Abstract
It is widely believed that the induction of a broadly neutralizing antibody (bNAb) response will be a critical component of a successful vaccine against HIV. A significant fraction of HIV-infected individuals mount bNAb responses, providing support for the notion that such responses could be elicited through vaccination. Infection of macaques with simian immunodeficiency virus (SIV) or SIV/HIV chimeric virus (SHIV) has been widely used to model aspects of HIV infection, but to date, only limited bNAb responses have been described. Here, we screened plasma from 14 R5-tropic SHIV-infected macaques for broadly neutralizing activity and identified a macaque with highly potent cross-clade plasma NAb response. Longitudinal studies showed that the development of broad and autologous NAb responses occurred coincidentally in this animal. Serum-mapping studies, using pseudovirus point mutants and antigen adsorption assays, indicated that the plasma bNAbs are specific for epitopes that include carbohydrates and are critically dependent on the glycan at position 332 of Env gp120. The results described herein provide insight into the development and evolution of a broad response, suggest that certain bNAb specificities may be more rapidly induced by immunization than others, and provide a potential model for the facile study of the development of bNAb responses.
Understanding the complex interplay of HIV-1 and the immune system in infected individuals may inform HIV-1 vaccine design. A series of studies have demonstrated that roughly 5–30% of HIV-1–infected individuals develop broadly neutralizing antibodies (bNAbs) over time (1–3), dependent upon the criteria used to define breadth and potency of neutralization. When passively administered, bNAbs have been shown to protect against infection by chimeric simian HIV (SHIV) challenge in macaque models (4–10). However, no immunogens developed to date have succeeded in eliciting significant bNAb responses. Understanding the development of such responses during natural infection may provide important clues for designing more appropriate immunogens.
A number of studies have characterized the antibody specificities mediating plasma neutralization breadth and potency in HIV-1–infected individuals (1, 11–13). Furthermore, a few longitudinal studies have examined the factors associated with the development of breadth, and although there are some inconsistencies, it has been suggested that broad neutralization correlates with time postinfection, plasma viremia levels, CD4+ T-cell counts at set-point, and binding avidity to the envelope protein (1, 14, 15). Though these studies have provided some insight into the factors associated with the development of bNAbs, detailed longitudinal studies involving bNAbs of different specificities would greatly improve our understanding of the evolution and maturation of broad responses.
SHIVs express the HIV envelope glycoprotein and can therefore be used to evaluate HIV-1 Env-specific neutralizing antibody (NAb) responses. However, although the SHIV/macaque model has been extensively used to evaluate vaccine efficacy, the ability of SHIV-infected macaques to mount highly potent bNAb responses has not yet been demonstrated. Developing a SHIV that is capable of eliciting broad and potent NAb responses, and tracking the evolution of this immune response, might provide unprecedented insight into the factors associated with the development of bNAbs.
Recently, a pathogenic R5-tropic SHIV with the env from a molecularly cloned derivative of HIV-1Ada (pHIV-1AD8) was developed by serial passage of viral “swarms” in macaques (16). To determine whether any of the SHIVAD8-infected macaques developed bNAbs, we screened plasma from 14 infected animals for neutralizing activity. Of these, one macaque displayed extraordinarily potent cross-clade plasma NAb responses. Neutralization assays were carried out using samples taken at serial time points, and indicated that the development of broad plasma neutralization was unusually rapid and coincided with the development of autologous NAbs. Furthermore, serum-mapping studies suggested that the bNAbs interact with carbohydrates and are critically dependent on the N332 glycan. The results described herein suggest considerable promise for the SHIV/macaque model in the dissection of bNAb responses to HIV.
Results
Analysis of Plasma Neutralization Breadth.
