Abstract
Trace conditioning is valued as a simple experimental model to assess how the brain associates events that are discrete in time. Here, we adapted an olfactory trace conditioning procedure in Drosophila melanogaster by training fruit flies to avoid an odor that is followed by foot shock many seconds later. The molecular underpinnings of the learning are distinct from the well-characterized simultaneous conditioning, where odor and punishment temporally overlap. First, Rutabaga adenylyl cyclase (Rut-AC), a putative molecular coincidence detector vital for simultaneous conditioning, is dispensable in trace conditioning. Second, dominant-negative Rac expression, thought to sustain early labile memory, significantly enhances learning of trace conditioning, but leaves simultaneous conditioning unaffected. We further show that targeting Rac inhibition to the mushroom body (MB) but not the antennal lobe (AL) suffices to achieve the enhancement effect. Moreover, the absence of trace conditioning learning in D1 dopamine receptor mutants is rescued by restoration of expression specifically in the adult MB. These results suggest the MB as a crucial neuroanatomical locus for trace conditioning, which may harbor a Rac activity-sensitive olfactory “sensory buffer” that later converges with the punishment signal carried by dopamine signaling. The distinct molecular signature of trace conditioning revealed here shall contribute to the understanding of how the brain overcomes a temporal gap in potentially related events.
Keywords: learning and memory, olfaction, cAMP, Rho GTPase
In trace conditioning, the conditional stimulus (CS) and the unconditional stimulus (US) are separated in time by a stimulus-free interval (1). This so-called “trace interval” can last for a fraction of a second in eyeblink conditioning but many seconds in fear conditioning, which poses a challenging question: how does the brain overcome this temporal gap to form the association between the CS and US (2)? Intriguingly, trace conditioning in mammals engages neural substrates fundamentally different from delay conditioning, where the CS precedes but also temporally overlaps with the US (3). Early evidence comes from lesion studies with experimental animals showing that acquisition of trace conditioning requires intact hippocampal formation (4, 5) and medial prefrontal cortex (6), whereas delay conditioning can occur even with the entire forebrain removed (7, 8). Later studies involving human subjects further validate the involvement of different brain circuits in these two conditioning variants and even suggest, more surprisingly, that conscious awareness might be a prerequisite for trace but not delay conditioning (9, 10). It is then hypothesized that the participation of hippocampus and neocortex, as well as the associated higher cognitive function, is necessary in trace conditioning to maintain a representation of the CS or CS/US contingency so as to bridge the temporal gap (11, 12). However, little is known about what form this representation takes and how it eventually converges with the US.
Pavlovian conditioning has also been extensively studied in invertebrate animals (13). In Drosophila, one of the best-studied paradigms is olfactory differential aversive conditioning (14), wherein fruit flies smell two odors [normally 3-octanol (OCT) and 4-methycyclohexanol (MCH)], one (CS+) associated with negative reinforcement but the other (CS−) not. A simultaneous conditioning procedure is frequently used (Fig. 1A) in which the 1-min CS+ odor exposure cooccurs with the US punishment, composed of twelve 1.5-s pulses of 60 V electric shock distributed in a 1-min period (15). Studies over the past three decades have substantiated the mushroom body (MB) as a major site where learning-related plasticity takes place (16). This brain locus is the third-order olfactory area in insects where the CS and US combine (17). Information about the CS reaches the MB via the projection neurons of the antennal lobe (AL), the insect equivalent of the olfactory bulb (18). The reinforcement signal from the US is conveyed via the dopamine neurons, which also form synapses with the MB (19). In a simplified molecular model, the CS+ and US converge on Rutabaga adenylyl cyclase (Rut-AC), which in turn triggers the cAMP/PKA signaling cascade that drives synaptic plasticity and learned behavior (20–22). The neural circuits processing the CS and US information are now being studied at single-cell resolution (e.g., 19, 23, 24) and an increasing repertoire of learning/memory-related molecules are being identified (e.g., 25, 26). The abundant knowledge of this conditioned behavior makes the fruit fly an attractive model to study trace conditioning (14, 27, 28).
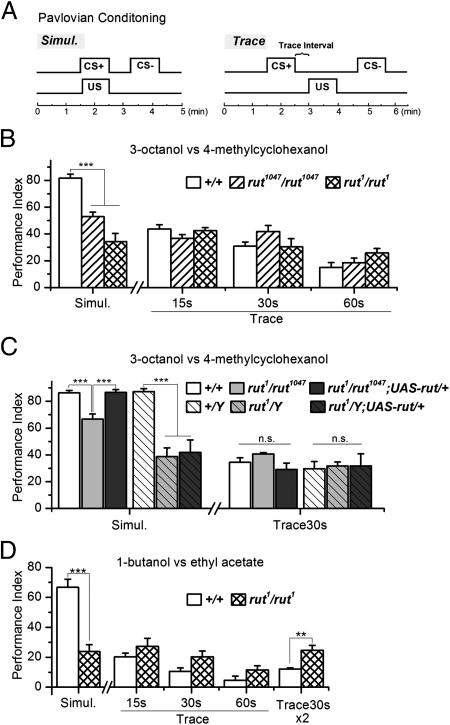
Fig. 1.
