Abstract
Large conductance, voltage- and calcium-gated potassium (BK) channels regulate several physiological processes, including myogenic tone and thus, artery diameter. Nongenomic modulation of BK activity by steroids is increasingly recognized, but the precise location of steroid action remains unknown. We have shown that artery dilation by lithocholate (LC) and related cholane steroids is caused by a 2× increase in vascular myocyte BK activity (EC50 = 45 μM), an action that requires β1 but not other (β2–β4) BK accessory subunits. Combining mutagenesis and patch-clamping under physiological conditions of calcium and voltage on BK α- (cbv1) and β1 subunits from rat cerebral artery myocytes, we identify the steroid interaction site from two regions in BK β1 transmembrane domain 2 proposed by computational dynamics: the outer site includes L157, L158, and T165, whereas the inner site includes T169, L172, and L173. As expected from computational modeling, cbv1+rβ1T165A,T169A channels were LC-unresponsive. However, cbv1 + rβ1T165A and cbv1 + rβ1T165A,L157A,L158A were fully sensitive to LC. Data indicate that the transmembrane domain 2 outer site does not contribute to steroid action. Cbv1 + rβ1T169A was LC-insensitive, with rβ1T169S being unable to rescue responsiveness to LC. Moreover, cbv1 + rβ1L172A, and cbv1 + rβ1L173A channels were LC-insensitive. These data and computational modeling indicate that tight hydrogen bonding between T169 and the steroid α-hydroxyl, and hydrophobic interactions between L172,L173 and the steroid rings are both necessary for LC action. Therefore, β1 TM2 T169,L172,L173 provides the interaction area for cholane steroid activation of BK channels. Because this amino acid triplet is unique to BK β1, our study provides a structural basis for advancing β1 subunit–specific pharmacology of BK channels.
Keywords: MaxiK channel, vascular smooth muscle, bile acids, KCNMB1, molecular dynamics
Large conductance, voltage- and calcium-gated potassium (BK) channels control a wide variety of physiological processes, including neuronal firing, neurotransmitter release, neurosecretion, tuning of cochlear hair cells, immunity, and myogenic tone (1, 2). In most tissues, BK channels are heterooligomers resulting from the association of four channel-forming α subunits (encoded by Slo1 or KCNMA1) and one type of four possible two-transmembrane domain (TM) accessory β subunits: β1, β2, β3, or β4, which are encoded by KCNMB1, -2, -3, and -4, respectively (3). Remarkably, these β subunit types are differentially distributed across tissues (1, 4). The resulting variety of BK channel complexes corresponds with distinct biophysical and pharmacological properties, which allows BK channels to serve a distinct function in each particular tissue where they are expressed.
Modulation of BK channel activity by physiological steroids is increasingly recognized (5–7). Moreover, some steroids differentially activate BK channels that differ in subunit composition. For example, micromolar levels of 17β-estradiol or lithocholate (LC) activate heteromeric, β1-containing but not homomeric α-channels (5, 7, 8). In turn, dehydroepiandrosterone activates β2-containing channels much more effectively than β4-containing channels, with the opposite subunit preference for corticosterone (6). Understanding the molecular bases of BK β-steroid interactions will enable us to precisely define the role of BK channels in steroid modulation of tissue-specific physiology and disease. Moreover, such knowledge will allow investigators to design pharmacological agents that target BK channels in a tissue-specific manner. Unfortunately, steroid interaction sites in BK subunits remain to be structurally defined.
Naturally occurring bile acids are steroids derived from the cholane steroid nucleus (C24). Epidemiological, empirical, clinical, and experimental data indicate that bile acids are found in circulation at high enough levels (high micromolar) during human disease to cause smooth muscle relaxation and artery dilation and thus, reduce systemic blood pressure. Moreover, bile acids contribute to ileal vasodilation, which facilitates fat absorption (9, 10). We have shown that vasodilation in response to LC and other bile acids is independent of vascular endothelium and circulating factors but occurs by steroid activation of smooth muscle BK channels (7, 11). Notably, LC fails to activate BK channels and induce vasodilation in KCNMB1 KO mice (7). Furthermore, LC selectively activates recombinant β1-containing but not β2-, β3-, or β4-containing BK channels (8). These findings underscore the key role of β1 subunits in cholane steroid action. Moreover, using BK complexes that included β-chimeras made of LC-sensitive (β1) and LC-insensitive (β4) subunits, we identified β1 TM2 as the protein region that senses steroid presence leading to channel activation (12). Computational modeling and systematic structure–activity relationship (SAR) studies of LC and related monohydroxysterols allowed us to hypothesize the existence of LC-sensing areas in BK β1 TM2 (13). Whether these putative docking regions play a role in BK–steroid interaction and eventual increase in channel activity has remained unknown.
In the present study, we used mutagenesis and computational modeling to identify the specific amino acid residues and nature of chemical interactions in BK β1 TM2 that determine LC interaction with BK channels resulting in channel activation. We probed steroid action on BK α (cbv1) and rβ1 subunits cloned from the same cell type [i.e., rat cerebral artery myocytes (AY330293 and FJ154955)] using a heterologous expression system where the recombinant channel phenotype matches the phenotype of the native channel, including its LC sensitivity (7, 8). Our study pinpoints T169, L172, and L173 within the inner region of BK β1 TM2 as the key set of residues necessary for steroid action. Furthermore, computational dynamics and systematic mutagenesis indicate that LC docking occurs by hydrogen bonding between T169 and the steroid α-hydroxyl at C3 and hydrophobic interactions between L172,L173 and the steroid rings. Thus, our study identifies a steroid sensing site in an ion channel auxiliary subunit. Because the triplet T169,L172,L173 is unique to BK subunits of type β1 and common to many species, including humans, these findings open the possibility of using LC as a starting point in designing agents that selectively target β1-containing BK channels and thus, relax smooth muscle, with possible applications in the pharmacotherapy of asthma, vasospasm, and systemic hypertension.
