Abstract
Globally, inadequate water supply, sanitation, and hygiene (WASH) are major contributors to mortality and burden of disease. We aimed to quantify the role of WASH in the risk of Schistosoma hematobium, Schistosoma mansoni, and hookworm infection in school-aged children; to estimate the population attributable fraction (PAF) of helminth infection due to WASH; and to spatially predict the risk of infection. We generated predictive maps of areas in West Africa without piped water, toilet facilities, and improved household floor types, using spatial risk models. Our maps identified areas in West Africa where the millennium development goal for water and sanitation is lagging behind. There was a generally better geographical coverage for toilets and improved household floor types compared with water supply. These predictions, and their uncertainty, were then used as covariates in Bayesian geostatistical models for the three helminth species. We estimated a smaller attributable fraction for water supply in S. mansoni (PAF 47%) compared with S. hematobium (PAF 71%). The attributable fraction of S. hematobium infection due to natural floor type (PAF 21%) was comparable to that of S. mansoni (PAF 16%), but was significantly higher for hookworm infection (PAF 86%). Five percent of hookworm cases could have been prevented if improved toilet facilities had been available. Mapping the distribution of infection risk adjusted for WASH allowed the identification of communities in West Africa where preventive chemotherapy integrated with interventions to improve WASH will yield the greatest health benefits.
Water supply, sanitation, and hygiene (WASH) have been called the greatest medical advances since 1840 and have been classified the “forgotten foundations of health” (1, 2). As a result, the international community has set a global benchmark to halve, by 2015, the proportion of people with inadequate access to WASH, as part of the seventh millennium development goal (MDG) (3). In sub-Saharan Africa, 6% of the total disability-adjusted life years (DALY) in 2000 was attributable to poor WASH (4, 5). Three hundred thirty million people in sub-Saharan Africa are without access to adequate water supply, and 565 million have inadequate access to sanitation (3).
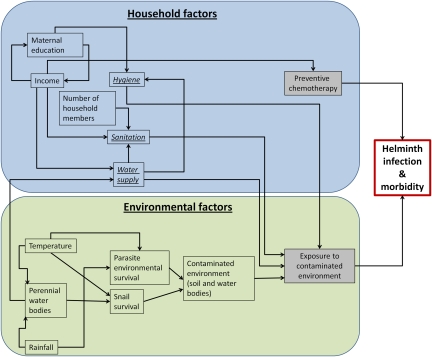
Inadequate WASH has well known independent risk factors for major diarrhea-causing infections and helminth infections such as schistosomiasis and soil-transmitted helminthiases (STH) (6–8) and is also indirectly associated with acute respiratory tract infections, trachoma, and undernutrition, particularly in preschool and school-aged children (9–11). Systematic reviews suggest that rates of diarrhea can be reduced as a result of improved water supply by 15–17% and sanitation by 32–37% (7, 12). Although there have been surprisingly few intervention studies, available evidence suggests that improved WASH reduces childhood morbidity associated with helminth infections (13–19). Knowledge of the epidemiology of helminth infections (Fig. 1) suggests that the contribution of WASH to the overall helminth burden is likely to be high, but this has never been quantified.
Fig. 1.
Proximal and distal contributors to helminth infections.
Current control of schistosomiasis and STH focuses on reducing morbidity by implementing preventive chemotherapy with praziquantel and albendazole in schools or entire communities (20). Although preventive chemotherapy has a high and immediate impact on morbidity, rapid reinfection means that it has limited impact on transmission and on the long-term control of the burden of infection (21). Current debate on the sustainability of helminth control argues that preventive chemotherapy should be viewed as a necessary component of a comprehensive integrated intervention package, including health education, intermediate host snail control (in the case of schistosomiasis), and interventions to improve WASH (22, 23).
The most recent information regarding WASH coverage in sub-Saharan Africa shows considerable regional disparities (3). The disease burden due to WASH is likely to be highly geographically variable, even in the highly endemic areas of sub-Saharan Africa (5). Modern geographical risk prediction methods highlight this geographical variability in helminth infections and are being used as control tools for targeting helminth interventions (24). The inclusion of modifiable factors such as the availability of WASH into these models would identify those communities where WASH is most needed and allow the contribution of WASH to the overall helminth burden to be quantified.
In this paper, we describe unique water and sanitation data from national surveys in three contiguous countries in western Africa (Burkina Faso, Ghana, and Mali). We map the spatial variability in selected WASH indicators and determine the contribution that water and sanitation make to the overall burden of helminth infections in school-aged children.
Results
Geographical Variation in Household WASH Indicators.
