Abstract
Taste and most sensory inputs required for the feedback regulation of digestive, respiratory, and cardiovascular organs are conveyed to the central nervous system by so-called “visceral” sensory neurons located in three cranial ganglia (geniculate, petrosal, and nodose) and integrated in the hindbrain by relay sensory neurons located in the nucleus of the solitary tract. Visceral sensory ganglia and the nucleus of the solitary tract all depend for their formation on the pan-visceral homeodomain transcription factor Phox2b, also required in efferent neurons to the viscera. We show here, by genetically tracing Phox2b+ cells, that in the absence of the protein, many visceral sensory neurons (first- and second-order) survive. However, they adopt a fate—including molecular signature, cell positions, and axonal projections—akin to that of somatic sensory neurons (first- and second-order), located in the trigeminal, superior, and jugular ganglia and the trigeminal sensory nuclei, that convey touch and pain sensation from the oro-facial region. Thus, the cranial sensory pathways, somatic and visceral, are related, and Phox2b serves as a developmental switch from the former to the latter.
Keywords: neuronal differentiation, Brn3a/Pou4f1
The activity of the viscera (including the respiratory, digestive, and cardiovascular organs) is regulated by a core set of reflex circuits passing through the hindbrain. The sensory pathway consists of first-order visceral sensory neurons (including chemoreceptors, baroreceptors, osmoreceptors, and neurons innervating the taste buds) located in three cranial ganglia (geniculate, petrosal, and nodose) and their target neurons in the hindbrain that form the nucleus of the solitary tract (nTS, for Nucleus Tractus Solitarius). The efferent pathway consists in the preganglionic (or VM for visceromotor) and postganglionic neurons of the sympathetic, parasympathetic, and enteric nervous systems, and the branchiomotor (BM) neurons (respiratory in fish and closely related ontogenetically and phylogenetically to VM neurons). All these neuronal types (except preganglionic sympathetic neurons, located in the spinal cord) depend for their differentiation on a dedicated homeobox gene, Phox2b, which is therefore a master gene of the visceral nervous system (VNS) (1).
The mode of action of Phox2b differs between the motor and sensory neurons of the VNS. In the motor pathways (i.e., in BM and VM neurons of the hindbrain and in autonomic ganglion cells), Phox2b is switched on in dividing progenitors (2, 3). In its absence, progenitors either switch fate and produce another type of neurons (as in the case of VM neuron progenitors, which prematurely produce serotonergic neurons in Phox2b knockout embryos) (4), or die early on, before any neuronal differentiation has occurred (as in the case of autonomic ganglia) (2).
In the sensory pathways, the fate of neurons in the absence of Phox2b has been only superficially characterized. First-order visceral sensory neurons of the geniculate, petrosal, and nodose ganglia are born in epibranchial placodes (5). The placodal cells express Phox2a (the paralogue of Phox2b) and switch on Phox2b as they form the ganglia (6) and become postmitotic (7). In Phox2b null embryos, the cells still form ganglia but lose Dopamine-β-hydroxylase expression at embryonic day (E)9.5 and show attenuated expression of the tyrosine kinase receptor Ret at E10.5 (2). At E11.5, they are capable of projecting fibers to the periphery (8). At E13.5, the ganglion cells are fewer than in the wild type (9), they have turned off the Phox2b locus as assessed by lacZ expression from the Phox2b locus in Phox2bLacZ/LacZ embryos, but still express peripherin (9). Therefore, a contingent of epibranchial ganglion cells acquire a neuronal identity in the absence of Phox2b and survive, at least until midgestation.
In the hindbrain, relay sensory neurons of the nTS are born from E9.5 to E13.5 (10) in the domain of dA3 progenitors, which extends from rhombomere (r) 4 to r7 (11, 12) and switch on Phox2b postmitotically (9). In homozygous Phox2b null embryos, nTS precursors switch on Lmx1b and Rnx/Tlx3 and start migrating ventrally (9)—as they do in heterozygous or wild-type embryos (9, 12). However, at E12.5, most become undetectable using LacZ as a reporter (9). At the end of gestation, a loss of tissue is detected where the nTS should be, but no sign of cell death precedes it, and some LacZ-positive cells are found misplaced in the ventrolateral medulla (9), hinting at a fate switch.
Here, we reexamine the fate of prospective first- and second-order sensory visceral neurons (i.e., epibranchial and nTS neurons) in Phox2b null mutants, using a Phox2b::Cre transgene to permanently label cells once they have switched on the Phox2b locus.
Results
Survival of Visceral Sensory Neurons in Phox2b Null Mutants.
To follow the fate of visceral sensory neuronal precursors, we produced a mouse in which Phox2b+ cells and their progeny are irreversibly labeled by the action of the cre recombinase (SI Materials and Methods). When the Phox2b::Cre line was crossed with a conditional YFP reporter (13), the Phox2b::Cre;ROSAstoploxYFP progeny (henceforth Phox2bCreYFP) showed YFP expression in all cells that express or have expressed Phox2b, or descend from Phox2b+ progenitors, in both the central nervous system (CNS) and peripheral nervous system (PNS) (Fig. S1). In the CNS, labeled cells included the (BM/VM) neurons (Fig. S1A), the nTS and area postrema (Fig. S1A), noradrenergic centers (Fig. S1 A and B), and serotonergic neurons (Fig. S1C) (strengthening earlier evidence that they arise from the same progenitors as BM/VM neurons; ref. 4). In the PNS, YFP+ cells included all epibranchial (Fig. S1D) and autonomic (Fig. S1 D and E) ganglia.
We then analyzed the fate of Phox2b+ cells and their progeny in the absence of Phox2b, by introducing the Phox2bCreYFP genotype in a Phox2b null background, i.e., in Phox2bCreYFP;Phox2bLacZ/LacZ (henceforth Phox2bCreYFPLacZ/LacZ) embryos.
