Abstract
The K+ channel KCa3.1 is required for Ca2+ influx and the subsequent activation of CD4 T cells. The class II phosphatidylinositol 3 kinase C2β (PI3KC2β) is activated by the T-cell receptor (TCR) and is critical for KCa3.1 channel activation. Tripartite motif containing protein 27 (TRIM27) is a member of a large family of proteins that function as Really Interesting New Gene (RING) E3 ubiquitin ligases. We now show that TRIM27 functions as an E3 ligase and mediates lysine 48 polyubiquitination of PI3KC2β, leading to a decrease in PI3K enzyme activity. By inhibiting PI3KC2β, TRIM27 also functions to negatively regulate CD4 T cells by inhibiting KCa3.1 channel activity and TCR-stimulated Ca2+ influx and cytokine production in Jurkat, primary human CD4 T cells, and Th0, Th1, and Th2 CD4 T cells generated from TRIM27−/− mice. These findings provide a unique mechanism for regulating class II PI3Ks, and identify TRIM27 as a previously undescribed negative regulator of CD4 T cells.
Keywords: Ca2+ signaling, phosphatidylinositol-3 phosphate, T helper cells, tripartite motif containing protein 27 knockout
PI3Ks play critical roles in the regulation of a variety of biological process (1). In general, members of this family have been divided into three classes (I, II, III) based on sequence homology and substrate specificity (2–4). Most of the previous work on PI3Ks in lymphocyte activation have focused on the class I PI3Ks (p110 α, β, γ, and δ), which are responsible for the acute rise in PI(3,4,5)P3 following antigen-receptor activation (5, 6). Studies in knockout mice have demonstrated that p110δ and p110γ play partly redundant functions in T-cell activation, and are most important for T-cell receptor (TCR) signaling by peripheral T cells, as well as for T-cell development and survival (7–11). Although the exact role for PI(3,4,5)P3 in T-cell activation is still controversial, recruitment and activation of a number of plekstrin-homology containing proteins by PI(3,4,5)P3 is critical. For example, recruitment of the Tek family kinase ITK to the plasma membrane via binding of it's plekstrin-homology domain to PI(3,4,5)P3 has been shown to contribute to Ca2+ influx, integrin activation, and synapse formation (12).
Mammals have three class II PI3Ks: PI3KC2α, PI3KC2β, and PI3KC2γ (1, 3). Although PI3KC2α and PI3KC2β have a wide tissue distribution and are both expressed in lymphocytes, PI3KC2γ has a more restricted pattern of expression and is absent from lymphocytes. Unlike the class I PI3Ks, the class II PI3Ks do not contain regulatory subunits (3, 13). Rather, upstream activation of class II PI3Ks is likely mediated via their extended N and C termini. Agonist-induced relocalization of a constitutively active class II PI3K to the plasma membrane via interaction of their N and C termini with adaptor signaling molecules, such as Grb2 in EGFR signaling (14), or with membrane-associated complexes, such as clathrin or intersectin, have been previously described (15, 16). In addition, some studies have shown agonist-induced increase in kinase activity (4, 17, 18).
Recently, we found that the class II PI3KC2β plays an important and unexpected role in CD4 T-cell activation (19). These studies demonstrated that activation of PI3KC2β, but not PI3KC2α, following TCR stimulation functions to recruit PI3KC2β to the immunological synapse, leading to the generation of PI(3)P, which is subsequently required for the histidine phosphorylation and activation of KCa3.1 by nucleoside diphosphate kinase B (NDPK-B) (19, 20). Activation of KCa3.1, as well as another K+ channel Kv1.3, has been shown to play critical roles in CD4 T-cell activation (21–25). By mediating the efflux of K+, these channels function to maintain a negative membrane potential, which is critical for sustained calcium entry into these cells via calcium release-acti-vated Ca2+ channels. Increased cytosolic Ca2+ then mediates the transcriptional activation of a number of genes critical for T-cell activation (26–28).
To understand the mechanism whereby PI3KC2β is regulated in T cells, we screened for PI3KC2β interacting proteins by yeast two-hybrid and identified TRIM27 (also known as Ret finger protein) as a PI3KC2β interacting protein. TRIM family proteins are characterized by the presence of the tripartite motif, which consists of a ring finger, Zn2+ binding motifs referred to as “B boxes,” and a coil-coil domain (29, 30). TRIM family members have been shown to regulate a plethora of cellular pathways, including apoptosis, the cell cycle, and antiviral activity, and recent evidence has indicated that this family of proteins regulates some of these processes by functioning as a novel class of Really Interesting New Gene (RING) E3 ubiquitin ligases (29–31). We now show that by ubiquitinating PI3KC2β, TRIM27 inhibits PI3KC2β's kinase activity, resulting in decreased KCa3.1 channel activity and decreased TCR-stimulated Ca2+ influx and cytokine production, thereby identifying TRIM27 as a unqiue negative regulator of CD4 T cells.
Results
TRIM27 Associates with PI3KC2β in the Yeast Two-Hybrid and in Vivo.
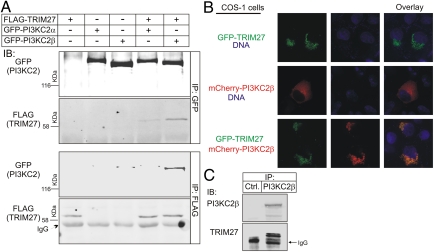
Four TRIM27 clones were identified that bound PI3KC2β in a yeast two-hybrid screen of a human CD4 T-cell library (Hybergenics). Although two clones encompassed full-length TRIM27, two clones contained only the carboxyl-terminal PRY-SPRY domain (also known as B30.2), indicating that these domains are sufficient for binding at least in vitro. To determine whether TRIM27 and PI3KC2β associate in cells, GFP-PI3KC2β or GFP-PI3KC2α was cotransfected with FLAG-TRIM27 in HEK293 cells and association was assessed by coimmunoprecipitation experiments. FLAG-TRIM27 coimmunoprecipitated with anti-GFP antibodies (Fig. 1A, lane 5), and GFP-PI3KC2β coimmunoprecipitated with anti-FLAG antibodies (Fig. 1A, lane 5). The association was specific because FLAG-TRIM27 only coimmunoprecipitated with GFP-PI3KC2β when both proteins were expressed (Fig. 1A, lanes 1 and 3). FLAG-TRIM27 also coimmunoprecipitated with the closely related class II PI3K, PI3KC2α (Fig. 1A, lane 4), although the association was decreased compared with PI3KC2β. In addition, endogenous TRIM27 coimmunoprecipitated with endogenous PI3KC2β, providing further validation that the association of the two proteins is physiologic (Fig. 1C).
