Abstract
Mutations in the PKHD1 gene, which encodes fibrocystin, cause autosomal recessive polycystic kidney disease (ARPKD). Unfortunately, the lack of specific antibodies to the mouse protein impairs the study of splicing, post-translational processing, shedding, and temporal and spatial expression of endogenous fibrocystin at the cellular and subcellular level. Here, we report using a knock-in strategy to generate a null Pkhd1 strain and a strain that expresses fibrocystin along with two SV5-Pk epitope tags engineered in-frame into the third exon, immediately C-terminal to the signal-peptide cleavage site in a poorly conserved region. By 6 mo of age, the Pkhd1-null mouse develops massive cystic hepatomegaly and proximal tubule dilation, whereas the mouse with epitope-tagged fibrocystin has histologically normal liver and kidneys at 14 mo. Although Pkhd1 was believed to generate many splice forms, our western analysis resolved fibrocystin as a 500 kD product without other forms in the 15–550 kD range. Western analysis also revealed that exosome-like vesicles (ELVs) secrete the bulk of fibrocystin in its mature cleaved form, and scanning electron microscopy identified that fibrocystin on ELVs attached to cilia. Furthermore, the addition of ELVs with epitope-tagged fibrocystin to wild-type cells showed that label transferred to primary cilia within 5 min. In summary, tagging of the endogenous Pkhd1 gene facilitates the study of the glycosylation, proteolytic cleavage, and shedding of fibrocystin.
Autosomal recessive polycystic kidney disease (ARPKD MIM ID #263200) is characterized by dilation of both the collecting ducts (CDs) in the kidney and hepatic fibrosis with or without nonobstructive biliary dilation, affecting 1:20,000 live births.1–3 The gene responsible for ARPKD is the polycystic kidney and hepatic disease gene (PKHD1: 472kb), which is encoded on chromosome 6p12.2, has 67 exons and a full length mRNA of 16,235bp (mouse mRNA is 12,928bp).4 Fibrocystin/polyductin, the product of the PKHD1 gene is a large type I membrane protein of 4074 amino acids with a pro-protein convertase site between residues 3617..3620.5 Fibrocystin has been localized to the primary cilium, the basal body and small (100nm diameter) membrane bound particles which are shed into urine and bile, known as PKD exosome–like vesicles (PKD–ELVs).6–10 It has been postulated that the mouse Pkhd1 and human PKHD1genes undergo extensive differential splicing and that many of the putative fibrocystin products are secreted as they have a signal peptide but no membrane anchor.11–13 If so, these splice forms could be partially functional or antagonistic to full length fibrocystin function and so mutations affecting a subset of splice forms are also thought to be responsible for some of the phenotypic variation seen in human ARPKD and the range of renal phenotypes in the available mouse models.14–18 However, there is debate as to the extent of PKHD1 and Pkhd1 splicing, since Pkhd1 has a relatively simple band pattern on northern blotting.4 Here we re-investigate the splicing of Pkhd1 at an mRNA level. Furthermore, to investigate splicing at a protein level we developed mouse models of ARPKD where the Pkhd1 gene is initially transcriptionally silenced by a STOP cassette flanked by two loxP sites, an LSL module.19 Upon removal of the LSL (Pkhd1LSL(−)/LSL(−)) with Cre recombinase the Pkhd1 gene reactivates, producing the Pkhd1Pk(+) allele, and expresses a form of fibrocystin with two SV5–Pk tags on its extreme N–terminus. This Pkhd1Pk(+)/Pk(+) mouse is phenotypically wildtype and produces epitope tagged PKD–ELVs in its urine and bile. The epitope tags are inserted into exon–3, an exon which has been shown to be present in 21 of 22 of the putative splice forms described by Boddu.13 If differential splicing occurs after exon–3 and these events alter the length of the protein, these forms of fibrocystin will be detectable upon western blotting of kidney or urine.
PKD–ELVs are thought to be shed from multivesicular bodies (MVB) and to interact with primary cilia in a rapid and specific manner.10 Such interactions have been observed in the embryologic node by Tanaka and in the maturation of male germ cells in the epididymis, where ELVs fuse with the flagellum (modified cilium) of the maturing sperm cells.20,21 However, there is no simple assay for PKD–ELV/primary cilium interactions. Here we show that Pkhd1Pk(+)/Pk(+) mouse urine can supply tagged PKD–ELVs that interact with WT primary cilia in vitro and be detected by immuno–scanning electron microscopy (ISEM). We further show that tagging of the endogenous Pkhd1 gene allows the monitoring of fibrocystin in its physiologically relevant context, in particular its glycosylation, proteolytic cleavage and shedding of fibrocystin on PKD–ELVs.
RESULTS
Investigation of PKHD1 Splicing
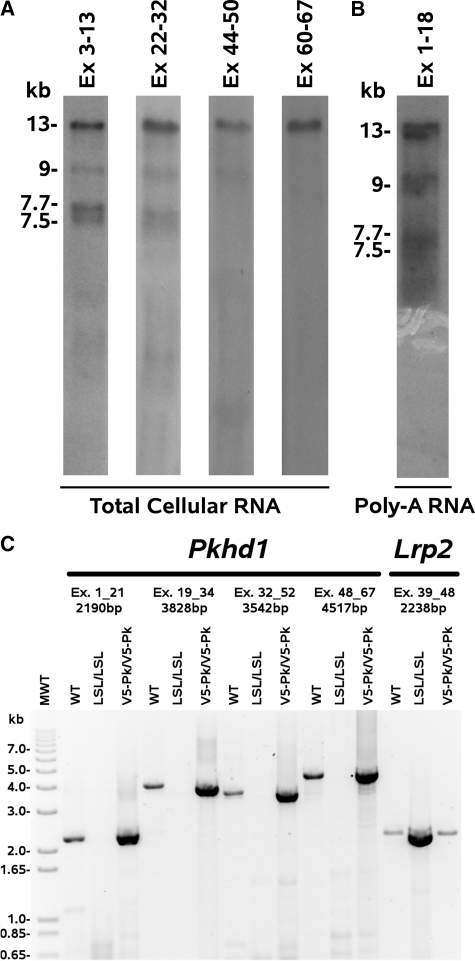
Pkhd1 is highly expressed in the kidney,4,11 and resolves as a major 13kb mRNA with three smaller minor species of 9, 7.7 and 7.5kb when probed with probes encompassing exons 3–13, and 22–32. A probe encompassing exons 44–50 detects the 9kb product weakly and not the 7.7 and 7.5kb species. However, a probe encompassing exons 60–67 detects only the longest 13kb product (Figure 1A). The 9, 7.7 and 7.5kb species appear to be polyadenylated as they are detected on northern blots of poly–A selected mRNA (Figure 1B). We investigated the possibility that the smaller mRNAs of 9, 7.7 and 7.5kb represent differentially spliced forms of Pkhd1, but RT–PCR on mouse kidney poly–A mRNA showed that overlapping RT–PCR products from exons 1–21, 19–34, 32–52, 48–52 and 48–67 only produced products of the predicted size, with only very faint products of different molecular mass. As there is only one Pkhd1 promoter,18 these data suggests that there is one full–length form of Pkhd1 mRNA at 13kb and that the smaller forms are the products of premature poly–adenylation. We sequenced all of the RT-PCR products and showed that they were 100% identical to the published full length cDNA sequence NM_153179 GI:126157465. There was no evidence of heterogeneity, deletions or insertions implying that there was no differential splicing. However, it remains possible that there are rare minor splice forms not delineated by this RT-PCR analysis.