Results
Two Putative LC Docking Areas Are Identified in the BK β1 Subunit TM2 Domain.
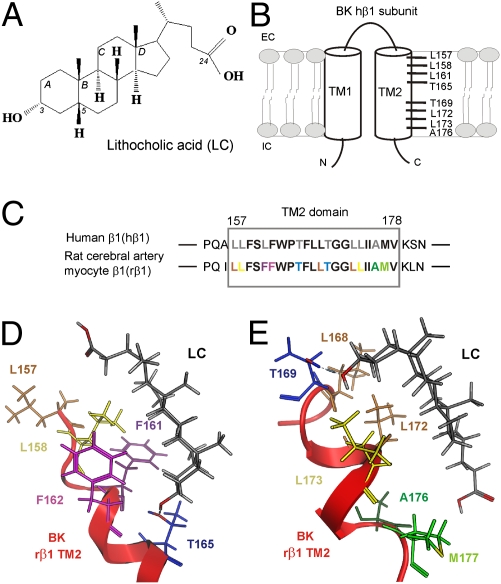
Systematic SAR studies of LC (Fig. 1A) and related steroids on BK channel function (11, 13) along with computational modeling (13) led us to advance two sites in human BK β1 TM2 for LC docking. These sites differ in their location along the TM2 α-helix longitudinal axis but satisfy a general model by which hydrogen bonding occurs between LC C3 α-hydroxyl and a polar TM2 amino acid, whereas hydrophobic interactions occur between LC steroidal rings and a few TM2 nonpolar residues. Thus, the outer site includes the polar residue T165 and hydrophobic residues L157,L158, whereas the inner site includes polar T169 and hydrophobic L172,L173 (Fig. 1B). To identify which of the two sites is necessary and/or sufficient for cholane steroid activation of BK channels, we decided to probe LC action on mutagenized BK channels constructed from α + β1 subunits cloned from the same cell type and species. Therefore, the recombinant channel complex mimics as much as possible the native channel. Thus, we cloned and expressed BK subunits from arterial myocytes (cbv1: AY330293; rβ1: FJ154955) from an experimental model that is relevant to study LC action on β1-containing BK channels (i.e., rat middle cerebral arteries) (7, 8).
Fig. 1.
Computationally predicted interaction areas for LC in β1 subunit–containing BK channels. (A) Molecular structure of LC. (B) Schematic representation of BK channels human β1 subunit with computationally predicted interaction areas for LC in TM2 domain. (C) Amino acid sequence alignment of human BK β1 TM2 and rat cerebral artery myocyte BK β1 TM2 domains. Amino acids proposed for LC interaction areas in hβ1 and rβ1 are shown in gray and colored, respectively. Snapshots from the molecular dynamics (MD) simulation for LC interactions with the BK rβ1 TM2 domain outer (D) and inner (E) regions. Hydrogen bond between TM2 Thr165 or 169 and C3-hydroxyl of LC is indicated as blue dashed line.
Sequence alignment of hβ1 vs. rβ1 TM2 domains reveals that, of 22 amino acids contributing to the TM2 α-helix (4), only 1 amino acid is not identical: F161 in rβ1 corresponds to L161 (Fig. 1C). From a previous model of hβ1 (13), computational modeling of the two possible LC recognition sites in rβ1 TM2 reveals that substitution of Leu with Phe in position 161 causes spatial rearrangement of residues within the outer site: the bulky F161 is moved farther away from the LC steroid nucleus, whereas L157 underlies the LC side chain (Fig. 1D). Thus, the model seems to indicate that hydrophobic–hydrophobic interactions between L157 and LC side chain are necessary for successful steroid–BK channel interaction. In contrast, the L161F substitution did not affect the spatial orientation of the proposed LC docking residues within the β1 TM2 inner site (Fig. 1E). Thus, as previously proposed for hβ1 TM2, T165 from the outer site and T169 from the inner site should be able to establish hydrogen bonding with LC α-hydroxyl (Fig. 1 D and E) and allow steroid recognition, eventually leading to increased BK channel steady-state activity (NPo). To test these hypotheses, we evaluated the LC sensitivity of cbv1 + rβ1 channels after alanine substitution of T165 and T169 within rβ1 TM2. Based on our previous study, we used cbv1 + WT rβ1 and homomeric cbv1 channels as positive and negative controls, respectively (7, 8). Presence of functional β1 subunits within the BK channel complex was confirmed by the universally recognized slow activation kinetics of macroscopic current that results from the functional coupling of accessory subunits of the β1-type to the BK channel-forming proteins complex (SI Materials and Methods, Fig. S1, and Table S1) (1, 4). LC was perfused onto the intracellular side of inside-out membrane patches (Materials and Methods) with free Ca2+i = 10 μM and Vm = −20 to −40 mV. These conditions of Ca2+i and voltage approach those conditions faced by native BK channels in cerebral artery myocytes during contraction (14, 15). As expected, 150 μM LC (EC90 for LC to activate native cerebral artery myocyte BK channels) (7) routinely failed to increase cbv1 NPo (Fig. 2 A and D). In contrast, cbv1 + WT rβ1 NPo was robustly increased by LC, reaching 170% of control (i.e., under perfusion with vehicle-containing solution) values (Fig. 2 B and D). Notably, the EC50 for LC action on cbv1 + WT rβ1 recombinant channels is 40.4 ± 3.8 μM, which is similar (P > 0.05) to the EC50 for LC activation of native BK channels in freshly isolated rat cerebral artery myocytes (7) (Fig. S2). The similarity in apparent affinities of LC action on recombinant and native cerebrovascular BK channels is consistent with previous studies (7, 8) and buttresses the idea that LC modifies BK NPo by acting on biological targets common to the different proteolipid environments, such as the BK subunits themselves.