We previously reported that some SHIVAD8-infected rhesus monkeys generated autologous NAbs, which generally correlated with levels of set-point viremia (16). Here, plasma samples from 14 SHIVAD8-infected macaques were tested for the development of autologous NAbs using either replication-competent (RC) or pseudovirus targets in the TZM-bl cell system (Table S1). Consistent with the previous study, we were only able to demonstrate evidence of virus neutralization in five of 14 infected animals. In two of the five macaques, partial neutralization was detected at a plasma dilution of 1:20. In two of the three remaining monkeys (CJ58 and CJ8B), high NAb titers declined over time to levels <1:100 by year 3 postinfection (Table S1). A single macaque (CE8J) generated high, sustained autologous NAb titers against both RC and pseudovirus targets in the TZM-bl assay.
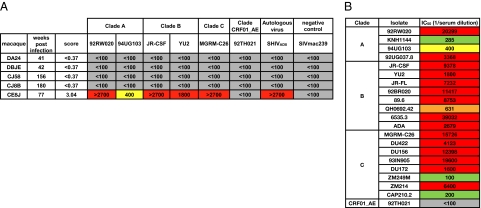
Plasma from the five SHIVAD8-infected macaques, which had generated autologous NAbs, were next tested for neutralizing activity against a cross-clade HIV-1 pseudovirus panel containing tier 2 isolates, that had previously been shown to be predictive of neutralization breadth and potency against a larger number of isolates (Fig. 1A) (2). Only macaque CE8J developed broad and potent plasma-neutralizing responses. A plasma sample, collected at 77 wk postinfection from macaque CE8J, neutralized five of six HIV-1 pseudoviruses from the cross-clade panel, and notably, four of these viruses were neutralized with exceptional potency (IC50s > 1/1500 plasma dilution). Based on these results, we then tested the macaque CE8J plasma for neutralization against a larger cross-clade pseudovirus panel containing 21 tier 2 isolates; 71% of the viruses were neutralized with extraordinary potency (IC50s >1/1500 plasma dilution), confirming the remarkably broad and potent activity (Fig. 1B).
Fig. 1.
Cross-clade neutralizing activity of macaque plasma. (A) Plasma from 5 SHIVAD8-infected macaques were tested for neutralizing activity against a cross-clade tier 2 pseudovirus panel, the autologous virus (SHIVAD8), and a negative control virus (SIVmac239). Values represent the plasma dilution at which 50% neutralization was detected. Scores were designated based on an algorithm incorporating breadth and potency (2). (B) Plasma from macaque CE8J was tested against a larger cross-clade pseudovirus panel containing 21 isolates. Boxes are color coded as follows: gray, IC50 < 1:100; green, 1:100 < IC50 < 1:300; yellow, 1:300 < IC50 < 1:500; orange, 1:500 < IC50 < 1:1,000; red, IC50 > 1:1,000.
To determine the component of the CE8J plasma responsible for the broad neutralizing activity, we adsorbed the plasma IgG with either Protein A-Sepharose beads or blank control beads and tested the depleted fractions for neutralizing activity. We observed that no neutralizing activity was present in the Protein A-depleted fraction (Fig. S1), demonstrating that the IgG component of the plasma mediates the broad neutralizing activity.
Evolution of Plasma Neutralization Breadth and Potency in Macaque CE8J.
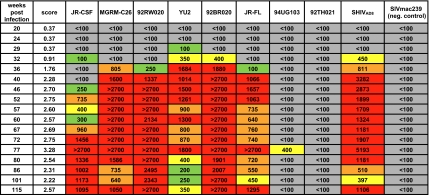
To investigate the development of plasma neutralization breadth and potency in macaque CE8J, we tested 17 plasma samples taken at serial time points (between 20 and 115 wk postinfection) for neutralizing activity. Potent cross-clade plasma neutralization developed between 32 and 36 wk postinfection (Fig. 2). Interestingly, potent neutralizing responses against the autologous virus also developed between these time points. The plasma neutralization potency against most isolates generally either plateaued or increased slightly until 80 wk postinfection, at which point there was a substantial drop in potency against most isolates (Fig. 2 and Fig. S2). Notably, the diminished neutralization potency may have resulted from the sharp decline in memory CD4+ T cells and/or spike in viral load, which also occurred at this time point (Fig. S3) (16).