Figure 1.
Pkhd1 shows minimal differential splicing. Northern blotting and RT–PCR of mouse kidney mRNA for Pkhd1 and Lrp2 (megalin): 0.5% agarose, MOPS formaldehyde northern blots, (A) Mouse kidney total cellular RNA probed with probes to mouse Pkhd1 exons 1–13, 22–32, 44–50 and 60–67. The full length product is 13kb, whereas the smaller forms are at 9, 7.7 and 7.5kb. (B) Mouse kidney poly–A mRNA probed with a probe to mouse Pkhd1 exons 1–18, showing that the Pkhd1 and its smaller forms are poly-adenylated. (C) Reverse transcription PCR on poly–A selected mRNA from WT, Pkhd1del2/del2, Pkhd1LSL(−)/LSL(−) and Pkhd1Pk(+)/Pk(+) kidney. PCRs were performed from exons 1 to 21, 19 to 34, 32 to 52 and 48 to 67 and generated species with the expected molecular mass when compared with the mouse sequence of fibrocystin [gi 126157465 ref NM 153179.3 ]. As a loading control we amplified Lrp2 to show that an excess of Pkhd1LSL(−)/LSL(−) mRNA was used in these assays and that the Pkhd1 in the Pkhd1LSL(−)/LSL(−) mouse is completely transcriptionally silent.
Generation of A Pkhd1 Null Mouse and An Epitope Tagged Pkhd1Pk(+)/Pk(+) Mouse
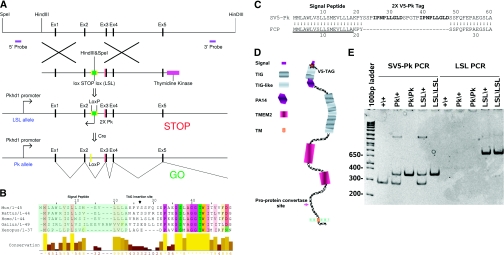
To investigate differential splicing at a protein level we generated a Pkhd1 gene with an epitope tag at its extreme N–terminus. To produce mice that were completely null for Pkhd1 mRNA we inserted a loxP flanked transcriptional STOP cassette into intron–2. The loxP STOP loxP (LSL) cassette contains a puromycin acetyltransferase (pac) gene and four copies of an SV–40 viral transcriptional termination sequence that terminates all Pkhd1 transcripts in intron 2.19 The LSL cassette can be removed by the action of the Cre recombinase, leaving an SbfI site and a SalI flanked loxP site in intron-2 (54bp in length), 369bp 5′ to the beginning of exon–3, allowing the gene to restart. This results in the expression of an epitope tagged form of fibrocystin that has two SV5–Pk tags encoded in–frame in exon–3. These tags, 26 amino acids in length, were inserted into one of the most evolutionary nonconserved regions of fibrocystin and, hence, were predicted not to influence the function of the protein (Figure 2B). Furthermore, once the signal peptide is cleaved, the two SV5–Pk tags are predicted to be on the extreme N–terminus of the protein, an ideal position from which to detect the native protein in tissue sections and monitor proteolytic processing, glycosylation and putative splice forms, by western blotting (Figure 2D). Exon–3 was also chosen as it ought to be present in 95% of putative splice forms.13
Figure 2.
The strategy used to produce the Pkhd1LSL(−)/LSL(−) and Pkhd1Pk(+)/Pk(+) mouse. (A) A loxP flanked puromycin N–acetyltransferase (pac) and SV–40 derived transcriptional STOP cassette was inserted into Intron–2 of the Pkhd1, LSL. This terminated all Pkhd1 transcripts after this point. Two SV5–Pk epitope tags where also inserted in frame into exon–3 of the Pkhd1 gene. (B) A T–Coffee alignment of the signal peptides and first 27 amino acids of five vertebrate fibrocystins.29 The insertion site for the tag was chosen so that it was in a region of poor overall conservation and at the extreme N–terminus of the mature protein (once the signal peptide is removed in the endoplasmic reticulum). (C) The protein sequence of the signal peptide, SV5–Pk tag and mature fibrocystin junction in the Pkhd1Pk(+)/Pk(+) mouse. (D) Schematic diagram of the fibrocystin protein showing the SV5–Pk tag site and the pro–protein convertase site. (E) Diagnostic PCRs for the detection of the Pkhd1Pk(+) and Pkhd1LSL(−) alleles (Concise Methods).
Comparison of Pkhd1LSL(−)/LSL(−) and Pkhd1Pk(+)/Pk(+) Mice
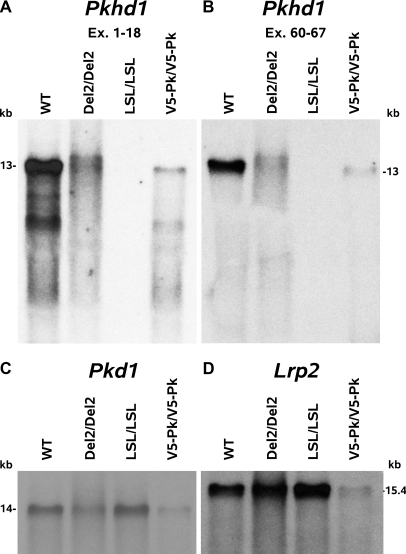
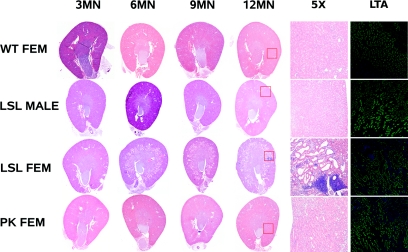
Northern blotting and RT–PCR analysis indicates that the Pkhd1LSL(−)/LSL(−) mouse makes no fibrocystin mRNA (Figures 1 and 3). The Pkhd1LSL(−)/LSL(−) mouse, on an F10 inbred BALB/cJ or C57BL/6J background, develops cysts and fibrosis in the liver, histologically visible at 1 mo of age, and females develop PT dilation at 6 mo of age, (Figures 4 and 5). Male mice are protected from kidney cyst development in a manner similar to the Pkhd1del2/del2 mice described by Woollard (Pkhd1del2/del2 male mice never develop PT dilation).15 The LSL was removed in the germline oocyte expression of Cre recombinase (the GDF–9–iCre transgenic mouse),22 to yield the Pkhd1Pk(+) allele (the LoxP site and epitope tags were confirmed by sequencing). The Pkhd1Pk(+)/Pk(+) mouse produces a 13kb the 9kb, 7.7kb and 7.5kb products identical in length to the WT products on northern blotting (Figure 3) and is phenotypically normal with no liver or kidney pathology at all at 14 mo, showing that the SV5–Pk tagged form of fibrocystin is functionally normal, (Figures 4 and 5).
Figure 3.