Trace conditioning of olfactory aversion in wild type and rut mutants. (A) Trace conditioning (Trace) differed from standard simultaneous conditioning (Simul.) only in an interstimulus trace interval between the CS+ odor and the US foot shock. (B) Learning performance after Simul. or Trace with various trace intervals (15, 30, and 60 s). Odors used were indicated. rut mutants showed learning defects in Simul. (ANOVA, P < 0.001), but normal performance in Trace (ANOVA, P > 0.1 for all intervals); n = 6. Error bars indicate SEM. (C) rut1/rut1047 transheterozygote also showed selective defect in Simul. learning. The defect was rescued by restoration of rut expression in rut1/rut1047; UAS-rut/+, but no increase in Trace performance was observed. Female and male flies were segregated after testing to obtain designated genotypes. n = 4 for Simul.; 6 for Trace30s. Error bars indicate SEM. ***P < 0.001; NS, nonsignificance. (D) In the training with hydrophilic odors, rut1 mutant also showed lower learning performance in Simul. (ANOVA, P < 0.001), but normal or even higher performance in Trace (ANOVA, P = 0.27, 0.06, 0.12 for 15, 30, and 60 s, respectively; P = 0.006 for Trace30s × 2 with intertrial interval of 5 min). n = 5. Error bars indicate SEM.
Here, we adapted our olfactory aversive trace conditioning procedure via insertion of an odor-free interval between the offset of CS+ and the onset of US (Fig. 1A). Single-trial training is sufficient to elicit considerable learning performance. The molecular underpinnings of the trace learning were found to be dramatically different from simultaneous conditioning, with respect to the requirement of Rut-AC and the involvement of Rac-mediated forgetting.
Results
rut Mutants Perform Normally in Trace Conditioning.
Learning performance generated after single-trial training with different conditioning procedures is shown in Fig. 1B. Consistent with previous reports (14, 27), trace conditioning elicits considerable conditioned aversion of the CS+ in wild-type flies. Learning becomes less efficient as the trace interval increases, but is still evident for intervals up to 60 s. Two rut mutants were tested along with the wild-type flies; rut1047 bears a P{Gal4} insertion (25), whereas rut1 carries a point mutation and is functionally null (29). Surprisingly, both rut mutants have completely normal performance in trace conditioning despite their severe learning defects in simultaneous conditioning (Fig. 1B). Likewise, rut1/rut1047 heterozygote shows a learning defect in simultaneous but not trace conditioning (Fig. 1C). Both rut1 and rut1047 have been equilibrated to the wild-type genetic background, so it is unlikely that the trace conditioning defect is masked by a second-site mutation. This assertion is further strengthened by the fact that restoration of rut expression by a upstream activation sequence (UAS)-rut transgene (30) rescues the learning defect in simultaneous conditioning, but does not further increase trace conditioning performance (compare the performance of rut1/rut1047 and rut1/rut1047; UAS-rut/+ in Fig. 1C). Thus, both wild-type and rut-deficient mutants acquire learning in the trace conditioning paradigm.
In the above experiments, we followed the conventional protocol (15) and used the hydrophobic odors, OCT and MCH. Galili et al. recently pointed out that MCH and possibly other hydrophobic odors are difficult to remove from the training apparatus by air flushing (28). To ensure the trace conditioning is not an artifact of residual odor, we monitored odor dissipation in the training tube via a photoionization detector (PID). Odor concentration follows a rapid decay after the termination of odor delivery, but the PID detected a trace amount of slowly decaying odor that took over 1 min to return to baseline (Fig. S1A). However, such a low level of residual odor at the time window relevant to the trace conditioning paradigm (≥30 s) is not sufficient to induce measurable learning performance in flies (Fig. S1B). Odors adhering to the fly cuticle or retained in the sensillum lymph are unlikely to be a problem as it is known that olfactory sensory neuron activity rapidly returns to baseline following odor offset (31). In addition, we validated the above rut results by training flies with a pair of hydrophilic odors, 1-butanol (BU) and ethyl acetate (EA). These two odors show much faster dissipation, decaying to baseline within 15 s (Fig. S1A), and the former is used by Galili et al. in their recent trace conditioning study (28). We observed a similar phenotype. rut1 mutant shows defect in simultaneous conditioning, but normal or even slightly higher learning performance in trace conditioning (Fig. 1D). This result, combined with those described above, demonstrates that Rut-AC is dispensable in trace conditioning, as opposed to its essential role in simultaneous conditioning (20, 30, 32).
Inhibition of Rac-Mediated Forgetting Enhances Trace Conditioning.
