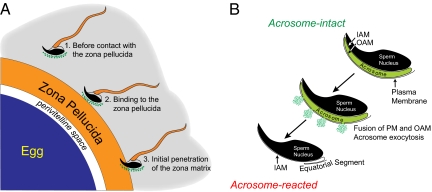
For successful mammalian fertilization, sperm penetrate the outer investments of female gametes in the ampulla of the oviduct and fuse with the egg plasma membrane. The “fertilizing” sperm traverse the cumulus mass surrounding ovulated eggs, bind temporarily, and then penetrate through the zona pellucida, a structured extracellular glycomatrix, to enter into the perivitelline space (Fig. 1A). Underlying the anterior plasma membrane of the mouse sperm head is the acrosome, a Golgi-derived subcellular organelle. Sperm that approach ovulated eggs in the female reproductive tract have an intact acrosome. Before fertilization, the outer acrosomal membrane fuses with the sperm plasma membrane resulting in exocytosis of acrosomal contents (Fig. 1B). Only acrosome-reacted sperm are observed in the perivitelline space between the egg plasma membrane and the inner aspect of the zona pellucida and only acrosome-reacted sperm fuse with the egg. Thus, at some point during sperm penetration through the investments of ovulated eggs, acrosome exocytosis must occur. But where (Fig. 1A)? This is the issue that concerns Inoue et al. (1) in their study in PNAS.
Fig. 1.
Sperm acrosome reaction. (A) The fertilizing sperm, capacitated by passage through the female reproductive tract, must undergo acrosome exocytosis before fusion with the egg in the perivitelline space. The site of this acrosome reaction has been variously described as occurring (i) while sperm pass through the cumulus oophorus (gray background) surrounding ovulated eggs; (ii) upon sperm binding to the surface of the extracellular zona pellucida; and (iii) during initial penetration of the zona matrix. (B) The acrosome is a Golgi-derived subcellular organelle that underlies the anterior plasma membrane of the sperm head (Upper Right). Upon mobilization of internal stores of calcium, the plasma membrane (PM) fuses with the outer acrosomal membrane (OAM), and acrosomal contents are released through resultant fenestrations (Center). Acrosome-reacted sperm within the perivitelline space have an exposed inner acrosomal membrane (IAM) and fuse to the egg via the residual equatorial segment of the plasma membrane (Lower Left).
Ovulated eggs in the ampulla of the oviduct are enmeshed in the cumulus oophorus composed of glycosaminoglycans interspersed with a gradient of residual cumulus cells from the ovarian follicle. Until recently, the prevailing model for mice was that acrosome-intact sperm pass through the cumulus oophorus and exocytosis is triggered by binding to the zona pellucida. Only acrosome-intact sperm are observed by EM on the surface of the zona pellucida and only acrosome-reacted sperm are observed in the perivitelline space (2). The presence of vesiculated acrosomal shrouds on the zona surface (3–5) and the reported ability of solubilized zonae pellucidae or isolated mouse ZP3 mouse to induce acrosome exocytosis (6) are consistent with this model. However, the persistence of acrosome-intact Acr-EGFP sperm on the surface of the mouse zona pellucida for several hours is seemingly at odds with gamete binding transducing a signal that triggers acrosome exocytosis, and raises the possibility that initial penetration into the zona matrix plays the more important role (7). Alternatively, a recent investigation that used live imaging of Acr-EGFP sperm to fertilize eggs in cumulus reports that, with few exceptions, the fertilizing sperm is already acrosome-reacted when it encounters the zona pellucida (8).
Earlier reports differ remarkably on the acrosome status of sperm within the cumulus mass, with some investigators observing acrosome-intact (9, 10) and others acrosome-reacted (8, 11) sperm. Further clouding the issue is the observation that some noneutherian mammals do not form a cumulus mass, and the efficacy of fertilizing cumulus-free compared with cumulus-intact eutherian eggs is variously reported as decreased or unchanged (12–14). With these disparate reports, the site for induction of acrosome exocytosis continues to intrigue and remains a worthy focus of investigation.
