Abstract
FCRLA is an intracellular B cell protein that belongs to the FcR-like family. Using newly generated FCRLA-specific antibodies, we studied the constitutive expression pattern of mouse FCRLA and monitored changes during an immune response and following in vitro B cell activation. All B cell subpopulations examined expressed FCRLA. However, the level of FCRLA expression is determined by the stage of B cell differentiation. Low expression of FCRLA is characteristic of naïve follicular and marginal zone B cells. High expression was detected in a small fraction of activated B cells scattered along migratory pathways in the lymphoid tissues. FCRLA-bright cells could be subdivided into two subpopulations, with high and low/undetectable level of intracellular immunoglobulins, which phenotypically resemble either plasma or memory B cells. High expression of FCRLA in subset(s) of terminally differentiated B-cells suggests that, being an ER protein, FCRLA may participate in the regulation of immunoglobulin assembly and secretion.
Keywords: FcR-like proteins, B cell differentiation, memory B cells, plasma cells
1. Introduction
Antigen-induced differentiation of B cells ultimately leads to generation of antibody-secreting plasma cells (PCs) and memory B cells, both highly heterogeneous cell populations. Depending on the B cell subset activated, tissue/cellular milieu, and the nature of the antigen, the differentiation process may generate cells differing in their maturity, functional capacity and lifespan [1–4]. Immature populations of antibody-secreting cells are called plasmablasts and pre-plasma cells, whereas terminally differentiated PCs can be both short- and long-lived [4, 5]. Similarly, several subpopulations of memory B cells have been described [6–9]. Because of the absence of unique markers, the relationships among the various PC and memory cell subpopulations described by different authors are not always clear-cut and are sometimes controversial. Better understanding of the late stages of B cell differentiation is important for definition of factors influencing long term immunity against pathogens as well as for understanding the genesis of immune disorders caused by autoantibodies.
Completion of the human and mouse genome sequencing projects resulted in the identification of numerous previously unknown genes, some of which are differentially expressed in B cells. One such gene, first called FCRL/FREB/FcRX and subsequently designated FCRLA, has been shown to be abundantly expressed in germinal center (GC) B cells in human tonsils [10–13]. The mature protein is composed of four domains the first three of which (D1-D3) resemble the extracellular Ig domains of FcγRI, although D1 is a highly degenerate Ig-like domain that is unlikely to assume the typical Ig domain structure. Based on protein sequence homology and genomic localization, FCRLA is related to the classical Ig-binding Fc receptors, but differs from other members of this family in several significant ways. There are no predicted N-linked glycosylation sites and, instead of a transmembrane domain, FCRLA has a unique carboxy terminal domain (D4) enriched in proline, serine and threonine residues. Most notably, human FCRLA is an intracellular rather than a plasma membrane protein. The function of FCRLA remains unclear, and the only available data come from studies of the human protein. We and others have shown that FCRLA is a resident endoplasmic reticulum (ER) protein that interacts with multiple isotypes of intracellular Igs [14, 15]. Until recently FCRLA expression at the protein level has only been studied in humans. It is highly expressed in GC cells in tonsils, primarily in the proliferating centroblasts, and has been shown to be up-regulated by peripheral blood B cells after in vitro stimulation [11]. These findings suggested that the protein may have a role in antigen-activated B cells. A later study has also shown moderate expression of human FCRLA in follicular and marginal zone B cells and its absence/low level expression in plasmacytomas and CD38-positive PCs (15, 16). In this study we extended our FCRLA expression studies to mice and have analyzed constitutive expression in conventional mice and SPF mice, as well as changes in expression following in vitro activation and during an in vivo immune response. We found that mouse naïve B cells express FCRLA at a low level. Significant up-regulation of FCRLA occurs in a small fraction of B cells (FCRLAbr) generated in response to antigenic challenge. The FCRLAbr cells can be further divided into two subsets, one with a high level of cytoplasmic Ig and the other with either low or undetectable cytoplasmic Ig. The phenotypic features of these cells only partially overlap with the typical characteristics of PCs and memory cells. Importantly, the FCRLAbr cells accumulate in the bone marrow of immunized mice, suggesting their possible involvement in long-term immunity.
