Abstract
Many investigators maintain that spermatozoa that have initiated the acrosome reaction (AR) before reaching the surface of the egg's zona pellucida (ZP) are unable to bind and penetrate the ZP. A recent study has revealed that most fertilizing mouse spermatozoa initiate the AR before contacting the ZP. We found that acrosome-reacted spermatozoa collected from the perivitelline space of Cd9-null mice (whose egg plasma membranes are incapable of fusing with spermatozoa) were able to pass through both the cumulus and ZP of WT mouse eggs and produced live offspring. This means that the spermatozoa we used had the ability to pass through the ZP at least twice. Apparently, some spermatozoa that had undergone the AR long before contact with the ZP remained capable of crossing the ZP and fertilizing eggs. Thus, the concept that acrosome-reacted spermatozoa are unable to bind to the ZP and have lost their fertilizing capacity must be reconsidered.
Keywords: fertilization, spermatozoon, oocyte, fusion, IZUMO1
A startling paper was published in 1984 by Kuzan et al. (1), who reported that spermatozoa collected from the perivitelline space (PVS) of fertilized rabbit eggs were able to enter other unfertilized eggs, which subsequently developed into two-cell embryos. This report, confirmed in the rabbit (2) but not in any other species, was largely ignored for two likely reasons. First, the report by Kuzan et al. (1) conflicted with a widely accepted concept that the fertilizing spermatozoon must begin its acrosome reaction (AR) on the zona pellucida (ZP) surface and that spermatozoa that have undergone the AR before contact with the ZP have lost their fertilizing capacity (3–5). Second, rabbit fertilization is unusual; the ZP of rabbit eggs, unlike in most other species, remains penetrable by spermatozoa even after fertilization, such that many spermatozoa continue to enter the PVS. In other species, including the mouse, cortical granule materials released from the egg after sperm–egg fusion quickly alter the biochemical characteristics of the ZP, such that it becomes refractory to penetration by excess spermatozoa. Thus, we seldom encounter superfluous spermatozoa in the PVS of fertilized eggs in most species.
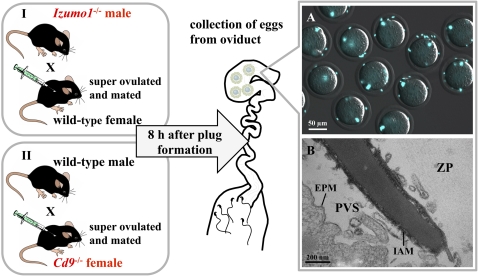
In the present study, two different methods were used to collect mouse spermatozoa from the PVS. First, we used transgenic (Izumo1−/−) male mice for mating. Spermatozoa of these mice are able to penetrate the ZP normally but are unable to fuse with oocytes (6). Because the eggs remain unactivated, the ZP remains penetrable to spermatozoa and many spermatozoa continue to enter the PVS. Second, we used transgenic (Cd9−/−) mice whose eggs allow sperm passage through the ZP but not sperm fusion (7–9). Thus, many superfluous spermatozoa accumulate in the PVS of these mice after natural mating or in vitro fertilization (Fig. 1A). That all mouse spermatozoa in the PVS are acrosome-reacted has been documented by EM (10, 11) (Fig. 1B) and immunocytochemistry (6). We report here that acrosome-reacted spermatozoa collected from the PVS of mouse eggs are able to fertilize fresh, cumulus-enclosed, zona-intact eggs. Furthermore, such fertilized eggs can develop into fertile offspring.
Fig. 1.
Preparation of spermatozoa accumulating in the PVS. Eggs from either B6D2F1 or Cd9−/− female mice were superovulated as indicated in Materials and Methods. One hour before the estimated ovulation time, both lines of female mice were mated with Izumo1−/− or B6D2F1 male mice, respectively. Eggs were collected from oviducts 8 h after plug formation. (A) Many spermatozoa accumulated in the PVS of Cd9−/− eggs because of impaired sperm–egg fusion. The spermatozoa were stained with Hoechst 33342 and viewed through a fluorescence microscope with phase-contrast optics. A mean of 5.5 spermatozoa per egg was able to penetrate the zona (n = 11). (Scale bar = 50 μm.) (B) Transmission electron micrograph of a spermatozoon within the PVS; the micrograph shows a longitudinal section of the anterior region of the sperm head. The inner acrosomal membrane (IAM) is exposed. Vesicle-like structures around the IAM are sections of egg's microvilli. EPM, egg-plasma membrane. (Scale bar = 200 nm.)