Inoue et al. (1) now provide evidence that acrosome-reacted mouse sperm can penetrate the zona pellucida and fertilize mouse eggs that develop to birth. These elegant studies take advantage of two genetically modified mouse lines. The first (Izumo I−/−) lacks a sperm surface protein and is unable to fuse with normal eggs (15); the second (Cd9−/−) lacks an egg plasma membrane protein and is unable to fuse with normal sperm (16). In each case, sperm accumulate in the perivitelline space and are acrosome-reacted. Using these sperm, the investigators report that acrosome-reacted sperm are able to fertilize normal eggs in cumulus. In a first experiment, normal female mice were mated to Izumo I−/− male mice. Eight hours after coitus, eggs were collected from the oviduct and sperm were mechanically released from the perivitelline space. The Izumo-null sperm were incubated with normal eggs in cumulus, and sperm were observed in the perivitelline space of five of 74 normal eggs (7%) 12 h after reinsemination.
These observations corroborate those from earlier studies in the rabbit and guinea pig. Normally, rabbit eggs accumulate multiple sperm in their perivitelline space because of a porous postfertilization block to zona penetration. Presumed to be acrosome-reacted, these sperm fertilize after reinsemination, albeit with only 24% efficiency (17). Likewise, acrosome-reacted guinea pig sperm are able to penetrate the zona pellucida and fertilize eggs in vitro (100%) as long as they maintain hyperactive motility (18). These results indicate that acrosome-reacted sperm can penetrate the zona pellucida and fertilize eggs, but the efficiency in rabbits and mice is surprisingly low. If these observations accurately reflect in vivo fertilization (i.e., sperm acrosome exocytosis before encountering the zona pellucida), a higher rate of fertilization might be anticipated, but could reflect the effect of decreased hyperactive motility, as noted with guinea pig sperm (18). Alternatively, there may be added complexity to gamete recognition and induction of acrosome exocytosis not captured under the reported experimental conditions.
It was not clear from the studies in rabbit and guinea pig (17, 18) if fertilization or formation of two-cell embryos (the endpoint of these experiments) reflected a physiological outcome. Therefore, to determine the developmental potential of eggs inseminated with acrosome-reacted sperm, Inoue et al. (1) recover sperm from the perivitelline space of Cd9-null females mated in vivo with normal males. After reinsemination of normal eggs in vitro, embryos were transferred to foster mothers, and two pups, one of which was cannibalized, were born. This confirms that fertilization with acrosome-reacted sperm
Inoue et al. confirm that acrosome-reacted sperm can penetrate the zona matrix and fertilize eggs.
results in live births and is consistent with the recent report that images Acr-EGFP sperm as they fertilize eggs in cumulus (8), and concludes that the fertilizing sperm undergo acrosome exocytosis within the cumulus before encountering the zona pellucida.
Collectively, these diverse observations raise the possibility that induction of acrosome exocytosis might be triggered differently depending on the conditions encountered by the fertilizing sperm. Sperm that reach the egg relatively soon after ovulation would pass through the cumulus mass, which could trigger acrosome exocytosis, thus preparing sperm for passage through the zona matrix (8). Late-arriving sperm might encounter a dissipating cumulus mass (or no cumulus mass in some species) and therefore rely on interactions with the zona pellucida to induce acrosome exocytosis. If correct, this formulation poses a number of intriguing questions. If the acrosome reaction occurs within the cumulus before the fertilizing sperm reaches the surface of the zona matrix, what molecular mechanisms account for observed taxon specificity in the induction of acrosome exocytosis (5)? If both acrosome-intact and acrosome-reacted sperm can bind to the surface of the zona pellucida (19, 20), are the same or different sperm macromolecules involved in binding, or is there redistribution of a common sperm receptor? Although the results of Inoue et al. (1) confirm that acrosome-reacted sperm can penetrate the zona matrix and fertilize eggs, our incomplete understanding of the site of acrosome exocytosis and its triggering mechanism(s), as well as the function of acrosomal contents, make the acrosome reaction a rich and compelling environment for future investigations.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
The authors declare no conflict of interest.
See companion article on page 20008.
