to Gαi, Gα12 and Gα13 proteins localized mainly to heavy fractions and were not detectable in caveolin-1–enriched fractions in control cells (Fig. 1A). Thus, Gαi and β-arrestins partition into caveolin-1–enriched fractions together with PAR1.
Fig. 1.
PAR1, Gαi, and β-arrestins cosegregate in caveolin-1–enriched fractions. Endothelial EA.hy926 cells were left untreated (Control) (A) or were treated at 37 °C with 20 nM APC for 5 min (B) or with 20 nM APC for 180 min (C). Cells were lysed, and caveolin-1–enriched fractions were isolated by detergent-free sucrose-gradient centrifugation. Aliquots representing each of the 12 fractions were immunoblotted with antibodies that recognize PAR1, β-arrestins (A1CT), Gαi, Gα12, Gα13, caveolin-1 (Cav-1), and EEA-1.
APC Signaling Is Mediated by β-Arrestins and Not by Gαi.
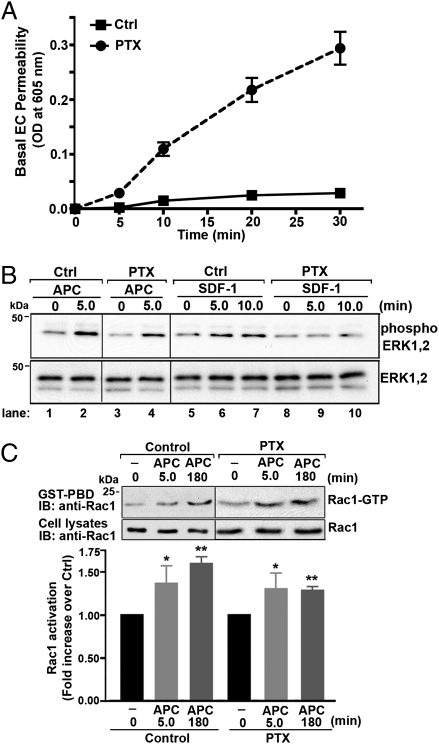
A previous study indicated that APC-activated PAR1 signals through Gαi based on the failure of APC to induce endothelial barrier protection in cells treated with pertussis toxin (PTX) (25), which ADP ribosylates and inactivates Gαi/o proteins. Compared with untreated control cells, however, PTX treatment caused a marked increase in basal endothelial barrier permeability (Fig. 2A), similar to that previously reported in bovine endothelial cells (13). Thus, in PTX-treated cells it is difficult to distinguish between the loss of APC-specific responses from global perturbation of the endothelial barrier. To assess the contribution of Gαi to APC-stimulated signaling responses in endothelial cells directly, we examined the activation of extracellular signal-regulated kinases-1 and -2 (ERK1,2). The extent of ERK1,2 activation induced by APC was comparable in control and PTX-treated cells (Fig. 2B, lanes 2 and 4), suggesting that Gαi is not essential for APC-stimulated ERK1,2 signaling. In contrast, however, activation of the Gαi-coupled C-X-C chemokine receptor 4 with stromal cell-derived factor-1 (SDF-1) resulted in increased ERK1,2 signaling (Fig. 2B, lanes 5–7). This increased signaling was virtually ablated in PTX-treated cells (Fig. 2B, lanes 8–10), confirming disruption of Gαi signaling by PTX. APC-stimulated Rac1 activation also was unperturbed in PTX-treated cells (Fig. 2C), consistent with Gαi-independent signaling. These findings suggest that in endothelial cells APC signals through a pathway independent of the Gαi protein.
Fig. 2.
APC-activated PAR1 signaling is independent of Gαi protein. (A) Confluent endothelial cell (EC) monolayers were preincubated with or without (Ctrl) 100 ng/mL PTX for 16 h at 37 °C, and basal endothelial barrier permeability was monitored over time. The data (mean ± SEM) are representative of three independent experiments. (B) Control (Ctrl) and PTX-pretreated endothelial cells were incubated with 20 nM APC or 100 ng/mL SDF-1α for various times at 37 °C, and the activation of ERK1,2 was determined by immunoblotting (IB). Membranes were reprobed with an anti-ERK1,2 antibody to control for loading. Data shown are representative of three independent experiments. (C) (Upper) Control and PTX-pretreated endothelial cells were stimulated with or without 20 nM APC for 5 min or 180 min at 37 °C and were lysed. Equivalent amounts of cell lysates were incubated with GST-PBD, and the amount of activated Rac1 was determined by immunoblotting. Total cell lysates were immunoblotted with an anti-Rac1 antibody as a control. (Lower) Data (mean ± SEM) are expressed as the fold over untreated control (Ctrl) from three independent experiments. The differences in Rac1 activity measured in APC-stimulated cells versus untreated control was significant (*P < 0.05; **P < 0.01).
We next determined whether APC signaling is mediated by β-arrestins. We first examined if APC-activated PAR1 associates with β-arrestins in endothelial cells. Endothelial cells were incubated with APC for various times and were lysed. Endogenous PAR1 was immunoprecipitated and β-arrestin association was assessed by immunoblot using the polyclonal antibody A1CT that detects both β-arrestin-1 and -2 isoforms (26). Endogenous β-arrestins appear to interact with PAR1 under unstimulated conditions (Fig. 3A, compare lanes 1 and 2). β-Arrestins remained bound to PAR1 following 5-min exposure to APC; after prolonged APC incubation β-arrestin association with PAR1 appeared to diminish (Fig. 3A, compare lanes 2–4). These findings suggest that endogenous PAR1 and β-arrestins exist in a preassembled complex and remain associated even after prolonged APC incubation. In contrast, thrombin stimulation caused an increase in β-arrestin association with PAR1 (Fig. 3A, compare lanes 5–6). Thus, APC and thrombin induce differential association of β-arrestins with activated PAR1, suggesting that β-arrestins may regulate distinct cellular responses depending on the activating protease.
Fig. 3.
