Abstract
We report here that mouse macrophages undergo receptor-interacting kinase-3 (RIP3)-dependent but TNF-α–independent necrosis when Toll-like receptors (TLR) 3 and 4 are activated by poly(I:C) and LPS, respectively. An adaptor protein, Toll/IL-1 receptor domain-containing adapter inducing IFN-β (TRIF/TICAM-1), which is dispensable for TNF-α–induced necrosis, forms a complex with RIP3 upon TLR3/TLR4 activation and is essential for TLR3/TLR4-induced necrosis. Mice without RIP3 or functional TRIF did not show macrophage loss and elevation of inflammatory cytokines when they were exposed to LPS. Necrosis in mouse macrophages induced by either TNFR or TLR3/TLR4 is executed by reactive oxygen species. Taken together, these data indicate that there are multiple upstream necrosis-initiating signaling pathways converging on the RIP3 during an innate immune response to viral and bacterial infections in mammals.
Keywords: necroptosis, bone marrow-derived macrophage
Cell death is vital for development and tissue homeostasis in multicellular organisms. Apoptosis, a prevalent form of programmed cell death, is executed by the activation of a set of intracellular cysteine proteases known as caspases. When the caspase activity is impaired or inhibited, certain cell types switch the death program to necrosis in response to the TNF-α family of cytokines, including TNF-α, TNF-related apoptosis inducing ligand, and CD95/Fas ligand (1). This form of cell death, also known as necroptosis, is dependent on the kinase activities of two receptor-interacting kinases RIP1 and RIP3, which form a pronecrotic complex with their homotypic interaction motif (RHIM) upon activation of TNFR and related receptors. Recently, a RIP3-dependent but RIP1-independent necrosis has been reported in cells infected by murine cytomegalovirus (2).
Necrosis/necroptosis plays prominent roles in various pathological conditions, such as retina detachment (3), ischemic injury (4), septic shock (5), and acute pancreatitis (6). Such a form of cell death has also been implicated in microbe infection-associated death. Many viruses encode caspase inhibitors to circumvent apoptosis, the predominant host-defensive mechanism to prevent virus replication. For example, vaccinia virus (VV) encodes B13R/Spi2 and cowpox virus expresses CrmA; both are potent caspase-1 and caspase-8 inhibitors. Therefore, instead of apoptosis, infection by VV causes necrosis in CD3+ or TNF-activated T cells as well as in TNF-treated mouse embryonic fibroblasts (7). RIP3−/− mice fail to initiate VV-induced tissue necrosis and inflammation (7). The murine cytomegalovirus encoded protein vIRA, a RHIM-containing protein that disrupts the RIP3-dependent necrosis in the host to promote viral propagation (2). These observations indicate that RIP3-dependent necrosis is a crucial part of immune defense against microbe infections.
Unlike TNF-induced necrosis, the molecular mechanisms of microbe-initiated necrosis remain elusive. Innate immune cells, such as macrophages, sense microbial infection and evoke antimicrobial responses via pattern-recognition receptors. The Toll-like receptors (TLR) are the best-characterized members of pattern-recognition receptor families. TLRs detect a variety of pathogen-associated molecular patterns, such as bacteria, viruses, fungi, and parasites (8, 9). Each TLR recognizes specific ligands with its extracellular N-terminal leucine-rich repeats; among these, TLR3 responds to viral double-strand RNA (dsRNA) or synthesized analog of dsRNA poly(I:C) (10), whereas TLR4 is activated by LPS (11, 12), a cell-wall component of Gram-negative bacteria. Engagements of TLR3 and TLR4 with corresponding ligands trigger the recruitment of Toll/IL-1 receptor domain-containing adaptor to elicit both inflammatory cytokine and type I IFN responses. Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF/TICAM-1) is the sole adaptor protein for TLR3, but TLR4 signals can be mediated by either TRIF or Myd88 (myeloid differentiation primary response gene) (13–15). Both TRIF-deficient and TRIFLPS2/LPS2 dominant-negative mutant mice are defective in activating IFN regulatory factor 3 (IRF3) nor able to induce type I IFNs in response to LPS or poly(I:C) (14, 15). In addition to these immune responses, TLR3/TLR4 signals have also been shown to facilitate necrosis in different cell types. When caspase-8 activation is suppressed by inhibitors, such as IETD-fmk, CrmA or zVAD-fmk (z-VAD), LPS induces necrosis instead of apoptosis in human macrophages (16). Similarly, in the presence of z-VAD-fmk, TLR4 ligation triggered mouse peritoneal macrophage death, which was ablated in RIP3-deficient cells (17). In combination with IFN-γ, poly(I:C) induced apoptotic death in human T-cell leukemia Jurkat cells, but a defect in caspase-8 or Fas-associated death domain (FADD) converted apoptosis to necrosis (18). Although these observations suggest that TLR3 and TLR4 are able to cause necrosis, how they do so remains unclear.
