Abstract
The machinery that conducts the light-driven reactions of oxygenic photosynthesis is hosted within specialized paired membranes called thylakoids. In higher plants, the thylakoids are segregated into two morphological and functional domains called grana and stroma lamellae. A large fraction of the luminal volume of the granal thylakoids is occupied by the oxygen-evolving complex of photosystem II. Electron microscopy data we obtained on dark- and light-adapted Arabidopsis thylakoids indicate that the granal thylakoid lumen significantly expands in the light. Models generated for the organization of the oxygen-evolving complex within the granal lumen predict that the light-induced expansion greatly alleviates restrictions imposed on protein diffusion in this compartment in the dark. Experiments monitoring the redox kinetics of the luminal electron carrier plastocyanin support this prediction. The impact of the increase in protein mobility within the granal luminal compartment in the light on photosynthetic electron transport rates and processes associated with the repair of photodamaged photosystem II complexes is discussed.
The primary processes of photosynthesis in cyanobacteria, algae, and higher plants are carried out within flattened vesicles, called thylakoids, which host the molecular complexes that conduct the light-driven reactions of photosynthesis and provide a medium for energy transduction. In higher plants and some green algae, the thylakoids are differentiated into two distinct morphological domains: cylindrical stacked regions ranging between 300 and 600 nm in diameter, coined grana, and unstacked membrane regions that interconnect the grana called stroma lamellae. The photosynthetic protein complexes are unevenly distributed between the two domains: most of photosystem II (PSII) and the major light-harvesting antenna complex II (LHCII) are localized in the appressed regions of the grana, whereas photosystem I (PSI) and ATP synthase are confined to nonappressed membrane regions, which include the stroma lamellae and grana end membranes and margins (refs. 1–4 and references therein).
The current study focuses on the thylakoid luminal compartment. This compartment forms a continuous aqueous space encased by the thylakoid membranes, which separate it from the chloroplast stroma. The major inhabitant of the thylakoid lumen in the granal membrane domains is the oxygen-evolving complex (OEC), which stabilizes the manganese catalytic center of PSII and optimizes the ionic environment for water oxidation (ref. 5 and references therein). The very high density of PSII in the granal membranes (6) implies that the space available for movement in the lumen of these thylakoids may be limited, depending on the thickness of this compartment. This impending constraint, in turn, raises the possibility that diffusion of proteins in the granal thylakoid lumen may be modulated by reversible changes in its thickness. Such changes may occur in response to alterations in the light environment. To test this possibility, we combined electron microscopy and light scattering analyses with models for the packing arrangement of OEC within the lumen, as well as with measurements of the redox equilibration in the high-potential chain, which is governed by diffusion of the small luminal protein plastocyanin (PC). The results obtained indicate that the grana lumen undergoes significant expansion in the light, considerably increasing the space available for protein diffusion in this compartment. We propose that the increase in available diffusion space facilitates PC-mediated electron transport from cytochrome b6f to PSI and the repair of photodamaged PSII complexes.
Results
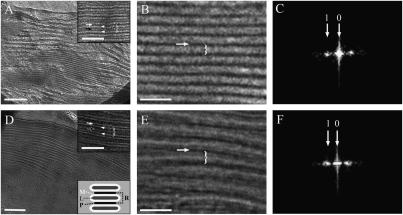
To optimally preserve the native structure of the thylakoid membranes, and to avoid complications arising from the use of contrasting agents, vitreous sections of unfixed, unstained, leaf samples were examined by cryotransmission electron microscopy (cryo-TEM). The preparation of frozen-hydrated leaf tissues for EM examination is technically challenging (1) and hitherto only one EM study has been conducted on such tissues (7). We nevertheless chose to perform the microscopic analyses on leaf samples, rather than on isolated chloroplasts or thylakoid membranes, as such samples represent the native state of the system most authentically. Fig. 1A shows an image of a chloroplast within a dark-adapted leaf. The measurements were performed on high-magnification images of individual granum stacks, such as the one depicted in Fig. 1B. The membrane bilayers of the stacked thylakoids were clearly discerned in the micrographs (Fig. 1 A and D, Insets). The thylakoid discs are separated from each other by a region corresponding to the aqueous stromal space, referred to as the partition gap. The thickness of the membrane bilayers and the width of the partition gaps were determined from intensity profiles along lines perpendicular to the layers in the stacks; the distance between adjacent partition gaps in the stacks (repeat distance) was derived from the power spectra of the images (Fig. 1 C and F). To derive the thickness of the thylakoid lumen (L) we subtracted the widths of one partition gap (P) and two thylakoid membrane bilayers (M) from the stacking repeat distance (R) (Fig. 1D, Lower Inset). The values obtained for the thickness of the membrane bilayers, stacking repeat distance, partition gap width, and granal thylakoid lumen thickness were 4.0 ± 0.2 nm, 16.3 ± 0.6 nm, 3.6 ± 0.4 nm, and 4.7 ± 0.8 nm, respectively. The same analysis was performed on samples derived from leaves that were illuminated (while still attached to the plant) with 500 μmol photons m−2⋅s−1 for 30 min (Fig. 1 D–F). The results revealed significant changes in the stacking repeat distance and the thickness of the membrane bilayer, the former increasing to 19.1 ± 0.5 nm and the latter decreasing to 3.3 ± 0.1 nm; the width of the partition gap was not significantly altered, measuring 3.3 ± 0.2 nm. Together, these values yield a luminal width of 9.2 ± 0.6 nm, 96% wider than that of dark-adapted granal thylakoids.
Fig. 1.
