Abstract
American Indians/Alaska Natives (AIANs) compose a heterogeneous population that includes geographically distinct tribal communities, many with high rates of ESRD. Regional features of dialysis care and mortality are unknown in this population. Here, we describe the structure of dialysis care and mortality of adult AIANs who initiated maintenance dialysis during 1995−2008 in different regions of the US. Overall, 13,716 AIANs received dialysis at 2054 facilities. Approximately 10% (n = 197) of these facilities provided care to two-thirds (n = 9011) of AIANs. AIANs from the Southwest and Alaska were concentrated in relatively few dialysis facilities whereas those in the Eastern US and Pacific Coast were distributed more diffusely. Despite comparably high rates of poverty, diabetes, and cardiovascular disease, annual mortality rates were lower in the Southwest (13.9%) compared with the Southern Plains (23.2%), Alaska (21.2%), Eastern US (20.0%), Northern Plains (20.8%), and Pacific Coast (22.0%). These regional differences were consistent over time and persisted after adjusting for sociodemographic and clinical variables and area-based poverty. In conclusion, regional differences in the structure of dialysis care and patient mortality exist among AIANs. Southwestern AIANs experience the highest concentration of dialysis care and the lowest mortality. Our findings suggest that an area-based approach examining the care structure of relatively few dialysis facilities may delineate determinants of these differences and improve the quality of care to many AIAN communities.
In the United States (US), rates of end-stage renal disease (ESRD) are over two-fold higher among American Indians and Alaska Natives (AIANs) compared with white Americans.1 Prior studies have reported clustering of ESRD and cardiovascular disease risk factors among some American Indian tribes in the Southwestern US.2–4 However, the few studies that have examined ESRD outcomes such as mortality and kidney transplantation among AIANs, have been limited primarily to specific geographic regions or tribal areas within the US.2,5,6
To date, no studies have comprehensively examined ESRD outcomes among AIANs nationally or compared specific determinants of dialysis mortality uniformly across regional AIAN communities. The extent to which differences in the structure and quality of dialysis care exist and how these factors might influence ESRD outcomes among diverse AIAN communities is likewise poorly understood. Thus, despite increasing numbers of AIANs on dialysis, the relative influences of individual-level factors such as obesity, diabetes and cardiovascular disease, and dialysis structural factors such as facility size, ownership, and achievement of performance measures on mortality are unknown.
To describe geographic differences in patient characteristics, dialysis care structure and mortality of AIANs, we examined data from two national registries that included information on all adults identified as AIAN who initiated dialysis during 1995−2008 and their dialysis facilities. Based on studies of AIANs in the general population, we hypothesized that dialysis-related mortality would be highest among AIANs in the Southern Plains and that any difference in mortality would be primarily attributable to differences in sociodemographic, comorbid and behavioral risk factors for death.7,8
RESULTS
Patient Characteristics
A total of 13,716 American Indians and Alaska Natives (AIANs) initiated dialysis during the study period. In contrast with the general US dialysis population, more AIAN women (53 percent) than men initiated dialysis during the study. Mean age at dialysis initiation among AIANs was 57.6 yr and did not appreciably differ across geographic regions (Table 1). However, the distribution of health insurance coverage differed substantially: AIANs from the Southwest had the highest rates of Medicaid and those from the Southern Plains had the highest rates of lacking health insurance coverage. Conversely, AIANs from the Pacific Coast had the highest rates of employer group health coverage at the time of dialysis initiation (P < 0.001).
Table 1.
Characteristics of American Indians and Alaska Natives initiating dialysis 1995–2008 by geographic region
| Individual-Level Characteristics† | Geographic Region |
||||||
|---|---|---|---|---|---|---|---|
| Southern Plains |
Alaska |
Southwest |
Northern Plains |
Eastern US |
Pacific Coast |
P-value* | |
| (n = 2,388) | (n = 139) | (n = 4,800) | (n = 2,830) | (n = 1,418) | (n = 2,142) | ||