β-Arrestins associate with PAR1 and mediate APC-activated PAR1 signaling in human endothelial cells. (A) Endothelial cells were incubated with or without 20 nM APC or 10 nM thrombin (Th) for various times at 37 °C, lysed, and immunoprecipitated (IP) with IgG or anti-PAR1 WEDE antibody. Immunoprecipitates were examined for the presence of β-arrestins using an anti–β-arrestin A1CT antibody or for PAR1 using an anti-PAR1 polyclonal antibody. Total cell lysates were immunoblotted with a β-arrestin A1CT antibody or with anti-actin antibody as controls. (B) Endothelial cells were transfected with 100 nM nonspecific (ns), β-arrestin-1 (β-Arr1), β-arrestin-2 (β-Arr2), or both β-arrestin-1 and -2 siRNAs. Cells were lysed, and equivalent amounts of cell lysates were immunoblotted with an anti-A2CT antibody (which detects β-arrestin-2 over β-arrestin-1), anti-A1CT (which detects β-arrestin-1 over β-arrestin-2), or anti-actin antibody to control for loading. The asterisk indicates a nonspecific band. (C) Serum-deprived endothelial cells transfected with nonspecific or β-arrestin siRNAs were incubated with 10 nM thrombin (Th) or 20 nM APC for 5 min at 37 °C. Cell lysates were prepared, and ERK1,2 activity was determined by immunoblotting using anti–phospho-ERK1,2 antibody. Membranes were reprobed for total ERK1,2 to control for loading. Data (mean ± SEM) are expressed as the fold over untreated control (Ctrl) and are representative of three independent experiments. The differences in ERK1,2 activation induced by agonist compared with untreated control were significant (*P < 0.05; **P < 0.01; ***P < 0.001).
To assess β-arrestin function, we used siRNAs specifically targeting the β-arrestin-1 and -2 isoforms and examined activation of ERK1,2 signaling, an effector pathway of the β-arrestin scaffold. Human endothelial EA.hy926 cells express both the β-arrestin-1 and -2 isoforms (Fig. 3B). The apparent differences between β-arrestin-1 versus β-arrestin-2 expression are likely caused by the greater affinity of the A1CT antibody for β-arrestin-1 versus the A2CT antibody for β-arrestin-2 (26), we therefore examined the function of both β-arrestin isoforms. Cells deficient in β-arrestin-1 expression displayed no apparent decrease in APC-induced ERK1,2 activation after 5 min of stimulation, as compared with control cells treated with nonspecific siRNA (Fig. 3B, lanes 3 and 6). However, the capacity of APC to stimulate ERK1,2 activation was markedly reduced in cells deficient in β-arrestin-2 or in both β-arrestin-1 and -2 isoforms (Fig. 3B, lanes 3, 9, and 12), suggesting that β-arrestin-2 is the predominant mediator of APC signaling. In contrast, thrombin-stimulated ERK1,2 activation was unperturbed in cells lacking either or both β-arrestin isoforms compared with control cells (Fig. 3C, lanes 2, 5, 8, and 11), indicating that thrombin-induced ERK1,2 signaling in endothelial cells is mediated by heterotrimeric G proteins and not by β-arrestins. These findings suggest that β-arrestins, and not Gαi, are important mediators of APC-activated PAR1 signaling in human endothelial cells.
APC-Induced Cytoprotective Signaling Is Mediated by β-Arrestins.
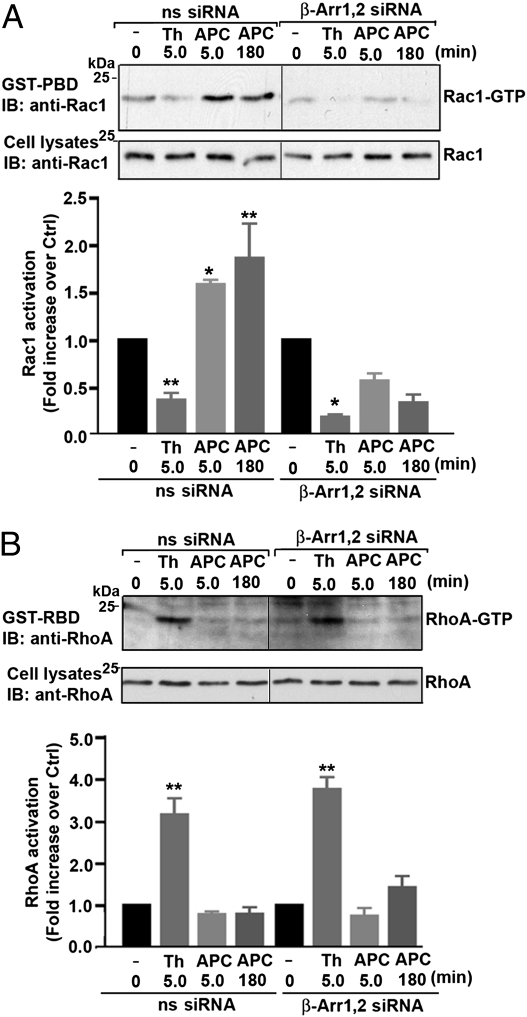
RhoA signaling is critical for thrombin-induced endothelial barrier dysfunction (10), whereas Rac1 is important for APC-promoted endothelial barrier protection (5, 11). To determine if β-arrestins mediate APC-induced endothelial barrier protection, we first examined the activation of endogenous RhoA and Rac1 in cells lacking both β-arrestin isoforms. In nonspecific siRNA control cells, APC stimulated a significant increase in Rac1 activation but not in RhoA signaling (Fig. 4 A and B). In contrast, thrombin induced robust RhoA activation and diminished Rac1 signaling in control cells treated with nonspecific siRNA (Fig. 4 A and B). These findings are consistent with the differential activation of PAR1 by APC versus thrombin, as previously reported (5). Remarkably, APC-stimulated Rac1 activity was ablated completely in cells deficient in β-arrestin-1,2 expression compared with control cells after agonist incubation for various times (Fig. 4A). In contrast to APC, however, thrombin-induced RhoA signaling was not affected in β-arrestin-1,2–depleted cells (Fig. 4B), indicating that β-arrestins are not essential for thrombin-stimulated RhoA activation. Thus, β-arrestins appear to link APC-activated PAR1 to Rac1 signaling, an important mediator of endothelial cell cytoprotective responses.
Fig. 4.
β-Arrestins are critical for APC-induced Rac1 activation but not for thrombin-induced RhoA activation. (A and B) Endothelial cells transfected with 100 nM nonspecific (ns) or β-arrestin siRNAs were stimulated with 10 nM thrombin (Th) or 20 nM APC for 5 min or 180 min at 37 °C. Cells were lysed. Equivalent amounts of cell lysates were incubated with GST-PBD or GST-RBD, and the amount of activated Rac1 or RhoA was determined by immunoblotting. Total cell lysates were immunoblotted with an anti-Rac1 or anti-RhoA antibody as a control. Data (mean ± SEM) are expressed as the fold increase over untreated control (Ctrl) of three independent experiments. The differences between Rac1 or RhoA activity observed in agonist-treated cells versus untreated control cells were significant (*P < 0.05; **P < 0.01).