Fig. 2.
Development of broad neutralization in macaque CE8J. Plasma samples collected from macaque CE8J at serial time points were tested for neutralizing activity against a cross-clade pseudovirus panel containing tier 2 viruses, the autologous SHIVAD8 virus, and a negative control virus (SIVmac239). Scores were designated based on an algorithm incorporating breadth and potency (2). Boxes are color coded as follows: gray, IC50 < 1:100; green, 1:100 < IC50 < 1:300; yellow, 1:300 < IC50 < 1:500; orange, 1:500 < IC50 < 1:1,000; red, IC50 > 1:1,000.
Dissecting the Antibody Specificities Mediating CE8J Plasma Neutralization Breadth.
We next sought to define the antibody specificities mediating broad plasma neutralization in macaque CE8J. As a first approach, we investigated whether the bNAb specificities reacted with recombinant gp120 by adsorbing the plasma with JR-FL gp120-coated beads and then testing the flow-through fraction for neutralizing activity against a cross-clade pseudovirus panel (Fig. S4). Indeed, the broad and potent plasma-neutralizing activity could be depleted on the gp120-coated beads, suggesting that gp120-specific antibodies were mediating the breadth of neutralization. Next, we examined the role of the variable loops in forming the epitopes recognized by the plasma bNAbs by performing plasma adsorptions with gp120JR-FL ΔV1/V2 and ΔV3-coated beads. Interestingly, the broad plasma-neutralizing activity could be partially adsorbed by gp120JR-FL ΔV1/V2 but not by gp120JR-FL ΔV3 (Fig. S4).
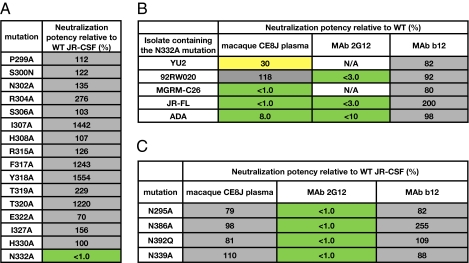
Because the results described herein suggested that the epitopes recognized by the bNAb specificities may be in proximity to or contiguous with the V3 loop, we next tested the plasma for neutralizing activity against a panel of JR-CSF mutants containing single point mutations in or adjacent to the V3 loop (Fig. 3A). Interestingly, we found that the N332A mutation, which results in the removal of a glycosylation site at the base of the V3 loop, completely abolished plasma-neutralizing activity against HIV-1JR-CSF. To determine whether this mutation also affected broad plasma-neutralizing activity, we tested the plasma for neutralization against a cross-clade panel of pseudoviruses incorporating the N332A mutation (Fig. 3B). Indeed, the plasma-neutralizing activity was significantly diminished against all of the mutant pseudoviruses on the panel except for 92RW020. The lack of plasma-neutralization sensitivity to 92RW020 N332A may suggest that the N332 glycan dependency is somewhat isolate-specific, as has been observed for other glycan-dependent and glycan-specific bNAbs (17). Notably, the N332 glycan is important for formation of the epitopes recognized by the glycan-specific bNAb 2G12 and the recently described PGT bNAbs (18, 19). Furthermore, serum-mapping studies have demonstrated that this glycan is critical for broad and potent serum-neutralizing activity in a significant proportion of HIV-1–infected humans (13, 14).
Fig. 3.