Georeferenced household-level data for three WASH indicators (i.e., source of drinking water and two sanitation indicators: type of toilet facility and floor type), maternal education, the number of members in the household, and residence were obtained from the most recent demographic health surveys (DHS) for Burkina Faso (2003), Ghana (2003), and Mali (2006). Individual-level variables significantly associated with all three WASH indicators were maternal education, the number of members in the household, and residence. Maternal age was associated with water supply and toilet facilities (Table 1).
Table 1.
Associations with sanitation indicators, estimated using the best-fitting geostatistical models
| Natural floor,† | No piped water,‡ | No toilet facility,‡ | |
| Variable | odds ratio (95% CrI) | odds ratio (95% CrI) | odds ratio (95% CrI) |
| Maternal education vs. no education | 0.30 (0.26, 0.34) | 0.25 (0.21, 0.28) | 0.23 (0.20, 0.27) |
| Maternal age in years* | 0.99 (0.94, 1.05) | 1.01 (1.05, 1.07) | 1.08 (1.03, 1.14) |
| No. members in household* | 0.86 (0.82, 0.90) | 0.91 (0.87, 0.97) | 0.88 (0.84, 0.92) |
| Rural vs. urban | 3.46 (3.03, 3.90) | 10.80 (9.58, 12.42) | 3.71 (3.16, 4.31) |
*Variables were standardized to have mean = 0 and SD = 1.
†Cubic model. CrI, credible interval.
‡Quadratic model.
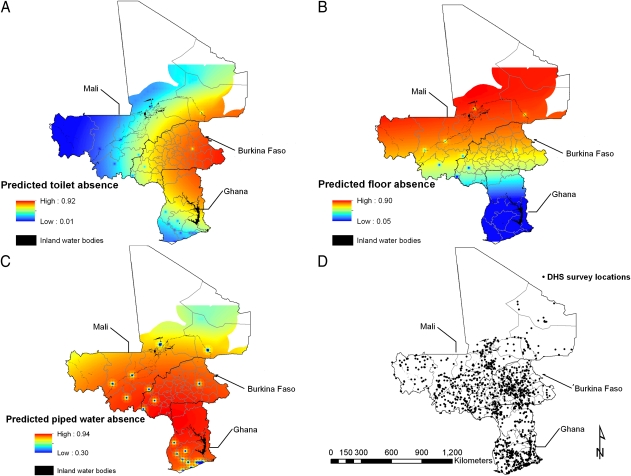
The predicted proportion of households with no toilet was highest in eastern Burkina Faso and northeastern Ghana (Fig. 2A). The predicted proportion of households having natural floor type was greatest (>90%) in a large area in central and northern Mali and in northern Burkina Faso (Fig. 2B). The predicted proportion of households with no piped water was high across most of the study area (Fig. 2C). Urban areas had improved WASH as shown by the small clusters in Fig. 2 A–C.
Fig. 2.
Predictive geographical distribution of toilet availability (A), household flooring (B), and piped water (C), based on the respective best-fitting models, and locations of demographic health surveys (D).
Geographical Variation in Helminth Infection Risk.
We used parasitological survey data from school-aged children in Burkina Faso (2007), Ghana (2008), and Mali (2007) collected by the Schistosomiasis Control Initiative (SCI) (25) (Fig. 3D) (SI Materials and Methods). Children's age and sex were significantly associated with risk of Schistosoma hematobium (Table 2), Schistosoma mansoni (Table 2), and hookworm (Table 3). The best-fitting model, as assessed by the deviance information criterion (DIC), for all infections included all individual-level, environmental covariates [distance to perennial waterbodies (DPWB), land surface temperature (LST), and normalized difference vegetation index (NDVI, a proxy for rainfall)] and WASH (water supply, sanitation, and floor type). The presence of a natural floor type greatly increased hookworm risk [odds ratio (OR) = 9.49]. A lack of water supply increased S. hematobium risk (OR = 4.18) and greatly decreased hookworm risk (OR = 0.02). Counterintuitively, a lack of sanitation decreased S. mansoni risk. Although not significant at the 5% level, natural floor type increased the risk of S. hematobium and S. mansoni, lack of water supply increased S. mansoni risk, and lack of sanitation increased hookworm risk.