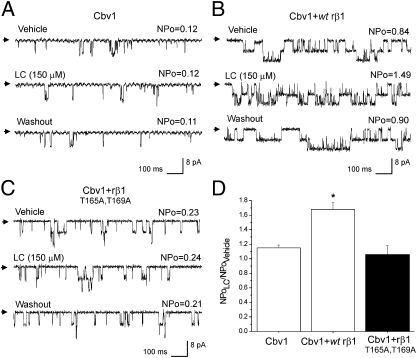
Fig. 2.
Heterologous expression of cbv1, cbv1 + WT rβ1, and cbv1 + rβ1T165A,T169A subunits renders BK channels differentially sensitive to LC. (A) Single-channel records from homomeric cbv1 channel. Here and in all other figures, records are obtained at Vm = −20 mV in symmetric Ca2+ = 10 μM. Arrows indicate baseline. LC at 150 μM and vehicle were tested on the same patch; each compound–vehicle pair was tested on different patches excised from different oocytes. (B) Records from cbv1 + WT rβ1 channel show robust, reversible increase in BK NPo evoked by 150 μM LC. (C) Records from cbv1 + rβ1T165A,T169A channel show lack of LC sensitivity. (D) Averaged responses to LC. *P < 0.05; significant from control (vehicle). Each bar represents average from n ≥ 4.
In contrast, cbv1 + rβ1T165A,T169A channels, although exhibiting the characteristic slow activation kinetics of macroscopic current that results from β1-BK channel-forming subunit coupling (1, 4) (Table S1), were totally insensitive to 150 μM LC (Fig. 2 C and D). This result indicates that polar T165 and/or T169 is required for LC activation of BK channels. It also supports the general model for LC docking onto BK β1 TM2 proposed above and satisfied by the two sites shown in Fig. 1 D and E.
After establishing the critical role of T165 and T169 within rβ1 TM2 in LC sensing, we addressed whether each of these threonines was sufficient/necessary to confer LC sensitivity to the BK channel complex.
Proposed LC Docking Site in the Outer TM2 Region of BK β1 Does Not Play a Role in LC Action.
LC application to cbv1 + rβ1T165A channels increased BK NPo to 165 ± 10% of control. This increase was not statistically different (P > 0.05) from LC action on cbv1 + WT rβ1 currents (Fig. 3 A and C), indicating that a threonine in position 165 is not necessary to provide LC sensitivity to the BK channel.
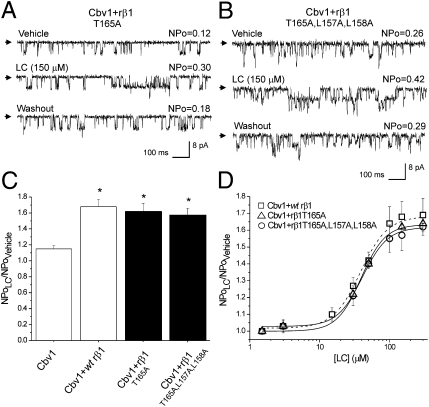
Fig. 3.
Ala substitutions in the proposed LC sensing site on the outer region of rβ1 TM2 domain do not ablate LC sensitivity of BK channels. Single-channel records from cbv1 + rβ1T165A (A) and cbv1 + rβ1T165A,L157A,L158A (B) channels show increases in BK NPo in response to LC. (C) Averaged responses to LC. *P < 0.05; significant from control (vehicle). (D) Concentration-response curves (CRCs) to LC on cbv1 coexpressed with WT rβ1, rβ1T165A, or rβ1T165A,L157A,L158A show similar characteristics: EC50 = 40.4, 39.3, and 40.8 μM, respectively, Emax ∼ 300 μM, and apparent Hill number (defined as the slope of the logit-log plot of LC action on channel steady-state activity) is ∼1.32 for each construct. Here and in all other figures, EC50 defines the LC required to obtain half-maximal increase in BK NPo by the steroid. Each point of CRC represents average from n ≥ 3.