2. Materials and Methods
2.1. Mice
Conventional BALB/c mice were housed within the animal facility at the Institute of Cytology and Genetics (SB RAS, Novosibirsk, Russia). Unless otherwise stated, 8–10-wk-old female mice were used. Specific pathogen-free (SPF) female mice were purchased from the Animal Breeding Facility, Branch of Shemyakin & Ovchinnikov Institute of Bioorganic Chemistry (Pushino, Russia). SPF CBA/J mice at 12 wk of age were used for in vitro mitogenic stimulation. To study FCRLA expression during an immune response in vivo, mice were immunized with rabbit RBC i.p. and in the rear paws, and their spleen, lymph nodes, bone marrow and PBMC were analyzed by immunohistochemical staining at 3, 7, and 14 days after immunization. All the studies were approved by the appropriate local ethical committee.
2.2. Northern blot analysis
The expression of FCRLA mRNA in mouse tissues was examined by Northern blot analysis. And EcoRI-NotI fragment of the mouse cDNA clone ms73c01 (I.M.A.G.E. clone 617184) was used as a probe. Poly(A)+ RNA from tissues of BALB/c mice was isolated from total RNA on oligo-dT cellulose (Sigma-Aldrich, St. Louis, MO, USA), fractionated (5 µg/line) on 1% agarose gels and vacuum blotted onto a Zeta-Probe nylon membrane (Bio-Rad Laboratories, Hercules, CA, USA). The blot was hybridized with either 32P-labeled Fcrla or β-actin probes under high stringency conditions following the Bio-Rad recommendations.
2.3. RT-PCR
The mouse T cell line EL4, B cell lines A20 and M12, macrophage cell line J774, pro-B cell line L1210, melanoma B16, and plasmacytomas NS1 and NS0 were maintained in RPMI 1640 supplemented with 50 µg/ml gentamicin, 2mM L-glutamine, and 10% FBS. Total RNA extracted from the cell lines was reverse transcribed with SuperScript II RNase H reverse transcriptase (Gibco-BRL, Grand Island, NY, USA) according to the manufacturer’s recommendations. The following gene-specific primer pair was used in the RT-PCR analysis of Fcrla expression: forward, 5’-ATGTCTGCCCTGCTGTGCTCC-3’ and reverse, 5’-GACCAGATGACCGAGGAGAGC-3’. The samples underwent denaturation at 94°C for 3 min followed by 30 cycles of amplification (94°C for 30 s, 68°for 30 s, 72°C for 1 min). A positive control (the Fcrla plasmid) and a negative control containing all the reagents except cDNA were included in every PCR analysis. The cDNA samples were additionally checked by applying RT-PCR analysis to β-actin. Oligonucleotides used as primers for PCR amplification of a mouse β-actin fragment were 5’-CGCGAGAAGATGACCCAGATC-3 ‘ and 5’-TTGCGATCCACATCTGCTGG-3’.
2.4. Rabbit antiserum
Recombinant mouse FCRLA protein was generated using an E. coli expression system. The pT7-ABPb and pT7-TZZb expression vectors were generously provided by Dr. S. Stahl (The Royal Institute of Technology, Stockholm, Sweden). The FCRLA fragment lacking the predicted leader peptide and the fourth domain was expressed as a part of the ABP- or TZZ-fused proteins. FCRLA-TZZ, which contains an S. aureus protein A-derived Ig binding motif, was affinity purified using rabbit IgG coupled to Sepharose 4B (Pharmacia Biotech, Uppsala, Sweden). A rabbit was immunized with the purified protein (three injections 200 µg each in complete Freund’s adjuvant, Sigma-Aldrich). The immunoglobulin fraction of antiserum was prepared by precipitation with (NH4)2SO4 (40% saturation).
2.5. Western blotting
Cells (5 × 104 per sample) were lysed for 5 min in a loading SDS buffer at 100°C and subjected to reducing 12 % SDS-PAGE. After electrophoresis, the separated proteins were transferred to nitrocellulose membrane Hybond-C (Amersham Biosciences, Piscataway, NJ, USA). The membrane was immuno-stained using FCRLA-specific Ab and HRP conjugated anti-rabbit IgG.
2.6. Transfections
293T cells were transiently transfected with the pCI-neo-Fcrla plasmid DNA using Unifectin 56 (IBCH, Moscow, Russia) according to the manufacturer’s protocol. After 72 hours, the cells and supernatants of transfectants were harvested and used for analysis of FCRLA expression as described above.