Ultrastructural analysis of thylakoid membranes in Arabidopsis chloroplasts. (A, B, D, and E) Low- (A and D) and high- (B and E) magnification cryo-TEM images of vitrified dark- (A and B) and light- (D and E) adapted leaf samples. The stacking repeat unit, which includes the thylakoid lumen, the two encasing membrane bilayers, and the width of one partition gap (white arrow), is marked by a brace. (A, Inset and D, Upper Inset). Defocus images of the grana in which the membrane bilayers (white arrowheads) are clearly discerned. The luminal space is marked by a black arrow. (D, Lower Inset). Scheme depicting the relation between the stacking repeat distance (R), membrane bilayers (M), lumen width (L), and partition gap (P); R = 0.5P + M + L + M + 0.5P. (C and F) Power spectra of grana from dark- (C) and light- (F) adapted leaves. [Scale bars, 200 nm (A and D); 50 nm (B and E, and Insets).]
The measurements described above were also performed on cryoimmobolized, freeze-substituted, and resin-embedded leaf samples (Fig. S1). This procedure is also known to preserve cellular structures and organelles with high fidelity (1, 8–10). The results obtained from this analysis likewise indicated an extensive (91%) expansion of the granal thylakoid lumen in the light. Here too, illumination of the leaves resulted in an increase of the stacking repeat distance, from 16.8 ± 0.4 nm to 18.6 ± 0.4 nm, although the width of the partition gap changed more significantly compared with the frozen-hydrated samples, from 4.2 ± 0.1 nm to 3.2 ± 0.1 nm. Due to the lower contrast, we could not determine the thickness of the lipid bilayers in the cryoimmobilized, freeze-substituted samples. Using the values obtained for the bilayer thickness from the measurements performed on the vitreous sections, we derived luminal width values of 4.6 ± 0.5 nm and 8.8 ± 0.4 nm, for dark- and light-adapted thylakoids, respectively.
In addition to the microscopic examinations, we also studied the effect of illumination by performing light-scattering measurements on isolated thylakoids (Fig. S2). The results obtained showed a reversible increase in the (90°) light scattering signal upon illumination (Fig. S2B). Addition of the uncoupler ammonium chloride during illumination led to a decrease in scattering (Fig. S2C). Experiments performed on dark-adapted thylakoids suspended in solutions containing different concentrations of sorbitol showed that the scattering signal decreased with increasing concentrations of the osmolyte, which should lead to contraction of the thylakoids (e.g., ref. 11). Altogether, the results are consistent with an overall expansion of the lamellar system in the light and suggest that it is correlated to changes in transthylakoid ΔpH. Previous studies revealed similar trends in light scattering but the changes were attributed to structural changes in the thylakoid membranes themselves (12) or to changes in LHCII xanthophyll composition occurring during nonphotochemical quenching (NPQ) (13, 14). Currently, we cannot distinguish between the contributions of the above processes to the observed changes in light scattering.
We then used the electron microscopy data to assess the consequences of the observed light-induced expansion of the granal thylakoid lumen on protein diffusion. As the major obstacle for diffusion in this compartment is the OEC, we generated models for its organization in the lumen of dark- and light-adapted granal thylakoids. (The contribution of the luminal part of cytochrome b6f to macromolecular crowding in the grana lumen is relatively minor, even if one assumes that it populates granal domains with a density about 50% of that of PSII) (3). Two basic arrangements of OEC were considered: A configuration in which opposing complexes directly face each other across the lumen and one in which a staggered configuration is adopted (Fig. 2). For the first arrangement to be realized, a minimal luminal width corresponding to twice the height of the OEC is required. Available structural data indicate that the OEC in higher plants has a height of about 4–5 nm (15). Thus, a minimum width of 8–10 nm is necessary if opposing complexes are to assume a face-to-face configuration. Our data indicate that, at least in Arabidopsis, this requirement is not met in dark-adapted leaves, implying a staggered arrangement of the OEC in the dark. Although expansion of the lumen in the light could, in principle, allow for a face-to-face configuration, adopting such an arrangement on a macroscopic level is unlikely, unless specific interactions stabilize this entropically unfavorable configuration. We therefore assumed a staggered configuration for both states. Such an arrangement is in line with electron tomography (16) and atomic force microscopy data (17) obtained on isolated spinach chloroplasts and grana thylakoids, respectively. However, our findings hold just as well if a face-to-face configuration is adopted in the light. In this case, the difference in available diffusion space in the lumen between dark- and light-adapted thylakoids is expected to be even larger.
Fig. 2.
Models of dark- (Left) and light-adapted (Right) granal thylakoids. The structures of PSII and plastocyanin (PC, small globule inside the lumen) are drawn to scale. In the dark, the measured luminal thickness does not allow a face-to-face arrangement of OECs residing on opposite membranes (Lower), indicating that they assume a staggered arrangement (Upper Left). Although expansion of the lumen in the light (Upper Right) may allow for OECs to arrange face to face, adopting such an arrangement upon illumination is unlikely (see text).