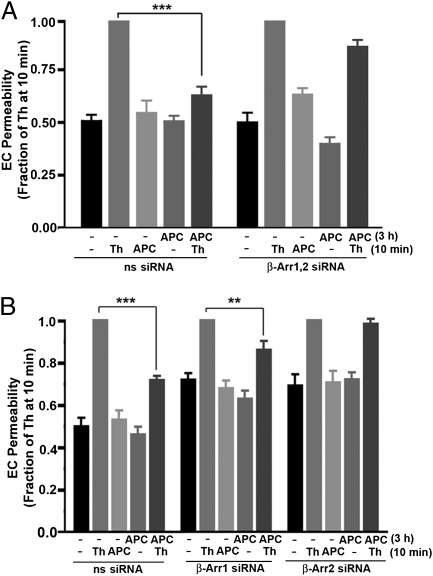
We next directly assessed the role of β-arrestins in APC-mediated endothelial barrier protection. Consistent with previous reports, preincubation of endothelial cells with APC for 30 min caused a significant (∼40%) inhibition of thrombin-induced endothelial barrier permeability, and ∼70% inhibition was observed after 180 min of APC exposure (Fig. S1A) (11). These findings indicate that APC pretreatment promotes endothelial barrier protection, as previously reported (5, 11). In nonspecific siRNA-treated cells, thrombin stimulated a significant increase in endothelial barrier permeability; this increase was virtually ablated in cells pretreated with APC for 3 h (Fig. 5A). However, in contrast to control cells transfected with nonspecific siRNA, APC failed to protect against thrombin-induced endothelial barrier permeability in cells lacking both β-arrestin-1 and -2 isoforms (Fig. 5A). In addition, basal endothelial barrier permeability was similar in control cells and cells transfected with β-arrestin siRNAs (Fig. S1B), indicating that β-arrestins regulate APC-induced endothelial barrier protection. To determine the function of the individual β-arrestin isoforms in APC-induced cytoprotection, endothelial barrier permeability was assessed in cells lacking only one β-arrestin isoform. In cells deficient in β-arrestin-1, pretreatment with APC caused a modest reduction in thrombin-stimulated endothelial barrier permeability compared with control cells transfected with nonspecific siRNA (Fig. 5B), suggesting a minor role for β-arrestin-1 in APC-promoted cytoprotection. In contrast, however, APC failed to inhibit thrombin-induced endothelial barrier permeability in cells lacking β-arrestin-2 expression (Fig. 5B). These findings indicate that β-arrestin-2 is the predominant mediator of APC-induced endothelial barrier protection.
Fig. 5.
APC-induced endothelial barrier protection is mediated by β-arrestins. (A and B) Serum-deprived endothelial cells (EC) transfected with 100 nM nonspecific (ns) or β-arrestin siRNAs were preincubated with or without 20 nM APC for 3 h at 37 °C. Cells were washed and then were stimulated with 10 nM thrombin (Th) or 20 nM APC for 10 min at 37 °C, and endothelial barrier permeability was assessed. Data (mean ± SEM) are expressed as the fraction of response compared with thrombin-induced permeability determined at 10 min from three separate experiments. The differences in thrombin-stimulated endothelial barrier permeability observed in APC-pretreated cells versus untreated control cells were significant (**P < 0.01; ***P < 0.001).
APC Stimulates Dvl-2 Coassociation with β-Arrestins and Polymerization.
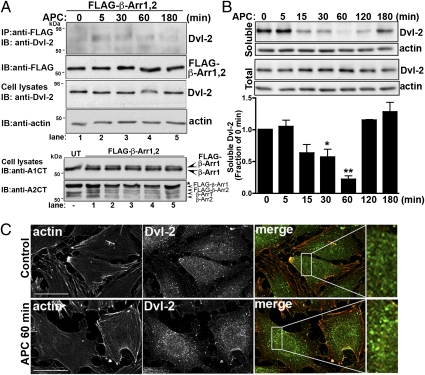
To delineate the mechanism by which β-arrestins mediate APC-induced cytoprotective signaling, we examined the role of Dvl-2. Dvl-2 is expressed abundantly in mammalian cells (27), binds to β-arrestins (21), and can mediate RhoA and Rac1 activation resulting in modulation of the actin cytoskeleton (28). We first determined whether Dvl-2 associated with β-arrestins after APC stimulation. Endothelial cells transiently coexpressing FLAG-tagged β-arrestins were treated with APC for various times, lysed, and immunoprecipitated with anti-FLAG antibody, and the presence of endogenous Dvl-2 was examined. In the absence of agonist, Dvl-2 displayed minimal interaction with β-arrestins in untreated transfected control cells (Fig. 6A, lane 1), whereas after 5 min incubation with APC endogenous Dvl-2 association with β-arrestins was detected (Fig. 6A, lane 2 and Fig. S2). Interestingly, the interaction of β-arrestin with Dvl-2 remained detectable for 30 min and appeared to diminish after 60 min of APC incubation (Fig. 6A, lanes 3–5). These findings suggest that APC activation of PAR1 induces β-arrestin and Dvl-2 interaction that is sustained for 30 min and is consistent with prolonged signaling induced by APC.
Fig. 6.