Broad neutralization of macaque CE8J plasma is critically dependent on the N332 glycan. (A) Neutralizing activity of macaque CE8J plasma against selected JR-CSF alanine mutants. (B) Neutralizing activity of macaque CE8J plasma against a cross-clade pseudovirus panel containing the N332A mutation. mAbs b12 and 2G12 are included for comparison. N/A, data are not available because 2G12 does not neutralize these isolates. (C) Neutralizing activity of macaque CE8J plasma against selected JR-CSF glycan mutants. mAbs b12 and 2G12 are included for comparison. Percent neutralization potency relative to the WT pseudovirus was calculated using the equation (IC50 variant/IC50 WT) × 100. Boxes are color coded as follows: gray, 50–2,000%, yellow, 10–50%; green, <10%.
Plasma bNAbs in Macaque CE8J Bind to the Glycan Shield of gp120.
Based on our results, we next investigated whether the plasma bNAbs bound to epitopes similar to that of 2G12, which recognizes a cluster of high-mannose glycans composed of N332, N339, and N392 (18). Mutation of individual glycans at positions 339 and 392 as well as 295 and 386 in JR-CSF pseudovirus had no effect on plasma-neutralizing activity (Fig. 3C), demonstrating that the plasma bNAb specificities bind to epitopes distinct from that recognized by 2G12. As a second approach, we adsorbed the plasma with TM-Pst1, a yeast glycoprotein displaying homogenous Man8GlcNAc2 glycans that has high affinity for 2G12 and can inhibit 2G12 neutralization of pseudoviruses (20). Interestingly, a large fraction of the broad and potent plasma-neutralizing activity could be adsorbed with TM-Pst1, suggesting that the broadly neutralizing plasma antibodies bind directly to Man8GlcNAc2 glycans (Fig. S5). We further investigated the role of glycans in recognition by the plasma bNAbs using the glucosidase analog N-butyldeoxynojirimycin (NB-DNJ), which blocks the removal of glucose residues attached to the D1 arm of the precursor Glc3Man9GlcNAc2 (21) and has been shown to inhibit 2G12 binding (18). Similar to results obtained for 2G12, treatment of pseudoviruses with NB-DNJ abolished CE8J plasma-neutralizing activity, suggesting that the terminal mannose of the D1 arm of Man9GlcNAc2 is important for formation of the epitopes recognized by the CE8J plasma bNAb specificities (Fig. S6). Together, these results indicate that the plasma bNAbs bind to glycan-specific epitopes, which may overlap with that of 2G12 and the recently described PGT MAb epitopes.
Evolution of the N332A-Sensitive, Broadly Neutralizing Specificity in the Plasma of Macaque CE8J.
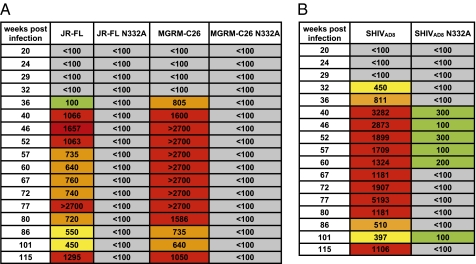
The broad plasma-neutralizing activity in macaque CE8J developed at 36 wk postinfection and was maintained after this time point, suggesting either rapid development of a single bNAb specificity (i.e., the N332A-sensitive specificity) or sequential development of broad and potent neutralizing antibodies with distinct specificities. To investigate this question, we tested the plasma samples collected at serial time points for sensitivity to the N332A mutation (Fig. 4A). Indeed, all of the serial plasma samples exhibited a loss of neutralization activity against the N332A-containing pseudovirus variants, indicating that the N332A-sensitive bNAb specificity was rapidly induced and subsequently maintained over time. A similar result was observed with the autologous SHIVAD8 virus containing an N332A mutation, suggesting that both the autologous and heterologous neutralizing antibody responses in this macaque are mediated by the N332A-sensitive specificity (Fig. 4B).
Fig. 4.