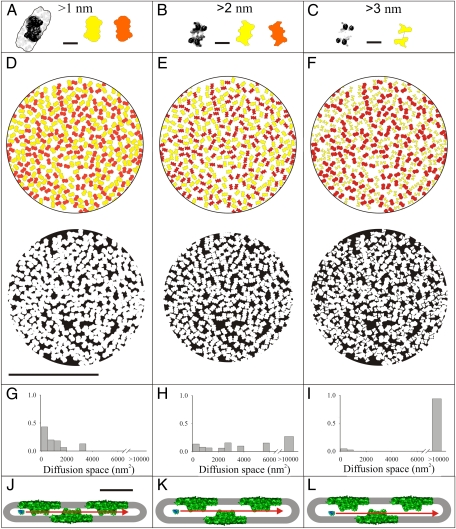
To generate density maps of the OEC complexes within the granal lumen, the positions and orientations of OECs were inferred from those of PSII complexes within the encasing membrane layers, extracted from tomographic reconstructions of frozen-hydrated grana stacks obtained from mildly solubilized thylakoid membrane preparations (18). Fig. 3 A–C shows contours of the OECs (15) as seen at different distances from the lipid bilayer. The density of the PSII complexes within each of the two membrane layers encasing the lumen is very high, averaging at 1,630 complexes per square micrometer. This value is within 5% of that determined from a freeze-fracture EM analysis of untreated (nonsolubilized) thylakoid membranes, which yielded a density of 1,720 complexes per square micrometer (19). An immediate consequence of the high PSII density is that the orientation of the oval contoured OEC complexes (Fig. 3A) cannot be purely random. Rather, a parallel orientation of neighboring complexes is imposed (Fig. 3D, Upper). Indeed, a next-neighbor angle distribution analysis revealed that the angles of 0° to 20° between adjacent OEC pairs in the thylakoid lumen are more prevalent than expected for an ensemble consisting of randomly oriented particles (Fig. S3). A similar tendency toward a parallel alignment was found for PSII complexes in isolated grana thylakoid membranes from spinach imaged by atomic force microscopy (17).
Fig. 3.
Models of OEC packing and available diffusion space in the lumen of dark- and light-adapted thylakoids. (A–C) Structure of the OEC (15) sliced near the plane or ∼2 or 3 nm away from the membrane bilayer surface (black and white) and corresponding projections (colored). The yellow and orange contours are mirror images of each other and represent OEC complexes that reside on opposite bilayers of one granum disk. (Scale bars, 10 nm.) (D–F, Upper) Predicted 2D density maps of OECs at the middle of the lumen of dark-adapted (D) or light-adapted (E) thylakoids, or ∼3 nm from one of the membranes in light-adapted thylakoids (F). The distributions were generated using the contours shown in A, B, and C, respectively. (D–F, Lower) Maps depicting the available diffusion area (black) for PC at the middle of the lumen of dark- (D) or light-adapted (E) thylakoids, or near one of the encasing membrane bilayers (F), as illustrated in J–L. Expansion of the lumen in the light allows PC to travel in the gap between an OEC and the opposing membrane surface, further (and substantially) increasing its available diffusion space (L). (G–I) Histograms of areas available for PC diffusion, as derived from the maps shown in D–F, Lower. For dark-adapted thylakoids (G), PC diffusion is restricted to small domains. The space available for PC diffusion increases considerably in light-adapted thylakoids (H) and becomes almost continuous once diffusion near the membrane surfaces is taken into account (L). [Scale bars, 250 nm (D–F); 20 nm (J–L).]
The expansion of the thylakoid lumen under light provides additional volume and paths for diffusion in this compartment. To estimate the change in the fraction available for diffusion, we generated density maps of OECs within the granal lumen of dark- and light-adapted thylakoids. The maps were constructed for two cases: (i) a particle moving along the midplane of the lumen (Fig. 3 D and E, Upper) or (ii) a particle displaced from the midplane and moving close to one of the two membranes that encase the lumen (Fig. 3F, Upper), which is applicable only to light-adapted thylakoids. These two cases are illustrated in Fig. 3 J–L. The probe chosen was the small luminal protein PC, which shuttles electrons from cytochrome b6f to PSI. The contours of the OECs were modified to account for the distance of the diffusing probe from the surface of the encasing membranes. For the case where the PC molecule was displaced from the luminal midplane, different contours were used for OECs associated with the nearby and distant membranes (orange- and yellow-colored particles in Fig. 3F).
Next, we estimated the diffusion area available for PC within the granal lumen. This 10.5-kDa protein is about 4 × 3 × 3 nm (20), implying that the gaps between adjacent OECs should not be smaller than 3 nm to allow for its passage. Therefore, the contours of OEC were expanded by 1.5 nm. The results are shown in Fig. 3 D–F, Lower. Evidently, diffusion of PC within the granal lumen of dark-adapted thylakoids is restricted to small, isolated domains (black regions in Fig. 3D, Lower). Analysis of the diffusion space (Fig. 3G) revealed that more than 85% of PC molecules are trapped in domains smaller than 40 × 40 nm2 and more than 50% are confined to domains smaller than 25 × 25 nm2. The only way for PC to escape these domains is via gaps between neighboring OECs, which are transiently formed by Brownian rearrangements of the PSII-LHCII network. The formation of such gaps is unlikely to be frequent; data derived from Monte Carlo simulations of PSII diffusion in granal thylakoid membranes (21) suggest that PSII traverses a distance of 3 nm in about 1 s. Fast, long-range diffusion of PC molecules within the lumen of appressed thylakoid domains is therefore highly unlikely. The situation is quite different for light-adapted thylakoids. As can be seen, the area available for diffusion of PC migrating at the midplane of the lumen (Fig. 3 E, Lower and H, Lower) or displaced from the midplane (Fig. 3 F, Lower and I, Lower) increases considerably, as the parts of the OECs encountered by the particles moving along this plane become smaller. Evidently, the gaps formed between OECs associated with one of the membrane faces and the opposing membrane upon expansion of the lumen (Fig. 3I) significantly enlarge the available diffusion space. The result is the emergence of continuous channels that enable long-range diffusion throughout the granum.