Fig. 1.
TRIM27 and PI3KC2β associate in vivo. (A) FLAG-TRIM27 was cotransfected with GFP-PI3KC2β or GFP-PI3KC2α in HEK293 cells and the ability of the two proteins to coimmunoprecipitate was assessed as indicated. (B) GFP-TRIM27 was cotransfected with mCherry-PI3KC2β in COS cells and colocalization was assessed by fluorescence. Magnification, 60×. (C) Lysates from 293 cells were immunoprecipitated with anti-PI3KC2β or a control antibody and then immunoblotted with anti-TRIM27 or anti-PI3KC2β antibodies.
To identify the subcellular localization of TRIM27 and PI3KC2β, mCherry-PI3KC2β and GFP-TRIM27 were expressed in COS cells and subcellular localization was assessed by fluorescence. When expressed alone, mCherry-PI3KC2β was predominantly cytosolic, although a portion was associated with the plasma membrane (Fig. 1B). In contrast, virtually all of the expressed GFP-TRIM27 was found localized to a poorly described subcellular vesicular compartment (Fig. 1B). Coexpression of mCherry-PI3KC2β together with GFP-TRIM27 resulted in recruitment of a portion of the coexpressed PI3KC2β to the TRIM27 subcellular compartment (Fig. 1B). Thus, these data indicate that TRIM27 and PI3KC2β associate in vivo and TRIM27 functions to localize a portion of PI3KC2β to a poorly described subcellular compartment, at least in COS cells.
To address the subcellular localization of TRIM27, GFP-TRIM27 overexpressing cells were incubated with rhodamine-transferrin to label the transferrin receptor, lysotracker to label lysosomes, or mitotracker to label mitochondria. These studies demonstrated that a portion of TRIM27 partially overlaps with the transferrin receptor and is likely in recycling endosomes, whereas none of the GFP-TRIM27 localized to the lysosome or mitochondria (Fig. S1).
TRIM27 Ubiquitinates PI3KC2β in Vivo.
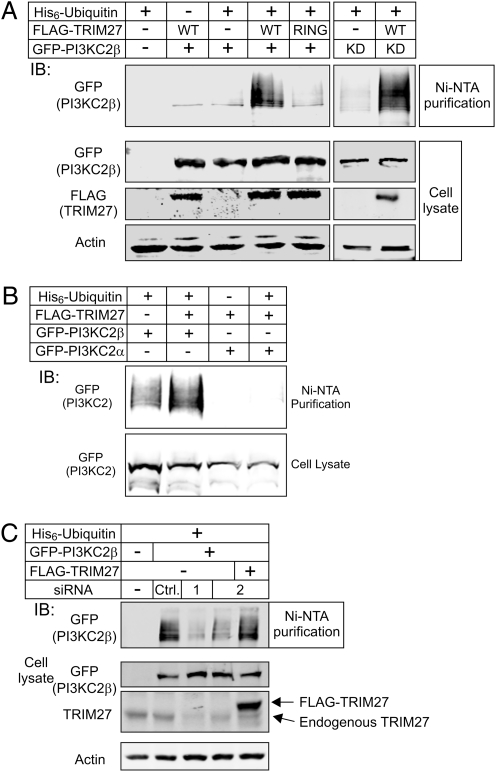
TRIM family members are a class of novel ring-finger E3 ligases that have been shown to ubiquitinate a number of different proteins (30–32). To assess whether TRIM27 ubiquitinates PI3KC2β, PI3KC2β was expressed with His-tagged ubiquitin either alone or together with TRIM27 and ubiquitinated PI3KC2β was assessed following purification with Nickel (Ni)-NTA beads. Although ubiquitinated PI3KC2β was detected when coexpressed with His-tagged ubiquitin alone, the amount of ubiquitinated PI3KC2β was further increased when coexpressed with TRIM27 (Fig. 2 A and B). Ubiquitination was specific because TRIM27 failed to ubiquitinate PI3KC2α (Fig. 2B). In addition, TRIM27-stimulated ubiquitination of PI3KC2β required TRIM27's RING domain; a TRIM27 RING mutant (RING MT) did not stimulate an increase in PI3KC2β ubiquitination (Fig. 2A) [RING, mutation of the zinc binding domain, as previously described (33)], even though PI3KC2β (RING MT) still bound and colocalized with TRIM27 in vivo. TRIM27 also stimulated the ubiquitination of a kinase dead PI3KC2β (KD, K805R) (Fig. 2A).
Fig. 2.
TRIM27 ubiquitinates PI3K-C2β. (A) FLAG-TRIM27(WT) or a FLAG-TRIM27 RING MT were cotransfected with GFP-PI3KC2β or GFP-PI3KC2β kinase dead (KD) together with His6-tagged ubiquitin in 293 cells as indicated. Following lysis, ubiquitinated proteins were purified using Ni-NTA agarose and ubiquitinated GFP-PI3KC2β was then quantitated by Western blot with anti-GFP antibodies. (B) Shown is the inability of TRIM27 to stimulate the ubiquitination of GFP-PI3KC2α using the protocol described in A. (C) 293 Cells were cotransfected with GFP-PI3KC2β and His6-tagged ubiquitin together with an siRNA to TRIM27 (siRNA-1, -2) or a control siRNA and ubiquitinated-GFP-PI3KC2β was assessed as described in A. siRNA-2 targets a sequence in the 3′ UTR of the endogenous TRIM27. siRNA-2–transfected cells were rescued by transfecting a TRIM27 full-length cDNA lacking the 3′ UTR.