To further test the role of the TM2 outer site in LC action, we introduced alanine to substitute for L157 and L158, the shorter alanine making it difficult for the TM2 outer site to make hydrophobic interactions with the LC steroid ring system (Fig. 1D). Lithocholic acid increased cbv1 + rβ1T165A,L157A,L158A NPo to ∼160% of control, a value that is indistinguishable from the value obtained with cbv1 + WT rβ1 channels. To further compare LC action on BK channel complexes containing WT rβ1 vs. rβ1 with amino acid substitutions in the TM2 outer site, we determined G/Gmax–V relationships in absence and presence of the steroid. Lithocholate (150 μM) evoked a parallel leftward shift in the G/Gmax–V plot of cbv1 + rβ1T165A,L157A,L158A macroscopic current over a wide voltage range of membrane voltages (from −60 to +70 mV) (SI Materials and Methods and Fig. S3). This outcome is identical to the outcome previously found with cbv1 + WT β1-complexes (7). Finally, all measured parameters of LC action on NPo from cbv1 + rβ1T165A and cbv1 + rβ1T165A,L157A,L158A channels, such as EC50, Emax, and apparent Hill number, were not significantly different from those parameters of cbv1 + WT rβ1 channels (P > 0.05) (Fig. 3D). The indistinguishable pharmacological profile of channels containing rβ1 TM2 outer site mutants vs. WT rβ1 when challenged with LC indicates that the proposed rβ1 TM2 outer site (Fig. 1D) does not play any significant role in LC activation of BK channels.
Proposed LC Docking Site in the Inner TM2 Region of BK β1 Provides Cholane Steroid Sensitivity to BK Channels.
Because the T165A,T169A double substitution totally abolished the LC sensitivity of rβ1 subunit-containing BK channels (Fig. 2 C and D), whereas cbv1 + rβ1T165A remained LC-sensitive (Fig. 3 A, C, and D), we hypothesized that the single, nonconserved rβ1T169A substitution would render BK channels LC-insensitive. Indeed, application of 150 μM LC onto cbv1 + rβ1T169A channels consistently failed to modify BK NPo (Fig. 4 A and D), despite the fact that the mutated β1 was still functionally coupled to cbv1 (Fig. S1B and Table S1). This result indicates that T169, a residue characteristic of BK β subunits of type 1, is necessary for LC recognition.
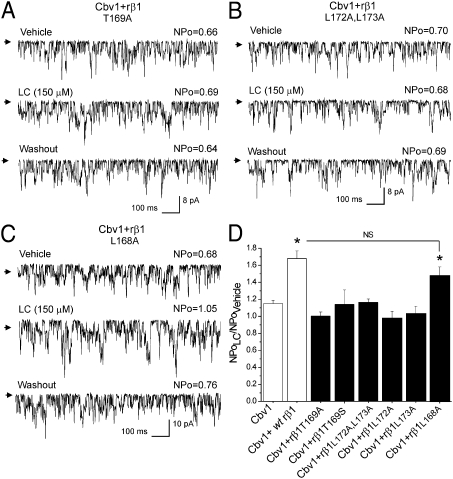
Fig. 4.
Amino acid substitutions in the proposed LC sensing site on the inner region of rβ1 TM2 domain ablate LC sensitivity. Records from cbv1 + rβ1T169A (A) and cbv1 + rβ1L172A,L173A (B) show LC-insensitive currents, whereas cbv1 + rβ1L168A (C) channels retain LC sensitivity. (D) Averaged responses to LC. NS, no significant difference (P > 0.05). *P < 0.05; significant from control (vehicle).
The suppression of LC action by a threonine to alanine substitution is consistent with our computationally proposed model, whereby threonine interacts with LC by hydrogen bonding to the α-hydroxyl located at C3 of the steroid at an average distance of 2.98 ± 0.60 Å over three separate molecular dynamics (MD) simulations (Fig. 1E and Table 1). This conclusion is also supported by previous SAR studies showing that deletion of the LC hydroxyl or its substitution with a bulky and ionized group (SO42−) ablates LC activation of BK channels (11, 13). Thus, to further test the importance of hydrogen bonding between steroid and position 169 within the β1 TM2, we next introduced the conserved substitution threonine to serine. These amino acids share the polar and uncharged hydroxyl in their side chain, but threonine possesses an extra methyl group. In only 3 of 11 patches, cbv1 + rβ1T169S channels were activated by LC, and average NPo values were not significantly larger than those values of cbv1 in the presence of the steroid (Fig. 4D). Diversity in LC sensitivity could be interpreted in terms of heterogeneous coupling between cbv1 and rβ1T169S subunits (i.e., effective and ineffective coupling would render LC sensitivity and LC resistance, respectively.) However, regardless of whether they were LC-sensitive or not, cbv1 + rβ1T169S currents consistently (11 of 11 patches) showed slow activation kinetics (Table S1), a characteristic of functional coupling between β1 and BK channel-forming subunits. As an alternative or complementary to the disrupted intersubunit-coupling hypothesis, diversity in the LC sensitivity of cbv1 + rβ1T169S constructs is very likely explained by the ligand–receptor interactions themselves, which differed from the interactions between LC and cbv1 + WT rβ1 as shown by MD simulations. First, throughout the course of MD simulations, the distances between the two heavy (oxygen) atoms of the C3 hydroxyl in LC and the hydroxyl group of the residue at position 169 are remarkably longer for serine than threonine; 4.10 vs. 2.98 Å average distances (Table 1). Thus, the cbv1 + rβ1T169S construct may have a reduced ability to form hydrogen bonds with LC C3 hydroxyl compared with cbv1 + WT rβ1 with its threonine at position 169. Second, according to our model for LC interaction with the β1 TM2 inner site (Fig. 1E), hydrophobic interactions by L172 and/or L173 are necessary for the steroid–protein interaction. Table 1 indicates that the rβ1 TM2 domain containing S169 is unlikely to form tight hydrophobic interactions with the LC steroid rings, because the distance between LC steroid rings and the nearest atom from L172 is two times as far in rβ1 T169S as in WT rβ1 TM2 (5.13 vs. 2.45 Å) (Table 1).