Here, using seven genetic mice models, we provide the detailed molecular characterization of TLR3- and TLR4-activated, RIP3-dependent necrosis in mouse bone marrow-derived macrophages. Our data indicated that the activation of these TLRs directly activates macrophage necrosis and inflammatory cytokine production through the adaptor TRIF.
Results
TLR3 and -4 Trigger Necrosis of Macrophages.
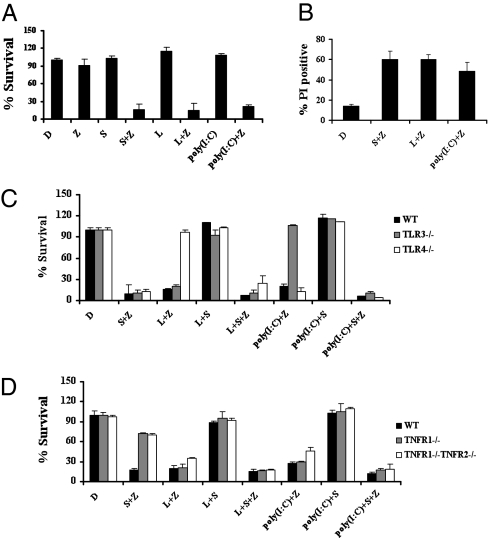
To investigate the necrotic death in response to TLR3 and TLR4 agonists, we obtained bone marrow-derived primary macrophages from C57BL/6 mice or gene-knockout mice in this genetic background. Consistent with previous findings in human cells, Smac mimetic, a small molecule compound mimicking the anti-IAP (anti-inhibitor of apoptosis protein) protein Smac/Diablo, caused massive necrosis in the presence of a pan caspase inhibitor z-VAD (Fig. 1 A and B). Notably, when treated with z-VAD, macrophages became highly susceptible to either poly(I:C) or LPS, both of which are agonists for TLR3 and TLR4, respectively.
Fig. 1.
TLR3 and TLR4 trigger necrosis of macrophages. (A) Bone marrow-derived macrophages isolated from wild-type mice were treated as indicated for 20 h. Cell viability was determined by measuring ATP levels. Data were represented as mean ± SD of duplicates. D, DMSO; L, LPS (20 ng/mL); poly(I:C) (50 μg/mL); S, Smac mimetic (100 nM); Z, z-VAD (10 μM). Identical concentrations were used in later experiments unless otherwise stated. (B) Bone marrow-derived macrophages from wild-type mice were treated as indicated for 16 h and then cells were analyzed for propidium iodide (PI) staining by flow cytometry. (C) Bone marrow-derived macrophages from wild-type, TLR3−/− and TLR4−/− mice were treated as indicated for 20 h. Cell viability was determined by measuring ATP levels. Data were represented as mean ± SD of duplicates. (D) Bone marrow-derived macrophages from wild-type, TNFR1−/−, and TNFR1−/−TNFR2−/− mice were treated as indicated for 20 h. Cell viability was determined by measuring ATP levels. Data were represented as mean ± SD of duplicates. All experiments were repeated at least three times with similar results.
As expected, macrophages from TLR3- and TLR4-deficient mice exhibited remarkable resistance to poly(I:C) and LPS, respectively. Their responses to Smac mimetic-induced necrosis were normal (Fig. 1C). Consistently, TAK-242 (19), a TLR4 antagonist, specifically blocked LPS-induced necrosis, but it had no effect on death induced by either Smac mimetics or poly(I:C) (Fig. S1).
Recognition of poly(I:C) and LPS by TLR3 and TLR4, respectively, induces NF-κB activation and the subsequent induction of cytokines, such as TNF-α (20), which may indirectly lead to TNF-α–dependent necrosis similar to Smac mimetic-induced necrosis (21). We sought to clarify the role of TNF signaling in TLR3/TLR4-induced death. As shown in Fig. 1D, poly(I:C)- or LPS-induced cell death was only slightly decreased in TNFR1−/− and TNFR1−/−TNFR2−/− macrophages compared with wild-type cells, suggesting that autocrine TNF-α plays a minor role in TLR3/TLR4-induced necrotic cell death. As expected, Smac mimetic/z-VAD–induced necrosis was abolished in both of these TNFR1 mutant macrophages. It is therefore apparent that TNFR and TLR3/TLR4 initiate necrotic death of macrophages through different pathways.
Multiple Upstream Necrotic Signals Converge at RIP3.