| Age, mean (SD), years | 57.9 (0.3) | 58.8 (1.4) | 56.9 (0.2) | 57.1 (0.3) | 58.3 (0.4) | 58.7 (0.3) | <0.001 |
| Age category, yrs, N (%) | <0.001 | ||||||
| 18–39 | 227 (10) | 17 (12) | 498 (10) | 291 (10) | 126 (9) | 214 (10) | |
| 40–49 | 372 (16) | 23 (17) | 832 (17) | 472 (17) | 226 (16) | 297 (14) | |
| 50–59 | 655 (27) | 25 (18) | 1.348 (28) | 793 (28) | 377 (27) | 580 (27) | |
| 60–69 | 677 (28) | 34 (25) | 1.268 (26) | 761 (27) | 389 (27) | 546 (26) | |
| ≥70 | 457 (19) | 40 (29) | 854 (18) | 513 (18) | 300 (21) | 505 (24) | |
| Female, N (%) | 1,222 (51) | 68 (49) | 2,618 (55) | 1,530 (54) | 778 (55) | 1,085 (51) | 0.006 |
| Health insurance‡, N (%) | |||||||
| employer group | 323 (14) | 20 (14) | 617 (13) | 381 (14) | 224 (16) | 439 (21) | |
| Medicare | 1,078 (45) | 64 (46) | 2,017 (42) | 1,247 (44) | 529 (37) | 1,001 (47) | |
| Medicaid | 727 (30) | 50 (36) | 2,020 (42) | 1,116 (39) | 579 (41) | 650 (30) | |
| no coverage | 355 (15) | 4 (3) | 246 (5) | 195 (7) | 61 (4) | 228 (11) | |
| other coverage | 631 (26) | 77 (55) | 1,797 (37) | 1,060 (38) | 508 (36) | 534 (25) | |
| Attributed cause of ESRD, N (%) | <0.001 | ||||||
| diabetes | 1,796 (75) | 48 (35) | 3,850 (80) | 2,140 (76) | 954 (67) | 1,273 (59) | |
| hypertension | 262 (11) | 19 (14) | 229 (5) | 180 (6) | 169 (12) | 418 (19) | |
| glomerulonephritis | 117 (5) | 30 (22) | 402 (8) | 211 (8) | 123 (9) | 172 (8) | |
| cystic disease | 31 (1) | 6 (4) | 12 (0) | 28 (1) | 13 (1) | 24 (1) | |
| other | 182 (8) | 36 (26) | 307 (6) | 271 (10) | 159 (11) | 255 (12) | |
| Clinical and laboratory measures, N (%) | |||||||
| diabetes | 1,734 (73) | 61 (44) | 3,767 (79) | 2,166 (76) | 996 (70) | 1,289 (60) | <0.001 |
| CVD§ | 966 (40) | 49 (35) | 1,389 (29) | 1,211 (43) | 479 (34) | 815 (38) | <0.001 |
| poor functional status | 197 (8) | 13 (9) | 211 (4) | 199 (7) | 91 (6) | 110 (5) | <0.001 |
| drug or tobacco use | 176 (7) | 23 (17) | 101 (2) | 277 (10) | 120 (8) | 166 (8) | <0.001 |
| cancer | 103 (4) | 9 (6) | 84 (2) | 111 (4) | 56 (4) | 83 (4) | <0.001 |
| BMI** >30 kg/m2 | 704 (31) | 39 (37) | 1,426 (30) | 984 (37) | 488 (40) | 680 (34) | <0.001 |
| serum albumin <3.5 g/dl | 1,416 (74) | 77 (58) | 2,644 (83) | 1,923 (82) | 824 (77) | 1,186 (71) | <0.001 |
| hemoglobin <10 g/dl | 1,403 (63) | 72 (55) | 1,818 (53) | 1,550 (58) | 711 (55) | 1,162 (58) | <0.001 |
| ESA†† | 460 (19) | 59 (43) | 925 (19) | 992 (35) | 480 (34) | 661 (31) | <0.001 |
| ZIP code-level characteristic | |||||||
| Residents living in poverty, N (%) | <0.001 | ||||||
| <5% | 52 (2) | 4 (4) | 81 (2) | 106 (4) | 58 (4) | 182 (9) | |
| 5–9% | 221 (10) | 47 (46) | 249 (5) | 450 (16) | 239 (18) | 379 (18) | |
| 10–14% | 412 (18) | 41 (41) | 362 (8) | 372 (14) | 356 (27) | 315 (15) | |
| 15–19% | 694 (31) | 2 (2) | 480 (10) | 342 (12) | 247 (18) | 302 (15) | |
| ≥20% | 862 (38) | 7 (7) | 3,403 (74) | 1,473 (54) | 437 (33) | 891 (43) | |
| Mean per capita income, $ (SD) | 15,103 (84) | 21,720 (458) | 10,918 (83) | 13,339 (113) | 17,826 (201) | 17,631 (147) | <0.001 |
| Percentage of adults with 4-yr college degree, % (SD) | |||||||
| 15 (8) | 23 (8) | 11 (9) | 16 (9) | 18 (12) | 18 (12) | <0.001 | |
*P-value for the global F-test statistic <0.001 (test of homogeneity).
†Due to rounding percentages may not total 100%;
‡Health insurance coverage may sum to greater than 100% in patients with multiple sources of coverage;
§Cardiovascular disease;
**Body mass index;
††Prescribed erythropoiesis-stimulating agent; SD = standard deviation.
Data are presented without imputation and reflect values at the time of initiation of renal replacement therapy; Overall, 24.9% of study subjects were missing data for serum albumin, 14.7% for hemoglobin, 5.3% for body mass index, 4.7% for residential poverty, and 1.2% for zipcode.
Overall, AIANs from the Southwest had the highest prevalence of diabetes and those from the Southern Plains had the highest prevalence of cardiovascular disease (Table 1). Relative to the general US dialysis population, the prevalence of diabetes was substantial among all AIANs: rates of diabetes were notably lower among AIANs from Alaska and the Pacific Coast compared with AIANs from other geographic regions. In addition, AIANs from the Southern Plains had the highest fraction of patients initiating dialysis with hemoglobin values below 10 g/dl, and along with Southwestern AIANs, were the least likely group to have received an erythropoiesis stimulating agent (P < 0.001 for all comparisons).