References
- 1.Inoue N, Satouh Y, Ikawa M, Okabe M, Yanagimachi R. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc Natl Acad Sci USA. 2011;108:20008–20011. doi: 10.1073/pnas.1116965108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saling PM, Sowinski J, Storey BT. An ultrastructural study of epididymal mouse spermatozoa binding to zonae pellucidae in vitro: Sequential relationship to the acrosome reaction. J Exp Zool. 1979;209:229–238. doi: 10.1002/jez.1402090205. [DOI] [PubMed] [Google Scholar]
- 3.Yanagimachi R, Phillips DM. The status of acrosomal caps of hamster sperm immediately before fertilization in vivo. Gamete Res. 1984;9:1–20. [Google Scholar]
- 4.VandeVoort CA, Yudin AI, Overstreet JW. Interaction of acrosome-reacted macaque sperm with the macaque zona pellucida. Biol Reprod. 1997;56:1307–1316. doi: 10.1095/biolreprod56.5.1307. [DOI] [PubMed] [Google Scholar]
- 5.Wakayama T, et al. Penetration by field vole spermatozoa of mouse and hamster zonae pellucidae without acrosome reaction. J Reprod Fertil. 1996;107:97–102. doi: 10.1530/jrf.0.1070097. [DOI] [PubMed] [Google Scholar]
- 6.Bleil JD, Wassarman PM. Sperm-egg interactions in the mouse: Sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev Biol. 1983;95:317–324. doi: 10.1016/0012-1606(83)90032-5. [DOI] [PubMed] [Google Scholar]
- 7.Baibakov B, Gauthier L, Talbot P, Rankin TL, Dean J. Sperm binding to the zona pellucida is not sufficient to induce acrosome exocytosis. Development. 2007;134:933–943. doi: 10.1242/dev.02752. [DOI] [PubMed] [Google Scholar]
- 8.Jin M, et al. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci USA. 2011;108:4892–4896. doi: 10.1073/pnas.1018202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherr GN, Lambert H, Meizel S, Katz DF. In vitro studies of the golden hamster sperm acrosome reaction: Completion on the zona pellucida and induction by homologous soluble zonae pellucidae. Dev Biol. 1986;114:119–131. doi: 10.1016/0012-1606(86)90388-x. [DOI] [PubMed] [Google Scholar]
- 10.Schroer SC, Yudin AI, Myles DG, Overstreet JW. Acrosomal status and motility of guinea pig spermatozoa during in vitro penetration of the cumulus oophorus. Zygote. 2000;8:107–117. doi: 10.1017/s0967199400000885. [DOI] [PubMed] [Google Scholar]
- 11.Cummins JM, Yanagimachi R. Sperm-egg ratios and the site of the acrosome reaction during in vivo fertilization in the hamster. Gamete Res. 1982;5:239–256. [Google Scholar]
- 12.Pavlok A, McLaren A. The role of cumulus cells and the zona pellucida in fertilization of mouse eggs in vitro. J Reprod Fertil. 1972;29:91–97. doi: 10.1530/jrf.0.0290091. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto H, Chang MC. Fertilization in vitro of mouse and hamster eggs after the removal of follicular cells. J Reprod Fertil. 1972;30:309–312. doi: 10.1530/jrf.0.0300309. [DOI] [PubMed] [Google Scholar]
- 14.Cross PC, Brinster RL. In vitro development of mouse oocytes. Biol Reprod. 1970;3:298–307. doi: 10.1093/biolreprod/3.3.298. [DOI] [PubMed] [Google Scholar]
- 15.Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–238. doi: 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- 16.Miyado K, et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science. 2000;287:321–324. doi: 10.1126/science.287.5451.321. [DOI] [PubMed] [Google Scholar]
- 17.Kuzan FB, Fleming AD, Seidel GE., Jr Successful fertilization in vitro of fresh intact oocytes by perivitelline (acrosome-reacted) spermatozoa of the rabbit. Fertil Steril. 1984;41:766–770. doi: 10.1016/s0015-0282(16)47847-7. [DOI] [PubMed] [Google Scholar]
- 18.Fleming AD, Yanagimachi R. Fertile life of acrosome-reacted guinea pig spermatozoa. J Exp Zool. 1982;220:109–115. doi: 10.1002/jez.1402200114. [DOI] [PubMed] [Google Scholar]
- 19.Myles DG, Hyatt H, Primakoff P. Binding of both acrosome-intact and acrosome-reacted guinea pig sperm to the zona pellucida during in vitro fertilization. Dev Biol. 1987;121:559–567. doi: 10.1016/0012-1606(87)90191-6. [DOI] [PubMed] [Google Scholar]
- 20.Morales P, Cross NL, Overstreet JW, Hanson FW. Acrosome intact and acrosome-reacted human sperm can initiate binding to the zona pellucida. Dev Biol. 1989;133:385–392. doi: 10.1016/0012-1606(89)90042-0. [DOI] [PubMed] [Google Scholar]