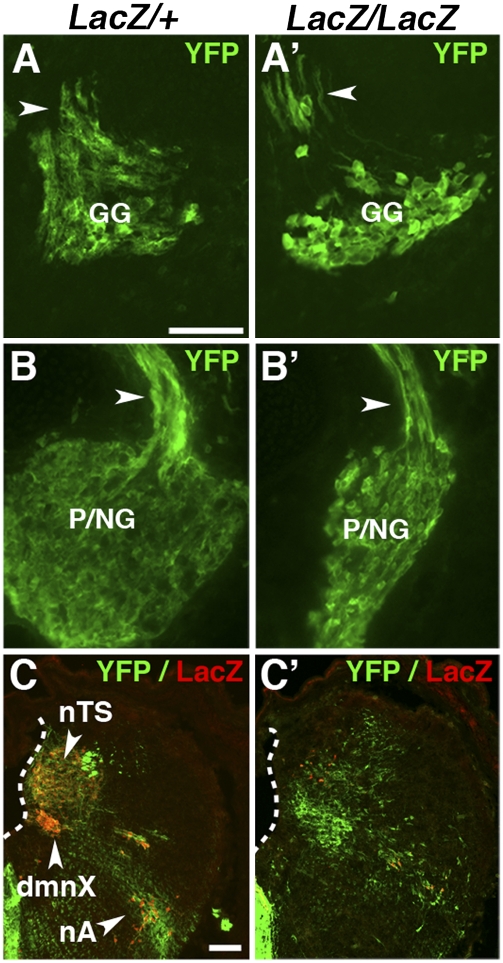
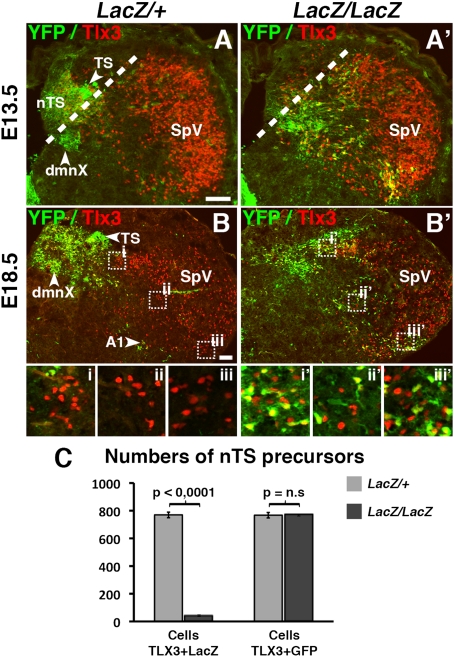
As expected from previous analyses (2, 3), autonomic ganglia and BM/VM neurons were undetectable by YFP expression in E13.5 Phox2bCreYFPLacZ/LacZ embryos (Fig. S2), whereas the epibranchial ganglia were present, albeit atrophic (Fig. 1 A–B′). Despite the absence of motor neurons, the nerve root of each ganglion contained YFP+ fibers (arrowhead in Fig. 1 A′ and B′), which were therefore sensory and attested to the capacity of the surviving ganglion cells to project to the CNS. In the caudal hindbrain, where all Phox2b+ (hence YFP+) interneurons normally contribute to the nTS (Fig. 1C), a large collection of YFP+ cells was preserved in the mutants (Fig. 1C′), albeit at a different location (see below), that was not detected by LacZ expression (Fig. 1C′). Thus, the disappearance of the LacZ signal in the nTS precursors of Phox2bLacZ/LacZ embryos (9) reflects the silencing of the Phox2b locus rather than cell death. These results demonstrate that precursors of the two components of the cranial visceral sensory pathways (i.e., epibranchial ganglia and the nTS) persist at least until midgestation in Phox2b null embryos.
Fig. 1.
Survival of afferent visceral neurons in the absence of Phox2b. (A–B′) Transverse sections of E13.5 Phox2bCreYFPLacZ/+ (A and B) and Phox2bCreYFPLacZ/LacZ (A′ and B′) embryos through the ganglia of the facial (A and A′), glossopharyngeal, and vagus nerves (B and B′) stained for YFP expression. The distal ganglia of the glossopharyngeal and vagus nerves (the petrosal and nodose ganglia) are fused at that age, forming the petrosal/nodose complex (P/NG). The roots of the ganglia contain YFP+ fibers in both genotypes (arrowheads). (C and C′) Transverse sections through the caudal hindbrain. Many YFP+ nTS precursors are present in the homozygous mutant, although they are misplaced and have lost LacZ expression. White outline, floor of the IVth ventricle. Note that the dorsal motor nucleus of the vagus nerve (dmnX) is absent in the homozygous mutant. (Scale bars: 100 μm.)
Changes in the Molecular Identity and Projections of Epibranchial Neurons in Phox2b Nulls.