Residential Zip Code Characteristics
The study population was distributed across 2201 residential zip codes in the US. Approximately 55 percent of AIANs resided in the most impoverished areas where at least one in five residents lived in poverty. These zip codes corresponded to areas with the lowest median per capita income and the lowest percentage of college graduates among the study sample. Residence in a poverty area was highest among AIANs from the Southwest and lowest among those from the Alaska (Table 1).
Dialysis Facility Characteristics
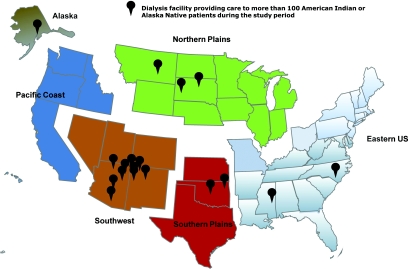
During the study period, approximately 2054 dialysis facilities across the US provided care to at least one AIAN patient; the total number of certified dialysis facilities in the US at the study mid-point, 2002, was 4379. Notable regional differences were evident in the structure and characteristics of dialysis facilities where AIANs initiated dialysis (Table 2). Overall, fewer than 5 percent (n = 197) of all US dialysis facilities provided care to nearly two-thirds (n = 9011) of the AIAN dialysis population. The concentration of dialysis care for AIANs was particularly high in the Southwest where 24 dialysis facilities provided care to nearly 60 percent (n = 2856) of the regional AIAN dialysis population. In contrast, the dialysis care of AIANs from the Eastern US and Pacific Coast was dispersed among 1210 dialysis facilities; only two facilities in these regions provided care to more than 100 AIAN patients during the study period (Figure 1).
Table 2.
Characteristics of dialysis facilities where American Indian and Alaska Native patients initiated dialysis
| Facility-Level Characteristics | Geographic Region |
|||||
|---|---|---|---|---|---|---|
| Southern Plains |
Alaska |
Southwest |
Northern Plains |
Eastern US |
Pacific Coast |
|
| (n = 283) | (n = 4) | (n = 217) | (n = 339) | (n = 358) | (n = 852) | |
| No. AIAN patients per facility, mean (SD) | 8 (17) | 32 (54) | 22 (44) | 8 (18) | 4 (6) | 2 (8) |
| Total no. of dialysis stations, mean (95% CI) | 18 (17, 18) | 20 (5, 35) | 17 (17, 17) | 13 (13, 13) | 18 (17, 19) | 20 (20, 21) |
| Total no. of dialysis patients, mean (95% CI) | 74 (71, 76) | 180 (166, 194) | 83 (82, 85) | 59 (57, 61) | 101 (97, 105) | 93 (90, 95) |
| Large chain dialysis organization,% (95% CI) | 47 (45, 49) | 34 (30, 36) | 45 (43, 46) | 21 (20, 23) | 43 (40, 45) | 45 (43, 47) |
| Home-based dialysis training,% (95% CI) | 31 (29, 33) | —– | 25 (24, 27) | 33 (31, 35) | 46 (44, 49) | 40 (38, 42) |
| Ownership category, % (95% CI) | ||||||
| for-profit | 72 (69, 75) | 49 (36, 62) | 68 (66, 69) | 35 (33, 38) | 60 (56, 64) | 68 (65, 71) |
| non-profit | 26 (23, 29) | 51 (38, 63) | 26 (24, 28) | 56 (53, 58) | 35 (31, 38) | 28 (26, 31) |
| non-federal or federal government | 2 (1, 4) | 0 (—) | 7 (6, 8) | 9 (7, 11) | 5 (3, 8) | 4 (3, 5) |
| Terminated, % (95% CI) | 3 (2, 3) | 1 (1, 2) | 2 (1, 2) | 3 (3, 4) | 2 (1, 2) | 1 (0, 1) |
| Hospital-based,% (95% CI) | 18 (16, 19) | 6 (2, 10) | 22 (21, 23) | 52 (50, 54) | 22 (20, 25) | 22 (20, 24) |
| Anemia management, % (95% CI) | ||||||
| <10 g/dl | 2 (2, 2) | 2 (2, 2) | 2 (2, 2) | 3 (2, 3) | 2 (2, 2) | 3 (3, 3) |
| 10–12 g/dl | 62 (61, 63) | 71 (70, 72) | 75 (75, 76) | 77 (76, 77) | 68 (67, 69) | 70 (69, 71) |
| >12 g/dl | 36 (35, 37) | 27 (26, 28) | 22 (22, 23) | 21 (20, 22) | 29 (29, 30) | 27 (26, 27) |
| URR ≥ 65 percent, % (95% CI) | 96 (96, 96) | 96 (95, 96) | 97 (97, 98) | 93 (93, 94) | 95 (94, 95) | 95 (95, 96) |
| Facility survival*, % (95% CI) | ||||||
| better than expected | 3 (3, 4) | — | 31 (30, 33) | 21 (19, 23) | 17 (15, 20) | 10 (9, 12) |
| as expected | 66 (64, 68) | 13 (8, 20) | 62 (60, 64) | 76 (74, 77) | 73 (71, 76) | 67 (65, 69) |
| worse than expected | 30 (28, 33) | 87 (80, 92) | 7 (6, 8) | 3 (3, 4) | 9 (8, 11) | 22 (20, 24) |
*This measure takes a facility's expected patient survival rate and compares it to the actual patient survival rate based on case-mix (i.e., age, race, sex, diabetes, dialysis vintage and comorbidity, available at www.medicare.gov/Dialysis) SD = standard deviation, CI = confidence interval, and URR = urea reduction ratio.