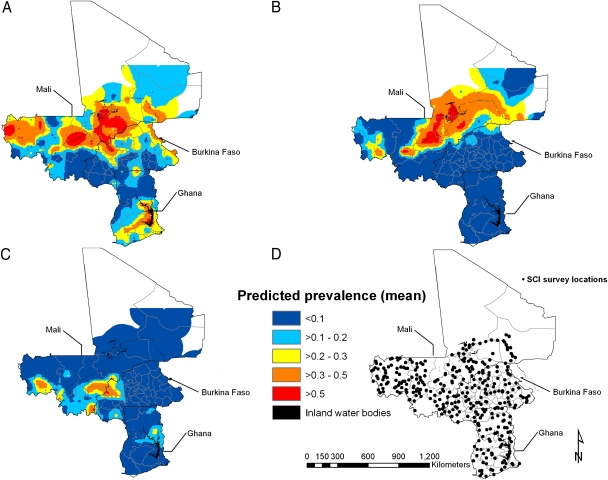
Fig. 3.
Predictive geographical risk of Schistosoma hematobium (A), Schistosoma mansoni (B) in boys aged 10–15 y, and hookworm (C) in boys aged 15–19 y, based on the respective best-fitting model-based geostatistical binomial model (model A), and location of parasite surveys (D).
Table 2.
Associations with prevalence of schistosomiasis in children in Burkina Faso, Ghana, and Mali, estimated using the best-fitting geostatistical models considering estimation uncertainty of sanitary indicators at parasitological survey locations
| S. hematobium, | S. mansoni, | |
| Variable | odds ratio (95% CrI) | odds ratio (95% CrI) |
| Female vs. male | 0.75 (0.70, 0.80) | 0.63 (0.52, 0.76) |
| Age 10–19 vs. 5–9 y | 1.48 (1.35, 1.62) | 1.55 (1.28, 1.86) |
| DPWB | 0.23 (0.18, 0.48) | 0.01 (0.0003, 0.46) |
| LST | 0.97 (0.53, 1.70) | 34.81 (0.84, 1248.88) |
| NDVI | 0.69 (0.47, 0.96) | 0.91 (0.50,1.77) |
| Natural floor vs. other floor type | 1.43 (0.29, 8.17) | 1.31 (0.17, 34.12) |
| No piped water vs. with piped water | 4.18 (1.22, 17.81) | 2.16 (0.06, 57.97) |
| No toilet facility vs. with toilet | 0.39 (0.09, 1.58) | 0.05 (0.003, 0.70) |
| φ, rate of decay of spatial correlation | 2.60 (1.86, 3.53) | 2.98 (1.26, 8.29) |
| σ2, variance of spatial random effect | 5.20 (3.97, 7.82) | 13.37 (8.14, 21.87) |
CrI, credible interval; DPWB, distance to perennial water body; LST, land surface temperature; NDVI, normalized difference vegetation index.
Table 3.
Associations with prevalence of hookworm infection in children in Burkina Faso, Ghana, and Mali, estimated using the best-fitting geostatistical models considering estimation uncertainty of sanitary indicators at parasitological survey locations
| Hookworm, | |
| Variable | odds ratio (95% CrI) |
| Female vs. male | 0.58 (0.51, 0.66) |
| Age 10–14 vs. 5–9 y | 1.46 (1.26, 1.73) |
| Age 15–19 vs. 5–9 y | 1.95 (1.45, 2.66) |
| DPWB | 1.22 (0.61, 3.13) |
| LST | 0.12 (0.06, 0.28) |
| NDVI | 1.90 (1.27, 2.77) |
| Natural floor vs. other floor type | 9.49 (6.17, 31.19) |
| No piped water vs. with piped water | 0.02 (0.001, 0.30) |
| No toilet facility vs. with toilet | 1.11 (0.14, 6.55) |
| φ, rate of decay of spatial correlation | 2.00 (0.81, 4.42) |
| σ2, variance of spatial random effect | 5.42 (3.64, 8.92) |
CrI, credible interval; DPWB, distance to perennial water body; LST, land surface temperature; NDVI, normalized difference vegetation index.
The spatial risk prediction maps for urinary (Fig. 3A) and intestinal schistosomiasis (Fig. 3B) show considerable geographical variability, particularly in the Niger River Basin (for S. hematobium and S. mansoni) and around the Lake Volta in Ghana (for S. hematobium). The predictive hookworm risk map shows that the risk of infection is highest in a large area straddling the borders between Mali and Burkina Faso and in a small area north of Lake Volta in Ghana (Fig. 3C).
The proportion of geographical variation explained by adding WASH indicators in helminth geographical models was 31% for S. hematobium, 7% for S. mansoni, and 14% for hookworm. Including WASH as random variables, as opposed to just considering WASH mean prediction, increased the mean SD across all unsampled locations by 2.6% in the S. hematobium model, 4.2% in the hookworm model, and 7.9% in the S. mansoni model.
Risk of Helminth Infections Attributable to WASH Indicators.
