The adaptor proteins β-arrestins 1 and 2 are ubiquitously expressed and were originally discovered for desensitizing G protein-mediated signal transduction by the cell-surface seven-transmembrane receptors (7TMRs or GPCRs) (1). 7TMRs constitute the largest family of cell-surface receptors, and their signaling regulates almost every aspect of mammalian physiology, including vision, olfaction, behavior, heart rate, blood pressure, inflammatory, and digestive processes. Although 7TMR signaling was originally thought to involve only G proteins, accumulating evidence indicates that β-arrestins not only block G protein signaling, but also facilitate receptor endocytosis and mediate G protein-independent signaling (2, 3). Studies have also illuminated receptor ligands called biased agonists that specifically activate β-arrestin signaling while blocking G proteins or vice versa (4). Such biased agonism can be exploited to develop better drugs acting at the 7TMRs with reduced side effects (5). In PNAS, Soh and Trejo report a cytoprotective β-arrestin-biased signaling pathway emanating from the 7TMR, protease activated receptor-1 (PAR1), which could be important in reducing sepsis-induced inflammation (6).
PAR1 is expressed in endothelial cells and is a central mediator of cellular responses generated during coagulation and anticoagulation (7). The serine protease thrombin binds PAR1, and cleaves its extracellular domain to form a tethered ligand to activate PAR1-mediated inflammatory responses. Anticoagulant protease activated protein C (APC; in this context, not to be confused with tumor suppressor gene adenomatous polyposis coli) activates a coreceptor, endothelial protein C receptor (EPCR), which functions with PAR1 (8, 9). APC-activated EPCR and PAR1 localize to membrane regions enriched in caveolin and promote cytoprotective pathways, thus decreasing mortality in animal models of sepsis (8, 10). Earlier studies have shown that APC and thrombin show different effects at the PAR1: thrombin does not engage a coreceptor, induces complete cleavage of PAR1, and promotes PAR1 internalization and degradation; APC engages the coreceptor EPCR, induces limited cleavage of PAR1, and does not promote receptor internalization and degradation (8, 11). Furthermore, thrombin is more potent than APC and promotes internalization of APC-bound PAR1 (11, 12). Previously, Trejo and coworkers reported that thrombin and APC stimulated different small GTPases, namely RHOA (by thrombin) and RAC1 (by APC), and only APC-induced effects required caveolin (11). Soh and Trejo have now compared PAR1-mediated signaling induced by thrombin or APC and show that thrombin-induced RHOA effects are G protein-dependent, whereas APC-stimulated RAC1 signaling is β-arrestin–dependent (Fig. 1). Moreover, APC functions as a β-arrestin–biased agonist, because it does not activate G protein signaling (6).
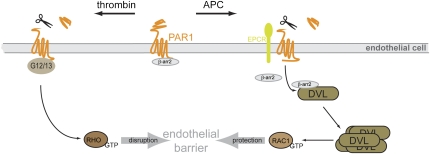
Fig. 1.
Schematic presentation of PAR1-mediated signaling pathways in endothelial cells. Thrombin binds PAR1 and activates heterotrimeric G12/13 proteins to regulate endothelial barrier disruption via the small GTPase RHOA. A second protease, APC, also acts by inducing proteolytic cleavage of PAR1 but requires the EPCR. APC induces the release of β-arrestin 2 (β-arr2) from PAR1 to associate with DVL2, which increases DVL polymerization. This is interpreted as DVL activation and is followed by GDP/GTP exchange at the small GTPase RAC1. β-Arrestin 2 and DVL2 interaction is necessary for the APC-induced endothelial barrier protection, which has therapeutic implications for the regulation of coagulation and anticoagulation. The discovery of the unexpected liaison between β-arrestin 2 and DVL downstream of PAR1 presents a unique signaling paradigm and opens the possibility for additional crosstalk between classical 7TMRs and WNT signaling.