Figure 1.
High concentration of dialysis facilities providing care to at least 100 American Indian and Alaska Native patients during 1995–2008 in the Southwest.
Following national trends, facilities associated with for-profit organizations provided the majority of dialysis care to AIANs except in the Northern Plains, where approximately half of the facilities were hospital-based compared with less than 25 percent in the other regions. With respect to clinical performance measures, dialysis facilities providing care to AIANs in the Southwest and Northern Plains had the highest fraction of patients with hemoglobin levels in the 10 to 12 g/dl range. In contrast, facilities providing care to AIANs from the Southern Plains had the highest fraction of patients with hemoglobin levels in excess of 12 g/dl. Achievement of target urea reduction ratio was universally high among facilities from all geographic regions (Table 2).
Mortality
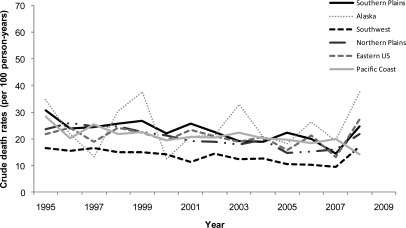
A total of 7927 (58%) patients died during 42,755 person-years of follow-up. Mean annual mortality rates from the time of dialysis initiation were lower among AIANs residing in the Southwest compared with the other geographic regions (Table 3). In general, crude mortality showed a modest decline over the duration of the study period with regional differences in mortality consistent over time (Figure 2). The lower mortality rate of AIANs in the Southwest compared with other regions was not attenuated in analyses adjusted for individual-level and area-based socioeconomic and clinical factors using the dialysis facility as the cluster variable (Table 3). Companion analyses stratified by age, sex, and diabetes status yielded similar results (P-value for Wald's test on interaction terms: 0.011, 0.275 and 0.194, respectively). Only Southwestern AIANs experienced consistently lower relative mortality compared with those from the Southern Plains across all age strata (data not shown).
Table 3.
Time to death among American Indians and Alaska Natives on dialysis by geographic region
| Region | Population at Risk No. | Deaths No. | Annual Rate, Percent per Year (95% CI) | Crude Model Hazard Ratio (95% CI) | Fully Adjusted Model* Hazard Ratio (95% CI) |
|---|---|---|---|---|---|
| Southern Plains | 2,388 | 1,533 | 23.2 (20.1, 24.1) | Referent | Referent |
| Alaska | 139 | 80 | 21.2 (20.2, 22.3) | 0.96 (0.76, 1.21) | 1.04 (0.87, 1.18) |
| Southwest | 4,800 | 2,429 | 13.9 (13.4, 14.4) | 0.59 (0.54, 0.64) | 0.58 (0.52, 0.65) |
| Northern Plains | 2,830 | 1,645 | 20.8 (19.8, 21.8) | 0.90 (0.83, 0.96) | 0.90 (0.82, 1.00) |
| Eastern US | 1,418 | 885 | 20.0 (18.0, 22.3) | 0.89 (0.82, 0.97) | 0.93 (0.83, 1.04) |
| Pacific Coast | 2,142 | 1,355 | 22.0 (20.9, 23.2) | 0.94 (0.87, 1.01) | 0.96 (0.87, 1.06) |
*Multiple imputation models adjusted for age, sex, health insurance coverage (employer group, Medicare, Medicaid, Medicare/Medicaid, other or no insurance), comorbid conditions (cardiovascular disease, insulin and non-insulin requiring diabetes, poor functional status, cancer, alcohol, tobacco and drug use, chronic lung disease), body size (body mass index: <18.5, 18.5–24.9, 25.0–29.9, ≥ 30.0 kg/m2), anemia (hemoglobin <10 g/dl), hypoalbuminemia (albumin <3.5 g/dl), zip code poverty (<5, 5–9, 10–14, 15–19, ≥ 20 percent of zip code residents living in poverty) using dialysis facility as the cluster variable.
Figure 2.
Consistently lower crude rates of death among AIANs in the Southwest relative to the other regions over the study period, 1995–2008.
Competing Risk: Kidney Transplantation
A total of 2515 (18%) patients were waitlisted and 1250 (50% of waitlisted patients) received a living or deceased donor kidney transplant during the follow-up period. Mean annual transplant rates from the time of dialysis initiation were higher among AIANs in the Northern Plains (4.6% [95% confidence interval: 4.2 to 5.1%]) compared with the Eastern US (3.0% [2.5 to 3.5%]), Southwest (2.2% [2.0 to 2.4%]), Southern Plains (2.4% [2.0 to 2.8%]) and Pacific Coast (3.3% [2.9 to 3.8%]). The relatively few transplants (n = 11) among AIANs in Alaska precluded meaningful estimates. In competing risks analysis the differential mortality rates were not attributable to differential rates of living or deceased donor transplantation (data not shown).