Pkhd1LSL(−)/LSL(−) mice are null for Pkhd1 mRNA whereas Pkhd1Pk(+)/Pk(+) mice have normal levels of Pkhd1 mRNA. Northern blots for Pkhd1, Pkd1 and Lrp2 (megalin): Northern blots of total mouse kidney RNA, run on a MOPS, formaldehyde, 0.5% agarose gel. WT, Pkhd1del2/del2, Pkhd1LSL(−)/LSL(−) and Pkhd1Pk(+)/Pk(+) kidney RNA probed with (A) the first 18 exons of Pkhd1, (B) the last 7 exons of Pkhd1, (C) a Pkd1 cDNA probe and (D) a Lrp2 megalin probe. The Pkhd1LSL(−)/LSL(−) mouse does not make a Pkhd1 product with either Pkhd1 probe but does synthesize Pkd1 and Lrp2 mRNA. The Pkhd1Pk(+)/Pk(+) kidney lane is somewhat under loaded, but the ratios of Pkhd1 and Lrp2/Pkd1 mRNA are similar to the control. This is reflected in the quantitative PCR in Supplemental Figure 2.
Figure 4.
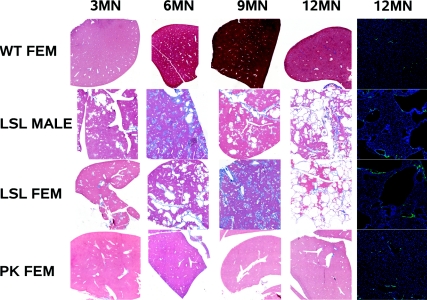
Female Pkhd1LSL(−)/LSL(−) mice develop proximal tubule dilatation and cysts, whereas male Pkhd1LSL(−)/LSL(−) mice and both sexes of Pkhd1Pk(+)/Pk(+) mice have cyst-free kidneys. Comparison of WT, Pkhd1Pk(+)/Pk(+) and Pkhd1LSL(−)/LSL(−) kidneys: Comparison of the renal phenotype in WT, male Pkhd1LSL(−)/LSL(−), female Pkhd1LSL(−)/LSL(−) and female Pkhd1Pk(+)/Pk(+) animals. Both Pkhd1LSL(−)/LSL(−) and Pkhd1Pk(+)/Pk(+) animals were inbred to F10 on a C57BL/6J background and compared with C57BL/6J WT mice. Only female Pkhd1LSL(−)/LSL(−) mice begin to develop PT dilation at 3 to 6 mo of age. This is confirmed by immunohistology with Lotus tetragonolobus lectin (LTL) which is specific for the PT (DAPI counter-stain). The female Pkhd1Pk(+)/Pk(+) mice have normal kidneys at 12 mo of age.
Figure 5.
Both sexes of Pkhd1LSL(−)/LSL(−) mice develop polycystic liver disease and fibrosis, whereas Pkhd1Pk(+)/Pk(+) mice always have normal livers. Comparison of WT, Pkhd1Pk(+)/Pk(+) and Pkhd1LSL(−)/LSL(−) liver: Comparison of the liver phenotype in WT, male Pkhd1LSL(−)/LSL(−), female Pkhd1LSL(−)/LSL(−) and female Pkhd1Pk(+)/Pk(+) animals. Both Pkhd1LSL(−)/LSL(−) and Pkhd1Pk(+)/Pk(+) animals were inbred to F10 on a C57BL/6J background and compared with C57BL/6J WT mice. Both male and female Pkhd1LSL(−)/LSL(−) mice develop liver cysts and fibrosis at 3 mo of age and this worsens with age until the liver is completely replaced by cysts at 12 mo of age. This is confirmed by staining with PCK–26 (IgG1 monoclonal antibody ab6401) anti–cytokeratins 5, 6, and 8, which shows that the cyst lining are epithelial in origin (DAPI counter-stain). Both sexes of the Pkhd1Pk(+)/Pk(+) mouse have normal livers at 12 mo and this has been confirmed to the 14 mo time point.
To ensure that the Pkhd1 gene in the WT and Pkhd1Pk(+)/Pk(+) mice had identical levels of expression and that the Pkhd1LSL(−)/LSL(−) mice were null for Pkhd1 mRNA, we developed a TaqMan single tube RT-PCR assay for mouse Pkhd1 (ABI catalog# Mm01233728_m1) and the house keeping gene mouse transferrin receptor (Tfrc) (ABI catalog # Mm00441941_m1). Both probes span exon-exon junctions (exons 2 to 3 in Tfrc and exons 48 to 49 in Pkhd1) and in the WT cross the Ct within one cycle of one another. Using the delta Ct technique we obtained Pkhd1/Trfc ratios of 1.64 SD ± 0.90 (n = 6), 1.15 SD ± 0.2 (n = 6) and 0.018 SD ± 0.01 (n = 6), from 1 mo old WT, Pkhd1Pk(+)/Pk(+) and Pkhd1LSL(−)/LSL(−) kidney respectively. The WT versus Pkhd1LSL(−)/LSL(−) was significant at P = 0.007, and Pkhd1Pk(+)/Pk(+) versus Pkhd1LSL(−)/LSL(−) at P = 3.286e−5 (Welch Two Sample t test). The WT versus Pkhd1Pk(+)/Pk(+) was NS P = 0.24, showing that the levels of Pkhd1 gene expression are similar in the WT and Pkhd1Pk(+)/Pk(+) mice and almost undetectable in the Pkhd1LSL(−)/LSL(−) mouse (Supplemental Figure 2).
Liver/body weight ratios between the Pkhd1LSL(−)/LSL(−) and Pkhd1Pk(+)/Pk(+) animals were significant at 6, 9 and 12 mo of age as were the kidney/body weight ratios at 9 and 12 mo (P < 0.01, Welch two–sample t–test). At 12 mo liver/body weight ratios were significantly increased in both sexes in the Pkhd1LSL(−)/LSL(−) mouse, female (P = 0.025) and male (P = 0.02). However, in the case of sex and kidney/body weight ratios the female, but not male, Pkhd1LSL(−)/LSL(−) mice had very significantly heavier kidneys than the female Pkhd1Pk(+)/Pk(+) mice (P = 0.0024) at 12 mo. The pancreas body weight ratios are NS at the P < 0.05 level for any time point. In total we examined 158 Pkhd1LSL(−)/LSL(−) mice and 90 Pkhd1Pk(+)/Pk(+) mice.