2.7. Intracellular staining and confocal microscopy
Transient transfections of COS-7 cells were performed with DEAE-dextran (Mr=500,000; Amersham-Pharmacia Plc, Bucks, UK). Cells were harvested 60 hours after the transfection, fixed with 2% PFA for 15 min on ice, and permeabilized in 0.2% saponin containing 1% FBS for 5 min at room temperature. Then cells were incubated with FCRLA-specific rabbit Ab and labelled with secondary anti-rabbit Alexa Fluor 647 conjugate (Molecular Probes, Eugene, OR, USA) and Alexa Fluor 488-conjugated cholera toxin B. Stained and washed cells were mounted onto microscope cover slips. The fluorescent signals were recorded and visualized with Olympus FLUOView500 laser scanning confocal microscope (Olympus, Hamburg, Germany). Images were further processed by ImageJ software (Wayne Rasband, NIH, Bethesda, MD, USA). To analyze intracellular localization of FCRLA, 293T cells were grown on coverslips and transiently transfected with pCI-neo-FCRLA. Forty eight hours after the transfection, double immunofluorescent staining with Abs against FCRLA and either p58K (Abcam, Cambridge, UK) to label Golgi or calnexin (BD Transduction Laboratories, Lexington, KY, USA) to label ER was performed. Confocal microscopic analysis was performed using an LSM 510 microscope (Carl Zeiss Inc, Jena, Germany).
2.8. Immunohistochemistry
Immunoperoxidase staining was performed to study the distribution of FCRLA-positive cells in mouse lymphoid tissues and mouse cell lines. Acetone fixed cryosections or cell smears were air dried, washed with PBS and blocked with 20% FBS. Endogenous peroxidase activity was blocked with 1% hydrogen peroxide and 0.03% NaN3 in PBS. After washing, unlabelled rabbit anti-mouse FCRLA Ab was applied for 1h followed by incubation with HRP labeled goat anti-rabbit Ab. Slides were developed with diaminobenzidine (AppliChem, Darmstadt, Germany) and examined on an Axioscope 2 Plus microscope using Axiovision software (Carl Zeiss Inc).
2.9. Immunofluorescence and Antibodies
Double immunofluorescent staining was performed using the following Abs: biotin-conjugated rat anti-mouse CD19 (1D3), FITC-conjugated rat anti-mouse B220 (RA3-6B2), biotin-conjugated hamster anti-mouse CD3e, biotin-conjugated rat anti-mouse CD138 (281–2), biotin-conjugated rat anti-mouse CD5, biotin-conjugated rat anti-mouse CD11b (all from BD Pharmingen, San Diego, CA, USA); FITC-conjugated goat anti-mouse IgG, biotin-conjugated goat anti-mouse IgM (Zymed Laboratories, San Francisco, CA, USA); biotin-conjugated rat anti-mouse IgD (Southern Biotechnology Associates, Birmingham, AL, USA), FITC-conjugated rat anti-mouse MOMA-1 (Serotec Ltd, Oxford, UK). Unlabeled primary Abs were detected using the following secondary reagents: FITC-conjugated goat anti-rabbit IgG (BD Pharmingen); Texas Red-conjugated goat anti-rabbit IgG, streptavidin-Texas Red and streptavidin-Alexa Fluor 488 conjugates (all from Molecular Probes). DAPI (Sigma-Aldrich Chemie, Munich, Germany) was used as a counterstain. Biotin labeled PNA was purchased from Sigma-Aldrich. Double immunofluorescent labeling was performed on 6–8 µm-thick frozen sections that were fixed in cold acetone, washed and blocked with 20% FBS for 30 min. Sections were incubated for 1h at room temperature or overnight at 4°C with pairs of primary Abs (e.g. polyclonal rabbit anti-FCRLA Ab together with biotin-conjugated goat anti-mouse IgM). Sections were then washed and incubated with appropriate secondary reagents (e.g. goat anti-rabbit and streptavidin in the example given above) labeled with contrasting green and red fluorochromes. The slides were mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA) and examined on an Axioscope 2 Plus microscope using AxioCam MRc digital camera and Axiovision software (Carl Zeiss Inc).
To establish mean percentages of FCRLAdl and FCRLAbr cells, cell smears of spleen, bone marrow or PBMC were stained for FCRLA and IgM or IgG and manual microscopic evaluation of 1000 cells per slide was performed. Additionally, at least 50 FCRLAbr cells were counted per slide to describe their phenotype. All counts were repeated on two separate slides. Each experimental group consisted of at least four mice.