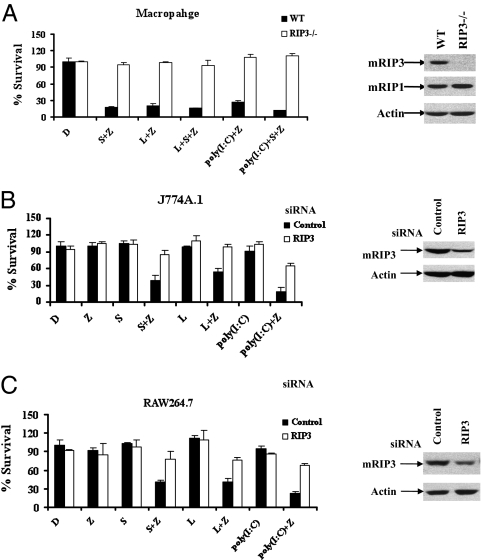
It is known that RIP3 plays an essential role in TNFR-mediated necrosis (7, 17, 21). Consistent with this notion, macrophages from RIP3-deficient mice were resistant to Smac mimetic-induced, TNF-dependent necrosis (Fig. 2A). Similarly, neither TLR3- nor TLR4-triggered necrotic death was observed in RIP3-deficient primary macrophages, indicating that RIP3 is also crucial for TLR3/TLR4-mediated necrosis. We further examined the requirement of RIP3 in TLR3/TLR4-induced necrotic death using two widely used macrophage cell lines, J774A.1 and RAW 264.7. As shown in Fig. 2 B and C, both cell lines behaved similarly to the primary macrophages and responded normally to Smac mimetic LPS or poly(I:C) plus z-VAD. However, reducing the endogenous RIP3 by RNAi provided effective protection against these necrosis-inducing agents, confirming that RIP3 confers a converging signaling molecule in TNFR- and TLR3/TLR4-induced necrotic pathways.
Fig. 2.
Multiple upstream necrotic signals converge at RIP3. (A) Bone marrow-derived macrophages isolated from wild-type and RIP3−/− mice were treated as indicated for 20 h. Cell viability was determined by measuring ATP levels. Data were represented as mean ± SD of duplicates. Cell lysates collected from indicated macrophages were subjected to Western blot analysis for RIP3, RIP1, and β-actin levels. (B) J774A.1 cells were transfected with control or RIP3 siRNA oligos. Forty-eight hours posttransfection, cells were treated as indicated for an additional 24 h, and then cell viability was determined by measuring ATP levels. Data were represented as mean ± SD of duplicates. Cell lysates were collected 48 h posttransfection, and 30-μg aliquots thereof were subjected to Western blot analysis. z-VAD was used at 15 μM. LPS and poly(I:C) were used at 50 ng/mL and 10 μg/mL, respectively. (C) RAW264.7 cells were transfected with control or RIP3 siRNA oligos. Forty-eight hours posttransfection, cells were treated as indicated for an additional 24 h, and then cell viability was determined by measuring ATP levels. Data were represented as mean ± SD of duplicates. Cell lysates were collected 48 h posttransfection, and 30-μg aliquots thereof were subjected to Western blot analysis. z-VAD and poly(I:C) were used at 40 μM and 20 μg/mL, respectively. All experiments were repeated at least three times with similar results.
Neither Inhibition of NF-κB Activation nor a Defect in IRF3 Has a Significant Effect on TLR3/TLR4-Mediated Necrotic Signals.
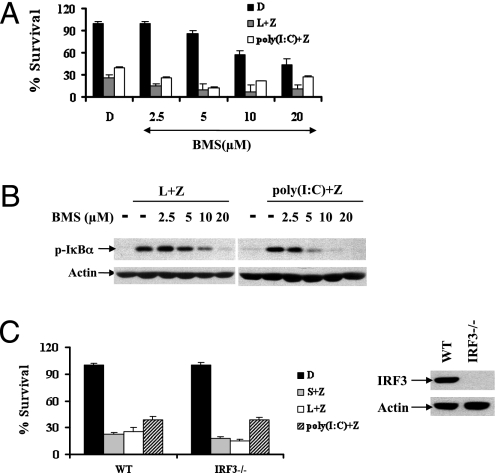
Each TLR selectively recruits specific adaptor proteins, thereby activating the corresponding immune signals. MyD88 is a common adapter shared by all known TLRs, except for TLR3, whereas TRIF is exclusively used by TLR3 and TLR4 (8, 9). Consistently, LPS triggered NF-κB activation in both wild-type and TRIF-mutant macrophages, but a lack of functional TRIF completely abrogated NF-κB activation in response to poly(I:C) (Fig. S2). Although critical for necrosis to occur, z-VAD did not affect NF-κB activation under these conditions, indicating that NF-κB does not affect necrosis. Consistently, the IκB kinase inhibitor BMS-345541 did not rescue TLR3/TLR4-induced necrosis, even though it efficiently blocked NF-κB activation during necrosis (Fig. 3 A and B).