The estimated population attributable fraction (PAF) of S. hematobium due to no piped water was 71% and to a natural floor type was 21%. The estimated PAF of S. mansoni due to no piped water was 47% and to a natural floor type was 16%. The estimated PAF of hookworm due to no toilet was 5% and to a natural floor type was 86%.
Discussion
We have investigated the role of geographical variation in WASH in driving the distribution of major helminth infections in an endemic region of Africa. Our maps have shown important subnational geographical variation in WASH, something that national-level WASH summary statistics fail to convey. Importantly, we have also calculated the ranking of contributing factors for helminth infections of school-aged children with MDG relevance, including selected water supply, sanitation, and household floor type indicators.
Despite the greater investment in water supply compared with sanitation, our geographic WASH models show that most areas in West Africa are very poorly served by water supply (except in major urban centers), especially compared with the geographical distribution of toilet availability and improved household flooring (3). The availability of water supply and sanitation facilities in households in West Africa is significantly associated with maternal education, household overcrowding, and residence. These socioeconomic indicators are well-known risk factors for childhood helminth infection at small spatial scales (26).
Our results show the important role of WASH in the burden of helminth infections and that different indicators impact differently on different parasite species. For schistosome species, a significant reduction of infection burden would have been achieved by improving piped water availability (S. mansoni PAF, 47%; S. hematobium PAF, 71%). For hookworm, most gains would have been obtained from improving household flooring (PAF 86%). Overall, these results are consistent with the known epidemiology of these helminth species, in that transmission of schistosomiasis is facilitated by contact with water contaminated with infected intermediate host snails, and hookworm transmission occurs with contact with contaminated soil in the absence of proper sanitation and ineffective treatment of excreta or wastewater (8).
Previous studies have shown that the benefits of WASH are contingent upon active community participation and hygiene education (27–29). Our contradictory finding that inadequate sanitation decreases the risk of schistosomiasis could be partly explained by incorrect disposal of human excreta, even in the presence of adequate toilet facilities, resulting in persistent environmental contamination (27). Although we accounted for maternal education in the generation of mapped outputs of WASH, the observed effect between sanitation and schistosomiasis risk may be confounded by unmeasured factors (e.g., community participation in the planning, construction, operation, maintenance, and financing of WASH infrastructure). The negative association between unsafe water supply and hookworm infection is also unexpected and requires further investigation.
Important limitations should be noted from the DHS WASH datasets used, which are likely to be propagated through the modeling framework. Whereas SCI parasite surveys were carried out in 2007 and 2008, the input data from the DHSs were collected in different years (2003 for Burkina Faso and Ghana and 2006 for Mali). To assess relationships between helminth infection and potential contributors, we have assumed little temporal variation in WASH indicators in the three countries. This assumption could be an issue in areas of Burkina Faso and Ghana, where efforts to improve WASH had been undertaken. In Ghana, improvements in water and sanitation have been delivered since 2001 through the eyelid surgery, antibiotic treatment, facial cleanliness, and environmental change (SAFE) strategy for trachoma control (30, 31). However, these improvements were targeted to trachoma hotspots particularly in northern districts of Ghana and the capital Accra, so the latrine construction and provision of clean water were limited in size and geographical scope (32–34). Additionally, because comparable individual-level WASH data were not available from SCI surveys, the predicted prevalence of water supply, sanitation, and household floor type in a location was used as an imperfect proxy for the WASH status of children's households. Imprecise exposure measurement can lead to underestimation of the true effects, known as regression dilution bias (35). It is noteworthy that given these limitations, we were able to find a strong signal in the WASH prediction maps indicating that inadequate WASH contributes substantially to the burden of helminth infections in the region. Our approach also incorporated one significant advance in model-based geostatistical applications to helminth epidemiology, as it explicitly considered the uncertainty of mapped values of WASH surfaces. This approach contributed to a more appropriate handling of prediction uncertainty, thereby increasing the confidence in our helminth predictive maps.
PAF estimation is of public health relevance when the risk factors being investigated are the most proximal in a causal pathway, when important confounders have been considered in a regression model, and when there is consensus that the exposure is amenable to intervention (36). The WASH indicators and environmental factors included in our models are well known to be causally related to helminth infections, but do not represent the complete multifactorial nature of helminth infections (Fig. 1). Exposure to environmental health risks such as schistosomiasis and STH is determined by multiple technological, environmental, and behavioral factors (37). Adjusting our analysis for age, sex, and socioeconomic factors has improved our confidence in the statistical control of confounding, but future studies should consider the inclusion of household- and community-level waste disposal, hygiene behavior, water transportation, and storage methods currently not available in the SCI datasets.