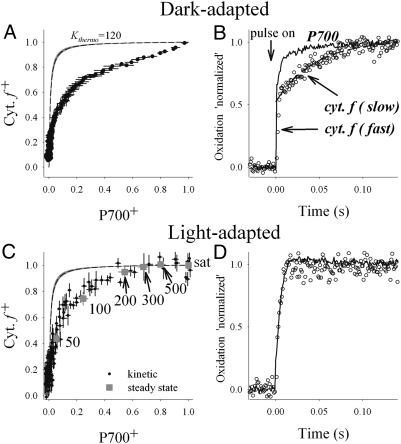
To test the predictions made above for the difference in PC mobility between dark- and light-adapted granal thylakoids, we monitored the redox equilibration within the high-potential chain (the part of the electron transport chain between cytochrome f and P700). By plotting the (dark) reduction kinetics of cytochrome f+ versus that of P700+ the apparent equilibrium constant (Kapp) can be deduced (Fig. 4 A and C). Deviation of Kapp from the thermodynamically expected constant (Kthermo), derived from the redox midpoint potential (EM) of the two redox components, can be used as a diagnostic tool for the presence of disequilibria that may be due to restricted PC diffusion (22). If diffusion of PC in the thylakoid lumen is sufficiently fast, Kapp is expected to be similar to Kthermo. On the other hand, if the mobility of PC is impeded, electrons arriving at cytochrome f+ would not be efficiently transferred to P700+, resulting in lowered cytochrome f oxidation levels (for a given P700+ level) and, hence, in divergence of Kthermo and Kapp.
Fig. 4.
Redox kinetics and equilibration between cytochrome f and P700 measured in dark-adapted (A and B) and light-adapted (C and D) leaves. (A) Equilibration plot of cytochrome f and P700 in dark-adapted leaves, generated from relaxation kinetic measurements following a 100-ms light pulse. The dashed line shows the theoretical equilibration curve (Kthermo), calculated using +352 and +472 mV for the redox midpoint potentials of cytochrome f and P700, respectively. The gray delineation of the line represents the range of values reported in the literature for the potentials (22). Error bars represent SE (n = 8). (B) Oxidation kinetics of cytochrome f and P700 induced by a saturating light pulse in dark-adapted leaves. The oxidation kinetics of P700 is monophasic, whereas that of cytochrome f is biphasic, exhibiting clearly distinguished slow and fast components. (C) Equilibration plot of cytochrome f and P700 in light-adapted leaves (black circles). Gray squares show the steady state oxidation levels in leaves exposed to different light intensities, in the absence of methylviologen. Sat, saturated light intensity. (D) Oxidation kinetics of cytochrome f and P700 in light-adapted leaves. Note that the slow cytochrome f component seen in dark-adapted leaves is no longer apparent. The absolute (nonnormalized) cytochrome f amplitudes are virtually identical for light- and dark-adapted samples.
Measurements conducted on dark-adapted leaves were performed following a 100-ms saturating light pulse. To allow unrestricted electron flow from PSI, methylviologen (MV) was added. The results are shown in Fig. 4A (filled circles). As reported previously for spinach (22), a clear deviation from the thermodynamic equilibrium constant (dashed line in Fig. 4A) is apparent, indicating restricted PC diffusion. In addition, the curve is clearly biphasic, with a fraction of about 30–40% of the cytochrome f population being characterized by a very small equilibrium constant (∼1) and the remaining equilibrating with a much larger constant (>20). A restricted diffusion of PC in the lumen is further supported by the biphasic oxidation kinetics of cytochrome f, induced by a saturating light pulse (Fig. 4B). Between 40 and 50% of the molecules are oxidized significantly slower than P700. Fitting of the slow phase with an exponential yielded a rate constant of 24 ± 6 ms−1. The presence of such a phase suggests that a significant fraction of the cytochrome b6f complexes is in poor redox contact with PSI. Given the similarity between the relative amplitudes of this fraction and the one corresponding to the slowly equilibrating cytochrome f species (see above), it seems likely that the two represent the same ensemble.
The presence of a slowly equilibrating population of cytochrome f molecules implies that oxidation of plasto(hydro)quinone (PQH2) by the corresponding cytochrome b6f complexes must also be attenuated in dark-adapted leaves. To verify this point, we modeled the oxidation behavior of the high-potential chain following a light pulse by numerically solving the differential equations that describe electron transfer from PQH2 to P700+. The results (Fig. S4) revealed that a slow cytochrome f component becomes apparent only when both PC-mediated electron transfer and the transport of electrons from PSII to the cytochrome b6f complex via PQH2 are impeded.
To study the redox equilibration dynamics between cytochrome f and P700 in light-adapted leaves, we performed two types of experiments. The first experiment was similar to the one described above for dark-adapted leaves, but the samples were illuminated for 5 min with 500 μmol quanta m−2⋅s−1 and, again, for 30 s, before each measurement (Fig. 4C, black circles). The dark interval between the 30-s preillumination and the 100-ms saturating pulse was 300 ms. In the second set of experiments, we probed the steady state oxidation levels of cytochrome f and P700 at different light intensities. The latter experiments were conducted in the absence of methylviologen, to avoid H2O2 poisoning. Before the measurements, the leaves were illuminated to activate the Calvin-Benson cycle, to allow unperturbed electron flux from PSI. The leaves were then exposed to different light intensities and the relative oxidation states of cytochrome f and P700 were measured (Fig. 4C, gray squares). In contrast to the dark-adapted samples, almost no deviations between Kapp and Kthermo were observed for P700 oxidation levels ranging from ∼50 to 100% or for light intensities ranging from 200 to 500 μmol photons m−2⋅s−1, indicating facilitated PC diffusion. In agreement with this observation, the slow cytochrome f oxidation phase seen in dark-adapted leaves during the light pulse was no longer apparent under these conditions (Fig. 4D). At lower P700 oxidation levels or light intensities, the data were similar to those measured for dark-adapted samples, suggesting that PC diffusion was restricted probably because of narrowing of the lumen at these light intensities. Alternatively, it may reflect restricted diffusion of plastoquinone molecules within the lipid phase under these conditions (see below).
Discussion
Electron microscopy data obtained in this study show that the granal thylakoid lumen expands in the light. Models generated for the organization of the OEC suggest that this expansion alleviates the restriction on protein mobility that prevails in the dark. Although these models do not represent the full complexity of the granal thylakoid lumen, their predictions are qualitatively supported by the results obtained from the redox-equilibration measurements, which indicate that diffusion of PC in the thylakoid lumen is facilitated under sufficiently high light intensities (>100 μmol photons m−2·s−1).