Fig. 3.
NF-κB activation and IRF3-dependent immune responses are dispensable for TRIF/RIP3-mediated necrotic signals. (A) Bone marrow-derived macrophages from wild-type mice were treated with DMSO or BMS at the indicated concentration 2 h before the treatment of DMSO (control), Smac mimetic/ z-VAD, LPS/z-VAD, or poly(I:C)/z-VAD for 20 h, cell viability was determined by measuring ATP levels. (B) Bone marrow-derived macrophages from wild-type mice were treated with DMSO or BMS at the indicated concentration 2 h before the treatment of DMSO (control), LPS/z-VAD, or poly(I:C)/z-VAD for 30 min. Cell lysates were collected and subjected to Western blot analysis for phosphorylated IκBα and β-actin levels. (C) Bone marrow-derived macrophages from wild-type and IRF3−/− mice were treated as indicated for 20 h. Cell viability was determined by measuring ATP levels. Data were represented as mean ± SD of duplicates. Cell lysates collected from the indicated macrophages were subjected to Western blot analysis for IRF3 and β-actin levels. All experiments were repeated at least twice with similar results.
In addition to NF-κB activation, TRIF also enables IRF3-mediated type I IFN response upon activation of TLR3 or TLR4. We then evaluated the possible role of IRF3-dependent innate immune response in TLR3- or TLR4-mediated necrosis. As shown in Fig. 3C, when cells were stimulated with Smac mimetic/z-VAD, Poly (I:C)/z-VAD, or LPS/z-VAD, no difference in cell survival was observed between the wild-type and the IRF3-deficient macrophages. Thus, IRF3-dependent signaling is dispensable for TNF as well as TLR3- or TLR4-triggered necrosis.
TRIF Activates RIP3 Through the RHIM Domain upon Activation of TLR3/TLR4.
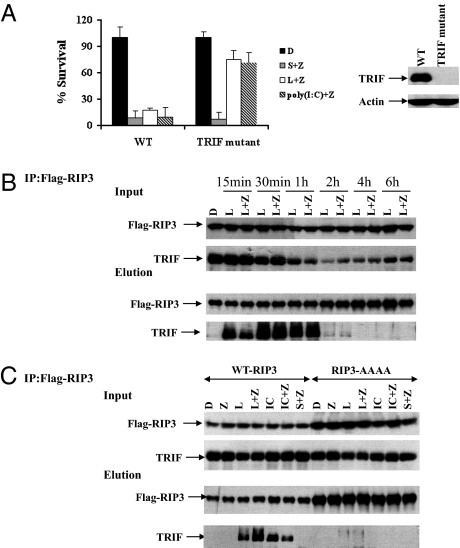
To investigate the role of TRIF in TLR3- or TLR4-induced necrosis, we analyzed macrophages from TRIFLPS2/LPS2 mutant mice (14). The TRIFLPS2/LPS2 mutant protein contains a mutated amino acid sequence at its carboxyl terminus, exhibiting a dominant-negative inhibitory effect. Antibody against the C-terminal peptide of TRIF detected the endogenous wild-type TRIF protein but not the TRIFLPS2/LPS2 mutant (Fig. 4A). The TRIFLPS2/LPS2 macrophages were unresponsive to necrotic cell death triggered by LPS/z-VAD or poly(I:C)/z-VAD, whereas they responded normally to Smac mimetic/z-VAD–induced necrosis. This behavior indicates that TRIF is specifically used for cellular necrosis in response to TLR3/TLR4.
Fig. 4.
TRIF is essential for TLR3/TLR4-induced necrosis and interacts with RIP3 through the RHIM domain. (A) Bone marrow-derived macrophages from wild-type and TRIF mutant mice were treated as indicated for 20 h. Cell viability was determined by measuring ATP levels. Data were represented as mean ± SD of duplicates. Cell lysates collected from the indicated macrophages were subjected to Western blot analysis for TRIF and β-actin levels. (B) J774A.1 cells stably expressing flag-tagged wild-type RIP3 were treated with DMSO (control), LPS, or LPS/z-VAD for the indicated time period. Cell lysates were immunoprecipitated with Flag beads as described in Methods. The immunocomplex was eluted with acid. The levels of Flag-RIP3 and endogenous TRIF in the immunocomplex were determined by Western blot analysis. (C) J774A.1 cells stably expressing flag-tagged wild-type or RHIM mutant RIP3 were treated as indicated for 40 min. Cell lysates were immunoprecipitated with Flag beads. The immunocomplex was eluted with acid. The levels of Flag-RIP3 and endogenous TRIF in the immunocomplex were determined by Western blot analysis. IC, poly(I:C). All experiments were repeated at least three times with similar results.