Evolution of the N332A-sensitive bNAb specificity in macaque CE8J plasma. (A) Plasma samples collected at serial time points were tested for neutralizing activity against JR-CSF and MGRM-C26 pseudovirus variants containing an N332A mutation. (B) Plasma samples taken at serial time points were tested for neutralizing activity against SHIVAD8 containing an N332A mutation. Boxes are color coded as follows: gray, IC50 < 1:100; green, 1:100 < IC50 < 1:300; yellow, 1:300 < IC50 < 1:600; orange, 1:600 < IC50 < 1:1,000; red, IC50 > 1:1,000.
Neutralization Escape SHIV Variant Emerges in Macaque CE8J.
The neutralization experiments described herein were all performed using pseudotyped virus prepared from a recently described pathogenic SHIVAD8 molecular clone carrying an env gene present in the swarm virus stock generated from animal CK15 at week 42 postinfection (22). To ascertain whether the neutralization sensitivity of the input swarm had changed during the 2-y infection of animal CE8J, the replication-competent SHIVAD8 inoculum (Lymph Node Virus) (16) or virus collected at the time of euthanasia at week 117 postinfection (W.117) were incubated with plasma collected at weeks 0, 50, and 87 and assayed for 28 h in TZM-bl cells. The input SHIVAD8 was neutralized by the week 50 and 87 plasma samples, whereas the week 117 virus swarm was resistant to neutralization at both of these time points. A comparison of env gene sequences in the SHIVAD8 inoculum with those present in the swarm virus at week 117 revealed consistent changes affecting all of the variable and the C3 regions of gp120 (Fig. S7) and is concordant with the neutralization escape properties of the virus circulating at weeks 50 and 87.
Discussion
Numerous studies have shown that ∼5–30% of HIV-1–infected donors develop broad and potent neutralizing serum, depending on the criteria used to define neutralization breadth and potency. Here, we observed that one of the 14 SHIVAD8-infected macaques tested (∼7%) developed broad and potent plasma-neutralizing activity, which is within the range that has been observed in humans. However, a larger number of infected macaques will need to be screened to make more robust comparisons of neutralization patterns in human and macaque sera. Importantly, macaque CE8J developed plasma-neutralizing activity with comparable breadth and potency as HIV-1 “elite neutralizers” (2), which, to our knowledge, represents the most broad and potent NAb response observed to date in the SHIV/macaque model. Therefore, the results demonstrate that the rhesus monkey immune system is capable of generating exceptionally potent bNAb responses during natural infection, further validating the use of the SHIV/macaque model to evaluate vaccine candidates.
Interestingly, macaque CE8J developed broad and potent responses by 9 mo postinfection, which is much faster than observed for most HIV-1 elite neutralizers, who generally only develop such responses 2–3 y or more postinfection. Also, in stark contrast to observations in HIV-1–infected humans, the autologous and broadly neutralizing responses in macaque CE8J developed simultaneously, and both responses appeared to be mediated by the N332A-sensitive bNAb specificity. The mechanisms underlying the development of bNAbs in a single SHIVAD8-infected monkey are currently under investigation and may relate to the quality and/or quantity of B-cell or CD4+ T-cell responses, or host genetics. Of note, many of the 14 SHIVAD8-infected animals in this study exhibited similar or higher levels of plasma viremia than macaque CE8J (Fig. S8), suggesting that the failure to develop broadly reactive neutralizing activity is likely not due to low levels of antigenic stimulation.
A common feature of SHIVAD8-infected macaques seen here is the relatively modest fraction that develop autologous NAbs. In our entire cohort of moneys infected with SHIVAD8 derivatives (swarm virus stocks prepared during infection or at the time of euthanasia or a pathogenic molecular clone [SHIVAD8MV]), only nine of 40 animals developed autologous NAbs (Table S2). When detected, neutralization was observed whether the target virus used in the assay was an exact match for the virus inoculated into an animal, and in some cases was not sustained over time. At present we have no explanation for the resistance of SHIVAD8-infected macaques to generate autologous NAbs, although the tier 3 status of the parental HIV-1Ada may be a contributing factor.