APC induces Dvl-2 association with β-arrestins and Dvl-2 polymerization. (A) Endothelial cells transfected with FLAG-tagged β-arrestin-1 and -2 were incubated with 20 nM APC for various times at 37 °C. Cells were lysed and immunoprecipitated with anti-FLAG M2 antibody. Immunoprecipitates were examined for the presence of endogenous Dvl-2 using an anti–Dvl-2 polyclonal antibody or for FLAG–β-arrestins using an anti-FLAG antibody. Cell lysates were immunoblotted with anti–Dvl-2 and anti-actin antibodies as a control. Untransfected (UT) and FLAG–β-arrestin cell lysates were immunoblotted with anti–β-arrestin A1CT or A2CT antibodies to detect individual β-arrestin isoform expression. The asterisk indicates a nonspecific band. These data are representative of three separate experiments. (B) Endothelial cells were incubated with 20 nM APC for various times at 37 °C. Cells were lysed, and fractions representing soluble proteins or total cell lysates were prepared and immunoblotted with anti–Dvl-2 antibody. Membranes were reprobed with an anti-actin antibody to control for loading. The data (mean ± SEM) are expressed as the fraction of untreated 0-min control and are representative of four independent experiments. The differences in soluble Dvl-2 detected in APC-stimulated cells versus untreated control cells were significant (*P < 0.05; **P < 0.01). (C) Serum-starved endothelial cells were left untreated (Control) or were treated with 20 nM APC for 60 min at 37 °C. Cells were fixed, processed, and stained for filamentous actin (red) and immunostained for Dvl-2 (green) and were imaged by confocal microscopy. Images shown are representative fields of three independent experiments. Magnifications of boxed areas in merged images are shown on the right. (Scale bars, 20 μm.)
Dvl-2 is a scaffold protein that recruits signaling effectors such as Rac1 to regulate the actin cytoskeleton and exists as a soluble protein that polymerizes upon activation, forming discrete puncta in the cytoplasm (29, 30). To determine whether APC-stimulated PAR1 signaling resulted in endogenous Dvl-2 activation, we examined Dvl-2 polymerization using detergent-extracted soluble fractions from cell lysates, as previously described (31, 32). In untreated control cells, Dvl-2 was detected mainly in the soluble cytosolic fraction that was comparable to the amount of Dvl-2 detected in total cell lysates (Fig. 6B). However, after incubation with APC for 15 min, the amount of Dvl-2 in the soluble fraction decreased significantly. This decrease persisted for 60 min and then soluble Dvl-2 amounts returned to basal levels after prolonged agonist stimulation (Fig. 6B). In contrast, the amount of Dvl-2 detected in total cell lysates remained constant over the time course of APC stimulation, indicating that the loss of Dvl-2 from the soluble fraction was not caused by Dvl-2 protein degradation (Fig. 6B). Thus, APC stimulation appears to alter Dvl-2 solubility. We next used immunofluorescent confocal microscopy to determine whether APC treatment resulted in endogenous Dvl-2 polymerization by examining the formation of discrete puncta in the cytoplasm of endothelial cells. In untreated control cells, endogenous Dvl-2 was found largely in the cytoplasm and appeared in a diffuse pattern with some cytoplasmic puncta (Fig. 6C). However, after incubation with APC for 60 min, endogenous Dvl-2 localized mainly to prominent puncta distributed throughout the cytoplasm (Fig. 6C), consistent with Dvl-2 polymerization, as previously reported (30). Together these findings suggest that APC activation of PAR1 results in activation of the Dvl-2 scaffold.
APC-Induced Dvl-2 Polymerization Is Mediated by β-Arrestins.
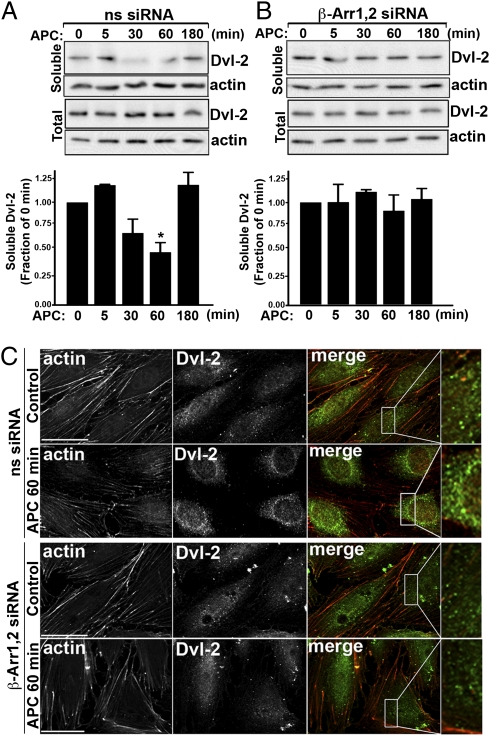
To determine whether Dvl-2 activation induced by APC requires β-arrestins, we examined the subcellular distribution of Dvl-2 in endothelial cells deficient in β-arrestin expression. In control cells transfected with nonspecific siRNA, loss of Dvl-2 from the soluble fraction was detected after 30 min of APC stimulation; this loss was sustained for 60 min, and then Dvl-2 returned to basal levels after 180 min of agonist incubation (Fig. 7A). In contrast, however, in cells lacking β-arrestin expression, APC failed to induce loss of Dvl-2 from the soluble fraction even after prolonged agonist exposure (Fig. 7B), suggesting that β-arrestins mediate APC-promoted Dvl-2 redistribution. We next examined the formation of endogenous Dvl-2 puncta in endothelial cells deficient in β-arrestin expression by immunofluorescent confocal microscopy. In control cells transfected with nonspecific siRNA, APC exposure resulted in an increase in prominent Dvl-2–positive puncta which were not evident in unstimulated control cells (Fig. 7C). Remarkably, however, APC failed to induce the formation of Dvl-2 cytoplasmic puncta in cells lacking β-arrestins (Fig. 7C). Thus, β-arrestins appear to link APC-activated PAR1 to activation of the Dvl-2 scaffold.
Fig. 7.
β-Arrestins mediate APC-induced Dvl-2 redistribution and polymerization. (A and B) Endothelial cells transfected with 100 nM nonspecific (ns) or β-arrestin siRNAs were incubated with 20 nM APC for various times at 37 °C and lysed. Cell fractions representing soluble proteins or total cell lysates were immunoblotted with an anti–Dvl-2 antibody. Membranes were reprobed with an anti-actin antibody as a control. Data (mean ± SEM) shown are expressed as the fraction of untreated 0-min control and are representative of three independent experiments. The difference in soluble Dvl-2 detected in APC-stimulated cells transfected with nonspecific siRNA was significant (*P < 0.05). (C) Endothelial cells transfected with 100 nM nonspecific or β-arrestin siRNAs were left untreated (Control) or were treated with 20 nM APC for 60 min at 37 °C. Cells were fixed, processed, and stained for filamentous actin (red) or immunostained for Dvl-2 (green) and imaged by confocal microscopy. Images shown are representative fields from three independent experiments. Magnifications of boxed areas in the merged images are shown on the right. (Scale bars, 20 μm.)
Dvl-2 Functions in APC-Induced Cytoprotective Signaling.