Unlike environmental covariates, WASH indicators are modifiable and have direct relevance to transmission control. Obtaining their effect sizes has allowed an objective assessment of the potential health impact of WASH interventions. Given that the three study countries cover a large area of West Africa, covering the full range of ecological zones from the wet, tropical coastal regions to the arid Sahel, we believe the study area is ideal for testing our research questions and presenting meaningful, generalizable findings. The generalizability of the observed effects to other areas in sub-Saharan Africa is also conditional on the level of stationarity of WASH-associated helminth infections. Spatial variation in effect sizes of different WASH indicators could be investigated by carrying out similar studies in the East African region and comparing the results to those reported in this study. For the West African countries included in our analysis, the results highlight priority areas for targeting integrated helminth control that includes WASH interventions. These results holds particularly true in the Niger River basin where we found considerable geographical heterogeneity in the distribution of schistosomiasis and hookworm burden compared with previous work in the region (24, 38, 39). The results of this study show that the areas of maximal S. hematobium and hookworm predicted risk (>50%) straddling the southern borders of Burkina Faso and Mali were much smaller than predicted in our previous work (24, 38). In contrast, the focus of maximal S. mansoni prevalence (>50%) predicted along the Niger basin in Mali and extending eastward was much larger than previously predicted (24, 38). Although we are not able to make a direct comparison with the results reported by Schur et al. (39) (due to differences in model structure and data used), the inclusion of WASH indicators has improved the ability of helminth prediction models to account for the geographical variation in infection risk compared with our previous work (24). Therefore, the analysis presented here also provides scope for improving spatial predictions of helminth infections using WASH variables as predictors.
We have demonstrated that including WASH in geographical disease risk models allows the quantification of the potential health impact of WASH interventions and improves our ability to explain the geographical variation in helminth infection risk of school-aged children. Our approach generated detailed maps that enable the identification of priority areas where preventive chemotherapy together with hygiene education and infrastructure programs on WASH could be targeted in West Africa.
Materials and Methods
WASH Data.
We restricted our analyses to Burkina Faso, Mali, and Ghana because these are the only countries in sub-Saharan Africa for which there were detailed DHS georeferenced WASH data and up-to-date extensive and representative helminth infection data. Data on source of drinking water, type of toilet facility, and floor type for households were extracted from the most recent, georeferenced DHS household survey datasets for Burkina Faso (2003), Ghana (2003), and Mali (2006) (SI Materials and Methods). The geographical unit of the DHS surveys was the cluster, which is usually a census enumeration area, and depending on the population size in rural areas may be a village or a group of villages and in urban areas may be a city block or an aggregate thereof. A total of 1,214 clusters (Fig. 2D) had complete geolocation. Within these clusters we had complete WASH and demographic information for 18,812 households, including 7,208 households in Burkina Faso, 2,740 households in Ghana, and 8,864 households in Mali. There was a significant difference between the proportion of households without piped water between Burkina Faso and the other countries (P = 0.025), but not between Ghana and Mali (P > 0.05) (Table S1). There was also a significant difference between all countries with respect to the prevalence of households without toilet facilities and the prevalence of households with natural floor type (e.g., earth, sand, dung, or mud) (P < 0.001).
Helminth Infection and Environmental Data.
We used data on S. hematobium, S. mansoni, and hookworm infections because these were the most prevalent helminth infections among those parasites looked for during the SCI surveys (25) (SI Materials and Methods). Across the three countries the mean prevalence of S. hematobium was 25.6%, that of S. mansoni was 3.3%, and that of hookworm was 5.9%.
A 5 × 5-km resolution rural/urban surface derived from the Global Rural–Urban Mapping Project beta product was obtained from the Center for International Earth Science Information Network of the Earth Institute at Columbia University (New York) (http://sedac.ciesin.columbia.edu/gpw/global.jsp). Although the DHS data contain information about whether the cluster is rural or urban, this classification has not been sufficiently consistent between countries. To evaluate rural/urban effects avoiding misclassification bias, we used a standardized rural–urban surface based on satellite remote sensing data. This was important for spatial prediction because we needed a rural–urban surface that covered the entire study area (the DHS rural–urban classification was measured only at the DHS locations). With that plan in mind, values of this surface were extracted for each DHS cluster using the geographical information system ArcGIS version 10.0 (ESRI) to define whether the residence was urban or rural. Data on LST and NDVI were obtained from the National Oceanographic and Atmospheric Administration's Advanced Very High Radiometer (40). The locations of large perennial inland water bodies were obtained from the Food and Agriculture Organization of the United Nations (http://www.fao.org/geonetwork/srv/en/main.home). These electronic datasets were imported into ArcGIS version 10.0 and values for LST, NDVI, and DPWB and WASH covariates were extracted for each parasitological survey location.