Early studies performed by Murakami and Packer on isolated thylakoid membranes indicated an overall contraction of appressed membrane domains upon illumination, including thinning of the membrane bilayers and shrinkage of the granal luminal space (12, 23). These studies also revealed that the above changes were accompanied by an increase in membrane hydrophobicity. The latter effect (which is expected to lead to a decrease in bilayer hydration level) and the reduction in membranes thickness were recently ascribed to alterations in LHCII conformation/organization and de-epoxidation of LHCII-bound violaxanthin to zeaxanthin associated with NPQ (24). Contraction of the thylakoid lumen was likewise used as the basis for a model suggesting that it is required for correct assembly of the OEC subunits within this compartment in the light (25). The micrographs we obtained also reveal thinning of the granal thylakoid membranes in the light which, given the illumination conditions we used, may arise from the aforementioned processes. However, they indicate that thinning is accompanied by expansion rather than contraction of the luminal compartment. This discrepancy likely reflects differences between the samples used in the two studies. The samples analyzed by Murakami and Packer were prepared for EM examination by chemical fixation, which is inherently slow (minutes) and involves substitution of water molecules at ambient temperatures. Consequently, samples prepared by this method are prone to artifacts, including deformation and shrinkage of membrane systems and extraction of lipids (see ref. 1 and references therein). The latter may lead to perforation of the thylakoids, resulting in changes in osmolarity, ionic content, and pH and, consequently, in their structural characteristics (10). These deleterious effects are circumvented entirely when untreated vitreous sections are examined by cryo-EM, or largely, when the samples are prepared by means of cryoimmobilization and freeze substitution, which were the methods we used in our study. In addition, our microscopic studies were performed on leaf preparations rather than on isolated thylakoids, thus preserving the native cellular milieu.
Linear electron transport involves two diffusion-controlled processes: transfer of electrons from PSII to cytochrome b6f within the lipid phase, via plastoquinone (PQ), and from cytochrome b6f to PSI in the thylakoid lumen, by PC. As we have shown, diffusion of PC within the granal thylakoid lumen is highly restricted in dark-adapted thylakoids. Diffusion of PQ in the lipid bilayer is likewise highly obstructed by the PSII and LHCII complexes in the granal thylakoid membranes (26, 27). The location of cytochrome b6f within the thylakoid membranes dictates the average diffusion distances for the two electron carriers and thus has a strong impact on the time scale of the two processes. Its exact localization in the thylakoid membranes of higher plants is under debate with some works suggesting that it is present in granal domains and others proposing that it is excluded from these regions (see ref. 2 and references therein). The results we obtained from the redox-equilibration measurements suggest that a significant fraction of the cytochrome b6f complexes resides within the granal thylakoid membranes and that the shuttling of electrons from these complexes to PSI limits electron transport in dark-adapted leaves. On the basis of the redox-equilibration curve (Fig. 4A) and the amplitude of the slowly oxidizing cytochrome f (Fig. 4B) observed in dark-adapted leaves, we estimate that this fraction comprises 30–50% of the cytochrome b6f population; the remaining 70–50% are likely localized to grana margins and/or stroma lamellar regions. These values are in agreement with previous estimates on the basis of fractionation analyses (28, 29).
The results obtained from the simulations of electron flow through the high-potential chain (Fig. S4) indicate that, for the slowly oxidizing cytochrome f component observed in dark-adapted plants to be present, the transport of electrons both to and from the cytochrome b6f complex must be attenuated. We propose that the decreased flow of electrons to cytochrome b6f in the dark is, at least in part, due to restricted diffusion of a subpopulation of PQ molecules within the thylakoid membranes, as was previously suggested (26, 27). This subpopulation likely corresponds to molecules trapped at the densely populated areas of the granum core; the mobility of molecules present at the grana margins is probably significantly higher. We therefore believe that the resulting heterogeneity of slowly and (more) rapidly diffusing PQ and PC molecules underlies the complex cytochrome f redox kinetics in the dark. We further propose that the restriction imposed on PQ diffusion in the dark is reduced in the light. The alleviation of this restriction, along with the pronounced facilitation of PC diffusion due to luminal expansion, leads to the disappearance of the slow cytochrome f component in the light-adapted leaves. We speculate that underlying the increase in the mobility of PQ in the light are alterations in PSII-LHCII macrostructure, which reconfigure the space available for its diffusion in the granal thylakoid membranes. Given that the PSII-LHCII assembly is known to undergo extensive light-dependent reorganizations, and given that the diffusion of PQ is expected to be highly sensitive to the details of the protein landscape in the membranes (6), such a scenario is not unlikely.
The basis for light-induced expansion of the thylakoid lumen is not clear, as various processes may affect luminal thickness. One possibility is that light-driven acidification of the lumen leads to an influx of anions, which, in turn, causes osmotic swelling of the lumen (11, 30). Recently, two chloride channels were identified in the thylakoid membranes of Arabidopsis (reviewed in ref. 31). These channels appear to be voltage gated (32), which raises the possibility that illumination leads to a proton motive force-controlled swelling of the lumen by chloride influx.