Results
When Izumo1−/− spermatozoa collected from the PVS of WT (B6D2F1) female mice were mixed with fresh cumulus-enclosed eggs (B6D2F1) and examined 12 h later, 7% of the eggs were penetrated by spermatozoa (Table 1, Exp. I), indicating that some PVS spermatozoa were able to penetrate the ZP (Fig. 2D).
Table 1.
Development of eggs fertilized by spermatozoa collected from the PVS
| Spermatozoa used for insemination of intact WT eggs | No. exp. | No. eggs from which PVS sperm were collected | No. eggs inseminated with PVS sperm | No. (%) eggs penetrated* | No. (%) two-cell embryos | No. pups born | |
| Exp. I | Izumo1−/− spermatozoa collected from PVS | 3 | 80 | 74 | 5 (7) | NA | NA |
| Exp. II | WT spermatozoa collected from the PVS of Cd9−/− eggs | 7 | 135 | 95 | 15 (16) | 15 (16) | 2 |
Exp., experiment; NA, not applicable (Izumo1−/− spermatozoa cannot fuse with an egg).
*Five eggs in Exp. I had spermatozoa in their PVS, but none had spermatozoa within their cytoplasm; therefore, they were not fertilized. Fifteen eggs in Exp. II each had two pronuclei and two polar bodies and were truly fertilized.
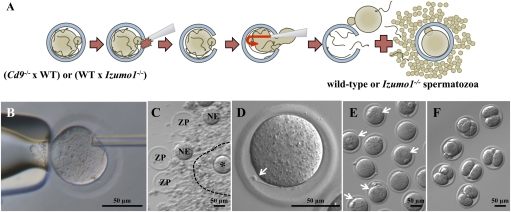
Fig. 2.
Recovery of spermatozoa from the PVS and subsequent fertilization in vitro. (A) Schematic diagram indicating the recovery of spermatozoa from the PVS and subsequent in vitro insemination and fertilization. (B) Cumulus cells and all the spermatozoa near and on the ZP were removed by hyaluronidase treatment and subsequent washings. A few spermatozoa on the ZP, if any, were individually immobilized (permanently) by damaging sperm tail plasma membrane by a single piezo pulse. A large slit was made in the ZP by applying several piezo pulses to the pipette before spermatozoa in the PVS were flushed out from the slit together with the ooplasm (Movie S1). (C) Second round of insemination was performed in a 4-μL drop of TYH medium. The spermatozoa released from the PVS were coincubated with a freshly prepared cumulus–oocyte complex. The mixture of empty ZP, naked eggs (NE), and zona-intact eggs (indicated by asterisk) within the cumulus, photographed 12 h after insemination, is shown. A broken line indicates a cumulus layer. (D) Izumo1−/− spermatozoon that had failed to fuse with the egg for the second time is indicated by an arrow. (E) Because the spermatozoa utilized for the assessment of second-round insemination using Cd9−/− eggs were WT spermatozoa, they could fuse with the eggs and formed pronuclei 12 h after insemination, as indicated by the arrows. (F) Embryos at the two-cell stage obtained from the fertilized eggs in E and incubated in potassium (K(+)) simplex optimized medium (kSOM) for another 8 h.
A second experiment was performed using WT (B6D2F1) spermatozoa collected from the PVS of Cd9−/− eggs. The results (Table 1, Exp. II) show that 16% of eggs were fertilized and developed into two-cell embryos (Fig. 2F). Two pups were born after transfer of 15 two-cell embryos to seven surrogate mothers. One pup was cannibalized by the surrogate mother, but the other (female) survived (Fig. 3 A and B), grew normally, and gave birth to healthy pups (Fig. 3C).
Fig. 3.
Production of healthy offspring from eggs fertilized by spermatozoa that had penetrated the ZP for the second time. (A) Black-eyed neonate (arrow) developed from a B6D2F1 egg fertilized by a PVS spermatozoon. Albino offspring were derived from concomitantly transplanted ICR two-cell embryos. (B) Seven-day-old mouse developed from an egg fertilized by a PVS spermatozoon (arrow). (C) This female mouse, derived from an egg fertilized by a PVS spermatozoon, was healthy and underwent a normal pregnancy.