To address whether endogenous TRIM27 ubiquitinates PI3KC2β, ubiquitination was assessed as above following transfection with an siRNA to TRIM27. Consistent with endogenous TRIM27 functioning as an E3 ligase for PI3KC2β, PI3KC2β ubiquitination was markedly decreased in cells transfected with two different siRNAs to TRIM27 (Fig. 2C, lanes 3 and 4); this was specific because a control siRNA did not inhibit (Fig. 2C, ctrl). In addition, the decrease in PI3KC2β ubiquitination in TRIM27 siRNA-transfected cells could be rescued by expressing exogenous TRIM27 that did not bind the TRIM27 siRNA (Fig. 2C, lane 5).
TRIM27 Stimulates K48 Polyubiquitination of PI3KC2β.
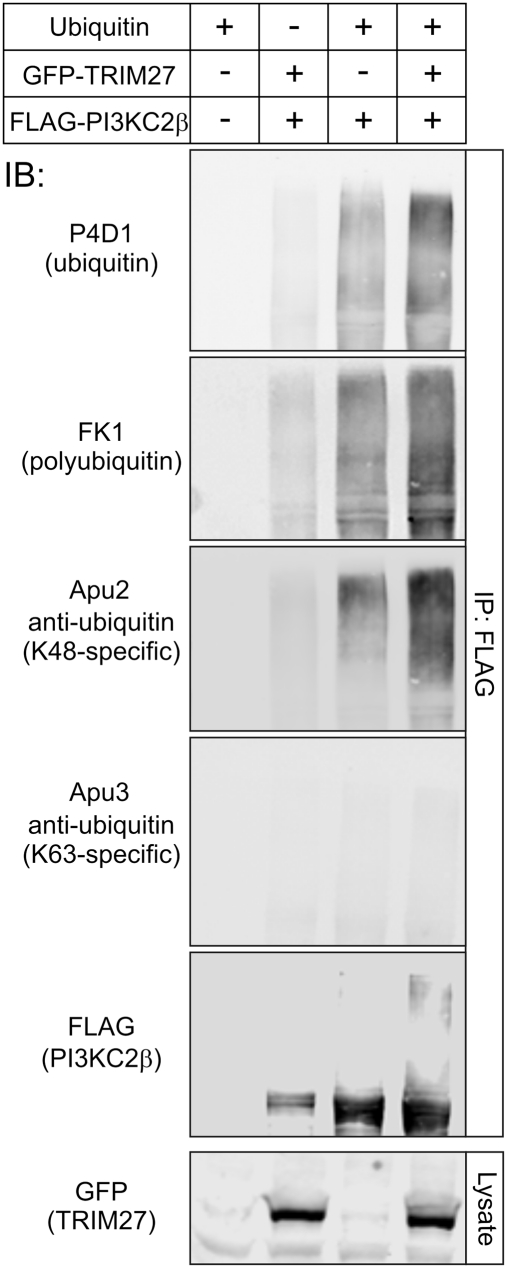
Ubiquitination occurs predominantly via K48 or K63 linkages and consists of the addition of a single ubiquitin (monoubiquitination) or the assembly of ubiquitin of various lengths that are assembled by the successive attachment of ubiquitin to lysines of previously conjugated ubiquitin (polyubiquitination) (34). Although ubiquitin contains seven lysine residues that have been shown to mediate ubiquitin conjugation, K48 and K63 linkages are the best studied. To assess whether TRIM27 mediates K48, K63, mono-, or polyubiquitination of PI3KC2β, Western blotting was performed using antibodies that distinguish between these various ubiquitin modifications (35). TRIM27-stimulated ubiquitination of PI3KC2β reacted with the Lys48-specific antibody (Millipore; clone Apu2) and polyubiquitin-specific antibody (Millipore; clone FK1), and did not react with the Lys63-specific antibody (Millipore; clone HWA4C4) (Fig. 3).
Fig. 3.
TRIM27 mediates K48 polyubiquitination of PI3KC2β. GFP-TRIM27 was cotransfected with FLAG-PI3KC2β and ubiquitin. FLAG-PI3KC2β was then immunoprecipitated with anti-FLAG antibodies and Western blotted with antibodies that recognize various ubiquitin modifications as indicated.
Ubiquitination of PI3KC2β Inhibits PI3KC2β Kinase Activity and Does Not Stimulate Degradation.