Analysis of Tagged Fibrocystin In Pkhd1Pk(+)/Pk(+) Mice
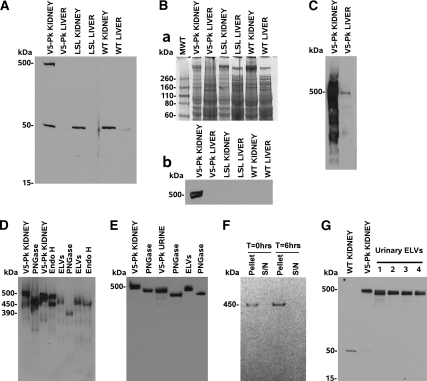
As endogenous mouse Ig interferes with the secondary western detection of mouse monoclonal antibodies applied to mouse tissues, we used a SV5–Pk1 antibody directly coupled to horseradish peroxidase (HRP)(MCA1360P Serotec) (Figure 6 A and B). A survey of Pkhd1Pk(+)/Pk(+) mouse tissues, by western blot, showed that fibrocystin expression was highest in the kidney followed by the pancreas, however we had difficulty detecting fibrocystin in liver tissue, perhaps because cholangiocytes make up only 1% of the liver cells and are much smaller than hepatocytes. Probing western blots using the SV5-Pk1 antibody as a primary and using an HRP conjugated anti-IgG2a antibody as a secondary with prolonged exposure showed that liver did have a weak signal compared with kidney (Figure 6C). Fibrocystin was also detectable in Pkhd1Pk(+)/Pk(+) bile. The spleen, thymus, colon, cerebrum, cerebellum, hypothalamus and spinal cord had undetectable levels of fibrocystin (data not shown). This agrees with the RT–PCR surveys, which suggest only kidney, pancreas, liver and lung make Pkhd1 mRNA.4 In this previous study we detected more fibrocystin mRNA in the liver than pancreas, perhaps because mRNA was degraded by pancreatic RNaseA. Western analysis of mouse kidney membrane in Pkhd1Pk(+)/Pk(+) mice showed that fibrocystin resolved as a 500 kD doublet with no smaller forms visible on western blotting (Figure 6 A and Bb). Upon N–linked deglycosylation with PNGase, this doublet resolved as a single species at 450 kD, implying that the 500 kD doublet is due to differential N–linked glycosylation (Figure 6 D and E). Exosome–like vesicles (ELVs) isolated from Pkhd1Pk(+)/Pk(+) mouse urine generate a single band at 450 kD which decreases in size to about 390 kD on deglycosylation with PNGase and is compatible with cleavage at the proprotein convertase site observed by Kaimori.5 This smaller cleaved form is not seen in kidney tissue implying that the mature form of fibrocystin is only present on PKD–ELVs (Figure 6 D, E, and G). To further show that the fibrocystin on PKD–ELVs is mature, we treated kidney membrane and PKD–ELVs with Endo H, which cleaves the chitobiose core of high mannose oligosaccharides from N-linked glycoproteins. This reduced about half the kidney membrane fibrocystin from 500 kD to 450 kD showing that about half of the kidney fibrocystin is in the ER (Figure 6D). The fibrocystin in PKD–ELVs on the other hand was reduced only 10 kD by Endo H treatment showing that it is mainly resistant and has complex post–Golgi carbohydrate. In summary, all of the PKD–ELV fibrocystin is proteolytically cleaved and has complex carbohydrates, both proxies for protein maturity. The idea that the Pkhd1 gene is extensively differentially spliced, led us to investigate whether there were any smaller forms of fibrocystin that were secreted into the urine. We were able to detect SV5-Pk tagged fibrocystin in unprocessed Pkhd1Pk(+)/Pk(+) mouse urine by western blotting and specifically searched for smaller forms in the 15–550 kD range (we can detect fibrocystin in this system by loading 30μl of mouse urine onto a gel and western blotting). We were unable to detect any forms smaller than 450 kD (Figure 6 E and F), indicating that there are no abundantly expressed, differentially spliced forms (containing exon-3) without trans–membrane anchors in the urine. The 7.5 and 7.7 kb species seen in northern blots of Pkhd1 would be predicted to make glycosylated protein products in the 280 to 350 kD range, but we did not observe these products. However, we cannot completely exclude the possibility of rare, smaller products, but these would be only a few percent of the abundance of the full length product. Next we investigated the idea that fibrocystin could be shed in a soluble form from ELVs. We pelleted 100μl aliquots of Pkhd1Pk(+)/Pk(+) urine at 100,000g for 1 h and compared the pellet to the supernatant. We also incubated 100μl aliquots of urine at 37 °C for 6 h in the absence of proteinase inhibitors and assessed whether fibrocystin could be shed. All of the urinary fibrocystin appeared to be closely associated with the pellet. To absolutely exclude the presence of smaller forms of fibrocystin, we prepared four separate preparations of Pkhd1Pk(+)/Pk(+) mouse ELVs and showed that these forms were not a feature of ELV fibrocystin, with all four preparations resolving at 450 kD (Figure 6G).
Figure 6.
SV5-Pk tagged fibrocystin is easily detected in the kidney, urine, and urinary ELVs. (A). A) 4–12% MOPS PAGE gel resolving from 15–550 kD, there is a 500 kD band in Pkhd1Pk(+)/Pk(+) kidney membrane preparation but no tagged material was seen in Pkhd1LSL(−)/LSL(−) or WT tissues. Note: there is an endogenous band at 50 kD which cross reacts with SV5–Pk1 antibody (this can be used as a loading control). (B) (a) Coomassie blue staining of a duplicate 4 to 12% MOPS PAGE gel in A; (b) 4% slab gel showing that fibrocystin runs as a doublet. (C) 3–8% Nu–PAGE gel comparing 30 μg of Pkhd1Pk(+)/Pk(+) kidney and liver membrane protein, probed with SV5-Pk1 and a HRP conjugated secondary antibody, the liver contains a small fraction of the kidney fibrocystin as biliary epithelial cells make up about 1% of hepatic cells. (D) 3–8% Nu–PAGE gel. Kidney membrane preparations were treated with PNGase and Endo H. PNGase results in fibrocystin resolving at 450 kD, whereas Endo H causes approximately half the kidney fibrocystin to resolve at 450 kD implying that about half of the fibrocystin is in the ER. In the case of Pkhd1Pk(+)/Pk(+) ELVs, fibrocystin shifts from 450 kD to 390 kD after treatment with PNGase (implying that it has undergone the pro–protein convertase cleavage event upon release from the cell). Upon treatment with Endo H there is a very slight decrease in size, c10 kD, implying that the bulk of the glycosylation is mature complex type carbohydrate (post–Golgi). (E) Distribution of fibrocystin in urine of the Pkhd1Pk(+)/Pk(+) mouse. We can use the SV5–Pk1 antibody to detect fibrocystin in unconcentrated urine and again we detected no smaller forms. (F) 200μl of fresh Pkhd1Pk(+)/Pk(+) mouse urine was harvested and 100μl brought to 1x Complete proteinase. This 100μl was centrifuged at 100,000g for 1 h at 4 °C and the supernatant carefully removed and the pellet resuspended. The remaining 100μl was then incubated at 37 °C for 6 h without proteinase inhibitors and the pellet and supernatant collected. A 3–8% MOPS western showed that all of the fibrocystin remained in the PKD–ELV fraction despite the prolonged incubation at 37 °C, showing that the cleaved fibrocystin is firmly attached to the PKD–ELVs. (G) To exclude the possibility of differential splice forms or other processed forms we ran four different ELV samples on a 4 to 12% gel.
Visualization of Pkhd1Pk(+)/Pk(+) Primary Cilia Using SEM
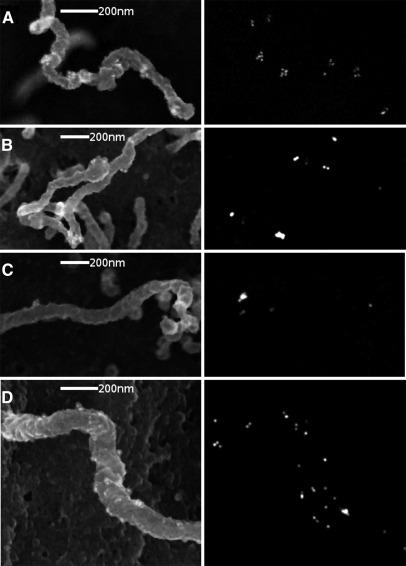
As fibrocystin is predicted to be a type–1 membrane protein and the N–terminal SV5-Pk tags are on the extracellular tip of the protein we used SEM to visualize the localization of the protein on the primary cilium and apical aspect of cultured renal epithelial cells. In previous experiments we used nano–gold and carbon coating to visualize exosome–primary cilia interactions.10 Although this gives excellent backscatter contrast between the carbon Z = 6 and gold Z = 79, the carbon must be applied to a high thickness, 22nm, which can obscure structure. To avoid this we coated with 1nm osmium, and were able to visualize small (50–100nm) SV5–Pk positive blebs which we interpret as ELVs attached to the membrane of the primary cilium (Figure 7 A–D).