2.10. In vitro mitogenic stimulation
Splenocytes from SPF CBA/J mice plated at a density of 1 × 106 per well in 24-well plates were cultured in RPMI 1640 medium supplemented with 50µg/ml gentamicin, 50µM β-mercaptoethanol (all from Sigma-Aldrich), 10% FBS and stimulated with 20 µg/ml of E. coli 055:B5 LPS (Sigma-Aldrich) or by a combination of anti-CD40 (5 µg/ml)/IL-4 (10 ng/ml) or anti-CD40 (5 µg/ml)/IL-4 (10 ng/ml)/anti-IgM (5 µg/ml). Supernatants and cells from cultures of stimulated and untreated splenocytes were collected at various time points. ELISA was used to determine levels of secreted IgM in supernatants. Smears of cultured splenocytes were prepared and stained for cytoplasmic IgM and FCRLA, as described above.
3. Results
3.1. The Fcrla gene is expressed in mouse lymphoid tissues and B cell lines
Northern blot analysis was performed to determine tissue distribution of Fcrla mRNA (Fig. 1A). Among the different tissues examined, spleen and peripheral blood leucocytes gave the strongest signals. Thymus and colon showed weak hybridization with the Fcrla probe. The Fcrla transcripts were undetectable in non-lymphoid tissues (skeletal muscle and brain), indicating immune cell-specific expression. Mouse tissues produced transcripts of two sizes, approximately of 2.0 and 2.3 kb. The 2.3-kb transcript was slightly more abundant in leukocytes; in all the other tissues the ratio was reversed. In addition, mouse leukocytes contained two larger transcripts, which probably represent nonprocessed or partially processed mRNA.
Fig. 1.
Analysis of Fcrla expression in mouse tissues and cell lines. (A) Northern blot analysis of FCRLA mRNA expression in different tissues. Fcrla cDNA was used as a probe and β-actin was to asses RNA abundance and integrity. (B) RT-PCR analysis of Fcrla expression in mouse cell lines. Total mRNA from each cell line was reverse transcribed and the resulting cDNA was amplified using Fcrla-specific primers.
Examination of a panel of mouse cell lines representing different lineages by RT-PCR showed no expression of Fcrla transcripts in the non-B lineage cells – T cell line EL4, macrophage cell line J774, and melanoma B16 (Fig. 1B). FCRLA expression was found in mature B cell lines (A20, M12) and plasmacytomas (NS0, NS1). A weak signal was also detected in the L1210 pro B cell line. B cell- specific expression of FCRLA was further confirmed at the protein level with the use of immunohistochemical and double immunofluorescent staining of mouse lymphoid tissues (paragraph 3.3). Using RT-PCR primers corresponding to the predicted 5' and 3' ends of the Fcrla transcript, we did not detect additional PCR fragments that could indicate alternative splicing of the mouse Fcrla transcript. This is in contrast to human FCRLA which has been shown to be expressed as multiple splice variants [10]. Further analysis of numerous FCRLA ESTs deposited in GeneBank indicates that the two major mRNA species observed in the Northern blots likely result from the use of two distinct polyadenylation sites.
3.2. FCRLA is an intracellular protein
Recombinant FCRLA was generated in E. coli, and rabbit antiserum was raised against this protein. The antiserum specifically recognized FCRLA as a 43 kDa protein in Western blots of FCRLA transfectants (Fig. 2A). Western blotting of lymphoid tissues showed that FCRLA was expressed in spleen, lymph nodes, and peritoneal cavity, and weakly in bone marrow and thymus (Fig. 2B). We also tested cell lysates of five different Ig-producing hybridomas, plasmacytomas and B cell lines A20, NS0, NS1, P815, which were found to contain FCRLA mRNA. With the exception of a weak signal in the A20 cells, FCRLA protein expression was undetectable in these lines by western blot (data not shown). Nevertheless, immunoperoxidase staining of cell smears of these cell lines revealed FCRLA-positive cells in the A20 B cell line and in all tested plasmacytomas/hybridomas. In each case, only a small proportion of cells was FCRLA-positive and the intensity of the staining was much weaker compared to that in transfected cells (data not shown).
Fig. 2.
Western blot analysis of FCRLA expression in (A) transfected 293T cells and (B) lymphoid tissues. Examined were (A) cell lysates of pCI-neo-Fcrla 293T cells transfectants under reducing (TF+β-ME) or non-reducing (TF-β-ME) conditions, and media conditioned by the FCRLA transfectants (TF-CM), and untransfected 293T cells (Control−) and (B) the indicated mouse tissues.