Table 1.
Computationally predicted distances between points of BK β1 TM2 and steroid (LC) interaction
| Distances (Å) |
|||
| Construct | Between C3 hydroxyl (oxygen) and T/S169 hydroxyl (oxygen) | Between LC steroid ring and nearest L/A172 atom | Between LC steroid ring and nearest L/A173 atom |
| wt rβ1 TM2 (T169) | 2.98 ± 0.60 | 2.45 ± 0.29 | 2.46 ± 0.27 |
| rβ1T169S | 4.10 ± 1.02 | 5.13 ± 1.66 | 2.34 ± 0.21 |
| rβ1L172A | 4.50 ± 1.35 | 5.38 ± 2.43 | 2.31 ± 0.22 |
| rβ1L173A | 4.33 ± 1.55 | 3.95 ± 2.49 | 3.15 ± 1.11 |
However, coexpression of cbv1 with rβ1L172A,L173A constructs rendered functional β1 subunit–containing BK channels (Table S1) that were totally insensitive to LC (Fig. 4 B and D). These data indicate that Leu-172 and/or -173 are indeed required to ensure robust activation of BK channels by LC. Thus, as suggested by MD simulations, hydrogen bonding by T169 and a tight coupling between the steroid concave hydrophobic hemisphere and long hydrophobic residue(s) (e.g., Leu) are both necessary for successful LC sensing by β1 TM2 and eventual BK current potentiation. Consistently, complexes made of cbv1 and either rβ1L172A or rβ1L173A were both LC-insensitive (Fig. 4D). Our MD simulations show increased contact distances between the steroid ring system and the side chain at position 172 for both L172A and L173A relative to WT (5.38 and 3.95 vs. 2.45 Å) as well as much greater standard deviations (SDs) during the simulations (L172A and L173A showed SDs greater than 2 Å in contrast to WT, which showed an SD less than 0.3 Å) (Table 1). Thus, L172 and L173 may be involved in steroid sensing and stabilization by van der Waals interactions with the steroidal ring system. In addition, both L172A and L173A constructs presented remarkable increase in distance between heavy (oxygen) atoms of Thr169 hydroxyl and C3-hydroxyl of LC (from 2.98 Å in WT TM2 domain up to 4.3–4.5Å in mutants) (Table 1). Although on numerous occasions, hydrogen bonding between T169 and the LC C3-hydroxyl was observed throughout the simulations, longer distances between critical polar groups preclude formation of steady hydrogen bonding.
Another hydrophobic residue that could contribute to stabilization of LC within the rβ1 TM2 inner recognition site is L168. According to the MD simulations, this residue appears occasionally in the vicinity of the LC A ring (Fig. 1E) and thus, may provide a site of hydrophobic interaction with the steroid molecule. However, expression of cbv1 + rβ1L168A rendered currents that were consistently LC-sensitive (Fig. 4 C and D). Whereas somewhat smaller, current activation by LC was statistically indistinguishable from the activation of cbv1 + WT rβ1 (P = 0.12) (Fig. 4D). This result indicates that a leucine at position 168 is not crucial for LC activation of BK channels.
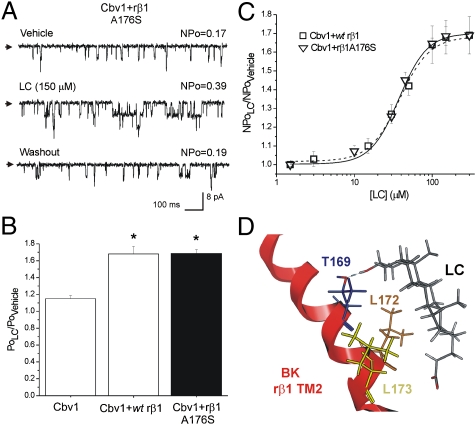
Finally, our computational model revealed the side chains of M177, A176 (Fig. 1E), and on very few occasions, I175 in the vicinity of the LC side chain. However, the LC side chain is considered to have a high degree of steric freedom, with a significant proportion of the carboxylate in ionized form. Moreover, we speculated that C24 carboxyl should be located at lipid bilayer–water interface for correct LC insertion into the membrane (13). Thus, the nature of the residue side chain at position 176 would not play a critical role in LC-BK channel interaction. Indeed, substitution of hydrophobic alanine at position 176 for polar serine failed to alter LC action: steroid activation of cbv1 + rβ1A176S constructs was indistinguishable from the activation of cbv1 + WT rβ1 (Fig. 5 A–C).
Fig. 5.
The nonconservative substitution A176S in the inner region of rβ1 TM2 domain does not ablate LC sensitivity of BK channels. Proposed model for the BK β1 TM2 domain-steroid (LC) interaction. (A) Unitary current records from cbv1 + rβ1A176S channels show increase in NPo in response to LC. (B) Averaged responses to LC. *P < 0.05; significant from control (vehicle). (C) CRCs for LC applied to cbv1 coexpressed with either WT rβ1 (dash line) or rβ1A176S show similar characteristics: EC50 = 40.4 and 37.4, respectively, Emax ∼ 300 μM, and apparent Hill number (defined as the slope of the logit-log plot of LC action on channel steady-state activity) is ∼1.32 for each construct. (D) Model of BK β1 TM2 domain steroid (LC) interaction. Hydrogen bond between TM2 Thr169 and C3-hydroxyl of LC is indicated as a blue dashed line.