Figure 7.
Fibrocystin is present on ELVs attached to the primary cilium. Immunolocalization of SV5–Pk tagged fibrocystin in cultured PT cells by ISEM: Primary Pkhd1Pk(+)/Pk(+) PT cells, the affected cell type in Pkhd1LSL(−)/LSL(−) mice, were cultured for 7 d and serum starved for 2 d, fixed in paraformaldehyde/glutaraldehyde and stained for SV5–Pk1 with 15nm protein A gold. (Left) Secondary electrons, (Right) Backscattered electrons (gold). (A–D) primary cilia with adherent PKD–ELVs staining with 15nm gold, there is also gold on the shaft of the primary cilium in D.
Use of the Pkhd1Pk(+)/Pk(+) Mouse To Develop An Assay for PKD–ELV/Primary Cilium Interactions
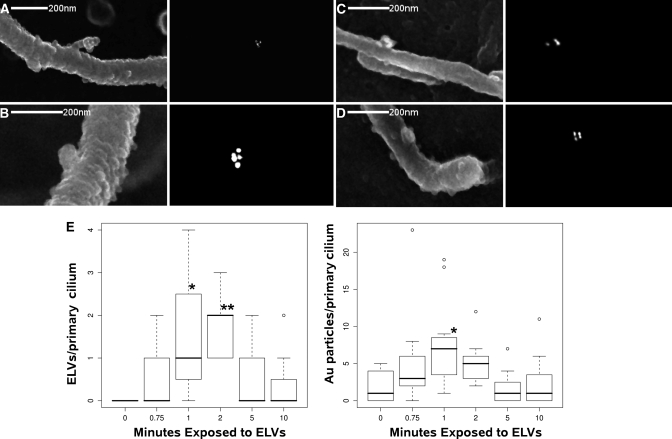
To develop a faster assay for ELV/primary cilium interactions, we used fresh urine from Pkhd1Pk(+)/Pk(+) mice as a probe onto primary PT renal epithelial cells from WT mice. These cells were >90% ciliated with primary cilia of 7.12 SD ± 3.05μm (n = 10). Control monolayers without the addition of ELVs or treated with WT urine, have only the occasional gold particle on their cilia membranes (1.9 SD ± 2.02 particles/cilium (the background)) and there are no gold positive ELVs adherent to the primary cilium. However, cells treated with Pkhd1Pk(+)/Pk(+) urine for 1, 2, 5 and 10 min have, 1.5 SD ± 1.5, 1.7 SD ± 0.7, 0.55 SD ± 0.68 and 0.36 SD ± 0.67 ELVs with one or more gold particles on them respectively (Figure 8) versus 0 at T = 0. This is reflected in the total amount of gold particles on the primary cilium 1.9 SD ± 2.02 at T = 0 to 7.63 SD ± 6.0, 5.2 SD ± 3.1, 1.6 SD ± 2.2 and 2.4 SD ± 3.6 at 1, 2, 5 and 10 min respectively. As described in Hogan et al. PKD–ELV–primary cilium interactions are fast, with the peak of SV5–Pk (ELV) deposition occurring at 1–2 min.10 By 10 min the primary cilia are cleared of SV5–Pk ELVs. This interaction appears to be specific and at early time points (1 and 2 min) the primary cilia extrude small 100nm by 20nm tubes, ciliary side branches, toward the SV5-PK positive ELVs (Figure 8A). The ELVs often appear to sit on the surface of these before integrating into the membrane at 5 min.
Figure 8.
PKD-ELVs interact with primary cilia and are cleared within 10 minutes. PKD–ELV/primary cilium interaction visualized by SEM: Primary WT PT cells are incubated with PKD–ELVs from Pkhd1Pk(+)/Pk(+) mouse urine. (A and B) WT PT primary cilia at 1 min with (A) a ciliary side branch interacting with SV5-Pk positive PKD–ELVs. (C and D) SV–Pk5 positive PKD–ELVs interacting with a primary cilia at 2 min. Left hand panels secondary electrons, right hand panels backscattered electrons – gold (SV5–Pk label, 15nm gold). (E) The number of exosomes positive for SV5–Pk gold adherent to WT primary cilia and the total amount of gold particles on primary cilium after adding 1:10 dilution of Pkhd1Pk(+)/Pk(+) urine, as a function of time. For adherent PKD–ELVs the time points 1 and 2 min were significant versus control (T = 0) at P = 0.0015, and P = 0.001 respectively (an ELV is defined as a 100nm vesicle attached to the primary cilium with >1 gold particle), for total gold per primary cilium the 1 min time point was significant versus control P = 0.038 (One–way ANOVA with Tukey's HSD, data presented as Tukey box plots).
DISCUSSION
Our studies show that extensive differential splicing is not a major feature of Pkhd1 at an mRNA or protein level with one main mRNA species and one protein predominating in both. The smaller minor 9, 7.7 and 7.5kb mRNA species are probably due to premature termination of the larger mRNA, and there is little evidence for smaller fibrocystin proteins in the kidney, bile or pancreas. We think that the observations that suggest that Pkhd1 and PKHD1 have many different splice forms may be due to the poor stability of the Pkhd1 transcripts. Differential splicing products might occur at a low level in Pkhd1 but could then be preferentially amplified by RT–PCR in a preparation lacking significant amounts of the longer full-length mRNA.11–13 Alternatively, as Pkhd1 is a large gene, 472kb, there may be a great deal of aberrantly spliced Pkhd1 RNA which is not represented in the mature poly-A RNA pool (as they often contain premature stop codons these aberrantly spliced transcripts may be degraded by nonsense mediated decay). In our hands, the use of total cellular RNA (nonpoly-A selected) leads to the generation of smaller aberrantly spliced products which are easily amplified but often contain premature stop codons.4
Removal of the Pkhd1LSL(−)/LSL(−) LSL cassette from the germline yields the Pkhd1Pk(+)/Pk(+) mouse which never develops liver disease and is phenotypically normal, showing the presence of two SV5–Pk tags in the N–terminus of fibrocystin have no influence on the function of the protein. Therefore, we are able to observe fibrocystin protein in its physiologic context without the problems associated with overexpression of cDNAs in transgenic animals. We are able to detect fibrocystin in untreated and unconcentrated Pkhd1Pk(+)/Pk(+) urine, using a directly labeled SV5–Pk1 HRP antibody, avoiding a common problem of secondary detection in mice where endogenous Ig overwhelms the true signal. Our data show that there is one fibrocystin polypeptide at the cellular level and is probably processed by a proprotein convertase on shedding on ELVs (at ANSERKRKR NC in the mouse sequence). The N–terminal portion remains attached to the ELV as shown by our fractionation data. Proteomics show that the C-terminal of fibrocystin is also present in the human PKD-ELV, running at a molecular weight of 55 to 60 kD and Kaimori suggests that the N and C fragments are disulfide linked (Figure 6G).5,10 The fact that only the PKD-ELV fibrocystin has undergone proteolytic processing and that it has complex Endo H resistant carbohydrate demonstrates that the mature, (and by implication, functional) form of fibrocystin only exists on the PKD–ELVs.