Access to Predialysis Medical Care
Counts of IHS clinics varied considerably by geographic region: Alaska had the highest (n = 197) and Eastern US the lowest (n = 32) number of IHS medical clinics. Intermediate counts of IHS clinics were observed in the Northern Plains (n = 125), Pacific Coast (n = 129), Southwest (n = 97) and Southern Plains (n = 53). Among patients with available data on the presence or absence of nephrology care before dialysis initiation (n = 3146), approximately 65% (n = 2036) reportedly received some predialysis care. In this subset of patients, AIANs residing on the Pacific Coast had the highest (73%) while those in the Southern Plains had the lowest (55%) likelihood of having received predialysis care. The fraction of patients receiving predialysis care in the other IHS regions was similar (65% in Alaska, 66% in Southwest, 66% in Northern Plains and 65% in Eastern US).
DISCUSSION
Over 30 yr ago, Donabedian proposed a model for assessing the quality of medical care based on structure, process, and outcome.9 According to Donabedian's model, process—the method by which healthcare is provided—is limited by the structure or environment in which it operates. We found marked regional differences in the structure of dialysis care among American Indians and Alaska Natives (AIANs) on dialysis. Compared with AIANs residing in other regions, the dialysis care of AIANs in the Southwest was highly concentrated in the hands of relatively few facilities. Although Southwestern AIANs suffered from comparably high rates of diabetes, cardiovascular disease, and poverty, they experienced substantially lower mortality than counterparts dialyzing in other geographic regions. Our study findings suggest that to further improve the quality of care to these relatively young AIAN patients, policymakers should consider an area-based approach that focuses on the structure of care at the level of the healthcare facility.
Although there is a paucity of national-level studies of chronic disease among AIANs, similar geographic patterns for cancer incidence and mortality have been described. For example, the highest rates of lung and colorectal cancer—two malignancies for which screening and risk factor management can lead to reduced incidence and mortality—occurred among AIANs in the Southern Plains while the lowest rates occurred in the Southwest.8 In our study, although regional differences in modifiable risk factors for death such as tobacco smoking and alcohol use7,8—often underascertained in observational studies—might account for the disparate mortality rates, differences in the structure of state- and local-level healthcare (e.g. Medicaid eligibility, capacity of local Indian Health Service facilities, municipal hospitals, and federally-funded clinics), which in turn influence access to general medical and subspecialty care, were likely to be operative.10 For example, AIANs in our study in the Southern Plains had the highest rates of lacking health insurance and the largest fraction of patients with low hemoglobin levels at dialysis initiation. They were also less commonly prescribed erythropoiesis stimulating agents compared with their counterparts in most other areas of the country. Moreover, among a subset of patients with available data on the presence of nephrology care before dialysis initiation, AIANs residing in the Southern Plains had the lowest (55%) while those on the Pacific Coast had the highest (73%) likelihood of having received nephrology care before dialysis initiation. Collectively, these data suggest that differences in access to and/or more willingness to utilize primary and subspecialty medical (“predialysis”) care might account for some of the regional discrepancies in mortality rates among AIANs on dialysis.11,12
Contrary to our original hypothesis, adjustment for conventional sociodemographic determinants of health disparities did not substantively attenuate regional differences in mortality and kidney transplantation observed among AIANs on dialysis. Although Southwestern AIANs had the highest rates of Medicaid and resided in the most impoverished communities, they experienced mortality rates that were 30 to 40 percent lower than counterparts in other geographic regions. As evidenced by dialysis facility-level clustering, it is plausible that the historical concentration of patients, cohort studies, and intervention trials among AIAN tribal communities in the Southwest may have led to heightened disease surveillance and more resources to deliver high-quality, culturally-competent general and subspecialty care to these populations.2–4,6 Regional differences in healthcare delivery structure (e.g., greater focus on chronic disease management compared with acute and subacute care), travel distance to access medical care including dialysis, and cultural hesitance to seek “Western” medicine for nonacute conditions may have also contributed to differential care delivery.10,11 These factors warrant further investigation.