The physiological benefit of the observed expansion of the granal thylakoid lumen and, along with it, the increase in the free diffusion space in this compartment, is straightforward. As we have shown, PC-mediated electron transport is considerably facilitated in light-adapted thylakoids. This would be clearly beneficial for both linear and cyclic electron transport and may serve a regulatory role if expansion of the lumen increases with the photon flux density. Such a gradation, at low light intensities (i.e., up to 100–200 μmol photons m−2⋅s−1), would be consistent with the results we obtained from the redox kinetic measurements shown in Fig. 4C. Another pertinent process that is likely to benefit from the increase in granal luminal thickness is the repair of damaged PSII complexes. Photoinduced damage of the D1 subunit of PSII occurs continuously, under all light intensities (33, 34), necessitating that impaired complexes be efficiently repaired. The repair of damaged PSII complexes involves release of the manganese from the manganese cluster and dissociation of OEC from the PSII core (35–37). Moreover, it was recently shown that subunits of the OEC are also prone to reactive oxygen species (ROS)-mediated damage (38) and, therefore, must be degraded and replenished. Clearly, disassembly of OEC would occur more readily in an expanded lumen, where it is less crowded. An increased luminal volume may also render damaged subunits more accessible to the luminal proteases that degrade damaged components during the repair cycle (39). Shuttling of newly synthesized OEC subunits from the stroma lamellae, where they are inserted into the thylakoids, to the granal thylakoids, is likewise expected to be facilitated by expansion of the lumen.
It is not clear what advantage, if any, a contracted grana lumen may have: this state may merely be a consequence of the shutdown of processes that take place in the light (and result in lumen expansion). One possibility could be a decrease in the accessibility of the grana lumen to proteins (e.g., luminally residing proteases) in the dark. Another possibility might be to minimize the formation of ROS at PSI under conditions where the light intensity increases abruptly following prolonged periods of dim light, e.g., upon clearing of the sky from cloudiness. Although often neglected, photoinhibition of PSI does occur and, in certain plants and under certain stress conditions, can be quite substantial. Moreover, as the repair of damaged PSI complexes is slow and inefficient, photoinhibition of PSI could be particularly harmful. The primary cause for photoinhibition of PSI is overreduction of its acceptor side, which leads to the formation of ROS that eventually destroy the (reduced) iron-sulfur centers in the complex (see refs. 40 and 41 for recent reviews on these topics). We propose that rapid, unobstructed diffusion of PC within the lumen during the lag time between the initiation of electron transport and reactivation of the Calvin-Benson cycle may lead to reduction of the NADP+ pool, resulting in transient shortage of electron acceptors and, therefore, in excessive electron pressure at the acceptor side of PSI. Such a buildup of potentially deleterious electron pressure on PSI may be avoided by attenuation of the transport of electrons from the grana to the stroma lamellar-residing PSI, by restricting PC diffusion in the thylakoid lumen. This would inevitably result in overreduction of PSII, but PSII is better equipped to manage excessive excitation pressures (by means of nonphotochemical energy dissipation) and is repaired much more rapidly than PSI (34, 42).
Methods
Electron Microcopy.
Dark-adapted or light-treated (500 μmol photons m−2⋅s−1, 30 min) Arabidopsis thaliana leaves were mildly degassed in 50 mM tricine buffer, pH 7.4, containing 15% dextran (40 kDa; Sigma-Aldrich). Leaf samples were then cryoimmobilized (7, 43) using an HPM 010 high-pressure freezer (Bal-Tec). For cryo-EM, the frozen samples were cut using a Leica EM FC6 cryomicrotome and the vitreous sections were transferred onto glow-discharged, carbon-coated, 200-mesh Quantifoil grids. The grids were loaded into a Gatan 626 cryoholder and examined under cryogenic conditions. Alternatively, the vitreous samples were freeze substituted and resin embedded as described (44). Plastic or vitreous thin sections were examined with an FEI Tecnai Spirit or T12 TEM, operating at 120 kV, and images were acquired with an FEI Eagle (2 k × 2 k) or TVIPS F224HD (2 k × 2 k) CCD camera.
Light Scattering.
Freshly isolated Arabidopsis thylakoids were suspended (at chlorophyll concentration of 20 μg/mL) in a sorbitol solution containing 15 mM NaCl, 5 mM MgCl2, 50 mM Hepes (pH 7.6). Unless otherwise stated, the concentration of sorbitol was 300 mM. Changes in 90° light scattering were recorded with a HoribaYvon Fluoromax 4 spectrofluorometer (λex = λem = 550 nm; bandwidth: 2 nm). Measurements involving the application of light (red; 1,000 μmol photons m−2⋅s−1) were conducted in the presence of 50 μM L634 methylviologen (MV).
Difference Spectroscopy.
Difference absorption kinetics of cytochrome f and P700 for intact Arabidopsis leaves was recorded with a home-built flash spectrometer. Cytochrome f redox kinetics was monitored following absorption changes at 545, 554, and 572 nm (22). P700 signals were derived by subtracting the absorption changes at 900 nm from those at 820 nm (22). For the measurements of dark-adapted leaves, saturating pulses (100 ms, 630 nm) were used to induce oxidation of the high-potential chain. MV was added (1 mM) to prevent electron backpressure at the PSI acceptor. For light adaptation, the plants were preilluminated for 10 min at 500 μmol photons m−2⋅s−1 to activate the Calvin-Benson cycle. This ensured an open PSI acceptor side as indicated by a stable P700 oxidation signal during a saturating pulse. MV was omitted from these measurements to avoid poisoning by H2O2. After activation of the Calvin-Benson cycle, the leaves were exposed to different light intensities and the steady-state oxidation levels of cytochrome f and P700 were derived. This was done by comparing the steady-state level with the ones corresponding to maximal and minimal oxidation levels (obtained following a 100-ms saturating light pulse and 100-ms dark period, respectively), measured immediately after the steady-state illumination. In control experiments, we measured the oxidation level of cytochrome f with and without a far-red background light (720 nm). (The application of the actinic light, which preferentially excites PSI, ensures a full oxidation of cytochrome f.) The level measured in the absence of the far-red light was 98 ± 7% of that measured in its presence. In addition, the maximal cytochrome f oxidation level measured for light-adapted plants (MV treated) was virtually the same as that measured for dark-adapted plants. The maximal normalized level (“1”) in Fig. 4, therefore, likely represents a fully oxidized cytochrome f population.