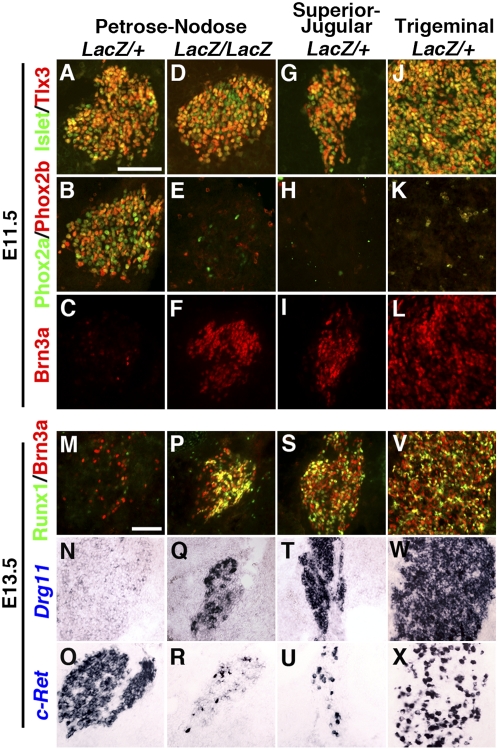
At E11.5, the molecular fingerprint of epibranchial ganglion cells [in the distal or geniculate ganglion of the facial nerve (Fig. S3) and in the petrosal and nodose ganglia (Fig. 2)] combined the homeobox genes Tlx3 (14) (Fig. 2A and Fig. S3 A and B), Islet1/2 (henceforth Islet) (15) (Fig. 2A and Fig. S3B), Phox2a (16), and Phox2b (6) (Fig. 2B and Fig. S3C). Expression of the POU-domain homeobox gene Brn3a/Pou4f1 was restricted to rare cells (Fig. 2C and Fig. S3D). In Phox2bCreYFPLacZ/LacZ embryos, the geniculate, petrosal, and nodose ganglion cells had changed their transcriptional code from Tlx3+/Islet+/Phox2b+/Phox2a+/Brn3a− to Tlx3+/Islet+/Phox2b−/Phox2a−/Brn3a+ (Fig. 2 D–F and Fig. S3 E–H), typical of the proximal ganglia of the VIIth, IXth, and Xth cranial nerves (Fig. 2 G–I and Fig. S3 A–H)—which convey sensibility of the external ear (17) or the pharynx (18) and references therein)—and of the trigeminal ganglia (Fig. 2 J–L)—which supply the somatosensory innervation of the face. Two days later, at E13.5, the mutant ganglia had aberrantly switched on Runx1, a target of Brn3a/b (19) (Fig. 2 M and P and Fig. S3 J and N), prolonged that of Drg11 (Fig. 2Q and Fig. S3O)—which is virtually shutdown at this stage in the controls (Fig. 2N and Fig. S3K) after its early wave of expression (20)—and converted that of Ret from homogenous (Fig. 2O and Fig. S3 L) to salt-and pepper (Fig. 2R and Fig. S3P). Again, this molecular profile was typical of somatic sensory neurons in the proximal ganglia of the same nerves (Fig. 2 S–U and Fig. S3 I–P) and in the trigeminal ganglia (Fig. 2 V–X). Both Runx1 and Drg11 are activated by Brn3a (21), and their ectopic or prolonged expression, respectively, in the visceral ganglia of Phox2b nulls, might follow from Brn3a derepression. Altogether these data show that in the absence of Phox2b, the visceral sensory neurons of cranial nerves VII, IX, and X acquire a molecular signature akin to that of somatic sensory neurons.
Fig. 2.
The visceral sensory ganglia of Phox2b null mutants adopt the molecular signature of somatic sensory ganglia. Transverse sections through the petrosal–nodose complex (A–F and M–R), the fused superior-jugular ganglia (G–I and S–U), and the trigeminal ganglion (J–L and V–X), at E11.5 or E13.5 as indicated on the left, of Phox2bLacZ/+ or Phox2bLacZ/LacZ embryos as indicated on top, stained with the antibodies and probes indicated on the left. (Scale bars: 100 μm.)
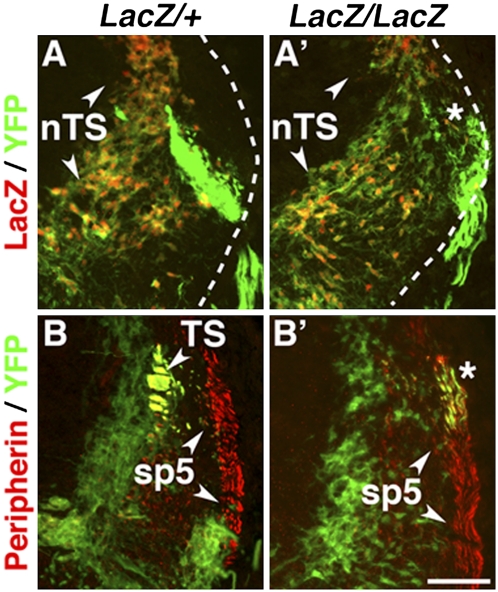
We next examined whether the molecular fate switch of visceral ganglionic neurons was matched by a congruent switch in their projection pattern. In wild-type E11.5 embryos, the central axons of epibranchial ganglia entered the CNS dorsally and reached the lateral border of the nTS (Fig. 3A) fasciculating there while veering to a rostro-caudal direction to form the solitary tract [tractus solitarius (TS)] (Fig. 3B). In contrast, in Phox2bCreYFPLacZ/LacZ embryos, YFP fibers accumulated at the surface of the medulla (asterisk in Fig. 3 A′ and B′), i.e., at the level of the spinal trigeminal tract (Fig. 3B′), and the solitary tract was absent (Fig. 3B′). Thus, the visceral neurons of Phox2b null mutants adopt a pattern of projections similar to that of somatic sensory neurons.
Fig. 3.
The visceral sensory ganglia of Phox2b null mutants adopt the central projection pattern of somatic sensory ganglia. Transverse sections through the hindbrain of E11.5 Phox2bCreYFPLacZ/+ (A and B) and Phox2bCreYFPLacZ/LacZ (A′ and B′) embryos at the level of the root of the vagus nerve (A and A′) and caudal to the vagal root (B and B′), stained with anti-GFP (green) and anti-LacZ (red) (A and A′) or anti-peripherin (red) (B and B′) antibodies. The central axons of epibranchial ganglia reach the nTS (A) and form the solitary tract (TS) (B) in the control, whereas they stay at the surface of the medulla (dotted line in A and A′) and join the spinal trigeminal tract in the mutant (asterisk in A′ and B′). Note that migrating nTS precursors (arrowheads in A and A′) are initially LacZ+/YFPweak and accumulate YFP as they migrate ventrally. nTS, nucleus of the solitary tract; TS, solitary tract; sp5, spinal trigeminal tract. (Scale bar: 100 μm.)
Change in Molecular Identity and Migration of nTS Neurons in Phox2b Nulls.