National efforts to improve diabetes and hypertension control among AIANs receiving care within Indian Health Service—the federal agency that provides comprehensive health services to approximately 1.9 million AIANs (nearly 60 percent of AIANs)—have yielded substantial reductions in ESRD incidence among AIANs.10 However, healthcare services are provided in over 700 Indian Health Service and tribal health care facilities scattered throughout 36 states. These facilities have a variety of healthcare structures and delivery models, and most are located in rural and isolated areas.13 Thus, examining local or area-based differences in health systems and delivery models (e.g., healthcare access, referral patterns, and performance measures) and patient-level determinants of mortality and disability (e.g., BP and diabetes control, body weight, physical activity, and tobacco use) may provide further insight into pathways responsible for the geographic differences found.14 Our study findings align with the observations of Narva and Sequist and highlight the long-term impact of structural changes in disease management on the quality of delivered care.10 Because over 40 percent of AIANs from the general population receive healthcare services outside of the Indian Health Service, area-based provider-training to better identify and manage AIAN patients at risk for progressive chronic kidney disease and disability might further improve care delivery and outcomes particularly to patients who reside in urban areas removed from large tribal communities.14,15
Overall, our results suggest that the dialysis care of AIANs is largely concentrated in the hands of relatively few dialysis facilities. This concentration of care was particularly evident among AIANs in the Southwest where 24 facilities provided dialysis care for nearly two-thirds of the regional AIAN dialysis population. Our findings also highlight variable achievement of quality measures by the dialysis facilities that care for AIANs (e.g., in anemia management and facility survival) suggesting that differences in facility-level performance might have influenced downstream outcomes such as dialysis-related mortality in these populations. The clustering of dialysis care for AIANs raises a parallel question of whether interventions targeted at a relatively small group of medical providers and facilities might improve care delivery and reduce geographic disparities in medical outcomes among the larger population of persons with nondialysis dependent chronic kidney disease.16
Strengths and Limitations
Our study's strengths included analysis of a national cohort of AIAN patients initiating dialysis with long-term follow-up for death and the inclusion of extensive patient-, dialysis facility-, and area-level characteristics. Our study also had several limitations. First, we used administrative data for determining AIAN race that has the potential for misclassification bias. Access to self-reported race data or certification of AIAN ancestry might have improved the specificity of our race ascertainment, but unfortunately these data were not available. However, we are unaware of recent studies using United States Renal Data System files that suggest the presence of differential misclassification of race according to a patient's geographic location.1 A report of 1265 ESRD patients categorized as “Native American” from 1983−86 found that approximately seven percent (n = 93) of these patients may have been misclassified based on surname review.17,18 Second, our results are potentially limited by residual confounding from underascertained comorbidities, such as cardiac disease or diabetes, based on the Medical Evidence Form.19 Given the magnitude of the differences in the relative rates of death and transplantation, it seems unlikely that our key findings were due to residual confounding alone. Third, we had limited power from small sample sizes to perform separate analyses by tribal area or community.20 Detailed data at the level of individual tribes may have uncovered specific determinants of mortality among certain tribal communities within the study cohort. Although we ascertained and incorporated zip code poverty as an estimate of neighborhood or contextual socioeconomic status, finer levels of geographic resolution may have magnified the associations of geography, socioeconomic factors, and outcomes in our study.21,22 Finally, our study results are subject to possible bias from regional differences in medical care and mortality before dialysis initiation. The inclusion of additional medical records (e.g., from IHS or other tribal health providers) may have enhanced our assessment of the quantity and quality of medical care received before, and during dialysis, but this information was not available.
Conclusions
We observed substantial geographic differences in the structure of dialysis care and in patient mortality of American Indians and Alaska Natives initiating maintenance dialysis. The dialysis care of AIAN patients, particularly those in the Southwest, was highly concentrated among relatively few dialysis facilities. Despite comparably high rates of poverty and traditional risk factors for death, AIANs receiving dialysis in the Southwest experienced substantially lower mortality than their counterparts from other regions. Area-based studies that examine the structure of care within large AIAN communities may help to delineate specific determinants of these differences and lead to interventions to further improve the quality of care for patients with earlier stages of chronic kidney disease.
CONCISE METHODS
Data Sources
We obtained individual patient and dialysis facility data from the United States Renal Data System (USRDS) Standard Analysis Files,1 and we extracted area-based socioeconomic data from the 2000 US Census at the level of the 5-digit zip code.23 We further linked patient-level data with dialysis facility performance measures from the Dialysis Compare database maintained by the Centers for Medicare and Medicaid Services using a crosswalk provided by USRDS. The facility performance measures corresponded to data from the year that the patient initiated dialysis at the facility.
Study Sample
We identified all persons aged 18 yr and older whose race was categorized as American Indian or Alaska Native and who initiated dialysis in the US between January 1, 1995 and September 30, 2008 (n = 13,716). We chose this starting point because before 1995 dialysis units and transplant centers were required to file the Medical Evidence Form only for Medicare-eligible patients.1
Outcome Variables
The primary outcome was time from dialysis initiation to death. Because the risk of death is modified by receipt of a kidney transplant, we censored patients at the time of living (n = 378), deceased (n = 871) or unknown (n = 1) donor transplantation, or at the end of the study observation period on September 30, 2008.
Primary Explanatory Variable
The primary explanatory variable for all analyses was the geographic region (Alaska, Eastern US, Northern Plains, Pacific Coast, Southern Plains, Southwest) in which the patient initiated dialysis based on residential information collected at the time of dialysis initiation. We chose these geographic divisions because they approximate the six main Indian Health Service regions (Figure 1). Approximately 40 percent of AIANs from the general population reside in designated tribal areas and nearly sixty percent receive medical care from Indian Health Service providers.13
Patient-Level Covariates
Additional patient-level sociodemographic covariates included age, sex, and health insurance coverage (Medicare, Medicaid, Medicare/Medicaid, employer group insurance, other insurance or no insurance). We examined the following comorbid conditions from the USRDS Medical Evidence form: cardiovascular disease, diabetes (insulin- or noninsulin-requiring), poor functional status (requiring assistance with daily activities, inability to ambulate or transfer, or institutionalized at an assisted living or nursing home facility), and active drug or tobacco use at the time of dialysis initiation. We further identified patients who were prescribed a erythropoiesis stimulating agent before dialysis initiation and those with low serum albumin (<3.5 g/dl) and hemoglobin concentrations (<10 g/dl).12 We divided patients into World Health Organization-designated categories of body mass index.24
Dialysis Facility-Level Covariates
Because the size, structure and performance of the dialysis facility are associated with patient mortality and these associations are modified by geographic location,11,25–27 we examined the following facility-level factors from the Centers for Medicare and Medicaid Services Dialysis Facility Compare and USRDS Facility files: anemia management (percent of facility patients with hemoglobin <10 g/dl, 10 to 12 g/dl or >12 g/dl), dialysis adequacy (percent of facility patients with urea reduction ratio ≥65%), survival category (better than expected, as expected, or worse than expected based on the facility's expected compared with the actual patient survival rate according to patient case mix, i.e. age, race, sex, diabetes, dialysis vintage and comorbidity, during the year surveyed),28 number of patients, number of dialysis stations, availability of home dialysis training, ownership type (for-profit, nonprofit, local or federal government), dialysis organization size (large versus small), prior history of facility closure or termination, and whether the facility was hospital-based or free-standing.1 Dialysis facility-level factors were extracted from the year in which each patient initiated dialysis at the specific facility. In the case where facility-level data were present for multiple years, we applied weights to the data based on the proportion of AIAN patients initiating dialysis in each year.