To assess the function of Dvl-2 in APC-induced cytoprotection, we examined Rac1 activation in endothelial cells lacking Dvl-2 expression. Endothelial cells transfected with Dvl-2 siRNA displayed a loss of endogenous Dvl-2 expression compared with control cells transfected with nonspecific siRNA (Fig. 8A, Inset). In contrast to cells treated with nonspecific siRNA, the capacity of APC to stimulate Rac1 activation was virtually abolished in endothelial cells deficient in Dvl-2 expression (Fig. 8A). However, ablation of Dvl-2 expression failed to affect thrombin-stimulated RhoA signaling (Fig. 8B), indicating that Dvl-2 functions selectively in cytoprotective signaling. To confirm a function for Dvl-2 in APC-stimulated cytoprotection, we examined endothelial barrier permeability in cells depleted of Dvl-2 expression (Fig. 8C). As expected, APC pretreatment virtually abolished thrombin-induced endothelial barrier permeability in control cells transfected with nonspecific siRNA (Fig. 8C). In contrast, however, APC failed to inhibit thrombin-induced endothelial barrier permeability in Dvl-2–depleted cells (Fig. 8C), suggesting that Dvl-2 is important for APC-promoted cytoprotection. Thus, Dvl-2 appears to function selectively in APC-mediated cytoprotective signaling and not in endothelial barrier-disruptive responses elicited by thrombin.
Fig. 8.
APC-induced cytoprotective signaling is mediated by Dvl-2. (A and B) Endothelial cells transfected with 50 nM nonspecific (ns) or Dvl-2 siRNAs were incubated with 10 nM thrombin (Th) or 20 nM APC for 5 or 180 min at 37 °C. Cells were lysed and incubated with GST-PBD or GST-RBD, and the amount of activated Rac1 or RhoA was detected by immunoblotting. Total cell lysates were immunoblotted with anti-Rac1 or anti-RhoA antibody as a control. Inset shows an anti–Dvl-2 and anti-actin antibody immunoblot of total cell lysates. The data (mean ± SEM) are expressed as the fold increase over untreated control (Ctrl) from three independent experiments. The differences between Rac1 or RhoA activation observed in agonist-treated cells versus untreated control cells were significant (*P < 0.05; **P < 0.01). (C) Serum-deprived endothelial cells (EC) transfected with 50 nM nonspecific or Dvl-2–specific siRNAs were preincubated with or without 20 nM APC for 3 h at 37 °C. Inset shows a total cell lysate immunoblot using the anti–Dvl-2 and anti-actin antibodies. Cells then were stimulated with 10 nM Th or 20 nM APC for 10 min at 37 °C, and endothelial barrier permeability was assessed. Data (mean ± SEM) are expressed as the fraction of thrombin-induced endothelial barrier permeability measured at 10 min and are representative of three independent experiments. The differences in thrombin-induced endothelial barrier permeability in cells transfected with nonspecific siRNA versus cells transfected with Dvl-2 siRNA was significant (**P < 0.01).
Discussion
In the present study we define a unique signaling pathway by which APC promotes PAR1 cytoprotective signaling in human endothelial cells that requires β-arrestin and Dvl-2 scaffolds. Cytoprotective signaling induced by APC requires PAR1 compartmentalization in caveolae (5, 6). We found that endogenous PAR1 and β-arrestins exist in a preassembled complex and localize to caveolar microdomains, consistent with a function in cytoprotective signaling. We further show that APC-promoted PAR1 cytoprotective signaling is mediated by β-arrestins rather than by the heterotrimeric Gαi protein. Remarkably, β-arrestins appear to link APC-activated PAR1 to the Dvl-2 scaffold; this association is critical for Rac1 activation and endothelial barrier protection. Thus, APC activation of PAR1 cytoprotective signaling occurs through caveolar microdomains and is mediated by β-arrestin and Dvl-2 scaffolds.
We show in cultured human endothelial cells that endogenous PAR1 differentially utilizes heterotrimeric G protein or β-arrestin signaling pathways depending on the activating protease. In the classic paradigm, activated GPCRs signal through heterotrimeric G proteins, and β-arrestins mediate receptor desensitization and internalization. However, several GPCRs have been shown to use β-arrestins as scaffolds to promote signaling to multiple effectors independent of heterotrimeric G proteins (33). Previous studies have demonstrated that thrombin activation of PAR1 promotes preferential coupling to Gαq and Gα12/13, resulting in RhoA activation, disassembly of adherens junctions, and increased endothelial barrier permeability (9, 10). We found that thrombin-stimulated RhoA and ERK1,2 signaling is unperturbed in endothelial cells lacking β-arrestins, consistent with the idea that thrombin-activated PAR1 signals preferentially through heterotrimeric G proteins and not through β-arrestins. In contrast, however, Rac1 and ERK1,2 signaling induced by APC activation of PAR1 was virtually abolished in β-arrestin–deficient endothelial cells but remained intact in cells in which Gαi was inactivated by PTX. Moreover, APC failed to protect β-arrestin–deficient cells from thrombin-induced endothelial barrier permeability. Thus, different activating proteases can bias PAR1 signaling toward heterotrimeric G protein or β-arrestin–dependent signaling pathways that result in differential regulation of endothelial barrier permeability.
The Wnt/Fz canonical signaling cascade induces β-catenin stabilization, whereas Wnt/Fz noncanonical signaling promotes Rho GTPase activation that regulates the actin cytoskeleton, cell morphology, and migration (28, 34). Dvl is a key mediator of both canonical and noncanonical Wnt/Fz signaling. In addition to its function in β-catenin stabilization induced by Wnt/Fz, Dvl appears to mediate β-catenin stabilization stimulated by classic GPCRs. Thrombin activation of PAR1 has been shown to promote β-catenin stabilization in endothelial cells and colorectal cancer cells (35, 36). In cancer cells, thrombin-induced stabilization of β-catenin occurs through Gα13-mediated recruitment of Dvl-1 (35). However, Gα12/13 proteins also are known to stabilize β-catenin directly by interacting with the cytoplasmic domain of cadherins, causing β-catenin dissociation and translocation to the nucleus (37). The parathyroid hormone receptor (PTH1R), a classic GPCR, also induces β-catenin stabilization through Dvl-2 (38). In this case, Dvl-2 binds directly to the PTH1R via a linear peptide motif K-(S/T)-XXX-W within the cytoplasmic tail, which is not present in PAR1. In addition to Dvl-mediated β-catenin stabilization induced by classic GPCRs, we now show that Dvl-2 functions downstream of PAR1 after stimulation by APC, which mediates activation of Rac1, an effector of noncanonical Wnt signaling. We found that APC activated PAR1-induced Rac1 signaling and endothelial barrier protection was impaired in endothelial cells deficient in Dvl-2 expression. Thus, classic GPCRs appear to use Dvl to differentially regulate several signaling effectors that also function in Wnt/Fz signaling pathways.