Discussion
This study shows that mouse spermatozoa in the PVS, like those in the PVS of fertilized rabbit eggs (1, 2), are able to fertilize other cumulus-invested eggs. Moreover, some mouse eggs thus fertilized developed into fertile offspring. Importantly, some spermatozoa without any remnant of the acrosomal cap (Fig. 1B) are capable of passing through both the cumulus and ZP and can fertilize eggs that develop into normal fertile offspring (Fig. 3C).
Obviously, not all PVS spermatozoa are fertile. In fact, only a few PVS spermatozoa could fertilize (Table 1). The reason for this is not clear, but it is possible that some PVS spermatozoa we used for in vitro insemination had entered the PVS just a few minutes previously. Some others could have entered the PVS several hours before. The spermatozoa that maintained hyperactivated motility even after their release from the PVS are likely those that reentered the ZP of other eggs.
Whether mammalian spermatozoa penetrate the cumulus and ZP mechanically or by chemical means (12, 13) is still the subject of debate. According to Valdivia and Barros (2), 26% of rabbit spermatozoa collected from the PVS have acrosin activity in their heads (in equatorial and postacrosomal regions). They suggested that such spermatozoa are able to reenter the ZP with the aid of this residual acrosin. Even though spermatozoa from Acrosin-KO mice can fertilize eggs (14), this enzyme and some other proteases (including PRSS21) on acrosome-reacted spermatozoa might work synergistically to assist sperm passage through the ZP (15).
According to Saling et al. (16), spermatozoa that undergo the AR before their contact with the ZP are unable to bind to it, and have therefore lost fertilizing potential. This was questioned by Jin et al. (17), who inseminated mouse eggs in vitro, video-recorded them continuously, and analyzed all images retroactively to see which spermatozoa actually fertilized the eggs. They found that most fertilizing spermatozoa moving in the cumulus had initiated the AR (as judged by loss of a fluorescent marker for the acrosomal matrix) and then penetrated the ZP without spending much time on its surface. Some spermatozoa began the AR on the ZP, but they were the exceptions rather than the rule. In the present study, PVS spermatozoa that had completed their AR (10) (Fig. 1B) were able to fertilize normal mouse eggs (Fig. 2F). Thus, the concept that acrosome-reacted spermatozoa are unable to bind to the ZP and have lost their fertilizing capacity must be reconsidered.
When rabbits and hamsters were mated and their eggs were examined during the process of fertilization using EM, spermatozoa with a reacted acrosomal carapace were seen on the ZP surface (also within the cumulus) (18, 19). These spermatozoa must have begun to react before or after contact with the ZP. Although the native ZP certainly has the ability to accelerate or induce the AR (20), it is no longer valid to consider only the ZP-mediated AR as physiological. As shown in this paper, some acrosome-reacted spermatozoa are certainly capable of fertilizing ZP-intact eggs. The assumption that the acrosomal carapace, consisting of vesiculated membranes with their underlying partly dispersed acrosomal contents, is essential for sperm binding to the ZP binding is no longer valid.
Under natural conditions, some eggs must be fertilized soon after ovulation, whereas some others are fertilized much later. As long as the eggs can develop into normal offspring, fertilization is considered normal regardless of the postovulatory age of the egg itself. When mating occurs after ovulation, the cumulus oophorus surrounding the eggs might have become loosened by the time spermatozoa make contact. In contrast, when mating occurs before ovulation, some fertilizing spermatozoa might have begun their AR within the oviduct or during their passage through the cumulus. Because mating in humans can occur irrespective of the time of ovulation, some spermatozoa resident in the female tract for a long time may undergo their AR before meeting eggs. As long as eggs develop into normal offspring, fertilization by such spermatozoa can be considered physiologically normal regardless of the site and time of the sperm AR.
Materials and Methods
Animals.
Izumo1-disrupted (Izumo1−/−) mice were produced as described (6). Cd9-disrupted (Cd9−/−) mice were a generous gift from E. Mekada (Research Institute for Microbial Diseases, Osaka University, Japan) (7). Hybrid B6D2F1 and Institute of Cancer Research (ICR) male and female mice were purchased from CLEA Japan. All animals were >8 wk old when used. Experiments were performed with the approval of Osaka University's Animal Care and Use Committee.