Zip Code-Level Covariates
We included a variable for poverty based on US Census estimates of the percentage of residents living in poverty within the zip code where each patient resided when they initiated maintenance dialysis (<5%, 5 to 9%, 10 to 14%, 15 to 19%, or ≥20% of the population).21,22,29 The US Census defines a “poverty area” as an area where at least 20 percent of residents are poor. Nationally, nearly one-fourth of AIANs reside in poverty compared with 13.2 and 8.6 percent of the general and non-Hispanic white populations, respectively.30
Statistical Analysis
We calculated mean annual crude event rates (per 100 person-years) over the duration of the study stratified by geographic region. We analyzed the associations of geographic region and time from dialysis initiation to death using proportional hazards (i.e. “Cox”) regression. Due to higher estimates of risk factors for death among Southern Plains AIANs relative to counterparts in other geographic regions, AIANs in this region served as the referent group for all analyses.7,8 We incorporated potential explanatory variables in the final adjusted model that were significant at the P < 0.05 level from bivariate analyses including patient- and dialysis facility-level variables described above, as well as proxies for residential (zip code) poverty. We used scaled Schoenfeld residual plots against time and estimated log (−log [survivor function]) versus time survival curves to assess the proportionality assumption and found no violations. We used the likelihood ratio test to assess for interactions between geographic (Indian Health Service) region with age, sex, and diabetes status. We found no evidence of collinearity among the vector of explanatory variables using the variance inflation factor, tolerance, and eigenvalue.31 To avoid bias caused by excluding patients with missing data, we performed multiple imputation using the Markov chain Monte Carlo method with five imputations for these variables.32 To address potential nonindependence of outcomes due to informative censoring, we performed additional analyses using competing-risks survival analysis for either outcome (death or kidney transplantation) using the method of Fine and Gray.33 Lastly, to account for potential correlations within dialysis facilities, we obtained robust sandwich estimates for the Cox model using the dialysis facility as the cluster variable.34,35 We confirmed model fit using Cox-Snell residuals. Two-tailed P-values <0.05 were considered statistically significant. All statistical analyses were conducted using Stata Statistical Software (Stata MP version 11.0, Stata Corp, College Station, Texas).
DISCLOSURES
None.
Acknowledgments
Y.N.H. received support from K23 DK 087900 and the Norman S. Coplon Extramural Grant Program of Satellite Healthcare. S.E.J. received support from NIH Diversity Supplement 3U01 HL064244. D.B. was supported by NIH grant for the Institute for Translational Health Science (Clinical Translation Sciences Award to the University of Washington, UL1RR025014).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. US Renal Data System, USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda MD, 2009. http://www.usrds.org/2009 last accessed on 15 April 2011 [Google Scholar]
- 2. Scavini M, Stidley CA, Paine SS, Shah VO, Tentori F, Bobelu A, Welty TK, MacCluer JW, Zager PG: The burden of chronic kidney disease among the Zuni Indians: The Zuni Kidney Project. Clin J Am Soc Nephrol May 2: 509–516, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Shah VO, Scavini M, Stidley CA, Tentori F, Welty TK, MacCluer JW: Epidemic of diabetic and nondiabetic renal disease among the Zuni Indians: The Zuni Kidney Project. J Am Soc Nephrol 14: 1320–1329, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Pasinski R, Pasinski M: End-stage renal disease among the Zuni Indians: 1973–1983. Arch Intern Med 147: 1093–1096, 1987 [PubMed] [Google Scholar]
- 5. Sequist TD, Narva AS, Stiles SK, Karp SK, Cass A, Ayanian JZ: Access to renal transplantation among American Indians and Hispanics. Am J Kidney Dis 44: 344–352, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG: Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA 296: 421–426, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Steele CB, Cardinez CJ, Richardson LC, Tom-Orme L, Shaw KM: Surveillance for health behaviors of American Indians and Alaska Natives-findings from the behavioral risk factor surveillance system, 2000–2006. Cancer 113: 1131–1141, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Perdue DG, Perkins C, Jackson-Thompson J, Coughlin SS, Ahmed F, Haverkamp DS, Jim MA: Regional differences in colorectal cancer incidence, stage, and subsite among American Indians and Alaska Natives, 1999–2004. Cancer 113: 1179–1190, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Donabedian A: The quality of medical care. Science. May 26 200: 856–864, 1978 [DOI] [PubMed] [Google Scholar]