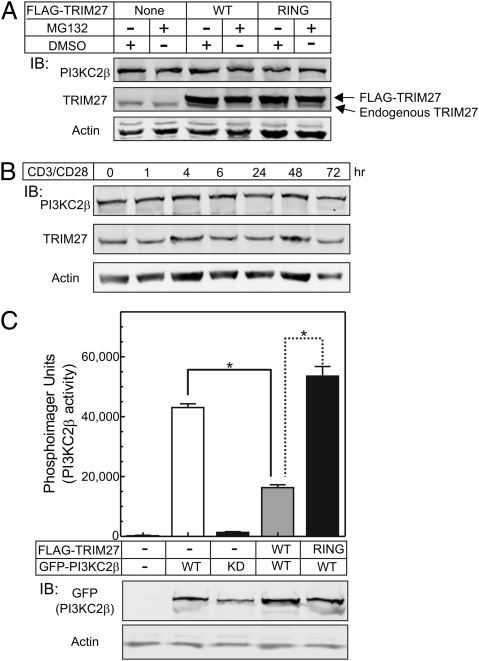
K48 polyubiquitination has been shown to mediate proteosomal degradation of modified proteins, although all non–K63-mediated ubiquitination can target proteins for degradation (36, 37). To determine whether TRIM27 stimulated the degradation of PI3KC2β, HEK293 cells were transfected with TRIM27(WT) or TRIM27(RING MT) and the amount of endogenous PI3KC2β was assessed by Western blot with or without treatment with the proteosome inhibitor MG132. These findings demonstrated that the total amount of PI3KC2β was similar between TRIM27 and control transfected cells, and the amount of PI3KC2β was unaffected by treatment with MG132 (Fig. 4A). In addition to our finding that TRIM27 ubiquitinates PI3KC2β, previous studies have demonstrated that TRIM27 also undergoes autoubiquitination (31). To assess whether either TRIM27 or PI3KC2β is degraded following a physiologic stimulus in T cells, the total amount of TRIM27 and PI3KC2β protein was assessed at various time points following TCR stimulation of Jurkat T cells with anti-CD3/CD28 antibodies (Fig. 4B). These findings also failed to demonstrate evidence for degradation of TRIM27 or PI3KC2β following TCR stimulation.
Fig. 4.
Ubiquitination of PI3KC2β inhibits PI3KC2β‘s enzymatic activity and does not stimulate PI3KC2β degradation. (A) FLAG-TRIM27(WT) or RING MT were transfected into 293 cells and levels of endogenous PI3KC2β was then assessed in by Western blot in cells that were either untreated or treated with the proteosome inhibitor MG132 48 h after transfection. (B) Jurkat T cells were stimulated with anti-CD3/CD28 antibodies and protein levels of endogenous TRIM27 and PI3KC2β were assessed by Western blot at various times after stimulation. (C) GFP-PI3KC2β (WT) or GFP-PI3KC2β (KD) were cotransfected with TRIM27 (WT) or TRIM27 (RING MT), and PI3KC2β kinase activity was determined on anti-GFP immunoprecipitates, as previously described by the authors (19). Shown (Lower) is an anti-GFP Western blot of lysates from transfected cells demonstrating equal levels of GFP-PI3KC2β expression. Plotted are means ± SE from two independent experiments performed in triplicate. *P < 0.01.
We next assessed whether TRIM27 ubiquitination of PI3KC2β affected PI3KC2β's kinase activity. GFP-PI3KC2β was cotransfected with or without FLAG-TRIM27(WT) or FLAG-TRIM27(RING MT) and PI3KC2β enzymatic activity was determined on anti-GFP immunoprecipitates. PI3KC2β enzymatic activity was significantly inhibited in cells cotransfected with TRIM27(WT), resulting in a >60% decrease in PI3KC2β enzymatic activity compared with cells transfected with GFP-PI3KC2β alone (Fig. 4C). In contrast, PI3KC2β enzymatic activity was not inhibited, and even slightly increased, in cells transfected with TRIM27(RING MT). The kinase activity detected was because of PI3KC2β and not an associated kinase, as enzymatic activity was not detected in anti-GFP immunoprecipitates of a kinase dead PI3KC2β (KD, K805R).
TRIM27 Negatively Regulates the K+ Channel KCa3.1 in Jurkat-KCa3.1 Cells and Primary Human CD4 T Cells by Inhibiting PI3KC2β.
Jurkat T cells that overexpress KCa3.1 (Jurkat-KCa3.1) were transfected with an siRNA to TRIM27 and KCa3.1 channel activity was assessed as previously described (19). siRNA knockdown of TRIM27 (Fig. S2A) resulted in about a twofold increase in KCa3.1 channel activity (Fig. S2 B–D). The increase in KCa3.1 channel activity was specific and caused by loss of TRIM27's E3 ligase activity because infection of TRIM27 siRNA transfected cells with a lentiviral construct expressing FLAG-TRIM27(WT), but not FLAG-TRIM27(RING MT), lacking the siRNA binding sequence, restored KCa3.1 channel activity to baseline (Fig. S2D). Similar experiments demonstrated that siRNA knockdown of TRIM27 in primary activated human CD4 cells also resulted in a significant increase in KCa3.1 but not Shk-sensitive Kv channel activity (Fig. S2E).
To demonstrate that TRIM27 mediates it's effect on KCa3.1 by inhibiting PI3KC2β, we assessed whether dialyzing PI3P into Jurkat-KCa3.1 cells transfected with GFP-TRIM27(WT) rescued KCa3.1 channel activity. Overexpression of TRIM27 resulted in a twofold inhibition of KCa3.1 channel activity. The decrease in KCa3.1 channel activity was a result of inhibition of PI3KC2β and decrease in PI3P because KCa3.1 channel activity was rescued by dialyzing GFP-TRIM27 transfected cells with PI3P. Rescue by PI3P was specific because neither PI4P, PI(4,5)P2, nor PI(3,4,5)P3 restored KCa3.1 channel activity in TRIM27 overexpressing cells (Fig. S2F).
TRIM27 Negatively Regulates TCR-Stimulated Ca2+ Flux and Cytokine Production in Jurkat-KCa3.1 Cells.
Consistent with the increase in KCa3.1 channel activity, TCR-stimulated Ca2+ influx was significantly increased in TRIM27 siRNA-transfected Jurkat-KCa3.1 cells (Fig. S3A). In addition, TRIM27 siRNA-transfected Jurkat-KCa3.1 cells secreted about 50% more IL-2 in response to treatment with phorbol myristate acetate and ionomycin (Fig. S3B). The increase in IL-2 production was dependent upon KCa3.1 channel activity because treatment with TRAM34, a specific KCa3.1 inhibitor (38, 39), blocked IL-2 production (Fig. S3B).
KCa3.1 Channel Activity and TCR-Stimulated Ca2+ Influx Is Increased in TRIM27−/− Th1 and Th2 CD4 T Lymphocytes.