Our study also demonstrates that a classic GPCR signals directly through β-arrestin and Dvl-2 scaffolds. Previous studies showed that, upon activation of Fz signaling, Dvl binds to the Fz4 receptor and promotes β-arrestin recruitment (21) to mediate various aspects of Wnt signaling, including Rac1 activation (34). In human endothelial cells, we show that β-arrestins link APC-activated PAR1 to the Dvl-2 scaffold. Thus, β-arrestins function upstream of Dvl-2 in APC-induced cytoprotective signaling. We further show that Dvl-2 is essential for APC-stimulated Rac1 activation but not for thrombin-induced RhoA signaling, consistent with a function for Dvl-2 in cytoprotective signaling. A previous study reported that Dvl-2 phosphorylation is important for β-arrestin recruitment and Wnt5A-stimulated Fz4 internalization (21). However, we failed to detect Dvl-2 phosphorylation after APC stimulation. In recent work, however, Bryja et al. (22) showed that Wnt5A-induced Rac1 activation is mediated by Dvl and β-arrestins and is inhibited by Dvl phosphorylation, although Dvl phosphorylation also promotes other noncanonical signaling responses. Thus, we propose that APC-induced cytoprotection mediated by Rac1 signaling is initiated by β-arrestin recruitment and activation of the Dvl-2 scaffold that are independent of Dvl phosphorylation. However, the mechanism by which APC promotes Dvl-2–dependent Rac1 activation is not known. We speculate that activated Dvl-2 may provide a platform that enhances effector binding (30), such as Rac1-specific guanine nucleotide exchange factors to promote Rac1 activation following APC stimulation.
This study describes a unique signaling pathway by which APC-activated PAR1 promotes cytoprotective signaling that is mediated by β-arrestin and Dvl-2 scaffolds. Our findings further indicate that PAR1 and β-arrestins are preassembled and coexist in caveolar microdomains. These results are consistent with the importance of PAR1 compartmentalization in caveolar microdomains for APC-induced cytoprotective signaling (5, 6). This work also indicates that the β-arrestin-2 isoform is the predominant mediator of APC-induced cytoprotective signaling and is consistent with previous studies demonstrating a protective role in vivo for β-arrestin-2 in mouse models of experimentally induced sepsis lethality (23, 24). APC activation of PAR1 signaling in vivo has also been shown to reduce mortality induced by sepsis in mouse models (2, 7). The mechanistic basis for a β-arrestin isoform-specific function in mediating APC-induced cytoprotective signaling is not known. In addition, APC-induced PAR1 cytoprotective signaling appears to involve crosstalk with sphingosine-1 phosphate (S1P) receptors (11, 39), but whether β-arrestins and/or Dvl-2 scaffolds facilitate transactivation of S1P and/or other receptors remains to be determined.
Materials and Methods
Reagents.
α-Thrombin was purchased from Enzyme Research Laboratories. Human APC was obtained from Hematologic Technologies. SDF-1α was purchased from R&D Systems, and PTX was obtained from Calbiochem. β-Arrestin-1 and -2 antibodies (A1CT and A2CT, respectively) were gifts from Robert Lefkowitz (Duke University Medical Center, Durham, NC). ERK1,2 and Dvl-2 antibodies were from Cell Signaling Technologies. RhoA, Gαi2, Gα12, Gα13, and Dvl-2 antibodies were from Santa Cruz Biotechnology. Rac1, caveolin-1, and EEA-1 antibodies were from BD Biosciences. The monoclonal anti-PAR1 WEDE-15 antibody was from Beckman Coulter, and the polyclonal anti-PAR1 rabbit antibody was generated as previously described (40). TRITC-conjugated phalloidin, anti-FLAG, and anti-actin antibodies were obtained from Sigma. HRP-conjugated goat anti-mouse and anti-rabbit secondary antibodies were from Bio-Rad. Alexa Fluor 488-conjugated donkey anti-goat IgG was from Molecular Probes. Glutathione-Sepharose 4B beads and protein A-Sepharose CL-4B beads were from GE Healthcare.
Cells and Transfections.
Human umbilical vein endothelial cell–derived EA.hy926 cells were provided by C. Edgell (University of North Carolina, Chapel Hill, NC) and were grown and maintained as previously described (5). Endothelial EA.hy926 cells were transfected with FLAG-tagged β-arrestin-1 and -2 using X-tremeGENE HP (Roche Applied Science), according to the manufacturer's instructions.
RNA Interference.
Sense and antisense RNA oligonucleotides were obtained from Dharmacon. EA.hy926 endothelial cells were plated at 3 × 105 cells per well in 24-well culture dishes and were transfected with 100 nM β-arrestin-1 (5′-CAUAGAACUUGACACAAAU-3′), 100 nM β-arrestin-2 (5′-GGACCGCAAAGUGUUUGUG-3′), or 50 nM Dvl-2 (5′-GGAAGAAAUUUCAGAUGAC-3′) or nonspecific (5′-CUACGUCCAGGAGCGCACC-3′) siRNAs using Oligofectamine or Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions.
Permeability Assay.
Endothelial barrier permeability was quantified by measuring the flux of Evans blue-bound BSA (Sigma) essentially as previously described (5).
RhoA and Rac1 Activity Assays.