- 10. Narva AS, Sequist TD: Reducing health disparities in American Indians with chronic kidney disease. Semin Nephrol 30: 19–25, 2004 [DOI] [PubMed] [Google Scholar]
- 11. McClellan WM, Wasse H, McClellan AC, Kipp A, Waller LA, Rocco MV: Treatment center and geographic variability in pre-ESRD care associate with increased mortality. J Am Soc Nephrol 20: 1078–1085, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kinchen KS, Sadler J, Fink N, Brookmeyer R, Klag MJ, Levey AS, Powe NR: The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 137: 479–486, 2002 [DOI] [PubMed] [Google Scholar]
- 13. US Department of Health and Human Services, Indian Health Service website. http://info.ihs.gov/FcltCnstr.asp last accessed on 2 September 2010 [Google Scholar]
- 14. Sequist TD, Cullen T, Bernard K, Shaykevich S, Orav EJ, Ayanian JZ: Trends in quality of care and barriers to improvement in the Indian health service. Journal of General Internal Medicine 26: 480–486, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ngo-Metzger Q, Sorkin DH, Phillips RS, Greenfield S, Massagli MP, Clarridge B, Kaplan SH: Providing high-quality care for limited English proficient patients: The importance of language concordance and interpreter use. J Gen Intern Med 22: 324–330, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL: Primary care physicians who treat blacks and whites. N Engl J Med 351: 575–584, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Newman JM, Marfin AA, Eggers PW, Helgerson SD: End state renal disease among Native Americans, 1983–86. Am J Public Health, 80: 318–319, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koepsell T, Weiss N. Epidemiologic Methods: Studying the Occurrence of Illness. New York: Oxford University Press; 2003 [Google Scholar]
- 19. Longenecker JC, Coresh J, Klag MJ, Levey AS, Martin AA, Fink NE, Powe NR: Validation of comorbid conditions on the end-stage renal disease medical evidence report: The CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol 11: 520–529, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Kasiske BL, London W, Ellison MD: Race and socioeconomic factors influencing early placement on the kidney transplant waiting list. J Am Soc Nephrol 9: 2142–2147, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Volkova N, McClellan W, Klein M, Flanders D, Kleinbaum D, Soucie JM, Presley R: Neighborhood poverty and racial differences in ESRD incidence. J Am Soc Nephrol 19: 356–364, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krieger N: Overcoming the absence of socioeconomic data in medical records: Validation and application of a census-based methodology. Am J Public Health 82: 703–710, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Bureau of the Census. [last accessed on 27 February 2010]. Poverty Areas available at http://www.census.gov/population/socdemo/statbriefs/povarea.html.
- 24. WHO, Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Consultation. WHO Technical Report Series Number 854, World Health Organization, Geneva: (1995) [PubMed] [Google Scholar]
- 25. Rodriguez RA, Sen S, Mehta K, Moody-Ayers S, Bacchetti P, O'Hare AM: Geography matters: Relationships among urban residential segregation, dialysis facilities, and patient outcomes. Ann Intern Med 146: 493–501, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Devereaux PJ, Schunemann HJ, Ravindran N, Bhandari M, Garg AX, Choi PT, Grant BJ, Haines T, Lacchetti C, Weaver B, Lavis JN, Cook DJ, Haslam DR, Sullivan T, Guyatt GH: Comparison of mortality between private for-profit and private not-for-profit hemodialysis centers: A systematic review and meta-analysis. JAMA 288: 2449–2457, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Lacson E, Jr., Wang W, Lazarus JM, Hakim RM: Hemodialysis facility-based quality-of-care indicators and facility-specific patient outcomes. Am J Kidney Dis 54: 490–497, 2009 [DOI] [PubMed] [Google Scholar]
- 28. US Government Site for Medicare Dialysis Facility Compare available at www.medicare.gov/Dialysis, last accessed on 11 May 2011
- 29. Patzer RE, Amaral S, Wasse H, Volkova N, Kleinbaum D, McClellan WM: Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol 20: 1333–1340, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. US Bureau of the Census: 2005–2009 American Community Survey 5-Year Estimates, last accessed on 10 May 2011 [Google Scholar]
- 31. Belsely DA, Kuh E, Welsch RE. Regression diagnostics: Identifying influential data and sources of collinearity. New York: Wiley; 1980 [Google Scholar]
- 32. Little RJA, Rubin DB. Statistical analysis with missing data. New York: J Wiley & Sons; 1987 [Google Scholar]
- 33. Fine JP: Regression modeling of competing crude failure probabilities. Biostatistics 2: 85–97, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Lin DY, Wei LJ: The robust inference for the Cox proportional hazards model. J Am Statist Assoc 84: 1074–1079, 1989 [Google Scholar]
- 35. Williams RL: A note on robust variance estimation for cluster-correlated data. Biometrics 56: 645–646, 2000 [DOI] [PubMed] [Google Scholar]