Another distinction between the two paradigms comes from the assessment of a molecular pathway mediating forgetting of early labile memory (25). Rac is a member of Rho family small G proteins, which play critical roles in neuronal actin cytoskeleton remodeling (33). We recently reported that inhibition of Rac activity lengthens early memory retention after simultaneous conditioning, but leaves initial learning unaffected (25). Intriguingly, when a temporal gap separates the CS+ and the US in trace conditioning, the same manipulation exerts a profound effect on learning.
We used the Gal4/Gal80ts system (32) to drive adult onset expression of a dominant-negative form of Drosophila Rac1, Drac1(N17) (34). As described previously (25), flies were raised in 18 °C; a 3-d heat-shock treatment at 30 °C was used to inactivate the ubiquitously expressed tubulin-Gal80ts (Gal80ts) and switch on Gal4-dependent transgene expression. Remarkably, inhibition of Rac activity throughout the adult brain (elav-Gal4/+; Gal80ts/+; UAS-Drac1(N17)/+) enhances the learning of trace conditioning compared with the two parental controls (Fig. 2A). Significant enhancement is obvious for trace conditioning at trace intervals of 15, 30, and 60 s. At an extremely long interval of 300 s, some residual learning at a score of ∼10 is still evident, which presumably arises from attraction to CS− via backward conditioning (27); however, no statistically significant differences were observed among groups, validating the enhancement is specific to trace conditioning. Heat-shock induction of transgene expression is a prerequisite of the enhancement, because no differences among genotypes were observed for uninduced groups kept at 18 °C (Fig. 2A).
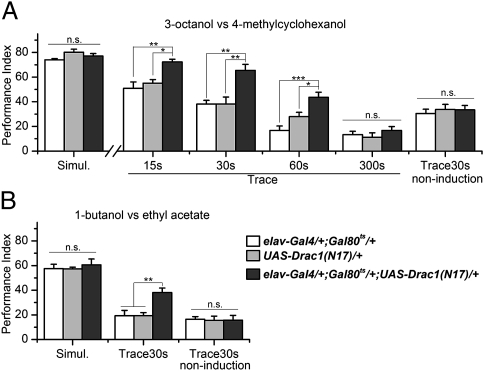
Fig. 2.
Drac1(N17) expression enhances trace conditioning. (A) Heat shock at 30 °C for 3 d was used to induce transgene expression. The learning performance of Drac1(N17)-expressing flies (elav-Gal4/+; Gal80ts/+; UAS-Drac1(N17)/+) was significantly higher than two similarly heat-shock–treated parental controls in Trace at trace intervals of 15, 30, and 60 s (ANOVA, P < 0.05), but not at the interval of 300 s (ANOVA, P > 0.15). Fly groups without heat-shock induction showed similar performance in Trace30s learning (ANOVA, P > 0.95). The Simul. data are from Shuai et al. (25) and are presented for ease of comparison. n = 6. Error bars indicate SEM. (B) Confirmation experiments that used hydrophilic odors for training (ANOVA, P < 0.01 for Trace30s; P > 0.95 for Simul. and Trace30s noninduction). n = 5 or 8. Error bars indicate SEM.
Drac1(N17)-expressing flies show normal task-relevant sensorimotor responses and acquisition of simultaneous conditioning (25); thus the observed trace conditioning enhancement is unlikely attributable to a superior ability in pairing foot-shock punishment with an ambient odor trace in the training tube. We performed three more control experiments below.
First, we trained flies with simultaneous conditioning but lowered odor concentration by further dilutions of 101-, 102-, and 104-fold (Fig. S2A). Drac1(N17)-expressing flies show performance largely comparable to the controls. A marginal yet statistically significant difference was observed compared with the elav-Gal4/+; Gal80ts/+ control in the 101-fold dilution, but not in the 102- or 104-fold dilution or in any comparison with the UAS-Drac1(N17)/+ control. Therefore, the detection or reinforcement of a weak odor is not significantly influenced by Drac1(N17) expression.
Second, we trained flies to avoid a specific concentration of one odor, i.e., to discriminate between regular concentration and 10-fold further dilution of MCH. PID measurements show that the very low level of residual odor after a 30-s air flushing does not provide information about the initial odor concentration (Fig. S1B). Therefore, we reasoned that trace conditioning of intensity discrimination should not be confounded by lingering odors. Accordingly, Drac1(N17)-expressing flies significantly outperformed controls when intensity discrimination was trained in a trace conditioning procedure, but not in a simultaneous conditioning procedure (Fig. S2B).
Third, we trained flies with a hydrophilic odor pair, BU and EA, which are cleared more rapidly from the training tube (Fig. S1A). Again, Drac1(N17) expression selectively enhances trace conditioning (Fig. 2B). The enhancement depends on heat-shock induction (Fig. 2B) and is not explained by differences in olfactory acuity of the hydrophilic odors (Table S1). Because we got consistent results with the hydrophobic and hydrophilic odor pairs, in later experiments we only used the hydrophobic odor pair (OCT and MCH), which generates higher learning scores.