GST-rhotekin Rho-binding domain (RBD) and p21-activated kinase (PAK-1)–binding domain (PBD) fusion proteins were transformed into BL21 (DE3) Escherichia coll; fusion proteins were induced and purified using standard techniques; and assays were conducted as previously described (5). Briefly, endothelial EA.hy926 cells grown in 60-mm dishes were serum starved overnight and were then treated or left untreated with agonists for various times at 37 °C. Cells were lysed in buffer containing 50 mM Tris-HCl (pH 7.4), 100 mM sodium chloride, 2 mM MgCl2, 1% (vol/vol) Triton X-100, 10% (vol/vol) glycerol containing 1 mM DTT, and protease inhibitors. Equivalent amounts of lysates were used in pull-down assays with GST PAK-PBD and GST Rhotekin-RBD bound to glutathione-Sepharose beads for 45 min at 4 °C. Beads were washed with lysis buffer, and GTP-bound Rac1 or RhoA was eluted in 2× SDS-sample buffer [125 mM Tris-HCl (pH 6.8), 20% (vol/vol) glycerol, 5% (wt/vol) SDS, 200 mM DTT, 0.01% (wt/vol) bromophenol blue], resolved by SDS/PAGE, transferred to PVDF membranes, and immunoblotted with an anti-Rac1 antibody or anti-RhoA antibody. Immunoblots were developed with enhanced chemiluminescence and analyzed with ImageJ (National Institutes of Health).
Immunoprecipitation Assays.
PAR1 was immunoprecipitated from endothelial EA.hy926 cells plated at 2 × 106 cells per well in six-well culture dishes and grown for 2 d. Serum-starved cells were washed, incubated with or without agonists at 37 °C, and lysed in buffer containing 50 mM Tris-HCl (pH 7.4), 100 mM sodium chloride, 5 mM EDTA, 50 mM sodium fluoride, 1% (vol/vol) Triton X-100 supplemented with 200 μM sodium orthovanadate, and protease inhibitors. Equivalent amounts of cell lysates then were incubated with anti-PAR1, WEDE-15, or mouse IgG control antibody. Immunoprecipitates were eluted with 2× SDS-sample buffer, resolved by SDS/PAGE, and transferred to membranes for immunoblotting. FLAG-tagged β-arrestins were immunoprecipitated from transfected EA.hy926 cells plated at 4 × 106 cells in 100-mm culture dishes. Cells were lysed as indicated above, and equivalent amounts of cell lysates were incubated with anti-FLAG M2 Affinity Gel (Sigma) or mouse IgG control overnight at 4 °C. Immunoprecipitates were eluted, analyzed by immunoblotting and were developed with SuperSignal West Femto (Pierce).
Protein Extraction Assays.
Endothelial EA.hy926 cells were plated in 60-mm dishes at 3 × 106 cells per well, were serum starved overnight, and then were incubated with or without APC for various times at 37 °C. Cells were lysed in buffer containing 50 mM Tris-HCl (pH 7.4), 100 mM sodium chloride, 2 mM MgCl2, 1% (vol/vol) Triton X-100, 10% (vol/vol) glycerol supplemented with 1 mM DTT, and protease inhibitors. Cell lysates were centrifuged at 20,800 × g for 30 min at 4 °C using an Eppendorf 5417R tabletop centrifuge (Eppendorf). The supernatant representing the soluble fraction was collected (31, 32), and protein concentrations were determined. Total cell lysates were collected in parallel using RIPA buffer containing 50 mM Tris-HCL (pH 8.0), 150 mM sodium chloride, 5 mM EDTA, 0.5% (wt/vol) sodium deoxycholate, 1% (vol/vol) Nonidet P-40, and 0.1% (wt/vol) SDS supplemented with protease inhibitors. Equivalent amounts of cell lysates were resolved by SDS/PAGE, and endogenous Dvl-2 was detected by immunoblotting.
Immunofluorescence Confocal Microscopy.
Endothelial EA.hy926 cells plated on glass coverslips were washed with cold PBS, fixed with 4% (wt/vol) paraformaldehyde at 4 °C, and processed for microscopy as described (17). Cells were permeabilized with 0.1% (vol/vol) Triton X-100 at room temperature for 15 min and incubated with goat polyclonal anti–Dvl-2 diluted in PBS containing 7% (vol/vol) FBS and 2% (wt/vol) BSA at room temperature for 60 min. Coverslips were washed with PBS containing 0.1% (vol/vol) Triton X-100 and incubated with Alexa Fluor 488-conjugated donkey anti-goat IgG and TRITC-conjugated phalloidin for filamentous actin. Samples were washed and mounted with FluorSave (Calbiochem). Fluorescent images of X-Y sections at 0.27 μm were captured using an Olympus spinning-disk (DSU) confocal microscope configured with a PlanApo 60× oil-immersion objective and a Hamamatsu ORCA-ER digital camera. Images were collected and processed using Slidebook 5.0 image analysis software (Intelligent Imaging Innovations Inc.), and the composite was generated using Adobe Photoshop CS3.
Purification of Caveolin-Enriched Membrane Fractions.
Endothelial EA.hy926 cells were grown to confluence in 150-mm plates, and caveolin-enriched fractions were prepared using detergent-free sucrose-gradient membrane fractionation, as previously described (41). Cells were washed twice with PBS and lysed in 150 mM sodium carbonate (pH 11.0) supplemented with protease inhibitors. Cell lysates were subjected to 10 strokes with a Dounce homogenizer, passed through an 18-gauge needle 10 times, and sonicated six times with 10-s bursts using a tip sonicator on ice (Branson Ultrasonics Corp). Cell extracts were diluted 1:2 with 80% (wt/vol) sucrose in MES-buffered saline (MBS), overlaid with 6 mL of 35% (wt/vol) sucrose/MBS followed by 4 mL of 5% (wt/vol) sucrose/MBS, and centrifuged at 220,000 x g for 20 h at 4 °C using a Beckman L7-65 ultracentrifuge with a SW41 rotor (Beckman Coulter). Twelve 1-mL gradient fractions were collected sequentially; aliquots were subjected to SDS/PAGE and immunoblotted with various antibodies.
Data Analysis.
Data were analyzed using Prism 4.0 software (GraphPad Software Inc.), and statistical significance was determined using InStat 3.0 (GraphPad). Group comparisons were made using two-way ANOVA and Bonferroni post tests.
Supplementary Material
Acknowledgments
We thank members of the J.T. laboratory for comments and advice. This work was supported by National Institutes of Health Grant HL073328 (to J.T.), a University of California Tobacco-Related Disease Research Program Exploratory Award (to J.T.), and a University of California Tobacco-Related Disease Research Program Postdoctoral Fellowship Award 20FT-0074 (to U.J.K.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 19849.
See Commentary on page 19839.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112482108/-/DCSupplemental.