TRIF contains a C-terminal RHIM motif capable of interacting with other RHIM domain-containing proteins, such as RIP1 and RIP3 (22). We then hypothesized that the RHIM-mediated interaction between TRIF and RIP3 might recruit RIP3 to a necrosis-inducing complex downstream of TLR3/TLR4. To test that theory, we generated J774A.1 cells stably expressing either wild-type RIP3 or the RHIM mutant form of RIP3. Endogenous TRIF was coprecipitated with RIP3 in cells that were treated with LPS or LPS/z-VAD as well as with poly(I:C) or poly(I:C)/z-VAD in a time-dependent manner, but no association between RIP3 and TRIF was detected under resting conditions or upon treatment with Smac mimetic/z-VAD (Fig. 4B and Fig. S3). These observations revealed that a RIP3/TRIF complex was specifically formed by the activation of TLR3 or TLR4, but not TNF signaling. In contrast to wild-type RIP3, RHIM mutant RIP3 failed to form a complex with TRIF upon activation of TLR3 or TLR4, despite a higher expression level (Fig. 4C).
LPS-Induced Macrophage Loss and Inflammatory Cytokine Production Were Mitigated in Either RIP3 Knockout or TRIFLPS2/LPS2 Mutant Mice.
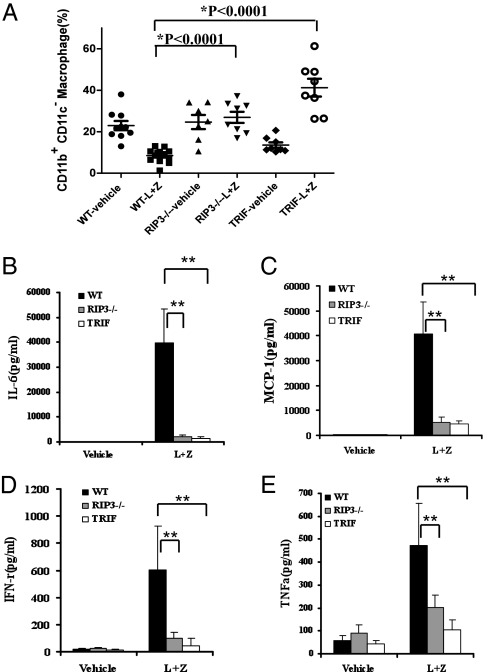
To examine TRIF/RIP3-mediated necrosis of macrophage in vivo, we challenged RIP3 knockout and TRIFLPS2/LPS2 mutant mice with LPS. Injection of high-dose LPS into mice is known to cause a systemic inflammatory responses and tissue injury associated with apoptotic and necrotic cell death. To study the role of RIP3 and TRIF in TLR4-indcued necrosis in vivo, we preinjected mice with z-VAD to alleviate apoptosis induced by LPS. In this model, the number of CD11b+ CD11c− intraperitoneal macrophages from wild-type mice was dramatically decreased compared with that from vehicle-treated mice (Fig. 5A). In contrast, no notable changes in intraperitoneal macrophages were detected in RIP3-deficient mice after the same treatment. Despite the finding that TRIFLPS2/LPS2 mutant mice originally had fewer intraperitoneal macrophages compared with those from wild-type mice, no loss in intraperitoneal macrophages was observed after treatment of LPS plus z-VAD. Moreover, inflammatory cytokines, including IL-6, MCP-1, IFN-γ, and TNF-α are highly induced in wild-type mice injected with LPS/z-VAD but the elevation of these cytokines was greatly mitigated in either RIP3-deficient or TRIFLPS2/LPS2 mutant mice (Fig. 5 B–E).
Fig. 5.
LPS-induced macrophage loss and inflammatory cytokine production were mitigated in either RIP3 knockout or TRIFLPS2/LPS2 mutant mice. Wild-type, RIP3-deficient, and TRIFLps2/Lps2 mutant mice were injected intraperitoneally with vehicle or z-VAD (20 mg/kg) 1 h before intraperitoneal injection with PBS or LPS (10 mg/kg). Resident peritoneal cells and blood were harvested at 24 h after the first injection, as described in Methods. CD11b+ CD11c− peritoneal macrophages were analyzed by flow cytometry (A) and the serum concentration of IL-6, MCP-1, IFN-γ, and TNF-α was measured (B–E). The cytokine data shown here are representative of at least five animals for each genotype. **P < 0.01 (t test).
Reactive Oxygen Species Accumulation Induced by RIP3/TRIF Signal Mediates TLR3/TLR4-Induced Necrotic Death.