Mushroom Body Is a Crucial Site for Trace Conditioning.
We found that the trace conditioning enhancement could be reproduced when Drac1(N17) expression was driven by the rut1047 Gal4 together with Gal80ts (Fig. 3A). Notably, enhancement reaches approximately the same level in the rut1047/Y hemizygote and in the rut1047/+ heterozygote. Thus, rut deficiency does not compromise the superior ability of transgenic mutants in trace conditioning learning, further supporting that Rut-AC is not involved. rut1047 Gal4 preferentially labels the MB (Fig. 3C). The result therefore also hints that Drac1(N17) expression in the MB is sufficient for the enhancement.
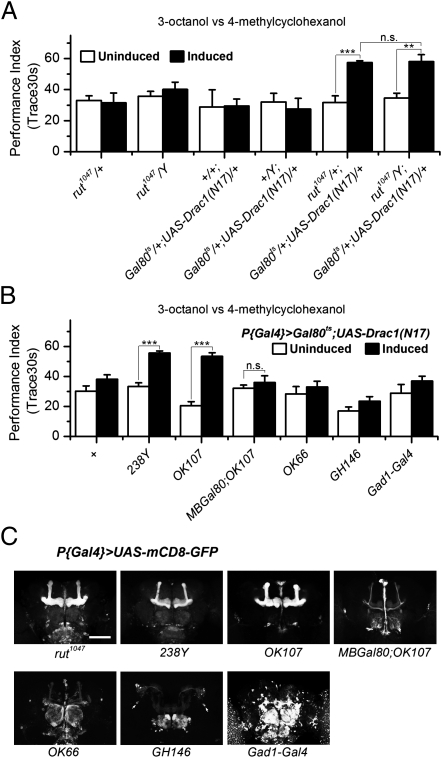
Fig. 3.
Drac1(N17) expression in the mushroom body is sufficient for the enhancement. (A) Trace enhancement was observed when induced expression of Drac1(N17) was driven by rut1047 Gal4 (ANOVA, P < 0.001 for rut1047/+, 0.01 for rut1047/Y, compared with the uninduced group). No statistically significant difference was detected when the enhancement in the rut1047/Y hemizygous background was compared with that in the rut1047/+ heterozygous background (ANOVA, P = 0.9). Female and male flies were segregated after testing to obtain designated genotypes. n = 4–12. Error bars indicate SEM. (B) Gal80ts; UAS-Drac1(N17) flies were crossed to wild-type flies (+) and the indicated Gal4 drivers. Trace enhancement after heat-shock induction was detected only when Drac1(N17) was expressed by the two MB Gal4s, OK107 and 238Y (ANOVA, P < 0.001). The enhancement was blocked when OK107 was combined with MBGal80 (ANOVA, P = 0.45). n ≥ 6. Error bars indicate SEM. (C) Gal4 expression patterns visualized by mCD8-GFP. rut1047 has primary expression in the MB; 238Y and OK107 presumably label all of the MB neurons; MBGal80 largely suppresses OK107 expression in the MB; OK66, local neurons of the AL, but faint expression in the MB is also visible; GH146, projection neurons of the AL and APL neurons, cell bodies of APL neurons are marked with arrowhead; Gad1-Gal4, GABAergic neurons. (Scale bar, 100 μm.)
We further confirmed this idea by using two pan-MB drivers, 238Y and OK107 (Fig. 3C) to induce Drac1(N17) expression specifically in the MB. Significant enhancement was observed compared with their respective uninduced control groups (Fig. 3B). Consistently, the enhancement associated with OK107 is blocked (Fig. 3B) by the introduction of the MBGal80 transgene (35), which specifically suppresses Gal4 activity in the MB (Fig. 3C). We also targeted Drac1(N17) expression to the AL with OK66 and GH146, which label local neurons (36) and projection neurons (37) of the AL, respectively (Fig. 3C). However, no differences were observed between induced and uninduced groups (Fig. 3B). It is worthwhile to note that GH146 also labels the anterior paired lateral (APL) neurons, a pair of GABAergic neurons that innervate the MB and suppress olfactory associative learning (38). The absence of enhancement (Fig. 3B) in GH146 and additionally in GABAergic Gad1-Gal4 (39), however, does not support a role for Drac1(N17) in the APL neurons. The mapping results thus suggest the MB as a predominant neuroanatomical locus in mediating the enhancement effect of Drac1(N17).
Dopamine Receptor in the Mushroom Body Supports Trace Conditioning.