Data Analysis Framework.
The analysis was conducted in two phases (Fig. S1) that are described in more detail in SI Materials and Methods.
Phase 1: Geographical risk prediction of household WASH indicators.
The variable “main floor material” was dichotomized into “natural floor” and “all other floor types”; the variable “source of drinking water” was dichotomized into “no piped water” and “all other water source types”; and the variable “toilet facility” was dichotomized into “no toilet facility” and “all other toilet facilities.” We developed logistic regression models of these three variables using the Bayesian statistical software WinBUGS version 1.4 (41). In all models the initial set of covariates was mother's age and education, number of members in the household, and residence (urban or rural). Four models were tested to capture spatial patterns in the data (Table S2): three models for geographical trend (a quadratic trend surface, model 1; a cubic trend surface, model 2; and a nonparametric trend surface using splines, model 3) and a model for local spatial variation in which a geostatistical random effect was included (model 4), on the basis of principles of model-based geostatistics (42). There was little second-order variation (local clustering) in the WASH variables to support the use of a geostatistical approach. We limited the order of the polynomials to cubic to avoid the instability associated with higher orders. The best-fitting model [based on the DIC (the lower the DIC was, the better the model fit to the data)] for floor type was model 2, whereas the best-fitting model for water supply and sanitation was model 1 (Table 1).
Phase 2: Geographical risk prediction of helminth infection in school-aged children.
We built model-based geostatistical models of S. hematobium, S. mansoni, and hookworm infection in school-aged children using WinBUGS version 1.4 (SI Materials and Methods). The initial set of covariates was the individual child's sex and age (categorized into 5–9 y, 10–15 y, and 16–19 y); the average values of LST, NDVI, and DPWB; and the posterior mean and variance of the WASH indicators (i.e., proportion of households without improved floor types, piped water, and toilet) extracted at each parasite survey location. Two WASH maps for each WASH indicator were generated and overlaid with the parasite survey locations: a map of the posterior mean WASH indicator and a map of the posterior variance WASH indicator. Each parasite location falls within a grid cell of these maps and the corresponding value was extracted for each point. The data for analysis included both sexes but the prediction map was generated for males only as this was the group with highest risk of infection.
This analysis the WASH indicators were added as random variables (using their mean and variance from the phase 1 model) instead of a fixed mean. This method incorporates the geographical uncertainty in WASH. The impact of incorporating this uncertainty was shown by comparing the overall SD in infection risk across the study area to the overall SD from the simpler model using mean WASH.
To assess the proportion of spatial variance in helminth infection accounted by WASH and to choose the best-fitting model for prediction, three models were tested for each helminth Table S3, Table S4, and Table S5: a model including all covariates (model A), a model with individual and environmental covariates only (model B), and a model with individual and WASH covariates only (model C). Model selection for prediction was based on the DIC (42). The ability of the final model to discriminate prevalence of natural floor type, water supply, and toilet availability and helminth infection thresholds was assessed using the area under the curve (AUC) of the receiver operating characteristic (ROC) (43). The proportion of geographical variation in helminth infections explained by WASH indicators was estimated by comparing the proportional decrease in the spatial variance parameter of the geostatistical random effect with a model containing only the environmental covariates.
Estimation of the PAF of Helminth Infection Due to WASH.
PAF estimates in this study represent the fraction of total helminth infections in the population that would not have occurred if WASH indicators were ideal, whereas the effects of other contributors (e.g., environmental variables) remain unchanged (44). PAF estimation for WASH indicators was based on the best-fitting model for each helminth (judged by the DIC). More details on estimation procedures are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank MEASURE DHS for permission to use DHS datasets from Burkina Faso, Ghana, and Mali. We thank the children, parents, teachers, head teachers, and district officials who participated in the SCI helminth surveys in Burkina Faso, Ghana, and Mali. We are grateful to the technicians and the staff of the national schistosomiasis control program in Burkina Faso, Ghana, and Mali who provided organizational and administrative support and undertook sample collection and microscopy during the field surveys.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106784108/-/DCSupplemental.