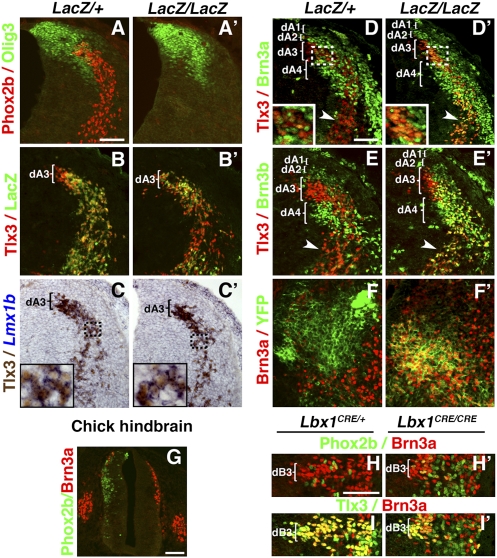
Precursors of the nTS are born from E9.5 to E13.5 (9) in the dA3 progenitor domain of the hindbrain (12) marked by the expression of the bHLH gene Olig3 (12) (Fig. 4A). As expected, Olig3 expression, restricted to dividing progenitors, was unaffected by the inactivation of Phox2b, which is switched on in postmitotic precursors (10) (Fig. 4 A and A′). In addition to Phox2b (Fig. 4 A and B) postmitotic nTS precursors, in the wild type, turn on Tlx3 (22,6) (Fig. 4 B and C), Lmx1b (9, 23) (Fig. 4C), and Drg11 (20) (Fig. S4A). In E11.5 Phox2b nulls, Tlx3 and Lmx1b expression was preserved (Fig. 4 B′ and C′) as reported (9), as well as that of Drg11 (Fig. S4A′). We examined the status of Pou4f1/Brn3a, and its paralogue Pou4f2/Brn3b, broadly expressed in dorsal interneurons of the hindbrain and spinal cord (12, 24) (Fig. S5). At E11.5, Brn3a and Brn3b were expressed in dA2 and dA4 neurons, whereas Brn3a alone was expressed in dA1 neurons (Fig. 4 D and E). The dA3 neurons had a unique status in that expression of Brn3a was fleeting and weak, soon extinguished along the ventral migration route (Fig. 4 D and D Inset) and that of Brn3b was undetectable (Fig. 4E). In contrast, in E11.5 Phox2bCreYFPLacZ/LacZ embryos, dA3 derivatives expressed Brn3a in a sustained way (Fig. 4D′) at least until E13.5 (Fig. 4 F and F′) and Brn3b was abnormally switched on (Fig. 4E′), making dA3 precursors, in that respect, indistinguishable from dA2 and dA4 precursors. Moreover, electroporation of Phox2b in the chick spinal cord led to down-regulation of Brn3a throughout the dorsal neural tube (Fig. 4G). Altogether, these data show that Phox2b directly or indirectly represses Brn3 genes, which mark other neuronal precursors born in the alar plate, including dB3 (Fig. 4 H and I) and dBLb precursors (Fig. S5) that normally contribute to the somatosensory principal and spinal nuclei of the trigeminal nerve (25). In Phox2b mutants, derepression of Brn3a/b combined with unchanged expression of Tlx3, Lmx1b, and Drg11 brought the code of dA3 interneuron precursors closest to that of dB3 interneuron precursors (11) (Fig. 4 H and I and Fig. S6). However, a distinction is introduced by the homeobox gene Lbx1, expressed by dB3 precursors in the wild type (11), but not by dA3 precursors in Phox2b nulls (Fig. S7). Removal of Lbx1 has been shown to trigger a reverse switch—bringing the dB3 code closest to that of dA3— through ectopic activation of Phox2b (11) and, as we now show, down-regulation of Brn3a (Fig. 4 H′ and I′ and Fig. S6). The latter might be a consequence of the former, as suggested by our Phox2b gain- and loss-of-function data.
Fig. 4.
Precursors of relay viscerosensory neurons adopt the molecular signature of relay somatosensory neurons in Phox2b null mutants. (A–F′) Transverse sections of the hindbrain at the level of r7 of Phox2bCreYFPLacZ/+ (A–F) and Phox2bCreYFPLacZ/LacZ mutants (A′–F′) at E11.5 (A–E′) or E13.5 (F and F′). (A and A′) Olig3+ dA3 progenitors, which give rise to postmitotic Phox2b+ neurons (A), keep Olig3 expression in the null mutants (A′). (B–C′) LacZ+ neurons born from dA3 coexpress Tlx3 and Lmx1b in heterozygous (B and C) and homozygous (B′ and C′) mutants. Note that LacZ expression is transient in the homozygotes (B′). (C Inset) High magnification of cells double-labeled for Tlx3 protein and Lmx1b mRNA. (D and D′) Brn3a, switched on in postmitotic neurons born in dA1–dA4, is extinguished in dA3 derivatives during their ventral migration in Phox2bLacZ/+ (arrowhead in D and D Inset) but not in Phox2bLacZ/LacZ embryos (arrowhead in D′ and D′ Inset). (E and E′) Brn3b is not expressed in dA3 derivatives of heterozygotes (arrowhead in E), but switched on in Phox2bLacZ/LacZ embryos (arrowhead in E′). (F and F′) At E13.5, Brn3a is still abnormally maintained in nTS precursors of Phox2bLacZ/LacZ. (G) Transverse section through a chicken spinal cord electroporated with Phox2b (on the left), showing down-regulation of Brn3a (red) in ectopic Phox2b+ cells (green). (H–I′) The dB3 cells express Brn3a (H and I) and Tlx3 (I) but not Phox2b (H) in Lbx1cre/+ heterozygotes. They lose expression of Brn3a (H′ and I′) as they acquire that of Phox2b (H′) in the homozygous Lbx1 nulls. (Scale bars: 100 μm.)