Preparation of Eggs with Many Spermatozoa in the PVS.
In method I (Fig. 1), WT (B6D2F1) female mice were induced to superovulate by consecutive injections of 5 IU of equine chorionic gonadotropin (eCG) and 5 IU of human chorionic gonadotropin (hCG) 48 h apart. At 12 h after the eCG injection (about the time of ovulation), female mice were allowed to mate with Izumo1−/− male mice, whose spermatozoa are able to pass through the ZP but are unable to fuse with the oolemma (6). The formation of a vaginal plug was examined every 30 min after the female mice were caged with the male mice. When eggs were collected from the female mice 8 h after plug formation, many spermatozoa were moving actively within the PVS. In method II (Fig. 1), Cd9−/− female mice were injected similarly with eCG and hCG before mating with WT (B6D2F1) male mice. Eggs collected 8 h after plug formation contained many spermatozoa in the PVS because the oolemma in this strain is unable to fuse with any type of spermatozoon (7–9).
Recovery of Spermatozoa from the PVS.
Eggs collected from oviducts of mated female mice were freed from cumulus cells by a 5-min treatment with 0.01% (wt/vol) hyaluronidase (350 units/mL; Sigma–Aldrich) in Toyoda, Yokoyama, Hoshi (TYH) medium (21), rinsed with TYH, and transferred to a 4-μL droplet of fresh TYH under mineral oil in a Petri dish. A large hole was made in the ZP using a capillary pipette attached to a piezoelectric actuator (22), and a small amount of the medium was injected into the PVS to flush out the spermatozoa (Fig. 2 A and B and Movie S1). Many spermatozoa displayed vigorous (hyperactivated) movement before and after release from the PVS (Movie S1). To collect PVS spermatozoa for the insemination, 10–25 eggs were used.
In Vitro Insemination of Cumulus-Intact Eggs Using PVS Spermatozoa.
Several cumulus–oocyte complexes were collected from the oviducts of B6D2F1 female mice 13 h after hCG injection. They were rinsed with TYH medium and transferred to a 4-μL droplet of TYH containing the spermatozoa released from the PVS. Because a mean of 5.5 spermatozoa was released from each of 10–25 eggs, the estimated sperm concentration in the medium was 1.4–3.1 × l04 per milliliter. Eggs were considered fertilized when two polar bodies and two pronuclei were seen 12 h after insemination (Fig. 2E). Those reaching the two-cell stage were mixed with albino eggs and transferred to the oviducts of surrogate (ICR) mice that had been mated with vasectomized mice 0.5 d previously.
EM.
Cd9−/− eggs containing PVS spermatozoa were collected from oviducts 8 h after coitus with B6D2F1 male mice and prepared for transmission EM by immersion in 2.5% (wt/vol) glutaraldehyde in 30 mM Hepes buffer (pH 7.3) containing 100 mM NaCl and 2 mM CaCl2 for 1 h at room temperature. Samples were then postfixed with 1% (wt/vol) OsO4 for 1 h, dehydrated in graded ethanol series, embedded in Epon 812 through propylene oxide, and thin-sectioned with an average thickness of 70 nm. Sections were stained with 5% (wt/vol) uranyl acetate solution for 20 min, followed by treatment with 0.4% (wt/vol) lead citrate solution for 3 min, and then examined using a JEM-1011 electron microscope (JEOL) at 80 kV.
Supplementary Material
Acknowledgments
We thank Dr. Eisuke Mekada at Osaka University for kindly giving us the Cd9-disrupted mouse line. We also thank Drs. J. Michael Bedford and Jim Cummins for critically reviewing the draft and thoughtful discussions. This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 19843.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116965108/-/DCSupplemental.