We were able to visualize SV5–Pk positive PKD–ELVs on primary cilia by ISEM (Figure 7), and to visualize fibrocystin staining on the primary cilium in the absence of visible ELVs. We were also able to show that urinary Pkhd1Pk(+)/Pk(+) PKD–ELVs are functional in our PKD–ELV/primary cilium interaction assay with PKD–ELVs interacting with WT primary cilia within one minute and being cleared from the primary cilium within 10 min. We also detected ciliary side branches emanating from primary cilia when challenged with diluted mouse urine. Occasionally, these were seen to interact with PKD–ELVs (Figure 8A). We are unsure as to the nature of these structures and as far as we know this is the first time they have been observed. We think that they are microtubule based short extensions which are actively stimulated by the proximity of PKD–ELVs. Further study of these structures will be difficult unless there is a way to induce them at will and in abundance, as they are relatively rare structures that are not suited for TEM.
A fast PKD–ELV/primary cilium interaction is necessary in the context of urine flowing through a tubule, and the rapid clearance of ELVs from the primary cilium may be due to fibrocystin integrating with the retrograde motors in the cilium. This ‘urocrine’ mechanism may be involved in transporting hydrophobic mediators and preformed complexes over relatively long distances. For example, urinary ELVs have been shown to contain polycystin–1 and 2, which are subunits of a large ligand gated Ca++ channel, and smoothened, the constitutionally active seven-membrane spanning receptor in the hedgehog pathway,10 raising the possibility of PKD–ELVs being involved in transmitting a range of signals. For example, alterations in fluid flow along the tubule may dislodge or induce the secretion of ELVs which interact with downstream cilia, inducing a flow dependent intracellular Ca++ flux. This would require a fast interaction between ELVs and primary cilia similar to the interaction observed in this study.
CONCISE METHODS
Generation of the Knockout Construct
A 11.526kb λ–phage extending from an endogenous HinDIII 1.174kb 5′ from the start of the first exon of Pkhd1 to a MboI (modified with an NotI adaptor) site 557bp 3′ of exon 5 was utilized and cloned into the kanamycin resistance vector pZero–2. This had a 3′ vector derived NotI site which was used to introduce a PGK promoter driven thymidine kinase gene for negative selection. The 5′ HinDIII site was deleted by the insertion of oligonucleotides creating a SrfI site. The region 369bp 5′ to 255bp 3′ of exon–3 was replaced by a β–lactamase cassette flanked with an SbfI site and a PacI site, using the recombineering Escherichia coli stain DY380.23 Next, the region between 369bp 5′ to 255 3′ of exon–3 was amplified with the 5′ end was modified to supply a SbfI site and SalI site, and the 3′ end to supply a PacI site. Then exon–3 was opened at an endogenous XhoI site and two mutually priming oligonucleotides were inserted to generate the sequence PIPNPLLGLDSPGTPIPNPLLGLDS encoding two copies of the SV5–Pk sequence described by Hanke.24 We then modified this construct by inserting the LSL cassette into the 5′ SalI site such that the pac gene was expressed in an antisense direction to Pkhd1. The LSL–SV5–Pk–Exon3 construct was then inserted SbfI PacI into the β–lactamase targeted phage to generate the final construct, removing the β-lactamase cassette. The final targeting construct has arms of 5.4kb and 6.2kb. SrfI was used to linearize the construct and it was used to create targeted ES clones as described by van Deursen,25 except that puromycin was used as a selective agent at 1.5 μg/ml, the recombination rate was 19.6%. Two independent clones went germline on a C57BL/6J background and were then inbred onto C57BL/6J and BALB/cJ backgrounds until the 10th generation. Both strains had identical phenotypes. To produce the Pkhd1Pk(+)/Pk(+) animal we crossed both strains onto GDF–9–iCre transgenic mice at generation F2 and then inbred the resultant Pkhd1Pk(+)/Pk(+) animals onto C57BL/6J to F10.22 We ensured that the LSL was appropriately removed by this manipulation leaving a single SbfI site and a SalI flanked loxP site in intron 2 as dictated by the original design. We confirmed that the Pkhd1LSL(−)/LSL(−) mice could not make Pkhd1 mRNA and that the gene was reactivated in Pkhd1Pk(+)/Pk(+) mice by northern blotting and RT–PCR.
PCR Diagnostics for Pkhd1LSL(−)/LSL(−) and Pkhd1Pk(+)/Pk(+) Mice
Genomic DNA was extracted from tail samples using the Puregene DNA Purification Kit (Qiagen). The Pkhd1LSL(−) allele was detected with primers TGGATGTGGAATGTGTGCGAG, and TTACGGCAACGGTGGTTTCTTCGG with cycling parameters of 32 cycles at 94 °C for 30 s, 65 °C for 30 s, and 72 °C for 30 s and the product was 341bp. The Pkhd1Pk(+) allele was detected with primers TTCTCTGGACCAATAATGCCTG and AAACCTACCGTCAAATACAACTGTG with cycling parameters of 30 cycles at 94 °C for 30s, 60 °C for 30 s, and 72 °C for 1 min and the product was 351bp.
Western Blotting
Mouse kidney membrane preparations were prepared by mincing and Dounce homogenization in 0.25M sucrose, 10mM Tris pH 7.5 with 200μM calcium chloride, with EDTA-free Complete 1x, proteinase inhibitors, 2.5ml per kidney. Nuclei and intact cells were removed by centrifugation at 500g for 5 min, the supernatant was then diluted four fold in 0.25M sucrose, 10mM Tris pH 7.5, 1mM EDTA with Complete, then centrifuged at 4000g for 15 min. The supernatant was underlayed by a 1M Sucrose cushion and ultracentrifuged at 30,000g for 1 h. The interface was collected diluted with the EDTA buffer to fill a 10 ml ultracentrifuge tube and spun at 100, 000g for 1 h. The pellet was recovered and resuspended in the EDTA buffer for long term storage in aliquots at −80 °C. WT, Pkhd1LSL(−)/LSL(−) and Pkhd1Pk(+)/Pk(+) urines were collected from mice in metabolic cages onto a single Complete (EDTA–free) mini tablet to ensure protease inhibition. Urine was precleared at 4000g for 15 min to remove debris. This urine could be used directly on western blotting. To prepare ELVs, precleared urine was centrifuged at 100,000g for 1 h at 4 °C for 1 h then the supernatant and pellet were collected. Western blotting was performed on Invitrogen, 10 well 4% polyacrylamide Tris–glycine gels to resolve 200–600 kD or 10 well 4–12% polyacrylamide MOPS gels to resolve between 15–600 kD. Blotting was performed with either invitrogen Tris–glycine transfer buffer or Nu–PAGE transfer buffer with 0.01% SDS onto Trans–Blot R® pure nitrocellulose (0.45μ) membrane at 15V for 4 h. The membranes were blocked in 20 mM Tris pH 7.5 0.15M NaCl and 0.05% Tween 20, with 5% non–fat milk added. A HRP conjugated SV5-Pk1 antibody (Serotec MCA1360P) was used to detect tagged protein at a concentration of 1:4000 at room temperature for 30 min then washed 3 times in TBS, before detection with SuperSignal R® West Femto substrate (Thermo scientific). Film was Kodak BIOMAX MS and exposures were 20 s to 2 min.