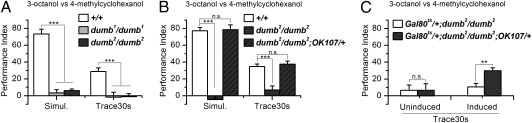
Dopaminergic neurons are believed to convey the reinforcement signal from foot shock (19, 24). Accordingly, mutants of the D1 dopamine receptor (dDA1), dumb1 and dumb2, show no learning of simultaneous conditioning (40). We report here that trace conditioning learning is abolished in these mutants as well (Fig. 4A), which suggests that trace conditioning also relies on dopamine signaling to transmit the US punishment information. Importantly, the piggyBac inserted in the first intron of the dDA1 gene in the dumb2 mutant contains UAS, which can produce functional dDA1 receptor in the presence of a Gal4 driver (40). Taking advantage of this property, we found that restoration of dDA1 expression in the MB with the OK107 driver fully rescued the deficit of dumb2 in trace conditioning (Fig. 4B). Moreover, the rescue effect was evident when expression was restored specifically in the adult MB (Fig. 4C). The localization of dDA1 function implies that in trace conditioning, the US signal is relayed to the MB, where it presumably converges with information about the now absent CS+.
Fig. 4.
Genetic lesions of D1 dopamine receptor abolish trace conditioning. (A) dumb1 and dumb2 mutants showed no learning in either Simul. or Trace (ANOVA, P < 0.001 compared with wild type). n = 6. Error bars indicate SEM. (B) Restoration of dDA1 expression in the MB (dumb2/dumb2; OK107/+) fully rescued the learning phenotype of dumb2 mutant in both paradigms (ANOVA, P > 0.95 compared with wild type). n = 4 for Simul.; 6 for Trace30s. Error bars indicate SEM. (C) Adult rescue of dumb2 mutant. Gal80ts; dumb2/dumb2; Ok107/+ were compared with Gal80ts; dumb2/dumb2 control that lacks the Gal4 driver. Learning performance in Trace was restored for flies subjected to 30 °C heat shock for 3 d (ANOVA, P < 0.01 for induced groups), but not for those kept at 18 °C (ANOVA, P > 0.95 for uninduced groups). n = 6 for uninduced; 5 for induced. Error bars indicate SEM.
Discussion
It has been postulated that trace and delay conditioning are two fundamentally different types of learning (11). Evidence is accumulating in mammals concerning the involvement of different brain systems (3). Here, we characterized trace conditioning in the fruit fly and used mutant analyses to show that it is distinct from the well-characterized simultaneous conditioning at the molecular level. These data complement the mammalian circuit-level studies and, more importantly, open up a molecular understanding of the internal trace that the brain uses to bridge the temporal gap.
Trace Conditioning of Olfactory Aversion in Drosophila.
Odor foot-shock pairing elicits robust learning in fruit flies (14). The current study adapted this assay to study trace conditioning simply by modifying the timing relationship between the CS+ odor and the US punishment. To mimic the widely used simultaneous conditioning paradigm (15), CS− presentation is kept at 45 s after the punishment. Single-trial training is sufficient to elicit considerable learning performance; the learning index for OCT and MCH is ∼35 for trace conditioning at a trace interval of 30 s. Although a portion of the score (∼10) might be attributed to attraction to the CS− via backward conditioning (27), the behavioral results clearly indicate a marked ability of fruit flies to associate events that are temporally discrete (14, 27, 28).
Residual odor is a great concern in olfactory trace conditioning, particularly in light of the hydrophobic nature of OCT and MCH (28). We conducted careful control experiments to show that air flushing for 30 s during the trace interval is sufficient to reduce residual odors in the training tube below the threshold for fruit fly learning. In addition, the results were replicated with a pair of hydrophilic odors (BU and EA) with much faster dissipation kinetics (28). Consistent phenotypes were observed for the rut1 mutant and Drac1(N17)-expressing flies using hydrophobic and hydrophilic odors. These experiments therefore addressed the concerns raised by the slower kinetics of hydrophobic odors. Galili et al. (28) excluded MCH from their recent trace conditioning study due to its slower dissipation. The apparent discrepancy in these results may arise from the fact that Galili et al. used a 10-times higher concentration of MCH and a trace interval of only 5 s, which together do not allow for sufficient odor decay.
Distinctive Mechanisms Support Trace and Simultaneous Conditioning.
One remarkable finding of the current study is that flies devoid of Rut-AC perform normally in trace conditioning. This result is interesting in view of the belief that dually regulated adenylyl cyclase plays a central role in invertebrate associative learning (16, 41). The function of Rut-AC is best described as a molecular coincidence detector that is synergistically activated by the CS-evoked calcium entry and the US-evoked G protein-coupled receptor activation (20–22). It has been hypothesized that the stimulus-free gap in trace conditioning can be bridged by the temporal integration property of Rut-AC (21, 42). However, our results disagree with this hypothesis. The normal or even higher performance of rut-deficient mutants suggests that CS–US association in trace conditioning may recruit separate molecular machineries or occur in a distinct group of neurons (26, 43). Also pertinent to our study is that cAMP levels in the prefrontal cortex negatively influence working memory performance (44). Therefore, whereas cAMP signaling is essential for some learning tasks, it is dispensable or even detrimental for others (45).