We next examined whether the changed molecular fingerprint of nTS precursors entailed a changed migration pattern. In r7, dA3 precursors normally migrate ventrally and forms the nTS in a region sandwiched dorsolaterally by the TS and ventromedially by the dorsal motor nucleus of the vagus nerve (dmnX) (Figs. 1C and 5 A and B). In contrast, in Phox2bCreYFPLacZ/LacZ E13.5 embryos, most nTS precursors overshoot the normal position of the nTS ventrally and laterally (Figs. 1 C and C′ and 5 A and A′). Using the Tlx3+/YFP+ signature, specific for nTS precursors in r7, we found that these cells were as numerous in the mutants as in the controls (Fig. 5C). At E18.5, they were further displaced in a fan-shaped pattern toward the pial surface, some cells settling at the dorsal or ventral edges of the spinal trigeminal nucleus (SpV) (Fig. 5 B, B′, and Insets). However, for the majority of cells, the final position, displaced toward the SpV but not identical to it, might reflect residual genetic differences between the fate-switched dA3 and SpV precursors, possibly related to the differential expression of Lbx1 or other unknown factors.
Fig. 5.
Altered migration of nTS precursors in Phox2b null mutants. (A–B′) Transverse section through the caudal hindbrain of Phox2bCreYFPLacZ/+ (A and B) and Phox2bCreYFPLacZ/LacZ (A′ and B′) mutants stained for YFP and Tlx3 at ages indicated on the left. The left edge of the image corresponds to the midline. (A and A′) At E13.5, the majority of YFP+ cells in Phox2bCreYFPLacZ/LacZ embryos (A′) have migrated beyond a ventro-lateral limit (dotted line) that encloses most of the wild-type nTS (A). (B and B′) At E18.5, the ectopic migration of nTS precursors in homozygous mutants is more pronounced, bringing them toward the edges of the spinal trigeminal nucleus (SpV). Close ups are shown of the dorsal (i and i′), medial (ii and ii′), or ventral (iii and iii′) edge of the Tlx3+/YFP— SpV, where Tlx3+/YFP+ cells are found in the null mutant. (C) Cell counts at E13.5 showing that the majority of dA3 derivatives (i.e., Tlx3+/YFP+ cells) survive in the Phox2bCreYFPLacZ/LacZ mutants, although they have lost LacZ expression. (Scale bars: 100 μm.)
Intrinsic Requirement for Phox2b in the Migration of nTS Cells and the Projection of Epibranchial Neurons.
Because epibranchial and nTS neurons are synaptic partners and depend both on Phox2b, the projection defects of the former or the migration defects of the latter in Phox2b nulls could be non–cell-autonomous.
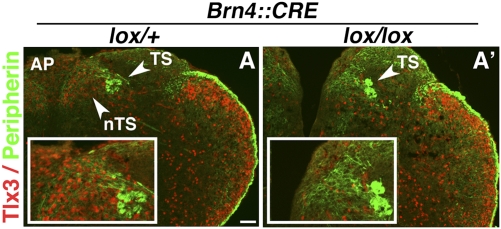
To address this issue, we restricted the inactivation of Phox2b to the CNS by partnering the Phox2b conditional null allele (8) with a Cre driven by the pan-CNS transcription factor Brn4 (26). In Brn4::Cre;Phox2blox/lox embryos, the TS was properly fasciculated and positioned (Fig. 6 A and A′), implying that it requires Phox2b cell-autonomously, but not in its target, the nTS. Conversely, the nTS had not formed at its proper location (Fig. 6 A, A′, and Insets) and underwent the same fate switch as in null embryos (Fig. S8 A–B′), implying that Phox2b has a cell-autonomous role in the nTS. One caveat is that the dmnX, whose precursors also depend on Phox2b (3) and migrate dorsally to settle ventral to the nTS in its caudal aspect (9), could play a role in the delimitation of the latter. To rule out this possibility, we deleted the dmnX by partnering the Phox2b floxed allele with a Nkx6.2::Cre allele (27), a combination that left the nTS morphologically intact (Fig. S8 C and C′). Altogether, these data show that Phox2b is required cell-autonomously in epibranchial ganglia for the formation of the TS and in nTS precursors for their proper spatial arrangement. In nTS formation, we cannot formally exclude an additional role for the TS, the specific deletion of which requires genetic tools currently unavailable.
Fig. 6.
Inactivation of Phox2b specifically in the CNS preserves the TS while disrupting the nTS. Transverse sections through the caudal hindbrain of Brn4::Cre;Phox2blox/+ (A) and Brn4::Cre;Phox2blox/lox (A′) embryos at E18.5. (Insets) Close up of the nTS region showing Tlx3+ nTS cells in the heterozygous but not in the homozygous mutant. Note that this region is invaded by YFP+ fibers coming from the TS in both genotypes, thus, even in the absence of the normal synaptic targets. (Scale bar: 100 μm.)
Discussion
Phox2b is a global determinant of visceral circuits (1, 28). Its mode of action, however, varies with neuronal types. In BM/VM progenitors, it acts as a stem cell fate switch, instructing them to produce BM/VM rather than serotonergic neurons (4). In autonomic ganglia, where differentiation proceeds alongside proliferation (29), Phox2b specifies generic and type-specific traits and is required for survival (2). In sensory neurons, we unveil a third mode of action, whereby Phox2b imposes a visceral fate on postmitotic precursors and suppresses a somatic one. Thus, throughout the evolution that gave rise to different types of visceral neurons, Phox2b was recruited to different nodes in the genetic architecture of cell differentiation, while maintaining control over a visceral program.
Ontogenetic Relatedness of Sensory Neurons.
Knowledge of the ontogeny of sensory neurons, both peripheral (i.e., first-order sensory neurons) and central (i.e., relay or higher order) is still fragmentary. Nevertheless, elements of a large-scale coherence have begun to emerge, consolidated by the present findings.