Our epitope-mapping studies indicate that the plasma bNAbs bind to an epitope critically dependent on the gp120 N332 glycan, perhaps overlapping that of 2G12 and/or the recently described PGT antibodies. Importantly, broad and potent serum-neutralizing activity in a significant proportion of HIV-1–infected donors has been mapped to N332A-sensitive epitopes (13, 14), and broad and highly potent monoclonal antibodies have recently been isolated from such donors (19). Given the rapid generation of bNAbs in this macaque, and the prevalence of bNAbs against N332A-sensitive epitopes in humans, it will be of interest to determine whether bNAbs against glycan epitopes are more rapidly elicited during natural infection and whether they can be more easily induced by vaccination. Future longitudinal studies on infected or vaccinated donors will reveal whether this is the case.
Materials and Methods
Animal Experiments.
Virus inoculations, phlebotomies, lymphocyte subset analysis, and plasma viral load determination were performed as described previously (16). All animal experiments were done at the National Institutes of Health in compliance with the guidelines of the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee.
Pseudovirus Production and Neutralization Assays.
Pseudoviruses incorporating single-alanine substitutions were generated by transfection of 293T cells with an Env-expressing plasmid and an Env-deficient genomic backbone plasmid (pSG3ΔEnv), as described previously (23). For generation of NB-DNJ–treated pseudoviruses, 2 mM of the glycosidase inhibitor was added at the time of transfection (17). Pseudoviruses were harvested 72 h posttransfection for use in neutralization assays. Neutralizing activity was assessed using a single round of replication pseudovirus assay and TZM-bl target cells, as described previously (24). Virus neutralization assays using replication competent virus stocks [the starting SHIVAD8 lymph node virus inoculum (16) and the virus recovered from macaque CE8J at the time of euthanasia at week 117 (W.117 virus)] were performed as described previously (16).
Serum Adsorptions.
Serum adsorptions with Protein A-Sepharose beads (GE Healthcare) were performed by incubating 20 μL plasma with ∼250 μL of a 50% slurry of Protein A-Sepharose beads for 2 h at room temperature. The beads were then pelleted by centrifugation, and the Protein A-depleted fraction was collected. Serum adsorptions with antigen-coupled beads were performed using tosyl-activated magnetic beads, as described previously (25). A total of 1 mg of gp120 or 1 mg of TM-Pst1 was used for bead coupling. Two rounds of adsorption were performed to ensure complete removal of antigen-specific antibodies. Functional antibodies were eluted from beads by exposing the beads to series of increasingly acidic conditions, as described (25).
ELISAs.
Ninety-six-well ELISA plates were coated overnight at 4 °C with 50 μL PBS containing 50 ng of goat anti-human IgG Fc (Pierce), 100 ng of gp120, or 100 ng TM-Pst1 per well. The wells were washed 4× with PBS containing 0.05% Tween-20 and blocked with 3% BSA at room temperature for 1 h. Serial dilutions of plasma or mAb were then added to the wells, and the plates were incubated at room temperature for 1 h. After washing 4×, goat anti-human IgG F(ab′)2 conjugated to alkaline phosphatase (Pierce), diluted 1:1,000 in PBS containing 1% BSA and 0.025% Tween-20, was added to the wells. The plate was incubated at room temperature for 1 h, washed 4×, and the plate was developed by adding 50 μL of alkaline phosphatase substrate (Sigma) to 5 mL alkaline phosphatase staining buffer (pH 9.8), according to the manufacturer's instructions. The optical density at 405 nm was read on a microplate reader (Molecular Devices).
Virus Isolation and Sequence Analysis.
The W.117 virus stock was prepared from peripheral blood mononuclear cell (PBMC) and lymph node samples collected from macaque CE8J at the time of euthanasia at week 117 postinfection. Lymph node and PBMC suspensions were cocultivated with ConA-stimulated PBMC from an uninfected animal as described previously (16). Entire env genes were amplified from the starting SHIVAD8 lymph node virus inoculum and W.117 virus by RT-PCR, using primer pairs described previously (16), and then cloned for sequence analysis.