References
- 1.Bartram J, Cairncross S. Hygiene, sanitation, and water: Forgotten foundations of health. PLoS Med. 2010;7:e1000367. doi: 10.1371/journal.pmed.1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferriman A. BMJ readers choose the “sanitary revolution” as greatest medical advance since 1840. BMJ. 2007 10.1136/bmj.39097.611806.DB. [Google Scholar]
- 3.WHO . Progress on Sanitation and Drinking-Water. WHO/UNICEF. Geneva: World Health Organization; 2010. [Google Scholar]
- 4.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Comparative Risk Assessment Collaborating Group Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 5.Prüss A, Kay D, Fewtrell L, Bartram J. Estimating the burden of disease from water, sanitation, and hygiene at a global level. Environ Health Perspect. 2002;110:537–542. doi: 10.1289/ehp.110-1240845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fewtrell L, et al. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: A systematic review and meta-analysis. Lancet Infect Dis. 2005;5:42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- 7.Clasen T, Schmidt WP, Rabie T, Roberts I, Cairncross S. Interventions to improve water quality for preventing diarrhoea: Systematic review and meta-analysis. BMJ. 2007;334:782. doi: 10.1136/bmj.39118.489931.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mara DD, Feachem RGA. Water- and excreta-related diseases: Unitary environmental classification. J Environ Eng. 1999;125:334–339. [Google Scholar]
- 9.Prüss A, Bos R, Gore F, Bartram J. Safe Water Better Health: Costs, Benefits, and Sustatinability of Interventions to Protect and Promote Health. Geneva: World Health Organization; 2008. [Google Scholar]
- 10.Emerson PM, et al. Role of flies and provision of latrines in trachoma control: Cluster-randomised controlled trial. Lancet. 2004;363:1093–1098. doi: 10.1016/S0140-6736(04)15891-1. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt WP, Cairncross S, Barreto ML, Clasen T, Genser B. Recent diarrhoeal illness and risk of lower respiratory infections in children under the age of 5 years. Int J Epidemiol. 2009;38:766–772. doi: 10.1093/ije/dyp159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waddington H, Snilstveit B, White H, Fewtrell L. Synthetic Review. New Delhi: International Initiative for Impact Evaluation; 2009. Water, sanitation and hygiene interventions to combat diarrhoea in developing countries; p. 119. Available at http://zunia.org/uploads/media/knowledge/3ie%20SR0011249554991.pdf. [Google Scholar]
- 13.Arfaa F, Sahba GH, Farahmandian I, Jalali H. Evaluation of the effect of different methods of control of soil-transmitted helminths in Khuzestan, southwest Iran. Am J Trop Med Hyg. 1977;26:230–233. doi: 10.4269/ajtmh.1977.26.230. [DOI] [PubMed] [Google Scholar]
- 14.Sahba GH, Arfaa F. The effect of sanitation on ascariasis in an Iranian village. J Trop Med Hyg. 1967;70:37–39. [PubMed] [Google Scholar]
- 15.Chandler AC. A comparison of helminthic and protozoan infections in two Egyptian villages two years after the installation of sanitary improvements in one of them. Am J Trop Med Hyg. 1954;3:59–73. doi: 10.4269/ajtmh.1954.3.59. [DOI] [PubMed] [Google Scholar]
- 16.Barbosa FS, Pinto R, Souza OA. Control of schistosomiasis mansoni in a small north east Brazilian community. Trans R Soc Trop Med Hyg. 1971;65:206–213. doi: 10.1016/0035-9203(71)90220-3. [DOI] [PubMed] [Google Scholar]
- 17.Esrey SA, Potash JB, Roberts L, Shiff C. Effects of improved water supply and sanitation on ascariasis, diarrhoea, dracunculiasis, hookworm infection, schistosomiasis, and trachoma. Bull World Health Organ. 1991;69:609–621. [PMC free article] [PubMed] [Google Scholar]
- 18.Wang LD, et al. A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med. 2009;360:121–128. doi: 10.1056/NEJMoa0800135. [DOI] [PubMed] [Google Scholar]
- 19.Asaolu SO, Ofoezie IE, Odumuyiwa PA, Sowemimo OA, Ogunniyi TA. Effect of water supply and sanitation on the prevalence and intensity of Ascaris lumbricoides among pre-school-age children in Ajebandele and Ifewara, Osun State, Nigeria. Trans R Soc Trop Med Hyg. 2002;96:600–604. doi: 10.1016/s0035-9203(02)90323-8. [DOI] [PubMed] [Google Scholar]
- 20.WHO . WHO Technical Report Series 912. Geneva: World Health Organization; 2002. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: Report of a WHO expert committee; pp. 1–57. [PubMed] [Google Scholar]
- 21.Clements ACA, et al. A comparative study of the spatial distribution of schistosomiasis in Mali in 1984-1989 and 2004-2006. PLoS Negl Trop Dis. 2009;3:e431. doi: 10.1371/journal.pntd.0000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Utzinger J, N'goran EK, Caffrey CR, Keiser J. From innovation to application: Social-ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop. 2011;120(Suppl 1):S121–S137. doi: 10.1016/j.actatropica.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Utzinger J, Bergquist R, Shu-Hua X, Singer BH, Tanner M. Sustainable schistosomiasis control—the way forward. Lancet. 2003;362:1932–1934. doi: 10.1016/S0140-6736(03)14968-9. [DOI] [PubMed] [Google Scholar]