Supplementary Material
Acknowledgments
We thank Egbert Boekema and Roman Kouřil for kindly providing the tomographic data used for modeling the photosystem II distributions. The photosystem II images shown in Figs. 2 and 3 (derived from ref. 15) were provided by Bertram Daum. The electron microscopy studies were conducted at the Irving and Cherna Moskowitz Center for Nano and Bio-Nano Imaging at the Weizmann Institute of Science. This study was supported in part by Washington State University (to H.K.); the Binational Agricultural Research and Development Fund (H.K. and Z.R.); and the Israel Science Foundation (1005/07), the Carolito Stiftung, and the Minerva Foundation (to Z.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1104141109/-/DCSupplemental.
References
- 1.Nevo R, Chuartzman SG, Tsabari O, Reich Z. Architecture and plasticity of thylakoid membrane networks. In: Wada H, Murata N, editors. Lipids in Photosynthesis. New York: Springer; 2009. pp. 295–328. [Google Scholar]
- 2.Dekker JP, Boekema EJ. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim Biophys Acta. 2005;1706:12–39. doi: 10.1016/j.bbabio.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Albertsson PA. A quantitative model of the domain structure of the photosynthetic membrane. Trends Plant Sci. 2001;6:349–358. doi: 10.1016/s1360-1385(01)02021-0. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JM. Insights into the consequences of grana stacking of thylakoid membranes in vascular plants: A personal perspective. Aust J Plant Physiol. 1999;26:625–639. [Google Scholar]
- 5.Barber J. Photosynthetic generation of oxygen. Philos Trans R Soc Lond B Biol Sci. 2008;363:2665–2674. doi: 10.1098/rstb.2008.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirchhoff H. Molecular crowding and order in photosynthetic membranes. Trends Plant Sci. 2008;13:201–207. doi: 10.1016/j.tplants.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Michel M, Hillmann T, Muller M. Cryo sectioning of plant material frozen at high pressure. J Microsc. 1991;163:3–18. [Google Scholar]
- 8.Vanhecke D, Graber W, Studer D. Close-to-native ultrastructural preservation by high pressure freezing. Methods Cell Biol. 2008;88:151–164. doi: 10.1016/S0091-679X(08)00409-3. [DOI] [PubMed] [Google Scholar]
- 9.McDonald KL, Auer M. High-pressure freezing, cellular tomography, and structural cell biology. Biotechniques. 2006;41:137–, 139, 141 passim. doi: 10.2144/000112226. [DOI] [PubMed] [Google Scholar]
- 10.Pfeiffer S, Krupinska K. New insights in thylakoid membrane organization. Plant Cell Physiol. 2005;46:1443–1451. doi: 10.1093/pcp/pci156. [DOI] [PubMed] [Google Scholar]
- 11.Cruz JA, Sacksteder CA, Kanazawa A, Kramer DM. Contribution of electric field (Δ psi) to steady-state transthylakoid proton motive force (pmf) in vitro and in vivo. control of pmf parsing into Δ psi and Δ pH by ionic strength. Biochemistry. 2001;40:1226–1237. doi: 10.1021/bi0018741. [DOI] [PubMed] [Google Scholar]
- 12.Murakami S, Packer L. Protonation and chloroplast membrane structure. J Cell Biol. 1970;47:332–351. doi: 10.1083/jcb.47.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilger W, Björkman O, Thayer SS. Light-induced spectral absorbance changes in relation to photosynthesis and the epoxidation state of xanthophyll cycle components in cotton leaves. Plant Physiol. 1989;91:542–551. doi: 10.1104/pp.91.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horton P, et al. Control of the light-harvesting function of chloroplast membranes by aggregation of the LHCII chlorophyll-protein complex. FEBS Lett. 1991;292:1–4. doi: 10.1016/0014-5793(91)80819-o. [DOI] [PubMed] [Google Scholar]
- 15.Nield J, Barber J. Refinement of the structural model for the Photosystem II supercomplex of higher plants. Biochim Biophys Acta. 2006;1757:353–361. doi: 10.1016/j.bbabio.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Daum B, Nicastro D, Austin J, 2nd, McIntosh JR, Kühlbrandt W. Arrangement of photosystem II and ATP synthase in chloroplast membranes of spinach and pea. Plant Cell. 2010;22:1299–1312. doi: 10.1105/tpc.109.071431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirchhoff H, Lenhert S, Büchel C, Chi L, Nield J. Probing the organization of photosystem II in photosynthetic membranes by atomic force microscopy. Biochemistry. 2008;47:431–440. doi: 10.1021/bi7017877. [DOI] [PubMed] [Google Scholar]
- 18.Kouřil R, Oostergetel GT, Boekema EJ. Fine structure of granal thylakoid membrane organization using cryo electron tomography. Biochim Biophys Acta. 2011;1807:368–374. doi: 10.1016/j.bbabio.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Kirchhoff H, et al. Low-light-induced formation of semicrystalline photosystem II arrays in higher plant chloroplasts. Biochemistry. 2007;46:11169–11176. doi: 10.1021/bi700748y. [DOI] [PubMed] [Google Scholar]
- 20.Guss JM, Harrowell PR, Murata M, Norris VA, Freeman HC. Crystal structure analyses of reduced (CuI) poplar plastocyanin at six pH values. J Mol Biol. 1986;192:361–387. doi: 10.1016/0022-2836(86)90371-2. [DOI] [PubMed] [Google Scholar]