To generate TRIM27−/− mice, the ES cell line 345D11 was purchased from The Center for Disease Modeling at The University of Toronto, which contained the exon-trapping plasmid pUPA located between exon 1 and 2 of TRIM27 on mouse chromosome 13 (Fig. S4), and TRIM27−/+ and TRIM27−/− mice were generated. TRIM27−/+ mice were backcrossed to C57BL/6, and studies were performed on mice that were backcrossed five generations. TRIM27 mice appeared normal and had normal numbers of peripheral blood, splenic and thymic CD4 and CD8 T lymphocytes, CD19 B cells, and FoxP3 regulatory T cells (Fig. S5). However, consistent with endogenous TRIM27 functioning to negatively regulate PI3KC2β enzyme activity, PI3KC2β enzyme activity detected in an immune complex kinase assay was about 1.6-fold increased in lymphocytes from TRIM27−/− mice compared with similar cells from TRIM27+/+ mice, but PI3KC2β protein levels were unchanged (Fig. S4D).
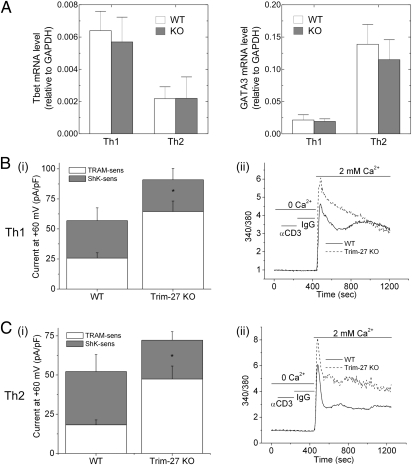
KCa3.1 is the predominant K+ channel expressed in Th0, Th1, and Th2 CD4 T cells and is required for both Ca2+ influx and cytokine production by these cells (40, 41). To assess whether KCa3.1 channel activity is also increased in TRIM27−/− Th1 and Th2 CD4 T lymphocytes, CD4 T cells isolated from spleens of TRIM27+/+ and TRIM27−/− mice were differentiated into Th1 and Th2 cells (40). TRIM27−/− CD4 T cells differentiated normally into Th1 and Th2 cells, as evidenced by similar expression of T-bet and GATA3, respectively, compared with TRIM27+/+ cells (Fig. 5A). Whole-cell patch-clamp experiments demonstrated that KCa3.1 channel activity was increased about twofold in TRIM27−/− Th1 and Th2 CD4 T cells compared with TRIM27+/+ cells, but Shk-sensitive Kv channel activity was similar between the two (Fig. 5 B and C). Consistent with the increase in KCa3.1 channel activity, both the acute rise and the plateau phase of Ca2+ influx was significantly increased in TRIM27−/− Th1 and Th2 cells following stimulation with anti-CD3 antibodies (Fig. 5 B and C).
Fig. 5.
KCa3.1 channel activity and TCR-stimulated Ca2+ influx is increased in TRIM27−/− CD4 Th1 and Th2 cells. (A) Real-time PCR of GATA-3 and T-bet expression in Th1 and Th2 differentiated cells. Bar graph summary of whole-cell patch-clamp experiments performed on (Bi) Th1 and (Ci) Th2 cells using a protocol similar to that described in Materials and Methods and Fig. S1 (n = 15 cells). Shown is the TRAM34 (KCa3.1) and Shk (Kv) sensitive current at +60 mV. *P < 0.005 for increase in TRAM-34 sensitive current between TRIM27−/− and WT cells. Anti-CD3 stimulated Ca2+ flux was assessed on (Bii) Th1 and (Cii) Th2 cells as described in Fig. 6A. All experiments shown are representative of at least three experiments performed on cells isolated from at least three separate mice.
Cytokine Production Is Increased in TRIM27−/− CD4 T Cells.
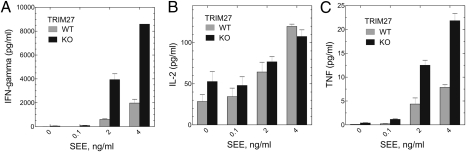
To assess whether increased Ca2+ influx leads to increased production of cytokines, TRIM27+/+ and TRIM27−/− CD4 T cells were stimulated with irradiated splenocytes and the superantigen staphylococcal enterotoxin E (SEE). In comparison with TRIM27+/+ cells, TRIM27−/− cells produced IFN-γ and TNF at lower concentrations of SEE, and also produced increased amounts of both cytokines at higher SEE concentrations (Fig. 6 A and C). In contrast, production of IL-2 was similar between TRIM27+/+ and TRIM27−/− cells (Fig. 6B).
Fig. 6.
Increased cytokine production by TRIM27−/− Th0 cells. TRIM27−/− and TRIM27+/+ CD4 T cells were stimulated for 3 d with antiCD3/CD28 antibodies and, after resting overnight, were restimulated with irradiated splenocytes at a ratio of 1:8 together with various concentrations of SEE. Cytokines were assessed in supernatants as indicated 72 h after stimulation. (A) IFN-γ, (B) IL-2, and (C) TNF-α. Plotted are means ± SE and are representative of two independent experiments performed in quadruplicates.
Proximal Signaling Molecules Are Activated Similarly in TRIM27−/− and TRIM27+/+ Th0 Cells.
Tyrosine phosphorylation of proximal signaling pathways downstream of the TCR should be unaffected in TRIM27−/− CD4 T cells, if TRIM27 primarily inhibits TCR signaling via direct ubiquitination of PI3KC2β. Anti-CD3/CD28 stimulation resulted in similar levels of total tyrosine phosphorylated proteins in TRIM27−/− and TRIM27+/+ cells (Fig. S6). In addition, activation of AKT and ERK MAP Kinase was similar between TRIM27−/− and TRIM27+/+ cells (Fig. S6).