PAR1 localized to caveolae is associated with β-arrestin in unstimulated cells and APC treatment does not augment PAR1–β-arrestin association, but upon sustained stimulation induces their dissociation. As β-arrestin is necessary for APC-induced activation of the small GTPase RAC1, which is crucial for the endothelial barrier protection, the authors investigated the underlying molecular mechanism connecting β-arrestin and RAC1 (6). Remarkably, it turned out that dishevelled 2 (DVL2), a phosphoprotein associated with the nonclassical 7TMR called frizzled (FZD), is the missing link (Fig. 1). Previous studies showed that β-arrestin is required for internalization of FZD4 by its ligand WNT-5A and also for WNT-induced β-catenin–dependent and -independent signaling in vitro and in vivo (13–15). DVL–β-arrestin interaction is also important for pathway decision for WNT-5A–induced RAC1 signaling (15). 7TMRs, such as PAR1, CysLT receptors, gonadotropin-releasing hormone, EP2, and FP prostanoid receptors are also capable of triggering stabilization of β-catenin (16). The point of convergence was glycogen synthase 3 activity, which is downstream of DVL in the WNT/β-catenin pathway. Interestingly, parathyroid hormone 1 receptors interact with DVL in a FZD-like mode, through a KS/TxxxW sequence in their C terminus (17). Nonetheless, the molecular mechanisms of the DVL–β-arrestin liaison and its importance for crosstalk between other receptor and signaling systems has remained obscure (18).
Soh and Trejo (6) have now identified a possibility for crosstalk between classic 7TMRs and WNT/FZD/DVL signaling through a β-arrestin 2–DVL2 interaction. In endothelial cells, APC triggers a dynamic association of β-arrestin 2 with DVL2, resulting in DVL2 polymerization. Loss-of-function approaches using siRNA show that (i) β-arrestin 2 is required for the APC-evoked DVL2 polymerization and (ii) DVL2 expression is necessary for the APC-induced activation of RAC1 and the subsequent endothelial barrier protection. In this scenario, RAC1, a β-catenin–independent WNT signaling component is localized downstream of DVL. The current finding that β-arrestin 2 and DVL coprecipitate from intracellular pools (6) is consistent with the previous report of their colocalization in membrane-free aggregates (14, 15).
The discovery of β-arrestin release from PAR1 receptors and the subsequent association with endogenous cytoplasmic DVL suggests that DVL can act as a signaling platform independent of plasma membrane-associated events. But how? Polyubiquitination of the polymerization-prone DIX domain of DVL, which can facilitate enhanced WNT–β-catenin signaling, could be a factor in engaging β-arrestin 2–RAC1 signaling (19). Although β-arrestin 2 expression is shown to be important for DVL polymerization and formation of subcellular aggregates, it is unclear how β-arrestin 2 and DVL interaction promote signaling. Previous studies have shown that, upon its ubiquitination, β-arrestin 2 stabilizes 7TMR signalosomes (3, 20), and the level of ubiquitinated β-arrestin 2 is diminished in soluble cellular fractions (20), which suggests that it could signal as a cytoplasmic protein aggregate in addition to being recruited to membrane compartments to activated 7TMRs. It is tempting to speculate that β-arrestin 2 ubiquitination could have an important role in mediating and/or stabilizing DVL polymerization and subsequent signaling. Moreover, DVL ubiquitination itself may involve β-arrestin 2, as it has been shown to function as an E3 ubiquitin ligase adaptor (3).
Despite the exciting findings, the exact effects of APC-induced β-arrestin–mediated polymerization of DVL2 on the downstream signaling targets remain unknown. How certain WNT/FZD combinations determine signaling specificity
β-arrestin 2–mediated effects could be important in reducing inflammation.
toward β-catenin–dependent or -independent pathways involving DVL remains a mystery. Coreceptors, such as LRP5/6, ROR1/2, or RYK, were previously shown as molecules that direct WNT/FZD/DVL-involving pathways toward RAC1 or β-catenin (18). Obviously, the APC-induced DVL-dependent RAC1 activation does not involve WNT coreceptors. The established view of DVL function puts this phosphoprotein at the center of so-called signalosomes (21), where FZD, LRP6, axin, and DVL are complexed to initiate WNT/β-catenin signaling from cell membranes. It would now be important to investigate if and how the β-arrestin–DVL interaction also inhibits glycogen synthase 3, thereby stabilizing β-catenin, and if these mechanisms could be distinguishable from the β-arrestin–DVL2 pathway toward RAC1.