- 21.Kirchhoff H, Tremmel I, Haase W, Kubitscheck U. Supramolecular photosystem II organization in grana thylakoid membranes: Evidence for a structured arrangement. Biochemistry. 2004;43:9204–9213. doi: 10.1021/bi0494626. [DOI] [PubMed] [Google Scholar]
- 22.Kirchhoff H, Schöttler MA, Maurer J, Weis E. Plastocyanin redox kinetics in spinach chloroplasts: Evidence for disequilibrium in the high potential chain. Biochim Biophys Acta. 2004;1659:63–72. doi: 10.1016/j.bbabio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Murakami S, Packer L. Light-induced changes in the conformation and configuration of the thylakoid membrane of Ulva and Porphyra chloroplasts in vivo. Plant Physiol. 1970;45:289–299. doi: 10.1104/pp.45.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson MP, et al. Photoprotective energy dissipation involves the reorganization of photosystem II light-harvesting complexes in the grana membranes of spinach chloroplasts. Plant Cell. 2011;23:1468–1479. doi: 10.1105/tpc.110.081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson JM, Chow WS, De Las Rivas J. Dynamic flexibility in the structure and function of photosystem II in higher plant thylakoid membranes: the grana enigma. Photosynth Res. 2008;98:575–587. doi: 10.1007/s11120-008-9381-3. [DOI] [PubMed] [Google Scholar]
- 26.Lavergne J, Bouchaud J-P, Joliot P. Plastochinone compartmentation in chloroplasts. II. Theoretical aspects. Biochim Biophys Acta. 1992;1101:13–22. [Google Scholar]
- 27.Kirchhoff H, Horstmann S, Weis E. Control of the photosynthetic electron transport by PQ diffusion microdomains in thylakoids of higher plants. Biochim Biophys Acta. 2000;1459:148–168. doi: 10.1016/s0005-2728(00)00143-2. [DOI] [PubMed] [Google Scholar]
- 28.Cox RP, Andersson B. Lateral and transverse organisation of cytochromes in the chloroplast thylakoid membrane. Biochem Biophys Res Commun. 1981;103:1336–1342. doi: 10.1016/0006-291x(81)90269-2. [DOI] [PubMed] [Google Scholar]
- 29.Anderson JM. Distribution of the cytochromes of spinach chloroplasts between the appressed membranes of grana stacks and stroma-exposed thylakoid regions. FEBS Lett. 1982;138:62–66. [Google Scholar]
- 30.Heber U, Heldt HW. The chloroplast envelope: Structure, function, and role in leaf metabolism. Annu Rev Plant Physiol. 1981;32:139–168. [Google Scholar]
- 31.Spetea C, Schoefs B. Solute transporters in plant thylakoid membranes: Key players during photosynthesis and light stress. Commun Integr Biol. 2010;3:122–129. doi: 10.4161/cib.3.2.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schönknecht G, Hedrich R, Junge W, Raschke K. A voltage-dependent chloride channel in the photosynthetic membrane of a higher plant. Nature. 1988;336:589–592. [Google Scholar]
- 33.Jansen MA, Mattoo AK, Edelman M. D1-D2 protein degradation in the chloroplast. Complex light saturation kinetics. Eur J Biochem. 1999;260:527–532. doi: 10.1046/j.1432-1327.1999.00196.x. [DOI] [PubMed] [Google Scholar]
- 34.Edelman M, Mattoo AK. D1-protein dynamics in photosystem II: The lingering enigma. Photosynth Res. 2008;98:609–620. doi: 10.1007/s11120-008-9342-x. [DOI] [PubMed] [Google Scholar]
- 35.Hundal T, Virgin I, Styring S, Andersson B. Changes in the organization of photosystem II following light-induced D1-protein degradation. Biochim Biophys Acta. 1990;1017:235–241. [Google Scholar]
- 36.Eisenberg-Domovich Y, Oelmüller R, Herrmann RG, Ohad I. Role of the RCII-D1 protein in the reversible association of the oxygen-evolving complex proteins with the lumenal side of photosystem II. J Biol Chem. 1995;270:30181–30186. doi: 10.1074/jbc.270.50.30181. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto Y. Quality control of photosystem II. Plant Cell Physiol. 2001;42:121–128. doi: 10.1093/pcp/pce022. [DOI] [PubMed] [Google Scholar]
- 38.Henmi T, Miyao M, Yamamoto Y. Release and reactive-oxygen-mediated damage of the oxygen-evolving complex subunits of PSII during photoinhibition. Plant Cell Physiol. 2004;45:243–250. doi: 10.1093/pcp/pch027. [DOI] [PubMed] [Google Scholar]
- 39.Adam Z, Rudella A, van Wijk KJ. Recent advances in the study of Clp, FtsH and other proteases located in chloroplasts. Curr Opin Plant Biol. 2006;9:234–240. doi: 10.1016/j.pbi.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Sonoike K. Photoinhibition of photosystem I. Physiol Plant. 2011;142:56–64. doi: 10.1111/j.1399-3054.2010.01437.x. [DOI] [PubMed] [Google Scholar]
- 41.Scheller HV, Haldrup A. Photoinhibition of photosystem I. Planta. 2005;221:5–8. doi: 10.1007/s00425-005-1507-7. [DOI] [PubMed] [Google Scholar]
- 42.de Bianchi S, Ballottari M, Dall'osto L, Bassi R. Regulation of plant light harvesting by thermal dissipation of excess energy. Biochem Soc Trans. 2010;38:651–660. doi: 10.1042/BST0380651. [DOI] [PubMed] [Google Scholar]
- 43.Studer D, Michel M, Müller M. High pressure freezing comes of age. Scanning Microsc Suppl. 1989;3:253–268, discussion 268–269. [PubMed] [Google Scholar]
- 44.Nevo R, et al. Thylakoid membrane perforations and connectivity enable intracellular traffic in cyanobacteria. EMBO J. 2007;26:1467–1473. doi: 10.1038/sj.emboj.7601594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.