Discussion
Even though a growing list of stimuli have been shown to activate class II PI3Ks, including chemokines, cytokines, and receptor tyrosine kinases, we still know very little regarding the exact role for class II PI3Ks in mediating biological output from these receptors, or the downstream signaling pathways that are activated (4, 13). We have previously shown that one function of the class II PI3KC2β is to generate the pool of PI3P required for activating KCa3.1 and, through this function, PI3KC2β is required for TCR stimulated Ca2+ influx in CD4 Th0, Th1, and Th2 cells (19, 40, 41). Thus, understanding the mechanisms for regulating PI3KC2β in the activation of KCa3.1 affords us a unique opportunity to both uncover fundamental mechanisms for regulating class II PI3Ks, as well as to identify new signaling molecules that modulate CD4 T-cell activation. We now demonstrate that TRIM27 functions as a unique negative regulator of PI3KC2β in CD4 T cells.
The TRIM family of proteins are composed of 74 family members in mammalian cells and are divided into 11 subgroups based on their carboxyl-terminal domain (29, 30). A consistent theme that has emerged over the past few years is the important role for TRIM family members in immune activation, and in particular, the regulation of innate immunity (30, 42). For example, TRIM25 promotes K63 ubiquitination of RIG-I, which has been shown to be critical to activate viral innate immunity (43). TRIM30α inhibits toll like receptor (TLR)-mediated activation of NF-κβ by targeting TAB2 and TAB3 for degradation (42), and TRIM21 has been shown to inhibit several IFN response factors (IRF) (44, 45). With regard to innate immunity, TRIM27 was shown to interact with and inhibit several IKK family members and TBK1 (TRAF family member-associated NF-κβ activator binding kinase), leading to inhibition of TLR activation of NF-κβ and IRF3 (46). We now uncover a critical role for TRIM27 to also negatively regulate the adaptive immune response. By inhibiting PI3KC2β, TRIM27 functions to down-regulate KCa3.1 channel activity, leading to decreased TCR-stimulated Ca2+ influx, proliferation, and cytokine production. This process is physiologically important, because we found that TRIM27 inhibits KCa3.1 in a number of different CD4 T cells, including Jurkat and activated primary human CD4 T cells. Moreover, we provide genetic evidence that TRIM27 functions to inhibit KCa3.1 in CD4 T cells that require KCa3.1 for activation; TCR-stimulated Ca2+ influx is increased in Th0, Th1, and Th2 cells generated from TRIM27−/− mice (40, 41). Thus, our findings reinforce the critical role for TRIM family proteins to regulate both the innate and adaptive immune response.
TRIM27 and other TRIM family members contain an N-terminal Zn-binding RING domain, which enable them to function as E3 ligases (29–31). Although TRIM27 inhibition of IKKε and TBK1 did not require its RING domain (46), TRIM27 inhibition of TCR signaling is mediated via the direct ubiquitination of PI3KC2β by TRIM27. This result is supported by our finding that overexpression of TRIM27(WT), but not a TRIM27(RING MT), inhibited PI3KC2β enzyme activity and rescued the increase in KCa3.1 channel activity following siRNA knockdown of TRIM27. In addition, the ability to rescue TRIM27 inhibition of KCa3.1 channel activity by dialyzing TRIM27 overexpressing cells with PI3P, but not other phosphoinositides, confirms that TRIM27 inhibits KCa3.1 by interfering with PI3P generation. We have previously shown that PI3P is required for KCa3.1 activation by enabling the histidine kinase, NDPK-B, to histidine phosphorylate the C terminus of KCa3.1, leading to its activation (20, 41). Thus, these findings, together with the demonstration that PI3KC2β enzyme activity is increased in TRIM27−/− lymphocytes, supports a model whereby direct ubiquitination of PI3KC2β by TRIM27 results in the inhibition of PI3KC2β's enzymatic activity leading to decreased levels of PI3P, resulting in decreased histidine phosphorylation and activation of KCa3.1 by NDPK-B (Fig. S7) (19, 40, 41, 47).
Despite the fact that the immune system has evolved a myriad number of mechanisms to turn itself off, redundant mechanisms for inhibition are often incomplete. This result is supported by the finding that disruption of even a single pathway is sufficient to lead to autoimmune disease under some circumstances (48). Our finding that TRIM27 is a unique negative regulator of CD4 T cells, when coupled with previous findings that TRIM27 may also negatively regulate innate signaling, places TRIM27 in a unique position to down-regulate the immune response at multiple levels.
Materials and Methods
Cells and Constructs.
Jurkat-KCa3.1 T cells (19) and human CD4 T cells were cultured in RPMI + 10% FBS. GFP-tagged PI3KC2α and PI3KC2β were kindly provided by J. Domin, Imperial College, London, UK. The PI3KC2β kinase dead mutant was generated by substituting lysine 850 to arginine and the TRIM27 RING mutant was generated by substituting C3/H1/C4/C5 in the cysteine-rich zinc binding domain to S3/Q1/S4/S5 (33). More details are available in SI Materials and Methods.
In Vivo Ubiquitination Assay.
GFP-tagged PI3KC2α or PI3KC2β were expressed with or without FLAG-TRIM27 together with His6-ubiquitin in HEK 293 cells. Transfected cells were then lysed in 6 M guanidinium and ubiquitinated proteins were purified using Ni-NTA beads followed by Western blotting with anti-GFP or anti-FLAG antibodies, as previously described (49). To assess whether TRIM27 stimulates K48, K63, poly-, or monoubiquitination of PI3KC2β, the Lys48-specific antibody (Millipore; clone Apu2), the polyubiquitin-specific antibody (Millipore; clone FK1), of the Lys63-specific antibody (Millipore; clone Apu3) (50, 51) were used to blot immuno-precipitated PI3KC2β.
Whole-Cell Patch-Clamp and Intracellular Ca2+ Activity.