The signaling paradigm discovered by Soh and Trejo (6) opens many intriguing possibilities for β-arrestin–dependent crosstalk between classic 7TMR signaling and the WNT/FZD pathways involving DVL. This intracellular communication network might allow fine-tuning of responses during embryonic development, stem cell regulation, adult tissue homeostasis, and the development of human disease. The work of Soh and Trejo (6) also reveals a specific molecular mechanism of β-arrestin–biased signal transduction involved in orchestrating cellular responses. By promoting DVL2 and RAC1 activity, β-arrestin 2 protects the endothelial cell barrier, thus preventing permeability and vascular leakage. Because APC signaling at the PAR1 can induce expression of protective genes such as the monocyte chemoattractant protein-1 (8), these β-arrestin 2–mediated effects could be important in reducing inflammation. This is consistent with the finding that β-arrestin 2–KO mice show an augmented inflammatory response toward experimentally induced polymicrobial sepsis (22). Mechanistic studies delineating β-arrestin–biased signaling such as that of APC-activated PAR1 not only provide a better understanding of the cellular consequences but would also stimulate new ideas for the development of drugs targeting distinct signaling pathways.
Acknowledgments
This work was supported by the Karolinska Institutet (G.S.), Swedish Research Council (G.S.), Swedish Cancer Society (G.S.), Knut and Alice Wallenberg Foundation (G.S.), National Institutes of Health Grant HL 080525 (to S.K.S.), and Grant-in-Aid from the American Heart Association (to S.K.S.).
Footnotes
References
- 1.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 2.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 3.Shenoy SK, Lefkowitz RJ. β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soh UJK, Trejo J. Activated protein C promotes protease-activated receptor-1 cytoprotective signaling through β-arrestin and dishevelled-2 scaffolds. Proc Natl Acad Sci USA. 2011;108:E1372–E1380. doi: 10.1073/pnas.1112482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 8.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 9.Riewald M, Ruf W. Protease-activated receptor-1 signaling by activated protein C in cytokine-perturbed endothelial cells is distinct from thrombin signaling. J Biol Chem. 2005;280:19808–19814. doi: 10.1074/jbc.M500747200. [DOI] [PubMed] [Google Scholar]
- 10.Bae JS, Yang L, Manithody C, Rezaie AR. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–3916. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo A, Soh UJ, Paing MM, Arora P, Trejo J. Caveolae are required for protease-selective signaling by protease-activated receptor-1. Proc Natl Acad Sci USA. 2009;106:6393–6397. doi: 10.1073/pnas.0810687106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludeman MJ, et al. PAR1 cleavage and signaling in response to activated protein C and thrombin. J Biol Chem. 2005;280:13122–13128. doi: 10.1074/jbc.M410381200. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, et al. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 14.Bryja V, Gradl D, Schambony A, Arenas E, Schulte G. Beta-arrestin is a necessary component of Wnt/beta-catenin signaling in vitro and in vivo. Proc Natl Acad Sci USA. 2007;104:6690–6695. doi: 10.1073/pnas.0611356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryja V, et al. Beta-arrestin and casein kinase 1/2 define distinct branches of non-canonical WNT signalling pathways. EMBO Rep. 2008;9:1244–1250. doi: 10.1038/embor.2008.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shevtsov SP, Haq S, Force T. Activation of beta-catenin signaling pathways by classical G-protein-coupled receptors: Mechanisms and consequences in cycling and non-cycling cells. Cell Cycle. 2006;20:2295–2300. doi: 10.4161/cc.5.20.3357. [DOI] [PubMed] [Google Scholar]
- 17.Romero G, et al. Parathyroid hormone receptor directly interacts with dishevelled to regulate beta-Catenin signaling and osteoclastogenesis. J Biol Chem. 2010;285:14756–14763. doi: 10.1074/jbc.M110.102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulte G, Schambony A, Bryja V. beta-Arrestins - scaffolds and signalling elements essential for WNT/Frizzled signalling pathways? Br J Pharmacol. 2010;159:1051–1058. doi: 10.1111/j.1476-5381.2009.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tauriello DV, et al. Loss of the tumor suppressor CYLD enhances Wnt/beta-catenin signaling through K63-linked ubiquitination of Dvl. Mol Cell. 2010;37:607–619. doi: 10.1016/j.molcel.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 20.Shenoy SK, et al. Ubiquitination of beta-arrestin links seven-transmembrane receptor endocytosis and ERK activation. J Biol Chem. 2007;282:29549–29562. doi: 10.1074/jbc.M700852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilic J, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 22.Fan H, et al. Beta-arrestin 2 negatively regulates sepsis-induced inflammation. Immunology. 2010;130:344–351. doi: 10.1111/j.1365-2567.2009.03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]