References
- 1.Coughlin SR. Molecular mechanisms of thrombin signaling. Semin Hematol. 1994;31(4):270–277. [PubMed] [Google Scholar]
- 2.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 3.Bernard GR, et al. Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 4.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 5.Russo A, Soh UJ, Paing MM, Arora P, Trejo J. Caveolae are required for protease-selective signaling by protease-activated receptor-1. Proc Natl Acad Sci USA. 2009;106:6393–6397. doi: 10.1073/pnas.0810687106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae JS, Yang L, Rezaie AR. Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proc Natl Acad Sci USA. 2007;104:2867–2872. doi: 10.1073/pnas.0611493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerschen EJ, et al. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204:2439–2448. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407(6801):258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin JN, et al. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J Biol Chem. 2005;280:25048–25059. doi: 10.1074/jbc.M414090200. [DOI] [PubMed] [Google Scholar]
- 10.Komarova YA, Mehta D, Malik AB. Dual regulation of endothelial junctional permeability. Sci STKE. 2007;2007:re8. doi: 10.1126/stke.4122007re8. [DOI] [PubMed] [Google Scholar]
- 11.Finigan JH, et al. Activated protein C mediates novel lung endothelial barrier enhancement: Role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 12.Camerer E, et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. 2009;119:1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson CE, Stasek JE, Schaphorst KL, Davis HW, Garcia JG. Mechanisms of pertussis toxin-induced barrier dysfunction in bovine pulmonary artery endothelial cell monolayers. Am J Physiol. 1995;268:L926–L934. doi: 10.1152/ajplung.1995.268.6.L926. [DOI] [PubMed] [Google Scholar]
- 14.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 15.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17(3):126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urban JD, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320(1):1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 17.Paing MM, Stutts AB, Kohout TA, Lefkowitz RJ, Trejo J. β-Arrestins regulate protease-activated receptor-1 desensitization but not internalization or down-regulation. J Biol Chem. 2002;277:1292–1300. doi: 10.1074/jbc.M109160200. [DOI] [PubMed] [Google Scholar]
- 18.Chen CH, Paing MM, Trejo J. Termination of protease-activated receptor-1 signaling by β-arrestins is independent of receptor phosphorylation. J Biol Chem. 2004;279:10020–10031. doi: 10.1074/jbc.M310590200. [DOI] [PubMed] [Google Scholar]
- 19.Goel R, Phillips-Mason PJ, Raben DM, Baldassare JJ. Alpha-thrombin induces rapid and sustained Akt phosphorylation by β-arrestin1-dependent and -independent mechanisms, and only the sustained Akt phosphorylation is essential for G1 phase progression. J Biol Chem. 2002;277:18640–18648. doi: 10.1074/jbc.M108995200. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs JJ, Hara MR, Davenport CL, Kim J, Lefkowitz RJ. Arrestin development: Emerging roles for beta-arrestins in developmental signaling pathways. Dev Cell. 2009;17(4):443–458. doi: 10.1016/j.devcel.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, et al. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 22.Bryja V, et al. Beta-arrestin and casein kinase 1/2 define distinct branches of non-canonical WNT signalling pathways. EMBO Rep. 2008;9:1244–1250. doi: 10.1038/embor.2008.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan H, et al. Beta-arrestin 2 negatively regulates sepsis-induced inflammation. Immunology. 2010;130:344–351. doi: 10.1111/j.1365-2567.2009.03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basher F, et al. Beta-arrestin 2: A negative regulator of inflammatory responses in polymorphonuclear leukocytes. Int J Clin Exp Med. 2008;1(1):32–41. [PMC free article] [PubMed] [Google Scholar]
- 25.Bae J-S, Yang L, Manithody C, Rezaie AR. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–3916. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. β-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci USA. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee YN, Gao Y, Wang HY. Differential mediation of the Wnt canonical pathway by mammalian Dishevelleds-1, -2, and -3. Cell Signal. 2008;20:443–452. doi: 10.1016/j.cellsig.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009;23(3):265–277. doi: 10.1101/gad.1760809. [DOI] [PubMed] [Google Scholar]
- 29.Nishita M, et al. Ror2/Frizzled complex mediates Wnt5a-induced AP-1 activation by regulating Dishevelled polymerization. Mol Cell Biol. 2010;30:3610–3619. doi: 10.1128/MCB.00177-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarz-Romond T, et al. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol. 2007;14:484–492. doi: 10.1038/nsmb1247. [DOI] [PubMed] [Google Scholar]
- 31.Harrington EO, Newton J, Morin N, Rounds S. Barrier dysfunction and RhoA activation are blunted by homocysteine and adenosine in pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1091–L1097. doi: 10.1152/ajplung.00421.2003. [DOI] [PubMed] [Google Scholar]
- 32.Potempa S, Ridley AJ. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol Biol Cell. 1998;9:2185–2200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: Biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulte G, Schambony A, Bryja V. beta-Arrestins—scaffolds and signalling elements essential for WNT/Frizzled signalling pathways? Br J Pharmacol. 2010;159:1051–1058. doi: 10.1111/j.1476-5381.2009.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turm H, et al. Protease-activated receptor-1 (PAR1) acts via a novel Galpha13-dishevelled axis to stabilize beta-catenin levels. J Biol Chem. 2010;285:15137–15148. doi: 10.1074/jbc.M109.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beckers CM, García-Vallejo JJ, van Hinsbergh VW, van Nieuw Amerongen GP. Nuclear targeting of beta-catenin and p120ctn during thrombin-induced endothelial barrier dysfunction. Cardiovasc Res. 2008;79:679–688. doi: 10.1093/cvr/cvn127. [DOI] [PubMed] [Google Scholar]
- 37.Meigs TE, Fields TA, McKee DD, Casey PJ. Interaction of Galpha 12 and Galpha 13 with the cytoplasmic domain of cadherin provides a mechanism for beta -catenin release. Proc Natl Acad Sci USA. 2001;98:519–524. doi: 10.1073/pnas.021350998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero G, et al. Parathyroid hormone receptor directly interacts with dishevelled to regulate beta-Catenin signaling and osteoclastogenesis. J Biol Chem. 2010;285:14756–14763. doi: 10.1074/jbc.M110.102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 40.Paing MM, Johnston CA, Siderovski DP, Trejo J. Clathrin adaptor AP2 regulates thrombin receptor constitutive internalization and endothelial cell resensitization. Mol Cell Biol. 2006;26:3231–3242. doi: 10.1128/MCB.26.8.3231-3242.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song KS, et al. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]