Several transcription factors are broadly, if sometimes transiently, expressed in primary and/or relay sensory neurons. In the PNS, Tlx3, Drg11, and Islet1 are expressed in all sensory ganglia, visceral and somatic (14, 20, 30, and this work). In the CNS, Tlx3, Drg11, and Lmx1b are expressed in the precursors of the nTS (9, 20, 22), spinal and principal trigeminal nuclei (11, 20, 31, 32), spinal dorsal horn interneurons (20, 23, 33). The expression of these transcription factors is neither strictly specific for sensory neurons [e.g., Tlx3 is expressed in sympathetic ganglia (14) and Lmx1b in serotonergic neurons (34)] nor inclusive of all sensory neurons, but largely biased toward them. None distinguishes somatic from visceral neurons (except for the shorter expression window of Drg11 in visceral than in somatic primary sensory neurons). The substantial overlap of transcriptional code among sensory neurons suggests that many are related in development and evolution and represent variations on a generic sensory neuronal type. Pan-sensory effector genes such as the noxious stimulus receptors TrpV1 (35) or Nav1.8 (36) also point to the existence of a generic sensory phenotype and might lie downstream of the shared transcription factors.
In this proposed generic type, two transcription factors set visceral and somatic neurons apart: Brn3a, expressed during the differentiation of all primary and many relay somatic sensory neurons (37–39) (and this work, although a definitive assessment will await lineage tracing with a Brn3a::Cre line)—but not visceral ones, except fleetingly in nTS precursors before onset of Phox2b expression—and Phox2b, symmetrically expressed in all primary and relay visceral sensory neurons (9)—but not somatic ones. Here, we show that this mutual exclusiveness reflects the repression of Brn3a by Phox2b. Thus, throughout cranial ganglia and dA neurons, Brn3a expression, and the attendant somatic identity, appear as a ground state on which Phox2b superimposes a visceral identity in dA3 interneurons and epibranchial ganglia. A reverse switch was reported in dB3 and dBLb interneurons, normally destined to a somatic fate (trigeminal sensory nuclei) (11, 25), upon inactivation of the homeobox gene Lbx1 that results in up-regulation of Phox2b and contribution to a visceral fate (nTS) (11). Thus, Lbx1 imposes a somatic identity by repressing Phox2b expression and the attendant visceral identity. As we show here, this action includes activation of Brn3a in dB3, most likely by rescuing it from Phox2b-mediated repression (Fig. 4 H′ and I′). We speculate that this Lbx1 > Phox2b > Brn3a/b repressive cascade reflects successive evolutionary events that sculpted the sensory architecture of the hindbrain from an original stock of somatic (i.e., Brn3+) sensory neurons.
Ontogenetic Relatedness of Connected Neurons.
A corollary of the ontogenetic relatedness of sensory neurons is that first-order sensory neurons (in the PNS) are transcriptionally matched with their targets, relay sensory neurons (in the CNS). This feature was previously noted for Drg11 (40), Phox2a (41), Phox2b (27), and Tlx3 (14), and appears to apply also to Brn3a, expressed in a fraction of SpV neurons (Fig. S5 E and F) as well as, subject to lineage tracing, the majority of their precursors (Fig. 4 H and I and Fig. S5 A–D). The visceral-to-somatic switch that Phox2b inactivation causes in parallel in the PNS and CNS further highlights this point: in the absence of Phox2b, the incoming sensory fibers, now growing from Brn3a+ cells, project to a new target that develops from Brn3a+ precursors, the SpV, instead of the nTS. Such a transcriptional match of connected neurons has been described in other parts of the nervous system as well. In the VNS, by far the most striking example so far, uninterrupted chains of 3–5 connected Phox2b+ neurons are common occurrences (1). Other examples include segments of the proprioceptive/vestibular/auditory pathways marked by Atoh1(42, 43), and amygdala neurons and their targets marked by Lhx6 (44).
Because the matching transcription factor is expressed before synapse formation, the former might control the latter (28). Another explanation could be that the connected neurons are sister cell types, which retain part of a common ancestral identity. For example, sensory ganglia and relay sensory neurons in the CNS could have arisen from an ancient split within a common pool of dorsal progenitors. The two explanations are not mutually exclusive, because an evolutionary relationship could underlie connectivity (45).
Materials and Methods
Phox2b::Cre BAC Construct.
A Phox2b::Cre BAC construct was obtained in which the coding sequence of Cre was fused to the first 82 amino acids of Phox2b by recombineering in BAC RP24-95M11, according to standard techniques (46). For the detailed strategy, see Dataset S1, SI Materials and Methods, and Fig. S1.
Mouse Strains.
The Phox2b::Cre BAC DNA was microinjected into fertilized eggs of B6D2 mice by SEAT (Centre National de la Recherche Scientifique). Two founder lines were obtained, one of which is used in this study (characterized in Fig. S1). Other transgenic lines used in this work were as follows: ROSAstoploxYFP (13), Phox2bLacZ/LacZ (2), Phox2blox/lox (8), Brn4::Cre (26), and Nkx6.2::Cre (27). All animal studies were done in accordance with the guidelines issued by the French Ministry of Agriculture and have been approved by the Direction Départementale des Services Vétérinaires de Paris.
Immunohistochemistry and In Situ Hybridization.
Staged embryos (vaginal plug was E0.5), or brains of E18.5 and P7 animals, were processed for immunohistochemistry, in situ hybridization, or combined immunohistochemistry and in situ hybridization on 12-μm-thick cryosections as described (41). A detailed protocol of whole-mount immunofluorescence is given in SI Materials and Methods. Primary antibodies used were as follows: chicken anti-GFP (Aves Laboratories; GFP-1020, 1/500), sheep anti-Th (Chemicon; AB1542, 1/500), rabbit anti-5-HT (Sigma; S5545, 1/500), rabbit anti-Phox2b (6) (1/500), guinea-pig anti-Phox2b (47) (1/500), rabbit anti-Phox2a (41) (1/500), anti-Islet1+2 (41) (Developmental Study Hybridoma Bank; 40.2D6 and 39.4D5, 1/100), rabbit and guinea-pig anti-Tlx3 (38) (1/10,000), guinea-pig anti-Lbx1 (1/5,000) (38), mouse anti-Brn3a (Millipore; MAB1585, 1/200), rabbit anti-Runx1 (kind gift from T. M. Jessell, Columbia University, New York, NY; 1/1000), rabbit anti-peripherin (Abcam; ab39374, 1/250), rabbit anti-Olig3 (48) (1/20,000), goat anti-Brn3b (Santa Cruz Biotechnology; SC-6026, 1/200). Antigen retrieval, by boiling for 10 minutes in sodium citrate (10 mM), was needed for optimal labeling with anti-Islet and anti-Brn3a antibodies. Probes used were as follows: Lmx1b (kind gift from Randy L. Johnson, MD Anderson Cancer Center, Houston, TX), c-Ret (kind gift from Vassilis Pachnis, National Institute for Medical Research, London, UK), Drg11 (kind gift from David J. Anderson, California Institute of Technology, Pasadena, CA).