Primary Cell Culture
Kidney tissue was sliced into 1 mm fragments and placed in a 1mg/ml collagenase type IV solution (Sigma) in dissection solution (Hanks Buffered Saline Solution (HBSS) with 10mM Glucose, 5mM Glycine, 1mM β–Alanine and 15mM HEPES) for a 30 min digestion at 37 °C. Digested fragments were sieved through a 250μm pore size nylon sieve and washed with dissection solution. Flow-through of tissue fragments were then transferred to an 80μm nylon sieve and washed with dissection solution. PT cells that were captured on the 80μm sieve were resuspended in dissection solution with 1% BSA in dissection solution and centrifuged for 5 min at 200g.26 Cells were then resuspended in cell culture media (DMEM/F12 without Phenol red with 15mM HEPES, 2.5mM L-glutamine, 10% Fetal Bovine Serum, 500nM Dexamethasone, 5 μg/ml Insulin, 5 μg/ml Transferrin, 50nM Sodium selenite, 0.55mM Sodium Pyruvate, and 10ml/L Nonessential amino acids) and plated onto BD biocoat type IV collagen glass slides (BD biosciences). These cells were >90% LTA positive.
Scanning Electron Microscopy (SEM)
PT explants were grown on poly–L–Lysine laminin coated glass coverslips until confluence, and then for another 7 d in 10% fetal bovine serum (FBS) containing medium which was then swapped for 0.4% FBS for another 3 d. For fixation cells were transferred to 0.5ml of serum free medium and cultured for 2 h, then 0.5ml of 37 °C, 2x fixative (8% paraformaldehyde and 0.4% glutaraldehyde, PBS) was added. Samples were blocked with 10% FBS in PBS. After rinsing with phosphate buffer 5 times, the samples were incubated overnight at 4 °C with SV5–Pk1 antibody diluted with blocking solution (1:10), washed 3x in blocking buffer, incubated for 1 h at room temperature with rabbit anti–mouse IgG diluted 1:1500 with blocking solution, washed 3x in blocking buffer, and then probed with protein A conjugated with 15nm gold particles (1:70 diluted with blocking solution) for 2 h. The samples were post–fixed in 1% glutaraldehyde for 10 min, incubated in OsO4 for 2 h, dehydrated, critical-point dried, and carbon coated to 22nm or osmium plasma coated to a depth of 1nm. Images were generated at 5–10 kV by a Hitachi S-4700 microscope (Hitachi, Pleasanton, California).
PKD–ELV/Primary Cilium Assay
Fresh urine from Pkhd1Pk(+)/Pk(+) mice was obtained by placing mice in a box with 96 well microtiter plates covering the bottom this separates urine from feces. Urine was gently spun at 1000g for 1 min to remove debris then diluted in serum free tissue culture medium and applied to WT ciliated PT cells and incubated for 1, 2, 5 or 10 min at 37 °C, before fixation in 2x fixation buffer and then stained for SV5-Pk and prepared for SEM as above. 15nm gold and 1nm Osmium coating was used.
Histology
Mice were anesthetized with 50mg/kg sodium pentobarbital. The left ventricle was cannulated and the inferior vena cava cut. The mice were perfused with PBS followed by a similar volume of 4% parafomaldehyde. Tissue was paraffin-embedded. Hemotoxylin/eosin and Mallory trichrome staining was performed according to standard procedures by the Mayo Clinic Histology laboratory. Slides were observed using a Zeiss AxioObserver (Carl Zeiss) microscope at a magnification of ×5 and ×40 magnification.
Immunofluorescence
Neonatal mouse kidneys were placed in 4% parafomaldehyde and embedded in paraffin. 4μm sections were taken, mounted on positively charged slides and dried for 20 min at 60 °C. The staining protocol is identical to that of Tammachote,27 with the addition of 0.1% Sudan Black B (Sigma) for 20 min after the series dehydration steps (to quench autofluorescence). Antigen retrieval was accomplished by either steaming in sodium citrate for 45 min or 1 μg/ml proteinase K treatment for 15 min at 37 °C. Slides were observed using a Zeiss AxioObserver (Carl Zeiss) microscope at a magnification of ×100.
RNA Analysis
Total cellular RNA was isolated using the acid phenol technique of Chomczynski.28 mRNA was then obtained from total RNA using PolyATtract mRNA Isolation System III (Promega) according to the manufacturer's instruction. 200–300ng of mRNA was reverse transcribed with Random Primers and Super-Script III Reverse Transcriptase (Invitrogen) using the manufacturers protocol. Amplification of fibrocystin was accomplished by generating primers from the cDNA sequence of fibrocystin using Macvector 8.1.1 (Accelrys). Primer sequences (see Table 1).
Table 1.
Oligonucleotides used in long RT-PCR
| Exon in Pkhd1 | Oligonucleotide |
|---|---|
| Ex. 1_21F | TCATTTGAGGCACAAGGCTGAC |
| Ex. 1_21R | GGAACCACGGGGCGGATG |
| Ex. 19_34F | CTCCAGGTCTGATGGGGTCC |
| Ex. 19_34R | CACAGGGAACTCTCTTCACAAAGG |
| Ex. 32_52F | AACTTCTTCATCGTGCCTCAGGTGC |
| Ex. 32_52R | TCCCCAGCCTTTTTCAACATCTTGC |
| Ex. 48_67F | AGATTGTCCCTGGCAGCGTC |
| Ex. 48_67R | GGTTTCTGGTGGAGCAGTATGG |
The megalin primers are forward primer 5-GAGGAGAATCCCAAGTGGTTCG −3 and reverse primer 5-ATGACTGCCTGATACACCGTCC-3. PCR was with Kapa Long–Range PCR (KAPABIOSYSTEMS).
Northern Blotting.
Northern blotting was done with 10 μg of total cellular RNA denatured in formaldehyde/formamide and run on a MOPS–formaldehyde 0.5% agarose gel, 50V overnight. The gels were washed in milliQ water 3 times and transfered onto Zeta–Probe Nylon blotting membranes. The blots were probed with the first 18 or the last 7 exons of Pkhd1, a Pkd1 or Lrp2 cDNA probe.
TaqMan Assay.
The expression studies of the selected genes are analyzed in a TaqMan probe-based quantitative real-time reverse transcriptase PCR (qRT-PCR) system. TaqMan probes and primers are purchased through Applied Biosystems. Specifically, products of ∼200bp or less will be amplified using Taqman gene Expression Master Mix (Applied Biosystems, Foster City, California) combined with specific TaqMan assay mix consists of a TaqMan MGB probe and two PCR primers. In this case, we developed a TaqMan single tube assay for mouse Pkhd1 (ABI catalog# Mm01233728_m1) and the house keeping gene, mouse transferrin receptor (Tfrc) (ABI catalog # Mm00441941_m1). Both probes span exon-exon junctions (exons 2 to 3 in Tfrc and exons 48 to 49 in the case of Pkhd1) and in the WT cross the Ct within a cycle of one another. Samples are run on a 7900 real-time PCR System (Applied Biosystems) at 95 °C for 10 min followed by 40 cycles of 15 sat 95 °C and 60 s at 60 °C. All reactions are performed in >triplicates to assure the accuracy of the assay. The values obtained for the target gene expression are normalized to endogenous control gene and quantified relative to the expression of control samples. For the calculation of relative quantification, the ΔΔCT method will be used to calculate the fold-differences in target genes between case samples and control samples.