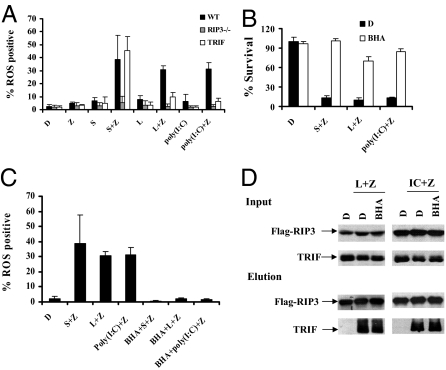
Accumulation of reactive oxygen species (ROS) is critical for TNF-α–mediated necrotic response in certain mouse cell lines (1). NADPH oxidase NOX1 and metabolic enzymes including glycogen phosphorylase, glutamate–ammonia ligase, and glutamate dehydrogenase 1 have been shown to mediate ROS generation (17, 23). We thereby monitored ROS production in macrophages stimulated with TNF-α and TLR3/TLR4. Increased ROS production was detected in the cells treated with Smac mimetic/z-VAD, Poly(I:C)/z-VAD, or LPS/z-VAD in wild-type macrophages, but such a response was blocked in RIP3-deficient cells. Although ROS accumulation was observed when TRIF mutant cells were treated with Smac mimetic/z-VAD, these cells exerted a significant defect in ROS accumulation in response to Poly(I:C)/z-VAD or LPS/z-VAD (Fig. 6A and Figs. S4–S6). Importantly, cell death induced by these necrotic stimuli was abolished in the presence of the ROS scavenger butylated hydroxyanisole (BHA), which efficiently suppressed ROS accumulation (Fig.6 B and C). Notably, BHA has no effect on the TRIF/RIP3 complex induced by activation of TLR3 or TLR4 (Fig. 6D). These observations indicate ROS accumulation is downstream of the TRIF/RIP3 signal and responsible for necrotic death in macrophages.
Fig. 6.
Activation of ROS accumulation by TRIF/RIP3 signal mediates TLR3/TLR4-induced necrosis, (A) Bone marrow-derived macrophages from wild-type, RIP3−/− mice, and TRIFLps2/Lps2 were treated as indicated for 5 h. ROS production was measured in the presence of CM-H2DCFDA by a flow cytometer. Data were represented as mean ± SD of three independent experiments. (B) Bone marrow-derived macrophages from wild-type mice were treated with DMSO or BHA (100 μM) 2 h before the treatment of DMSO (control), Smac mimetic/ z-VAD, LPS/z-VAD, or poly(I:C)/z-VAD for an additional 20 h. Cell viability was determined by measuring ATP levels. Data were represented as mean ± SD of duplicates. (C) Bone marrow-derived macrophages from wild-type mice were treated with DMSO or BHA 2 h before the treatment of DMSO (control), Smac mimetic/ z-VAD, LPS/z-VAD, or poly(I:C)/z-VAD for an additional 5 h. ROS production was measured in the presence of CM-H2DCFDA by a flow cytometer. Data were represented as mean ± SD of three independent experiments. (D) J774A.1 cells stably expressing flag-tagged wild-type RIP3 were treated with DMSO or BHA 2 h before the indicated treatment. Cell lysates were immunoprecipitated with Flag beads as described in Methods. The immunocomplex was eluted with acid. The levels of Flag-RIP3 and endogenous TRIF in the immunocomplex were determined by Western blot analysis. All experiments were repeated at least three times with similar results.
Discussion
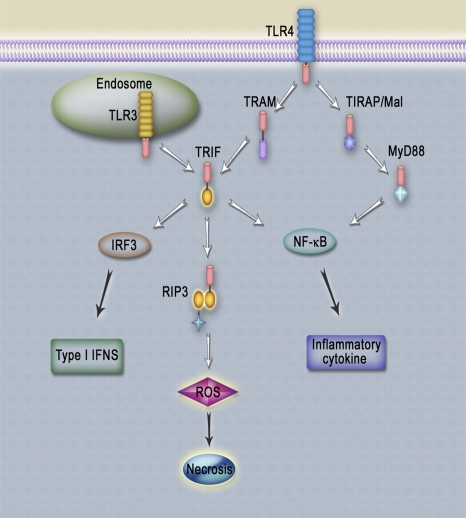
Macrophages play a crucial role in the host innate-immune response to pathogenic infections. Here, we have demonstrated that necrosis can be initiated via multiple distinctive upstream signaling pathways, including TNF-α, TLR3, and TLR4, in mouse macrophages. Engagement of TLR3 or TLR4 with poly(I:C) or LPS induces the interaction between TRIF and RIP3 through their RHIM domains (Fig. 7). In the presence of caspase inhibitor, z-VAD, TRIF/RIP3 elicits a downstream ROS accumulation and subsequently executes necrotic death independent of NF-κB activation and IRF3-dependent type I IFNs.