Supplementary Material
Acknowledgments
We thank T. M. Jessell and T. Müller for antibodies; Randy L. Johnson, Vassilis Pachnis, and D. J. Anderson for probes; C. Le Moal for handling of mouse strains; N. Vodovar and S. Chanet for advice on recombineering; E. B. Crenshaw for the Brn4::Cre mouse line; and P. Ernfors for the Nkx6.2::Cre mouse line. This work was supported by grants from the Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, Fondation pour la Recherche Médicale, and Agence Nationale pour la Recherche (to J.-F.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110416108/-/DCSupplemental.
References
- 1.Brunet J-F, Goridis C. Phox2b and the homeostatic brain. In: Gaultier C, editor. Genetic Basis for Respiratory Control Disorders. New York: Springer-Verlag; 2008. pp. 25–44. [Google Scholar]
- 2.Pattyn A, Morin X, Cremer H, Goridis C, Brunet J-F. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;399:366–370. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
- 3.Pattyn A, Hirsch M-R, Goridis C, Brunet J-F. Control of hindbrain motor neuron differentiation by the homeobox gene Phox2b. Development. 2000;127:1349–1358. doi: 10.1242/dev.127.7.1349. [DOI] [PubMed] [Google Scholar]
- 4.Pattyn A, et al. Coordinated temporal and spatial control of motor neuron and serotonergic neuron generation from a common pool of CNS progenitors. Genes Dev. 2003;17:729–737. doi: 10.1101/gad.255803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Douarin NM. Ontogeny of the peripheral nervous system from the neural crest and the placodes. A developmental model studied on the basis of the quail-chick chimaera system. Harvey Lect. 1984-1985;80:137–186. [PubMed] [Google Scholar]
- 6.Pattyn A, Morin X, Cremer H, Goridis C, Brunet J-F. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;124:4065–4075. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- 7.Blentic A, Chambers D, Skinner A, Begbie J, Graham A. The formation of the cranial ganglia by placodally-derived sensory neuronal precursors. Mol Cell Neurosci. 2011;46:452–459. doi: 10.1016/j.mcn.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Coppola E, et al. Epibranchial ganglia orchestrate the development of the cranial neurogenic crest. Proc Natl Acad Sci USA. 2010;107:2066–2071. doi: 10.1073/pnas.0910213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dauger S, et al. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development. 2003;130:6635–6642. doi: 10.1242/dev.00866. [DOI] [PubMed] [Google Scholar]
- 10.Pattyn A, Guillemot F, Brunet JF. Delays in neuronal differentiation in Mash1/Ascl1 mutants. Dev Biol. 2006;295:67–75. doi: 10.1016/j.ydbio.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Sieber MA, et al. Lbx1 acts as a selector gene in the fate determination of somatosensory and viscerosensory relay neurons in the hindbrain. J Neurosci. 2007;27:4902–4909. doi: 10.1523/JNEUROSCI.0717-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Storm R, et al. The bHLH transcription factor Olig3 marks the dorsal neuroepithelium of the hindbrain and is essential for the development of brainstem nuclei. Development. 2009;136:295–305. doi: 10.1242/dev.027193. [DOI] [PubMed] [Google Scholar]
- 13.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.logan C, Wingate RJT, McKay IJ, Lumsden A. Tlx-1 and TLx-3 Homeobox gene expression in cranial sensory ganglia and hindbrain of the chick embryo: Markers of patterned connectivity. J Neurosci. 1998;18:5389–5402. doi: 10.1523/JNEUROSCI.18-14-05389.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Begbie J, Ballivet M, Graham A. Early steps in the production of sensory neurons by the neurogenic placodes. Mol Cell Neurosci. 2002;21:502–511. doi: 10.1006/mcne.2002.1197. [DOI] [PubMed] [Google Scholar]
- 16.Valarché I, et al. The mouse homeodomain protein Phox2 regulates Ncam promoter activity in concert with Cux/CDP and is a putative determinant of neurotransmitter phenotype. Development. 1993;119:881–896. doi: 10.1242/dev.119.3.881. [DOI] [PubMed] [Google Scholar]
- 17.Folan-Curran J, Cooke FJ. Contribution of cranial nerve ganglia to innervation of the walls of the rat external acoustic meatus. J Peripher Nerv Syst. 2001;6:28–32. doi: 10.1046/j.1529-8027.2001.006001028.x. [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa T, Kuwahara S, Maeda S, Tanaka K, Seki M. Calcitonin gene-related peptide immunoreactive neurons innervating the soft palate, the root of tongue, and the pharynx in the superior glossopharyngeal ganglion of the rat. J Chem Neuroanat. 2010;39:221–227. doi: 10.1016/j.jchemneu.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Marmigère F, et al. The Runx1/AML1 transcription factor selectively regulates development and survival of TrkA nociceptive sensory neurons. Nat Neurosci. 2006;9:180–187. doi: 10.1038/nn1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rebelo S, et al. DRG11 immunohistochemical expression during embryonic development in the mouse. Dev Dyn. 2007;236:2653–2660. doi: 10.1002/dvdy.21271. [DOI] [PubMed] [Google Scholar]