- 24.Magalhães RJ, Clements AC, Patil AP, Gething PW, Brooker S. The applications of model-based geostatistics in helminth epidemiology and control. Adv Parasitol. 2011;74:267–296. doi: 10.1016/B978-0-12-385897-9.00005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fenwick A, et al. The Schistosomiasis Control Initiative (SCI): Rationale, development and implementation from 2002-2008. Parasitology. 2009;136:1719–1730. doi: 10.1017/S0031182009990400. [DOI] [PubMed] [Google Scholar]
- 26.Raso G, et al. An integrated approach for risk profiling and spatial prediction of Schistosoma mansoni-hookworm coinfection. Proc Natl Acad Sci USA. 2006;103:6934–6939. doi: 10.1073/pnas.0601559103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asaolu SO, Ofoezie IE. The role of health education and sanitation in the control of helminth infections. Acta Trop. 2003;86:283–294. doi: 10.1016/s0001-706x(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 28.Hunter PR, MacDonald AM, Carter RC. Water supply and health. PLoS Med. 2010;7:e1000361. doi: 10.1371/journal.pmed.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mara D, Lane J, Scott B, Trouba D. Sanitation and health. PLoS Med. 2010;7:e1000363. doi: 10.1371/journal.pmed.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yayemain D, et al. Achieving trachoma control in Ghana after implementing the SAFE strategy. Trans R Soc Trop Med Hyg. 2009;103:993–1000. doi: 10.1016/j.trstmh.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Emerson PM, Burton M, Solomon AW, Bailey R, Mabey D. The SAFE strategy for trachoma control: Using operational research for policy, planning and implementation. Bull World Health Organ. 2006;84:613–619. doi: 10.2471/blt.05.28696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodgers AF, Ajono LA, Gyapong JO, Hagan M, Emerson PM. Characteristics of latrine promotion participants and non-participants; inspection of latrines; and perceptions of household latrines in Northern Ghana. Trop Med Int Health. 2007;12:772–782. doi: 10.1111/j.1365-3156.2007.01848.x. [DOI] [PubMed] [Google Scholar]
- 33.Chen C, et al. Incremental cost of conducting population-based prevalence surveys for a neglected tropical disease: The example of trachoma in 8 national programs. PLoS Negl Trop Dis. 2011;5:e979. doi: 10.1371/journal.pntd.0000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dada AC. Packaged water: Optimizing local processes for sustainable water delivery in developing nations. Global Health. 2011 doi: 10.1186/1744-8603-7-24. 10.1186/1744-8603-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frost C, Thompson SG. Correcting for regression dilution bias: Comparison of methods for a single predictor variable. J R Stat Soc Ser A Stat Soc. 2000;162:173–189. [Google Scholar]
- 36.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ezzati M, Utzinger J, Cairncross S, Cohen AJ, Singer BH. Environmental risks in the developing world: Exposure indicators for evaluating interventions, programmes, and policies. J Epidemiol Community Health. 2005;59:15–22. doi: 10.1136/jech.2003.019471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clements AC, et al. Mapping the probability of schistosomiasis and associated uncertainty, West Africa. Emerg Infect Dis. 2008;14:1629–1632. doi: 10.3201/eid1410.080366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schur N, et al. Geostatistical model-based estimates of Schistosomiasis prevalence among individuals aged ≤ 20 years in West Africa. PLoS Negl Trop Dis. 2011;5:e1194. doi: 10.1371/journal.pntd.0001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hay SI, Tatem AJ, Graham AJ, Goetz SJ, Rogers DJ. Global environmental data for mapping infectious disease distribution. Adv Parasitol. 2006;62:37–77. doi: 10.1016/S0065-308X(05)62002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: Evolution, critique and future directions. Stat Med. 2009;28:3049–3067. doi: 10.1002/sim.3680. [DOI] [PubMed] [Google Scholar]
- 42.Diggle PJ, Moyeed RA, Tawn JA. Model-based geostatistics. Appl Stat. 1998;47:299–350. [Google Scholar]
- 43.Brooker S, Hay SI, Bundy DA. Tools from ecology: Useful for evaluating infection risk models? Trends Parasitol. 2002;18:70–74. doi: 10.1016/s1471-4922(01)02223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C. Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol. 1985;122:904–914. doi: 10.1093/oxfordjournals.aje.a114174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.