Another intriguing finding is that induced expression of dominant-negative Rac enhances the learning of trace but not simultaneous conditioning. Notably, no learning enhancement was observed in a number of simultaneous conditioning variants with altered training parameters, including lowered odor concentration and conditioned intensity discrimination in the current work, as well as reduced shock pulses and lowered shock voltage in our previous report (25). Thus, the differential effects are not explained by a ceiling effect or other ancillary factors. Trace conditioning testing was performed almost immediately (within 3 min) after the training, rendering a better retention of the acquired associative memory also unlikely. Trace conditioning becomes less efficient as trace interval increases, indicating that an inner trace of the odor gradually degrades with time. We therefore speculate that inhibition of Rac activity might preserve this transient “sensory buffer” so as to facilitate trace conditioning. In the learning of simultaneous conditioning, the co-occurrence of odor and shock makes it possible to process the CS and US information automatically, e.g., via simple convergence on coincidence detection molecules like Rut-AC; hence the requirement of an olfactory sensory buffer is superfluous, which explains the lack of enhancement from Rac inhibition. The above speculation is particularly attractive considering a recently established role of Rac in the forgetting of a cold-shock sensitive early associative memory (25). It appears that the perdurance of two short-lived memory forms, one registered after a passive olfactory experience and lasting tens of seconds and the other registered after an associative reinforcement and lasting several hours, are both sensitive to Rac signaling manipulation.
Mushroom Body May Hold a Sensory Buffer of the Odor.
Drac1(N17) takes effect in the MB, the center for olfactory learning and sensory integration in insects (46). The localization of the Drac1(N17) effect, combined with the full rescue of the dDA1 mutant phenotype in the MB, implies a possible trace conditioning model in which the MB bridges the temporal gap by holding a short-term sensory buffer of the odor, which later converges with the reinforcement signal carried by dopamine signaling. In accordance with this model, two recent studies in fruit fly (28) and honey bee (47) found no correlation between trace conditioning behavior and the postodor calcium response patterns in olfactory sensory neurons and projection neurons of the AL. Both studies pointed out the likelihood that the sensory buffer relevant to trace conditioning is in neurons downstream of the AL, most likely in the MB. Nonetheless, the AL may still retain odor information in biochemical signals other than calcium or in short-term synaptic plasticity (48, 49). The rapidly evolving molecular imaging techniques in fruit flies (50) may help to delineate the nature of the putative sensory buffer and how it interacts later with a biologically significant stimulus.
Another remaining puzzle is that both simultaneous and trace conditioning, although recruiting different molecular mechanisms, rely on the MB as a mutual crucial site. This seems at variance with the view from mammalian studies, where trace conditioning recruits neural circuits distinct from delay conditioning. Species or paradigm differences might explain the discrepancy, but it awaits to be fully addressed by future studies exploring whether brain regions outside the MB are additionally engaged in trace conditioning in fruit flies and, more importantly, whether various MB subdivisions (51) contribute differentially to these two conditioning variants.
Materials and Methods
Fly Stocks.
rut1 and rut1; UAS-rut were gifts from Josh Dubnau (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY); Gad1-Gal4 from Liqun Luo (Stanford University, Stanford, CA); and dumb1 and dumb2 from Kyung-An Han (University of Texas at El Paso, El Paso, TX). All of the other strains were extant stock in the laboratory and were described in a previous paper (25). We were not able to obtain flies homozygous for both dumb2 and OK107. In the dumb2 rescue experiment, dumb2/dumb2; OK107/+ were crossed to dumb2/dumb2 or Gal80ts/Gal80ts; dumb2/dumb2. Progeny genotypes were easily segregated after behavioral experiments on the basis of the bright red eye color of flies bearing OK107 driver.
Behavioral Assays.
Pavlovian conditioning of odor avoidance response was performed in a controlled environment room of 25 °C and 70% relative humidity as described (15).
During training, around 100 flies were loaded into a training tube covered with copper grid. Odors (Sigma-Aldrich/Fluka) were dissolved in heavy mineral oil (Fisher Scientific) and brought to the training tube by a moisturized air current bubbling through the odor vials at 750 mL/min. Normally, the dilution in (vol/vol) was: 1.5 × 10−3 for OCT, 1 × 10−3 for MCH, 2 × 10−3 for BU, and 2 × 10−3 for EA; further dilutions from these starting concentrations were indicated in some experiments. The odor source of BU and EA was replenished after every 8 min of odor presentation considering their faster run down. With this procedure, no apparent effects on behavioral scores were observed. In simultaneous conditioning, the two odors were presented to flies sequentially; each lasted for 60 s and was followed by flushing of fresh air for 45 s. The US (twelve 1.5-s pulses of 60 V electric foot shock at 5-s interpulse intervals) was present during the delivery of the first odor (CS+) but not the second (CS−). Trace conditioning differed from simultaneous conditioning only in the temporal relationship between CS+ and US, i.e., CS+ preceded US but a no-odor interval separated the offset of CS+ and onset of US. CS− delivery was maintained at 45 s following US. Odorless clean air bubbling through heavy mineral oil was delivered at 750 mL/min whenever there was no odor delivery. It is estimated that the training tube (inner volume of ∼15 mL) was refreshed every 1.2 s.