- 21.Dykes IM, Tempest L, Lee SI, Turner EE. Brn3a and Islet1 act epistatically to regulate the gene expression program of sensory differentiation. J Neurosci. 2011;31:9789–9799. doi: 10.1523/JNEUROSCI.0901-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian Y, et al. Formation of brainstem (nor)adrenergic centers and first-order relay visceral sensory neurons is dependent on homeodomain protein Rnx/Tlx3. Genes Dev. 2001;15:2533–2545. doi: 10.1101/gad.921501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian Y, Shirasawa S, Chen CL, Cheng L, Ma Q. Proper development of relay somatic sensory neurons and D2/D4 interneurons requires homeobox genes Rnx/Tlx-3 and Tlx-1. Genes Dev. 2002;16:1220–1233. doi: 10.1101/gad.982802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray PA. Transcription factors and the genetic organization of brain stem respiratory neurons. J Appl Physiol. 2008;104:1513–1521. doi: 10.1152/japplphysiol.01383.2007. [DOI] [PubMed] [Google Scholar]
- 25.Kim EJ, Battiste J, Nakagawa Y, Johnson JE. Ascl1 (Mash1) lineage cells contribute to discrete cell populations in CNS architecture. Mol Cell Neurosci. 2008;38:595–606. doi: 10.1016/j.mcn.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zechner D, et al. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 27.Baudet C, et al. Retrograde signaling onto Ret during motor nerve terminal maturation. J Neurosci. 2008;28:963–975. doi: 10.1523/JNEUROSCI.4489-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunet J-F, Pattyn A. Phox2 genes - from patterning to connectivity. Curr Opin Genet Dev. 2002;12:435–440. doi: 10.1016/s0959-437x(02)00322-2. [DOI] [PubMed] [Google Scholar]
- 29.Rohrer H, Thoenen H. Relationship between differentiation and terminal mitosis: Chick sensory and ciliary neurons differentiate after terminal mitosis of precursor cells, whereas sympathetic neurons continue to divide after differentiation. J Neurosci. 1987;7:3739–3748. doi: 10.1523/JNEUROSCI.07-11-03739.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Y, et al. A central role for Islet1 in sensory neuron development linking sensory and spinal gene regulatory programs. Nat Neurosci. 2008;11:1283–1293. doi: 10.1038/nn.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang C, et al. The transcription factor, Lmx1b, is necessary for the development of the principal trigeminal nucleus-based lemniscal pathway. Mol Cell Neurosci. 2010;44:394–403. doi: 10.1016/j.mcn.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding YQ, et al. Lmx1b controls the differentiation and migration of the superficial dorsal horn neurons of the spinal cord. Development. 2004;131:3693–3703. doi: 10.1242/dev.01250. [DOI] [PubMed] [Google Scholar]
- 33.Cheng L, et al. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat Neurosci. 2004;7:510–517. doi: 10.1038/nn1221. [DOI] [PubMed] [Google Scholar]
- 34.Ding YQ, et al. Lmx1b is essential for the development of serotonergic neurons. Nat Neurosci. 2003;6:933–938. doi: 10.1038/nn1104. [DOI] [PubMed] [Google Scholar]
- 35.Cavanaugh DJ, et al. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci. 2011;31:5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stirling LC, et al. Nociceptor-specific gene deletion using heterozygous NaV1.8-Cre recombinase mice. Pain. 2005;113:27–36. doi: 10.1016/j.pain.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Fedtsova NG, Turner EE. Brn-3.0 expression identifies early post-mitotic CNS neurons and sensory neural precursors. Mech Dev. 1995;53:291–304. doi: 10.1016/0925-4773(95)00435-1. [DOI] [PubMed] [Google Scholar]
- 38.Müller T, et al. The homeodomain factor lbx1 distinguishes two major programs of neuronal differentiation in the dorsal spinal cord. Neuron. 2002;34:551–562. doi: 10.1016/s0896-6273(02)00689-x. [DOI] [PubMed] [Google Scholar]
- 39.Gross MK, Dottori M, Goulding M. Lbx1 specifies somatosensory association interneurons in the dorsal spinal cord. Neuron. 2002;34:535–549. doi: 10.1016/s0896-6273(02)00690-6. [DOI] [PubMed] [Google Scholar]
- 40.Saito T, Greenwood A, Sun Q, Anderson DJ. Identification by differential RT-PCR of a novel paired homeodomain protein specifically expressed in sensory neurons and a subset of their CNS targets. Mol Cell Neurosci. 1995;6:280–292. doi: 10.1006/mcne.1995.1022. [DOI] [PubMed] [Google Scholar]
- 41.Tiveron M-C, Hirsch M-R, Brunet J-F. The expression pattern of the transcription factor Phox2 delineates synaptic pathways of the autonomic nervous system. J Neurosci. 1996;16:7649–7660. doi: 10.1523/JNEUROSCI.16-23-07649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bermingham NA, et al. Proprioceptor pathway development is dependent on Math1. Neuron. 2001;30:411–422. doi: 10.1016/s0896-6273(01)00305-1. [DOI] [PubMed] [Google Scholar]
- 43.Wang VY, Rose MF, Zoghbi HY. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron. 2005;48:31–43. doi: 10.1016/j.neuron.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 44.Choi GB, et al. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Arendt D. The evolution of cell types in animals: Emerging principles from molecular studies. Nat Rev Genet. 2008;9:868–882. doi: 10.1038/nrg2416. [DOI] [PubMed] [Google Scholar]
- 46.Wang S, Zhao Y, Leiby M, Zhu J. A new positive/negative selection scheme for precise BAC recombineering. Mol Biotechnol. 2009;42:110–116. doi: 10.1007/s12033-009-9142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dubreuil V, et al. Defective respiratory rhythmogenesis and loss of central chemosensitivity in Phox2b mutants targeting retrotrapezoid nucleus neurons. J Neurosci. 2009;29:14836–14846. doi: 10.1523/JNEUROSCI.2623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Müller T, et al. The bHLH factor Olig3 coordinates the specification of dorsal neurons in the spinal cord. Genes Dev. 2005;19:733–743. doi: 10.1101/gad.326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.