Fig. 7.
TLR3/TLR4-mediated signaling pathways. Engagement of TLR3 or TLR4 with poly(I:C) or LPS induces the interaction between TRIF and RIP3 through their RHIM domains. In the presence of caspase inhibitor, z-VAD, TRIF/RIP3 elicits downstream ROS accumulation and subsequently executes necrotic death independent of NF-κB activation and IRF3-dependent type I IFNs. TLR3/TLR4-mediated necrosis further triggers the induction of inflammatory cytokines.
RIP1 is a protein important for both cell survival and cell death. RIP1-deficient mice die within 3 d after birth, exhibiting a high rate of apoptosis in the lymphoid and adipose tissues (24). Unexpectedly, knockdown of RIP1 caused severe cell death in RWA264.7 cells (Fig. S7A), indicating that the precise amount of endogenous RIP1 is also critical for macrophage survival. Surprisingly, suppression of RIP1 by RNAi increased the macrophage sensitivity to Smac mimetic as well as LPS- or poly(I:C)-induced necrosis. However, the TLR3/TLR4-induced necrosis is effectively blocked by necrostatin-1, an inhibitor of RIP1 kinase (Fig. S7B). One possible explanation for this apparent paradox is that the RIP1-mediated signal, and possibly the induction of cFLIP [cellular FLICE (FADD-like IL-1β–converting enzyme)-inhibitory protein], is particularly important for macrophage survival (25). Recently, two independent groups have demonstrated that a cytosolic complex named Ripoptosome containing RIP1, FADD, caspase 8, and cFLIP, was formed upon IAP degradation and induced both apoptosis and necrosis (26, 27). cFLIPL was able to negatively regulate the formation of Ripoptosome, and cFLIPS protected Ripoptosome-mediated apoptosis but promoted necrosis in the presence of a caspase inhibitor. Although RIP3 RNAi was shown to slightly suppress Ripoptosome-mediated necrosis induced by combination of TLR3 agonist and IAP antagonist (26), we here demonstrated that RIP3-deficient primary macrophages were entirely resistant to TLR3-mediated necrosis in the absence of IAP antagonist.
Active caspase-8 is known to cleave RIP1 and RIP3 (28, 29), attenuating necrosis. Therefore, caspase-8 inhibitors, such as z-VAD, are required to initiate necrosis by inhibition of apoptosis. Although z-VAD has been shown to be essential for the stable formation of the RIP1/RIP3 complex in TNF-α–induced necrosis (7, 21), it is unnecessary for the recruitment of RIP3 to TRIF in TLR3/TLR4-mediated signals (Fig.4 B and C). Nevertheless, z-VAD is required for ROS accumulation in macrophages, indicating that z-VAD may facilitate an intermediate step between the TRIF/RIP3 complex and increased ROS production in the necrotic pathway. Because mitochondria are the main source for ROS generation and z-VAD has been shown to interact with mitochondrial adenine nucleotide translocase, it is plausible that z-VAD has an additional role in causing ROS production in the mitochondria (30). Further studies are required to understand the precise molecular mechanism through which TRIF/RIP3 controls ROS accumulation.
TLR3/TLR4 signals are known to play a critical role in orchestrating the antimicrobial pathogen response through the production of proinflammatory cytokines and type I IFNs. However, neither NF-κB activation nor IRF3-dependent innate immune responses are necessary for TLR3/TLR4-mediated necrosis. Both TRIF and RIP3 are not only essential for TLR3/TLR4-initiated necrosis in the cultured primary macrophages, but also required for macrophage loss and the production of proinflammatory cytokines, including IL-6, MCP-1, IFN-γ, and TNF-α in vivo after challenge with high-dose LPS and caspase inhibitor (Fig. 5). Thus, TLR3- and TLR4-directed necrosis mediated by the TRIF-RIP3 pathway seems to be an important part of the antimicrobial innate immune response.
Methods
Source of Animals.
RIP3−/− mice were generated as described previously (21) and crossed to C57BL/6 mice for 10 generations. Littermate RIP3+/+ mice were used as wild-type mice. TNFR1−/−(003242), TNFR1−/−TNFR2−/−(003243), TRIFLps2/Lps2(005037), TLR3−/−(005217), and TLR4−/−(007227) mice were purchased from The Jackson Laboratories. IRF3-deficient mice were kindly provided by Tadatsugu Taniguchi (University of Tokyo, Tokyo, Japan). Female mice at the age of 8 to 10 wk were used for the generation of bone marrow-derived macrophages and LPS challenge. All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee at National Institute of Biological Sciences.
Reagents.