In summary, our computational modeling and mutagenesis studies of the inner region of BK rβ1 TM2 indicate that the cluster T169,L172,L173 is necessary for LC activation of BK channels (Fig. 5D). Thus, the site provides steroid recognition by three critical points: hydrogen bonding between T169 and the LC C3 α-hydroxyl and tight hydrophobic interactions between L172, L173, and LC steroid rings. The fact that all three contacts are required highlights the amphiphilic character that agonists such as LC must have to activate BK channels by docking onto this site.
Discussion
Modulation of BK channel function by physiologically relevant steroids is increasingly recognized (5–7). Because BK accessory β subunit expression is highly tissue-specific (1), the fact that most physiological steroids can differentially activate BK complexes that contain different β subunits (6, 8) acquires special relevance. First, understanding the molecular basis of BK β-steroid interactions will enable us to define the precise role of BK channels in steroid modulation of tissue-specific physiology and disease. Second, such knowledge will allow investigators to design novel pharmacological agents that target BK channels and thus, alter biology in a tissue-specific manner. This research, however, has remained stalled, because steroid interaction sites in BK subunits have not been structurally defined. Here, we have identified and characterized in detail the cholane steroid recognition site on the BK β1 subunit.
To do so, we used a combination of computational modeling, mutagenesis, and patch-clamp electrophysiology. A similar approach has been previously used to document steroid-sensing domains in ion channel-forming subunits. Thus, a neurosteroid binding site on GABAA receptor (16), cholesterol recognition amino acid consensus on several membrane-spanning proteins (17), including the BK channel-forming cbv1 subunit,* and the critical role of Kir2.1 channel CD loop in cholesterol sensing (18) have been proposed. Our study, however, maps the steroid-sensing site not to the channel-forming subunit of the BK protein complex but to its accessory β1 subunit. More precisely, the cholane steroid recognition site responsible for LC activation of BK channels involves T169, L172, and L173 in the inner region of β1 TM2, a triplet that is characteristic of BK β subunits of type1.
Based on SAR studies (11, 13), we initially generated two computational models for LC docking, one located at the outer region and the other at the inner region of β1 TM2 (Fig. 1 D and E). Both models explain the significant difference in efficacy of several LC analogs to activate BK channels (13). For instance, neither model could successfully accommodate epialloLC, which was found totally ineffective in increasing BK NPo (13). Present mutagenesis data show that, although T169, L172, and L173 are all necessary for LC sensing and channel activation, none of these residues is sufficient to support steroid action (Figs. 4 and 5). In contrast, the TM2 β1 outer site, which includes L157, L158, and T165, plays no noticeable role in LC activation of BK channels (Fig. 3).
The combination of computational molecular dynamics and amino acid substitution with residues of variant length and/or chemical nature allowed us to pinpoint not only the residues that interact with the LC molecule resulting in BK current potentiation but also the steric requirements and nature of intervening chemical interactions between LC and the critical steroid sensing residues (Fig. 5D and Table 1). This information is of paramount importance to understand the selective sensing of cholanes vs. other physiological steroids and the design of nonsteroid pharmaceutical agents that selectively target β1-containing BK channels (see below).
Besides cholanes, several physiologically relevant steroids, including estrogens (5), androgens, and glucocorticoids (6), have been reported to increase the activity of β1-containing BK channels. Thus, the question arises whether these steroids could also be recognized by the steroid-sensing site identified in our study and thus, lead to increased BK activity. The structural constraints identified in our present study strongly suggest that this is not the case. The molecule of LC and other naturally occurring cholanes has a characteristic bean-like shape (19). This shape is particularly prominent in LC, with its C3 hydroxyl in α-configuration (i.e., pointing to the concave α-plane of the molecule) and a cis junction between steroidal rings A/B (Fig. 1 D and E). Suppression of either feature ablated BK channel activation by cholanes. Moreover, using closely related structural analogs of LC, we showed that the efficacy of cholanes to activate BK channels is a direct function of the ability of the steroid to match the LC molecule shape (13). Remarkably, none of the physiological steroids mentioned above that activate BK channels fulfill the two structural requirements or have a clearly defined bean-like shape of the molecule.
Another important feature of cholanes is the distance between their carboxylate at the terminal C24 in the side chain to their α-hydroxyl at C3 in ring A: ∼11 Å. Our model places the C24 carboxylate at the lipid bilayer–cytosolic aqueous interface, which allows the LC α-hydroxyl to hydrogen bond with T169, a residue that is absolutely necessary for cholane steroid action on BK channels (Fig. 4). The importance of such distance in activation of β1-containing BK channels is underscored by data from hydroxy-alkynoic acids specifically designed to mimic major features of LC: when the molecule is too long or too short, BK optimal activation could not be evoked (20). The presence of different functional groups and distances among them make it rather unlikely that physiological steroids other than cholanes would effectively reach β1 T169 and thus, activate BK channels by docking onto the site identified in the present study. However, systematic testing of steroids other than cholanes on BK channels where the LC sensing site is disrupted by the amino acid substitutions used in our study will certainly shed light on tissue-specific actions of physiologically relevant steroids by modification of BK channel function, meriting formal systematic testing in future studies.