Whole-cell patch clamping on activated CD4 T cells (47) and Jurkat-KCa3.1 T cells, PI3P rescue experiment and Ca2+ imaging were performed as previously described (19) and detailed in SI Materials and Methods.
Generation of TRIM27−/− Mice.
All procedures were approved by the Institutional Animal Use and Care Committee. ES cell line (clone ID 345D11, strain 129/ola) that contained an exon-trapping plasmid pUPA integrated between exon 1 and exon 2 of the TRIM27 gene was purchased from The Center for Modeling Human Disease at the University of Toronto. (http://www.cmhd.ca/genetrap/index.html). ES cells (strain 129/ola) were injected into C57BL/6 blastocyst by the transgenic facility at the New York University Langone Medical Center (New York) and chimeric mice were obtained. TRIM27+/− mice were backcrossed six generations with C57BL/6 and then used to generate TRIM27−/− C57BL/6 mice in these studies. CD4+ T cells were purified on MACS beads (Miltenyi Biotech) from WT or TRIM27−/− spleens and various CD4 T-cell subsets were generated, as previously described (52).
Cytokine Assays.
For cytokine assays, TRIM27−/− and TRIM27+/+ CD4 Th0 cells were stimulated for 3 d together with splenocytes in the presence of various concentrations of SEE. Cytokines were assessed in supernatants using the BD Cytometric Bead Array Cytokine Kit.
Supplementary Material
Acknowledgments
We thank Yan Deng at the Microscopy Core of New York University Langone Medical Center for help with the live-cell imaging system, and Ian Ahearn for help with the Mitotracker experiment. This work was supported in part by National Institutes of Health Grants R01GM084195 and R01AI052459 (to E.Y.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1111233109/-/DCSupplemental.
References
- 1.Vanhaesebroeck B, Vogt PK, Rommel C. PI3K: From the bench to the clinic and back. Curr Top Microbiol Immunol. 2010;347:1–19. doi: 10.1007/82_2010_65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falasca M, et al. The role of phosphoinositide 3-kinase C2alpha in insulin signaling. J Biol Chem. 2007;282:28226–28236. doi: 10.1074/jbc.M704357200. [DOI] [PubMed] [Google Scholar]
- 3.Foster FM, Traer CJ, Abraham SM, Fry MJ. The phosphoinositide (PI) 3-kinase family. J Cell Sci. 2003;116:3037–3040. doi: 10.1242/jcs.00609. [DOI] [PubMed] [Google Scholar]
- 4.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 5.Huang YH, Sauer K. Lipid signaling in T-cell development and function. Cold Spring Harb Perspect Biol. 2010;2:a002428. doi: 10.1101/cshperspect.a002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fruman DA. The role of class I phosphoinositide 3-kinase in T-cell function and autoimmunity. Biochem Soc Trans. 2007;35:177–180. doi: 10.1042/BST0350177. [DOI] [PubMed] [Google Scholar]
- 7.Okkenhaug K, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez-Borlado L, et al. Phosphatidylinositol 3-kinase regulates the CD4/CD8 T cell differentiation ratio. J Immunol. 2003;170:4475–4482. doi: 10.4049/jimmunol.170.9.4475. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki T, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 10.Swat W, et al. Essential role of PI3Kdelta and PI3Kgamma in thymocyte survival. Blood. 2006;107:2415–2422. doi: 10.1182/blood-2005-08-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webb LM, Vigorito E, Wymann MP, Hirsch E, Turner M. Cutting edge: T cell development requires the combined activities of the p110gamma and p110delta catalytic isoforms of phosphatidylinositol 3-kinase. J Immunol. 2005;175:2783–2787. doi: 10.4049/jimmunol.175.5.2783. [DOI] [PubMed] [Google Scholar]
- 12.Berg LJ, Finkelstein LD, Lucas JA, Schwartzberg PL. Tec family kinases in T lymphocyte development and function. Annu Rev Immunol. 2005;23:549–600. doi: 10.1146/annurev.immunol.22.012703.104743. [DOI] [PubMed] [Google Scholar]
- 13.Falasca M, Maffucci T. Role of class II phosphoinositide 3-kinase in cell signalling. Biochem Soc Trans. 2007;35:211–214. doi: 10.1042/BST0350211. [DOI] [PubMed] [Google Scholar]
- 14.Wheeler M, Domin J. Recruitment of the class II phosphoinositide 3-kinase C2beta to the epidermal growth factor receptor: role of Grb2. Mol Cell Biol. 2001;21:6660–6667. doi: 10.1128/MCB.21.19.6660-6667.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaidarov I, Smith ME, Domin J, Keen JH. The class II phosphoinositide 3-kinase C2alpha is activated by clathrin and regulates clathrin-mediated membrane trafficking. Mol Cell. 2001;7:443–449. doi: 10.1016/s1097-2765(01)00191-5. [DOI] [PubMed] [Google Scholar]
- 16.Das M, et al. Regulation of neuron survival through an intersectin-phosphoinositide 3′-kinase C2beta-AKT pathway. Mol Cell Biol. 2007;27:7906–7917. doi: 10.1128/MCB.01369-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown RA, Shepherd PR. Growth factor regulation of the novel class II phosphoinositide 3-kinases. Biochem Soc Trans. 2001;29:535–537. doi: 10.1042/bst0290535. [DOI] [PubMed] [Google Scholar]
- 18.Maffucci T, Falasca M. Phosphoinositide 3-kinase-dependent regulation of phospholipase Cgamma. Biochem Soc Trans. 2007;35:229–230. doi: 10.1042/BST0350229. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava S, et al. The class II phosphatidylinositol 3 kinase C2beta is required for the activation of the K+ channel KCa3.1 and CD4 T-cells. Mol Biol Cell. 2009;20:3783–3791. doi: 10.1091/mbc.E09-05-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava S, et al. Histidine phosphorylation of the potassium channel KCa3.1 by nucleoside diphosphate kinase B is required for activation of KCa3.1 and CD4 T cells. Mol Cell. 2006;24:665–675. doi: 10.1016/j.molcel.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Beeton C, et al. Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases. Mol Pharmacol. 2005;67:1369–1381. doi: 10.1124/mol.104.008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghanshani S, et al. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J Biol Chem. 2000;275:37137–37149. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- 23.Wulff H, Beeton C, Chandy KG. Potassium channels as therapeutic targets for autoimmune disorders. Curr Opin Drug Discov Devel. 2003;6:640–647. [PubMed] [Google Scholar]