To assay for learning performance, flies were allowed to choose between CS+ and CS− in a T maze for 120 s immediately after the training. Performance index (PI) was calculated (15) as the fraction of flies avoiding CS+ minus the fraction of flies avoiding CS−. To eliminate odor bias, each PI was averaged over two reciprocally trained groups, e.g., one associating shock with OCT, the other with MCH. PI was normalized to a range of 0–100, with 0 indicating no learning and 100 indicating perfect learning.
Statistics.
The data are shown as means ± SEM and analyzed by ANOVA followed by Bonferroni's post hoc test in Origin 8.0 software. *P < 0.05, **P < 0.01, ***P < 0.001; NS, nonsignificance (P > 0.05).
Supplementary Material
Acknowledgments
We thank J. Dubnau, L. Luo, K. A. Han, and the Bloomington Stock Center for fly stocks; G. C. Turner for the use of PID; J. Beshel for revising the manuscript; and H. Tanimoto for sharing an in-press manuscript. This work was supported by 973 Program Grants 2006CB500806 and 2009CB941301, National Institutes of Health Grants 1R01NS064331-01A2, Department of Defense Grant W81XWH-10-1-0450, and Dart Neuroscience (all to Y.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107489109/-/DCSupplemental.
References
- 1.Pavlov IP. In: Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. Anrep GV, editor. London: Oxford Univ Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shors TJ. Memory traces of trace memories: Neurogenesis, synaptogenesis and awareness. Trends Neurosci. 2004;27:250–256. doi: 10.1016/j.tins.2004.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodruff-Pak DS, Disterhoft JF. Where is the trace in trace conditioning? Trends Neurosci. 2008;31:105–112. doi: 10.1016/j.tins.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- 5.McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 6.Kronforst-Collins MA, Disterhoft JF. Lesions of the caudal area of rabbit medial prefrontal cortex impair trace eyeblink conditioning. Neurobiol Learn Mem. 1998;69:147–162. doi: 10.1006/nlme.1997.3818. [DOI] [PubMed] [Google Scholar]
- 7.Norman RJ, Buchwald JS, Villablanca JR. Classical conditioning with auditory discrimination of the eye blink in decerebrate cats. Science. 1977;196:551–553. doi: 10.1126/science.850800. [DOI] [PubMed] [Google Scholar]
- 8.Mauk MD, Thompson RF. Retention of classically conditioned eyelid responses following acute decerebration. Brain Res. 1987;403:89–95. doi: 10.1016/0006-8993(87)90126-0. [DOI] [PubMed] [Google Scholar]
- 9.Clark RE, Squire LR. Classical conditioning and brain systems: The role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- 10.Clark RE, Manns JR, Squire LR. Trace and delay eyeblink conditioning: Contrasting phenomena of declarative and nondeclarative memory. Psychol Sci. 2001;12:304–308. doi: 10.1111/1467-9280.00356. [DOI] [PubMed] [Google Scholar]
- 11.Clark RE, Manns JR, Squire LR. Classical conditioning, awareness, and brain systems. Trends Cogn Sci. 2002;6:524–531. doi: 10.1016/s1364-6613(02)02041-7. [DOI] [PubMed] [Google Scholar]
- 12.Wallenstein GV, Eichenbaum H, Hasselmo ME. The hippocampus as an associator of discontiguous events. Trends Neurosci. 1998;21:317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- 13.Carew TJ, Sahley CL. Invertebrate learning and memory: From behavior to molecules. Annu Rev Neurosci. 1986;9:435–487. doi: 10.1146/annurev.ne.09.030186.002251. [DOI] [PubMed] [Google Scholar]
- 14.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 15.Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 16.Davis RL. Olfactory memory formation in Drosophila: From molecular to systems neuroscience. Annu Rev Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- 17.Heisenberg M. Mushroom body memoir: From maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 18.Wilson RI, Mainen ZF. Early events in olfactory processing. Annu Rev Neurosci. 2006;29:163–201. doi: 10.1146/annurev.neuro.29.051605.112950. [DOI] [PubMed] [Google Scholar]
- 19.Claridge-Chang A, et al. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blum AL, Li W, Cressy M, Dubnau J. Short- and long-term memory in Drosophila require cAMP signaling in distinct neuron types. Curr Biol. 2009;19:1341–1350. doi: 10.1016/j.cub.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomchik SM, Davis RL. Dynamics of learning-related cAMP signaling and stimulus integration in the Drosophila olfactory pathway. Neuron. 2009;64:510–521. doi: 10.1016/j.neuron.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gervasi N, Tchénio P, Preat T. PKA dynamics in a Drosophila learning center: Coincidence detection by rutabaga adenylyl cyclase and spatial regulation by dunce phosphodiesterase. Neuron. 2010;65:516–529. doi: 10.1016/j.neuron.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Turner GC, Bazhenov M, Laurent G. Olfactory representations by Drosophila mushroom body neurons. J Neurophysiol. 2008;99:734–746. doi: 10.1152/jn.01283.2007. [DOI] [PubMed] [Google Scholar]