As mentioned above, T169 seems to be unique to BK β subunit of type 1 and common to several species, including humans (4). Thus, the structural constraints for LC docking onto the BK β1 TM2 site open a venue for the rational design of activators of β1-containing BK channels. Considering the key role of BK β1 in determining myogenic tone, such compounds would potentially be beneficial within a wide clinical spectrum of prevalent human conditions associated with spasm/constriction of smooth muscle, including bronchial asthma, systemic hypertension, and cerebral vasospasm.
Materials and Methods
cRNA Preparation and Injection into Xenopus laevis Oocytes.
Cbv1 (AY330293) and rβ1 (FJ154955) cDNA cloning, cRNA preparation, and ion current phenotyping were performed as described (21, 22). Targeted mutations were introduced into WT BK β1 cDNA inserted in pOX using overlap-extension PCR and the Quickchange kit with pfu polymerase (Stratagene) following the manufacturer's instructions. Presence of targeted mutations and absence of unintended mutations in the PCR-amplified regions of all constructs were verified by automated sequencing (Molecular Resource Center, University of Tennessee Health Science Center). cRNA of the β1-mutants was prepared as described for WT rβ1.
cRNA was dissolved in diethyl polycarbonate-treated water at 10 (cbv1) and 30 (rβ1) ng/μL; 1-μL aliquots were stored at −70 °C. Oocytes were removed from Xenopus laevis (Xenopus Express), cRNA-injected, and prepared for patch-clamping as described elsewhere (8, 23). The interval between cRNA cytosolic injection and patch-clamping was 36–48 h.
Electrophysiology Data Acquisition and Analysis.
Currents were recorded from excised, inside-out patches. Bath and electrode solutions contained 130 mM Kgluconate, 5 mM EGTA, 1.6 mM HEDTA, 2.28 mM MgCl2 ([Mg2+]free = 1 mM), 15 mM Hepes, 5.22 mM CaCl2 ([Ca2+]free = 10 μM), pH 7.35. Free Ca2+ was calculated with MaxChelator Sliders (Stanford University) and validated experimentally using Ca2+-selective and reference electrodes (Corning).
Patch-recording electrodes were made as described previously (23). An agar bridge with gluconate as the main anion was used as ground electrode. Solutions were applied onto the cytosolic side of excised patches using an automated, pressurized DAD12 system (ALA) through a micropipette tip with an internal diameter of 100 μm. Experiments were carried out at room temperature (20–22 °C).
Ionic current was recorded using an EPC8 amplifier (HEKA) at 1 kHz. Data were digitized at 5 kHz using a Digidata 1320A A/D converter and pCLAMP 8.0 (Molecular Devices). As an index of channel steady-state activity, we used the product of the number of channels in the patch (N) and channel open probability (Po). NPo was obtained using a built-in option in Clampfit 9.2 (Molecular Devices) from ≥30 s of gap-free recording under each condition.
Plotting, fitting, and statistical analysis of the data were conducted using Origin 7.0 (Originlab) and InStat 3.0 (GraphPad). Statistical analysis was conducted using one-way ANOVA and Bonferroni multiple comparison test; significance was set at P < 0.05. Data are expressed as mean ± SEM; n = number of patches (each patch was obtained from a different oocyte).
Chemicals and Compound Application.
5β-Cholanic acid-3α-ol (LC) was purchased from Steraloids. All other chemicals were purchased from Sigma. On the day of the experiment, a stock solution (333 mM) of LC was freshly made in DMSO by sonication for 5 min. Steroid-containing stock solution was diluted 1/10 in 95% ethanol and further diluted with bath solution to final cholane concentration (150 μM). The DMSO/ethanol vehicle in bath solution (0.05/0.45% final concentrations) was used as control perfusion.
Computer Modeling of Steroid–BK rβ1 Complexes.
Computer modeling of LC (nonionized form) 3D structure was performed as described (13). Rat cerebral artery myocyte BK rβ1 (FJ154955) protein sequence was aligned with Homo sapiens hβ1 (U38907). The polypeptides extending from BK rβ1 residue L157 to V178, which correspond to BK rβ1 TM2 (4), were modeled as an ideal α-helix and optimized with the AMBER99 force field to a root mean square gradient (RMSG) of 0.1 kcal·mol−1·Å−1 (24). The dielectric constant for the model was set at three, and this value was appropriate for the membrane lipid interior (25). LC was manually placed onto the two proposed docking sites in BK rβ1 TM2. Then, the two LC-BK rβ1 complexes were optimized with computational energy minimization routine using the MM94 force field (26). A similar procedure was used to generate molecular complexes between LC and BK rβ1s that contained T169S, L172A, or L173A substitutions. For each complex, at least three MD simulations were performed using MM94 force field (26). Each MD simulation was run for 500 ps using 1-ps steps. Starting LC-BK rβ1 complexes were heated from 0 to 300 °K within the first 100 ps of the simulation, with stability and structural changes of each complex over time being assessed throughout the 101–500 ps (production phase). All procedures were performed using MOE 2010.10 (Chemical Computing Group).
Supplementary Material
Acknowledgments
The authors thank Maria Asuncion-Chin for excellent technical assistance. Thanks to the Chemical Computing Group for the MOE program (A.L.P.). This work was supported by University of Tennessee Health Science Center Neuroscience Institute Postdoctoral Fellowships (to A.N.B. and A.K.S.) and National Institutes of Health Grant HL10463-01 (to A.M.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
*Singh A, Bukiya A, Dopico A. Biophysical Society 55th Annual Meeting, abstr 3158.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112901108/-/DCSupplemental.