- 24.Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov. 2009;8:982–1001. doi: 10.1038/nrd2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol Rev. 2009;231:59–87. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feske S. ORAI1 and STIM1 deficiency in human and mice: Roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev. 2009;231:189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winslow MM, Crabtree GR. Immunology. Decoding calcium signaling. Science. 2005;307:56–57. doi: 10.1126/science.1108163. [DOI] [PubMed] [Google Scholar]
- 29.Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of ‘single protein RING finger’ E3 ubiquitin ligases. Bioessays. 2005;27:1147–1157. doi: 10.1002/bies.20304. [DOI] [PubMed] [Google Scholar]
- 30.Ozato K, Shin DM, Chang TH, Morse HC., 3rd TRIM family proteins and their emerging roles in innate immunity. Nat Rev Immunol. 2008;8:849–860. doi: 10.1038/nri2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Napolitano LM, Jaffray EG, Hay RT, Meroni G. Functional interactions between ubiquitin E2 enzymes and TRIM proteins. Biochem J. 2011;434:309–319. doi: 10.1042/BJ20101487. [DOI] [PubMed] [Google Scholar]
- 32.Miller AT, et al. Production of Ins(1,3,4,5)P4 mediated by the kinase Itpkb inhibits store-operated calcium channels and regulates B cell selection and activation. Nat Immunol. 2007;8:514–521. doi: 10.1038/ni1458. [DOI] [PubMed] [Google Scholar]
- 33.Borden KL. RING fingers and B-boxes: Zinc-binding protein-protein interaction domains. Biochem Cell Biol. 1998;76:351–358. doi: 10.1139/bcb-76-2-3-351. [DOI] [PubMed] [Google Scholar]
- 34.Malynn BA, Ma A. Ubiquitin makes its mark on immune regulation. Immunity. 2010;33:843–852. doi: 10.1016/j.immuni.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newton K, et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 2008;134:668–678. doi: 10.1016/j.cell.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 36.Clague MJ, Urbé S. Ubiquitin: same molecule, different degradation pathways. Cell. 2010;143:682–685. doi: 10.1016/j.cell.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Xu P, et al. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137:133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wulff H, Gutman GA, Cahalan MD, Chandy KG. Delineation of the clotrimazole/TRAM-34 binding site on the intermediate conductance calcium-activated potassium channel, IKCa1. J Biol Chem. 2001;276:32040–32045. doi: 10.1074/jbc.M105231200. [DOI] [PubMed] [Google Scholar]
- 39.Wulff H, et al. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: A potential immunosuppressant. Proc Natl Acad Sci USA. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di L, et al. Inhibition of the K+ channel KCa3.1 ameliorates T cell-mediated colitis. Proc Natl Acad Sci USA. 2010;107:1541–1546. doi: 10.1073/pnas.0910133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di L, et al. Nucleoside diphosphate kinase B knock-out mice have impaired activation of the K+ channel KCa3.1, resulting in defective T cell activation. J Biol Chem. 2010;285:38765–38771. doi: 10.1074/jbc.M110.168070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi M, et al. TRIM30 alpha negatively regulates TLR-mediated NF-kappa B activation by targeting TAB2 and TAB3 for degradation. Nat Immunol. 2008;9:369–377. doi: 10.1038/ni1577. [DOI] [PubMed] [Google Scholar]
- 43.Gack MU, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 44.Espinosa A, et al. Loss of the lupus autoantigen Ro52/Trim21 induces tissue inflammation and systemic autoimmunity by disregulating the IL-23-Th17 pathway. J Exp Med. 2009;206:1661–1671. doi: 10.1084/jem.20090585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshimi R, et al. Gene disruption study reveals a nonredundant role for TRIM21/Ro52 in NF-kappaB-dependent cytokine expression in fibroblasts. J Immunol. 2009;182:7527–7538. doi: 10.4049/jimmunol.0804121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zha J, et al. The Ret finger protein inhibits signaling mediated by the noncanonical and canonical IkappaB kinase family members. J Immunol. 2006;176:1072–1080. doi: 10.4049/jimmunol.176.2.1072. [DOI] [PubMed] [Google Scholar]
- 47.Srivastava S, et al. Phosphatidylinositol-3 phosphatase myotubularin-related protein 6 negatively regulates CD4 T cells. Mol Cell Biol. 2006;26:5595–5602. doi: 10.1128/MCB.00352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marrack P, Scott-Browne J, MacLeod MK. Terminating the immune response. Immunol Rev. 2010;236:5–10. doi: 10.1111/j.1600-065X.2010.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campanero MR, Flemington EK. Regulation of E2F through ubiquitin-proteasome-dependent degradation: stabilization by the pRB tumor suppressor protein. Proc Natl Acad Sci USA. 1997;94:2221–2226. doi: 10.1073/pnas.94.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furukawa M, Andrews PS, Xiong Y. Assays for RING family ubiquitin ligases. Methods Mol Biol. 2005;301:37–46. doi: 10.1385/1-59259-895-1:037. [DOI] [PubMed] [Google Scholar]
- 51.Bloom J, Pagano M. Experimental tests to definitively determine ubiquitylation of a substrate. Methods Enzymol. 2005;399:249–266. doi: 10.1016/S0076-6879(05)99017-4. [DOI] [PubMed] [Google Scholar]
- 52.Izcue A, et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.