- 24.Aso Y, et al. Specific dopaminergic neurons for the formation of labile aversive memory. Curr Biol. 2010;20:1445–1451. doi: 10.1016/j.cub.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shuai Y, et al. Forgetting is regulated through Rac activity in Drosophila. Cell. 2010;140:579–589. doi: 10.1016/j.cell.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 26.Tan Y, Yu D, Pletting J, Davis RL. Gilgamesh is required for rutabaga-independent olfactory learning in Drosophila. Neuron. 2010;67:810–820. doi: 10.1016/j.neuron.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanimoto H, Heisenberg M, Gerber B. Experimental psychology: Event timing turns punishment to reward. Nature. 2004;430:983. doi: 10.1038/430983a. [DOI] [PubMed] [Google Scholar]
- 28.Galili DS, Lüdke A, Galizia CG, Szyszka P, Tanimoto H. Olfactory trace conditioning in Drosophila. J Neurosci. 2011;31:7240–7248. doi: 10.1523/JNEUROSCI.6667-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levin LR, et al. The Drosophila learning and memory gene rutabaga encodes a Ca2+/Calmodulin-responsive adenylyl cyclase. Cell. 1992;68:479–489. doi: 10.1016/0092-8674(92)90185-f. [DOI] [PubMed] [Google Scholar]
- 30.Zars T, Fischer M, Schulz R, Heisenberg M. Localization of a short-term memory in Drosophila. Science. 2000;288:672–675. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]
- 31.de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 32.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 33.Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- 34.Luo L, Liao YJ, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- 35.Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron. 2007;53:103–115. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, et al. Blockade of neurotransmission in Drosophila mushroom bodies impairs odor attraction, but not repulsion. Curr Biol. 2003;13:1900–1904. doi: 10.1016/j.cub.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Davis RL. The GABAergic anterior paired lateral neuron suppresses and is suppressed by olfactory learning. Nat Neurosci. 2009;12:53–59. doi: 10.1038/nn.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ng M, et al. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36:463–474. doi: 10.1016/s0896-6273(02)00975-3. [DOI] [PubMed] [Google Scholar]
- 40.Kim YC, Lee HG, Han KA. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci. 2007;27:7640–7647. doi: 10.1523/JNEUROSCI.1167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abrams TW, Kandel ER. Is contiguity detection in classical conditioning a system or a cellular property? Learning in Aplysia suggests a possible molecular site. Trends Neurosci. 1988;11:128–135. doi: 10.1016/0166-2236(88)90137-3. [DOI] [PubMed] [Google Scholar]
- 42.Ito I, Ong RC, Raman B, Stopfer M. Olfactory learning and spike timing dependent plasticity. Commun Integr Biol. 2008;1:170–171. doi: 10.4161/cib.1.2.7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brembs B, Plendl W. Double dissociation of PKC and AC manipulations on operant and classical learning in Drosophila. Curr Biol. 2008;18:1168–1171. doi: 10.1016/j.cub.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 44.Wang M, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 45.Dash PK, Moore AN, Kobori N, Runyan JD. Molecular activity underlying working memory. Learn Mem. 2007;14:554–563. doi: 10.1101/lm.558707. [DOI] [PubMed] [Google Scholar]
- 46.Strausfeld NJ, Hansen L, Li Y, Gomez RS, Ito K. Evolution, discovery, and interpretations of arthropod mushroom bodies. Learn Mem. 1998;5:11–37. [PMC free article] [PubMed] [Google Scholar]
- 47.Szyszka P, et al. Mind the gap: Olfactory trace conditioning in honeybees. J Neurosci. 2011;31:7229–7239. doi: 10.1523/JNEUROSCI.6668-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stopfer M, Laurent G. Short-term memory in olfactory network dynamics. Nature. 1999;402:664–668. doi: 10.1038/45244. [DOI] [PubMed] [Google Scholar]
- 49.Galán RF, Weidert M, Menzel R, Herz AV, Galizia CG. Sensory memory for odors is encoded in spontaneous correlated activity between olfactory glomeruli. Neural Comput. 2006;18:10–25. doi: 10.1162/089976606774841558. [DOI] [PubMed] [Google Scholar]
- 50.Davis RL. Traces of Drosophila memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keene AC, Waddell S. Drosophila olfactory memory: Single genes to complex neural circuits. Nat Rev Neurosci. 2007;8:341–354. doi: 10.1038/nrn2098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.