References
- 1.Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel beta-subunits. News Physiol Sci. 2002;17:156–161. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- 2.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 3.Lu R, et al. MaxiK channel partners: Physiological impact. J Physiol. 2006;570:65–72. doi: 10.1113/jphysiol.2005.098913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem. 2000;275:6453–6461. doi: 10.1074/jbc.275.9.6453. [DOI] [PubMed] [Google Scholar]
- 5.Valverde MA, et al. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science. 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- 6.King JT, et al. Beta2 and beta4 subunits of BK channels confer differential sensitivity to acute modulation by steroid hormones. J Neurophysiol. 2006;95:2878–2888. doi: 10.1152/jn.01352.2005. [DOI] [PubMed] [Google Scholar]
- 7.Bukiya AN, Liu J, Toro L, Dopico AM. Beta1 (KCNMB1) subunits mediate lithocholate activation of large-conductance Ca2+-activated K+ channels and dilation in small, resistance-size arteries. Mol Pharmacol. 2007;72:359–369. doi: 10.1124/mol.107.034330. [DOI] [PubMed] [Google Scholar]
- 8.Bukiya AN, Vaithianathan T, Toro L, Dopico AM. Channel beta2-4 subunits fail to substitute for beta1 in sensitizing BK channels to lithocholate. Biochem Biophys Res Commun. 2009;390:995–1000. doi: 10.1016/j.bbrc.2009.10.091. [DOI] [PubMed] [Google Scholar]
- 9.Pak JM, Lee SS. Vasoactive effects of bile salts in cirrhotic rats: In vivo and in vitro studies. Hepatology. 1993;18:1175–1181. [PubMed] [Google Scholar]
- 10.Bomzon A, Ljubuncic P. Bile acids as endogenous vasodilators? Biochem Pharmacol. 1995;49:581–589. doi: 10.1016/0006-2952(94)00428-o. [DOI] [PubMed] [Google Scholar]
- 11.Dopico AM, Walsh JV, Jr, Singer JJ. Natural bile acids and synthetic analogues modulate large conductance Ca2+-activated K+ (BKCa) channel activity in smooth muscle cells. J Gen Physiol. 2002;119:251–273. doi: 10.1085/jgp.20028537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bukiya AN, Vaithianathan T, Toro L, Dopico AM. The second transmembrane domain of the large conductance, voltage- and calcium-gated potassium channel beta(1) subunit is a lithocholate sensor. FEBS Lett. 2008;582:673–678. doi: 10.1016/j.febslet.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bukiya AN, McMillan J, Parrill AL, Dopico AM. Structural determinants of monohydroxylated bile acids to activate beta 1 subunit-containing BK channels. J Lipid Res. 2008;49:2441–2451. doi: 10.1194/jlr.M800286-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508:199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pérez GJ, Bonev AD, Nelson MT. Micromolar Ca(2+) from sparks activates Ca(2+)-sensitive K(+) channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol. 2001;281:C1769–C1775. doi: 10.1152/ajpcell.2001.281.6.C1769. [DOI] [PubMed] [Google Scholar]
- 16.Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 17.Epand RM. Cholesterol and the interaction of proteins with membrane domains. Prog Lipid Res. 2006;45:279–294. doi: 10.1016/j.plipres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Epshtein Y, et al. Identification of a C-terminus domain critical for the sensitivity of Kir2.1 to cholesterol. Proc Natl Acad Sci USA. 2009;106:8055–8060. doi: 10.1073/pnas.0809847106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carey MC. In: Sterols and Bile Acids. Danielsson H, Sjovall J, editors. Amsterdam: Elsevier Science; 1985. pp. 346–347. [Google Scholar]
- 20.Patil S, Bukiya AN, Li W, Dopico AM, Miller D. Design and synthesis of hydroxy-alkynoic acids and their methyl esters as novel activators of BK channels. Bioorg Med Chem Lett. 2008;18:3427–3430. doi: 10.1016/j.bmcl.2008.03.080. [DOI] [PubMed] [Google Scholar]
- 21.Jaggar JH, et al. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res. 2005;97:805–812. doi: 10.1161/01.RES.0000186180.47148.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bukiya AN, Liu J, Dopico AM. The BK channel accessory beta1 subunit determines alcohol-induced cerebrovascular constriction. FEBS Lett. 2009;583:2779–2784. doi: 10.1016/j.febslet.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dopico AM, Anantharam V, Treistman SN. Ethanol increases the activity of Ca(++)-dependent K+ (mslo) channels: Functional interaction with cytosolic Ca++ J Pharmacol Exp Ther. 1998;284:258–268. [PubMed] [Google Scholar]
- 24.Wang J, Cieplak P, Kollman PA. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J Comput Chem. 2000;21:1049–1074. [Google Scholar]
- 25.Cornell BA, Krishna G, Osman PD, Pace RD, Wieczorek L. Tethered-bilayer lipid membranes as a support for membrane-active peptides. Biochem Soc Trans. 2001;29:613–617. doi: 10.1042/bst0290613. [DOI] [PubMed] [Google Scholar]
- 26.Halgren TA. Merck molecular force field. J Comput Chem. 1996;17:490–641. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.