References
- 1.Kuzan FB, Fleming AD, Seidel GE., Jr Successful fertilization in vitro of fresh intact oocytes by perivitelline (acrosome-reacted) spermatozoa of the rabbit. Fertil Steril. 1984;41:766–770. doi: 10.1016/s0015-0282(16)47847-7. [DOI] [PubMed] [Google Scholar]
- 2.Valdivia M, Barros C. The participation of acrosin in sperm penetration through the zona pellucida in rabbits. Biol Reprod. 1997;56(Suppl 1):164. (abstr) [Google Scholar]
- 3.Bleil JD, Wassarman PM. Sperm-egg interactions in the mouse: Sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev Biol. 1983;95:317–324. doi: 10.1016/0012-1606(83)90032-5. [DOI] [PubMed] [Google Scholar]
- 4.Saling PM. Mammalian sperm interaction with extracellular matrices of the egg. Oxf Rev Reprod Biol. 1989;11:339–388. [PubMed] [Google Scholar]
- 5.Florman HM, Ducibella T. Knobil and Neill's Physiology of Reproduction. 3rd ed. Vol 1. St. Louis, MO: Elsevier, Academic Press; 2006. Fertilization in mammals; pp. 55–112. [Google Scholar]
- 6.Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–238. doi: 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- 7.Miyado K, et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science. 2000;287:321–324. doi: 10.1126/science.287.5451.321. [DOI] [PubMed] [Google Scholar]
- 8.Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C. Severely reduced female fertility in CD9-deficient mice. Science. 2000;287:319–321. doi: 10.1126/science.287.5451.319. [DOI] [PubMed] [Google Scholar]
- 9.Kaji K, et al. The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat Genet. 2000;24:279–282. doi: 10.1038/73502. [DOI] [PubMed] [Google Scholar]
- 10.Stefanini M, Oura C, Zamboni L. Meiotic cleavage of the mammalian ovum. J Cell Biol. 1969;43:138a. (abstr) [Google Scholar]
- 11.Toshimori K. Penetration of the mouse sperm head through the zona pellucida in vivo: An electronmicroscope study at 200KV. Biol Reprod. 1982;26:475–481. doi: 10.1095/biolreprod26.3.475. [DOI] [PubMed] [Google Scholar]
- 12.Yanagimachi R. Mammalian Fertilization. New York: Raven Press; 1994. [Google Scholar]
- 13.Bedford JM. Mammalian fertilization misread? Sperm penetration of the eutherian zona pellucida is unlikely to be a lytic event. Biol Reprod. 1998;59:1275–1287. doi: 10.1095/biolreprod59.6.1275. [DOI] [PubMed] [Google Scholar]
- 14.Baba T, Azuma S, Kashiwabara S, Toyoda Y. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J Biol Chem. 1994;269:31845–31849. [PubMed] [Google Scholar]
- 15.Kawano N, et al. Mice lacking two sperm serine proteases, ACR and PRSS21, are subfertile, but the mutant sperm are infertile in vitro. Biol Reprod. 2010;83:359–369. doi: 10.1095/biolreprod.109.083089. [DOI] [PubMed] [Google Scholar]
- 16.Saling PM, Sowinski J, Storey BT. An ultrastructural study of epididymal mouse spermatozoa binding to zonae pellucidae in vitro: Sequential relationship to the acrosome reaction. J Exp Zool. 1979;209:229–238. doi: 10.1002/jez.1402090205. [DOI] [PubMed] [Google Scholar]
- 17.Jin M, et al. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci USA. 2011;108:4892–4896. doi: 10.1073/pnas.1018202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bedford JM. Sperm capacitation and fertilization in mammals. Biol Reprod Suppl. 1970;2:128–158. [PubMed] [Google Scholar]
- 19.Yanagimachi R, Phillips DM. The status of acrosomal caps of hamster spermatozoa immediately before fertilization in vitro. Gamete Res. 1984;9(1):1–19. [Google Scholar]
- 20.Tollner TL, Yudin AI, Cherr GN, Overstreet JW. Real-time observations of individual macaque sperm undergoing tight binding and the acrosome reaction on the zona pellucida. Biol Reprod. 2003;68:664–672. doi: 10.1095/biolreprod.102.009175. [DOI] [PubMed] [Google Scholar]
- 21.Toyoda Y, Yokoyama M, Hoshi T. Studies on the fertilization of mouse eggs in vitro I: In vitro fertilization of eggs by fresh epididymal sperm. Jpn J Anim Reprod. 1971;16:147–151. [Google Scholar]
- 22.Yamagata K, et al. Sperm from the calmegin-deficient mouse have normal abilities for binding and fusion to the egg plasma membrane. Dev Biol. 2002;250:348–357. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.