Ethical Treatment of Animals
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Mayo Clinic, Rochester, MN (“Production of mouse models for autosomal recessive polycystic kidney disease [ARPKD],” IACUC: A35808). There was no recovery surgery and all efforts were made to minimize suffering.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This study was funded by the National Institutes of Health (NIH) grant 5R01DK065056–02 ‘Analysis of ARPKD by Targeted Manipulation of Pkhd1’ and the PKD Foundation grant ‘Analysis of the Interaction of Polycystin–1 positive exosome–like vesicles with the Primary Cilium’ as well as the Mayo Translational PKD Center (MTCP), Molecular Genetics and Proteomics Core. We would also like to thank Dave Tuveson for the gift of pBS.DAT-LoxStop plasmid which contains the LSL cassette.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Tagged Fibrocystin Sheds Its Secrets,“ on pages 2148–2150.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Zerres K, Mucher G, Becker J, Steinkamm C, Rudnik-Schoneborn S, Heikkila P, Rapola J, Salonen R, Germino GG, Onuchic L, Somlo S, Avner ED, Harman LA, Stockwin JM, Guay-Woodford LM: Prenatal diagnosis of autosomal recessive polycystic kidney disease (ARPKD): Molecular genetics, clinical experience, and fetal morphology. Am J Med Genet 76: 137–144 1998 [PubMed] [Google Scholar]
- 2. Jorgensen MJ: The ductal plate malformation. Acta Pathol Microbiol Scand Suppl: 1–87, 1977 [PubMed] [Google Scholar]
- 3. Zerres K, Rudnik-Schoneborn S, Steinkamm C, Becker J, Mucher G: Autosomal recessive poly-cystic kidney disease. J Mol Med 76: 303–309, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC: The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet 30: 259–269, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Kaimori JY, Nagasawa Y, Menezes LF, Garcia-Gonzalez MA, Deng J, Imai E, Onuchic LF, Guay-Woodford LM, Germino GG: Polyductin undergoes notch-like processing and regulated release from primary cilia. Hum Mol Genet 16: 942–956, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoder BK, Hou X, Guay-Woodford LM: The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13: 2508–2516, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan S, Bacallao R, Torra R, LaRusso NF, Torres VE, Harris PC: Cellular and subcellular localization of the ARPKD protein: Fibrocystin is expressed on primary cilia. Hum Mol Genet 12: 2703–2710, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Wang S, Luo Y, Wilson PD, Witman GB, Zhou J: The autosomal recessive polycystic kidney disease protein is localized to primary cilia, with concentration in the basal body area. J Am Soc Nephrol 15: 592–602, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Menezes LFC, Cai Y, Nagasawa Y, Silva AMG, Watkins ML, Silva AMD, Somlo S, Guay-Woodford LM, Germino GG, Onuchic LF. Polyductin: The PKHD1 gene product, comprises isoforms expressed in plasma membrane, primary cilium, and cytoplasm. Kidney Int 66: 1345–1355, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, Huang BQ, Leontovich AA, Beito TG, Madden BJ, Charlesworth MC, Torres VE, Larusso NF, Harris PC, Ward CJ: Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol 20: 278–288, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Onuchic LF, Furu L, Nagasawa Y, Hou X, Eggermann T, Ren Z, Bergmann C, Senderek J, Esquivel E, Zeltner R, Rudnik-Schoneborn S, Mrug M, Sweeney W, Avner ED, Zerres K, Guay-Woodford LM, Somlo S, Germino GG: PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet 70: 1305–1317, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagasawa Y, Matthiesen S, Onuchic LF, Hou X, Bergmann C, Esquivel E, Senderek J, Ren Z, Zeltner R, Furu L, Avner E, Moser M, Somlo S, Guay-Woodford L, Buttner R, Zerres K, Germino GG: Identification and characterization of PKHD1, the mouse orthologue of the human ARPKD gene. J Am Soc Nephrol 13: 2246–2258, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Boddu R, Germino GG, Onuchic LF, Guay-Woodford LM: Intragenic motifs may regulate Pkhd1/PKHD1 transcriptional complexity. American Society of Nephrology: [TH-PO684], 2009 [Google Scholar]
- 14. Moser M, Matthiesen S, Kirfel J, Schorle H, Bergmann C, Senderek J, Rudnik-Schneborn S, Zerres K, Buettner R: A mouse model for cystic biliary dysgenesis in autosomal recessive polycystic kidney disease (ARPKD). Hepatology 41: 1113–1121, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Woollard JR, Punyashtiti R, Richardson S, Masyuk TV, Whelan S, Huang BQ, Lager DJ, van-Deursen J, Torres VE, Gattone VH, LaRusso NF, Harris PC, Ward CJ: A mouse model of autosomal recessive polycystic kidney disease with biliary duct and proximal tubule dilatation. Kidney Int 72: 328–336, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Garcia-Gonzalez MA, Menezes LF, Piontek KB, Kaimori J, Huso DL, Watnick T, Onuchic LF, Guay-Woodford LM, Germino GG: Genetic interaction studies link autosomal dominant and recessive polycystic kidney disease in a common pathway. Hum Mol Genet 16: 1940–1950, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gallagher AR, Esquivel EL, Briere TS, Tian X, Mitobe M, Menezes LF, Markowitz GS, Jain D, Onuchic LF, Somlo S: Biliary and pancreatic dysgenesis in mice harboring a mutation in PKHD1. Am J Pathol 172: 417–429, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams SS, Cobo-Stark P, James LR, Somlo S, Igarashi P: Kidney cysts, pancreatic cysts, and biliary disease in a mouse model of autosomal recessive polycystic kidney disease. Pediatr Nephrol 23: 733–741, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, Mercer KL, Grochow R, Hock H, Crowley D, Hingorani SR, Zaks T, King C, Jacobetz MA, Wang L, Bronson RT, Orkin SH, De-Pinho RA, Jacks T: Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 5: 375–387, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Tanaka Y, Okada Y, Hirokawa N: FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature 435: 172–177, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Sullivan R, Saez F, Girouard J, Frenette G: Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells Mol Dis 35: 1–10, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Lan ZJ, Xu X, Cooney AJ: Differential oocyte-specific expression of Cre recombinase activity in GDF-9-iCre, Zp3cre, and Msx2Cre transgenic mice. Biol Reprod 71: 1469–1474, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Lee EC, Yu D, de Velasco JM, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG: A highly efficient escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73: 56–65, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Hanke T, Szawlowski P, Randall RE: Construction of solid matrix-antibody-antigen complexes containing simian immunodeficiency virus p27 using tag-specific monoclonal antibody and tag-linked antigen. J Gen Virol 73 (Pt 3): 653–660, 1992 [DOI] [PubMed] [Google Scholar]
- 25. van Deursen J: Gene targeting in mouse embryonic stem cells. Methods Mol Biol 209: 145–158, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Terryn S, Jouret F, Vandenabeele F, Smolders I, Moreels M, Devuyst O, Steels P, Kerkhove EV: A primary culture of mouse proximal tubular cells, established on collagen-coated membranes. Am J Physiol Renal Physiol 293: F476–F485, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Tammachote R, Hommerding CJ, Sinders RM, Miller CA, Czarnecki PG, Leightner AC, Salisbury JL, Ward CJ, Torres VE, Gattone VH, Harris PC: Ciliary and centrosomal defects associated with mutation and depletion of the meckel syndrome genes MKS1 and MKS3. Hum Mol Genet 18: 3311–3323, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 29. Notredame C, Higgins DG, Heringa J: T-coffee: A novel method for fast and accurate multiple sequence alignment. J Mol Biol 302: 205–217, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.