The Smac mimetic compound was synthesized as previously described (31). LPS, BHA, propidium iodide, and BMS-345541 were obtained from Sigma. Necrostatin-1 and z-VAD were purchased from Alexis Biochemicals and Bachem, respectively. Poly(I:C) and TAK-242 were from InvivoGen. H2DCFDA was from Invitrogen. Cell Titer-Glo Luminescent Cell Viability Assay kit was from Promega. The following antibodies were used for Western blotting: RIP1 (BD Biosciences), RIP3 (Prosci), Flag (Sigma), IRF3 (Cell Signaling), and β-actin (Sigma). Mouse TRIF polyclonal antibodies were generated against the C-terminal peptide of mouse TRIF.
Flow Cytometric Analyses of CD11b+ CD11c− Peritoneal Macrophages and Cytokines.
Wild-type, RIP3-deficient, and TRIFLps2/Lps2 mutant mice were injected intraperitoneally with vehicle or z-VAD (20 mg/kg) 1 h before intraperitoneal injection with PBS or LPS (10 mg/kg). Animals were killed at 24 h after the first injection, resident peritoneal cells were harvested by lavage of the peritoneal cavity with 8 mL PBS, and blood was harvested. CD11b+ CD11c− peritoneal macrophages were analyzed by flow cytometry. The serum concentration of multiple cytokines was measured by CBA kit (BD Biosciences).
Supplementary Material
Acknowledgments
We thank Dr. Tadatsugu Taniguchi (University of Tokyo) for providing IRF3-deficient mice; Dr. Zhi Gang and other members of the Antibody Center at the National Institute of Biological Sciences, Beijing, for generating the polyclonal TRIF antibody; Cai Zhang, Cuilin Shi, Xiao Wang, Guangbo Tao, and Yinan Gong for technical assistance; Dr. Libin Shang and Dr. Lai Wang for their critical reading of the manuscript; and Ning Yang for help with the image of the model. This work was supported by National High Technology Projects 863 (2008AA022318) from the Chinese Ministry of Science and Technology, a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions \x{ff08}PAPD, National Natural Science Foundation of China Grant 31171330, and Natural Science Foundation of Jiangsu Province Grant BK2011287.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116302108/-/DCSupplemental.
References
- 1.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 2.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trichonas G, et al. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc Natl Acad Sci USA. 2010;107:21695–21700. doi: 10.1073/pnas.1009179107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Degterev A, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1(2):112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 5.Cauwels A, Janssen B, Waeytens A, Cuvelier C, Brouckaert P. Caspase inhibition causes hyperacute tumor necrosis factor-induced shock via oxidative stress and phospholipase A2. Nat Immunol. 2003;4:387–393. doi: 10.1038/ni914. [DOI] [PubMed] [Google Scholar]
- 6.Mareninova OA, et al. Cell death in pancreatitis: Caspases protect from necrotizing pancreatitis. J Biol Chem. 2006;281:3370–3381. doi: 10.1074/jbc.M511276200. [DOI] [PubMed] [Google Scholar]
- 7.Cho YS, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 9.Coll RC, O'Neill LA. New insights into the regulation of signalling by toll-like receptors and nod-like receptors. J Innate Immun. 2010;2:406–421. doi: 10.1159/000315469. [DOI] [PubMed] [Google Scholar]
- 10.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 11.Poltorak A, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino K, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 13.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11(11):115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 14.Hoebe K, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Temkin V, Liu H, Pope RM. NF-kappaB protects macrophages from lipopolysaccharide-induced cell death: the role of caspase 8 and receptor-interacting protein. J Biol Chem. 2005;280:41827–41834. doi: 10.1074/jbc.M510849200. [DOI] [PubMed] [Google Scholar]
- 17.Zhang DW, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 18.Kalai M, et al. Tipping the balance between necrosis and apoptosis in human and murine cells treated with interferon and dsRNA. Cell Death Differ. 2002;9:981–994. doi: 10.1038/sj.cdd.4401051. [DOI] [PubMed] [Google Scholar]
- 19.Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 2008;584(1):40–48. doi: 10.1016/j.ejphar.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 20.Akira S, Takeda K. Functions of toll-like receptors: Lessons from KO mice. C R Biol. 2004;327:581–589. doi: 10.1016/j.crvi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 21.He S, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Meylan E, et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 23.Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Kelliher MA, et al. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 25.Oberst A, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feoktistova M, et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tenev T, et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Chan FK, et al. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278:51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- 29.Feng S, et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19:2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Temkin V, Huang Q, Liu H, Osada H, Pope RM. Inhibition of ADP/ATP exchange in receptor-interacting protein-mediated necrosis. Mol Cell Biol. 2006;26:2215–2225. doi: 10.1128/MCB.26.6.2215-2225.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, et al. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.