Supplementary Material
Acknowledgments
We thank C. Corbaci, C. Williams, and S. M. Eagol for figure preparation. This study was funded by the International AIDS Vaccine Initiative through Neutralizing Antibody Consortium, National Institute of Allergy and Infectious Diseases, National Institutes of Health Grants AI33292 (to D.R.B.) and AI084817 (to I.A.W.); the Ragon Institute of Massachusetts General Hospital, Massachusetts Institute of Technology, and Harvard University; and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117531108/-/DCSupplemental.
References
- 1.Sather DN, et al. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol. 2009;83:757–769. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simek MD, et al. Human immunodeficiency virus type 1 elite neutralizers: Individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doria-Rose NA, et al. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J Virol. 2009;83:188–199. doi: 10.1128/JVI.01583-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hessell AJ, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hessell AJ, et al. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parren PW, Burton DR. The antiviral activity of antibodies in vitro and in vivo. Adv Immunol. 2001;77:195–262. doi: 10.1016/S0065-2776(01)77018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mascola JR, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura Y, et al. Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: Implications for HIV-1 vaccine development. Proc Natl Acad Sci USA. 2003;100:15131–15136. doi: 10.1073/pnas.2436476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baba TW, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 10.Shibata R, et al. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 11.Gray ES, et al. Antibody specificities associated with neutralization breadth in plasma from human immunodeficiency virus type 1 subtype C-infected blood donors. J Virol. 2009;83:8925–8937. doi: 10.1128/JVI.00758-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, et al. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J Virol. 2006;80:1414–1426. doi: 10.1128/JVI.80.3.1414-1426.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker LM, et al. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS Pathog. 2010;6:e1001028. doi: 10.1371/journal.ppat.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray ES, et al. and the CAPRISA002 Study Team The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J Virol. 2011;85:4828–4840. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Gils MJ, Euler Z, Schweighardt B, Wrin T, Schuitemaker H. Prevalence of cross-reactive HIV-1-neutralizing activity in HIV-1-infected patients with rapid or slow disease progression. AIDS. 2009;23:2405–2414. doi: 10.1097/QAD.0b013e32833243e7. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura Y, et al. Generation of the pathogenic R5-tropic simian/human immunodeficiency virus SHIVAD8 by serial passaging in rhesus macaques. J Virol. 2010;84:4769–4781. doi: 10.1128/JVI.02279-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doores KJ, Burton DR. Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16. J Virol. 2010;84:10510–10521. doi: 10.1128/JVI.00552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scanlan CN, et al. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1&rarr2 mannose residues on the outer face of gp120. J Virol. 2002;76:7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker LM, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luallen RJ, et al. A yeast glycoprotein shows high-affinity binding to the broadly neutralizing human immunodeficiency virus antibody 2G12 and inhibits gp120 interactions with 2G12 and DC-SIGN. J Virol. 2009;83:4861–4870. doi: 10.1128/JVI.02537-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubbard SC, Ivatt RJ. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- 22.Shingai M, Yoshida T, Martin MA, Strebel K. Some human immunodeficiency virus type 1 Vpu proteins are able to antagonize macaque BST-2 in vitro and in vivo: Vpu-negative simian-human immunodeficiency viruses are attenuated in vivo. J Virol. 2011;85:9708–9715. doi: 10.1128/JVI.00626-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pantophlet R, et al. Fine mapping of the interaction of neutralizing and nonneutralizing monoclonal antibodies with the CD4 binding site of human immunodeficiency virus type 1 gp120. J Virol. 2003;77:642–658. doi: 10.1128/JVI.77.1.642-658.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, et al. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat Med. 2007;13:1032–1034. doi: 10.1038/nm1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.