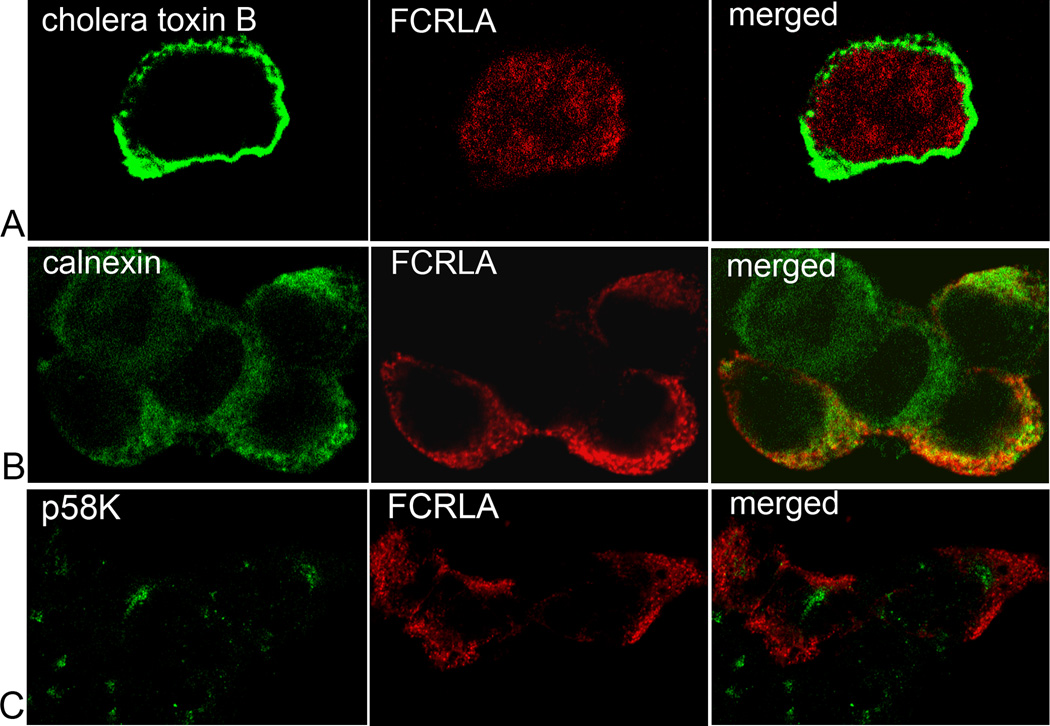
A unique feature of human FCRLA as compared with other FCRL-family members is its intracellular localization. Similarly, we found that mouse FCRLA accumulated intracellularly in transfected 293T cells. The protein was not detected in the media conditioned by the transfectants (Fig. 2A). Double immunofluorescent staining with cholera toxin B, which binds to cell surface GM1 gangliosides, and the FCRLA-specific rabbit antiserum demonstrated that FCRLA is not a cell surface protein (Fig. 3A). The diffuse pattern of FCRLA cytoplasmic staining resembled that of ER localized proteins. Indeed, confocal double immunofluorescence microscopy with Abs against FCRLA and calnexin, a marker of the ER, demonstrated their overlapping staining (Fig. 3B). Based on the results of double staining with the Golgi marker p58K, FCRLA does not transit to the Golgi region (Fig. 3C). These results are consistent with our findings on the human protein [15].
Fig. 3.
Intracellular localization of FCRLA. COS-7 cells were transiently transfected with pCI-neo-Fcrla, encoding full length mouse FCRLA. Cells were harvested 48h post-transfection and co-stained with anti-FCRLA (red) and either (A) Alexa Fluor 488-conjugated cholera toxin B, (B) anti-calnexin or (C) anti-p58K (green). After mounting, cells were analyzed by confocal microscopy.
3.3. Two subpopulations of FCRLA-positive cells
Although the rabbit polyclonal FCRLA-specific antiserum was an efficient reagent for immunostaining of cell smears and tissue sections, it was not applicable for detection of intracellular FCRLA by flow cytometry. For this reason, immunohistochemical staining of spleen and lymph node cryosections and cell smears was used for the phenotypic characterization of FCRLA-positive cells. In conventional mice, these cells were found in all lymphoid tissues examined including bone marrow, spleen, lymph node, thymus, peripheral blood, and peritoneal cavity. Examination of numerous peroxidase and fluorescence stained specimens led to the conclusion that the cells expressing FCRLA can be subdivided into two populations: small, weakly stained cells and cells of various size with intensely stained cytoplasm, which will be referred to here as dull (FCRLAdl) and bright (FCRLAbr), respectively (Fig. 4A). This subdivision has been confirmed by quantitative evaluation of staining intensity of FCRLA-positive cells using AxioVision software.
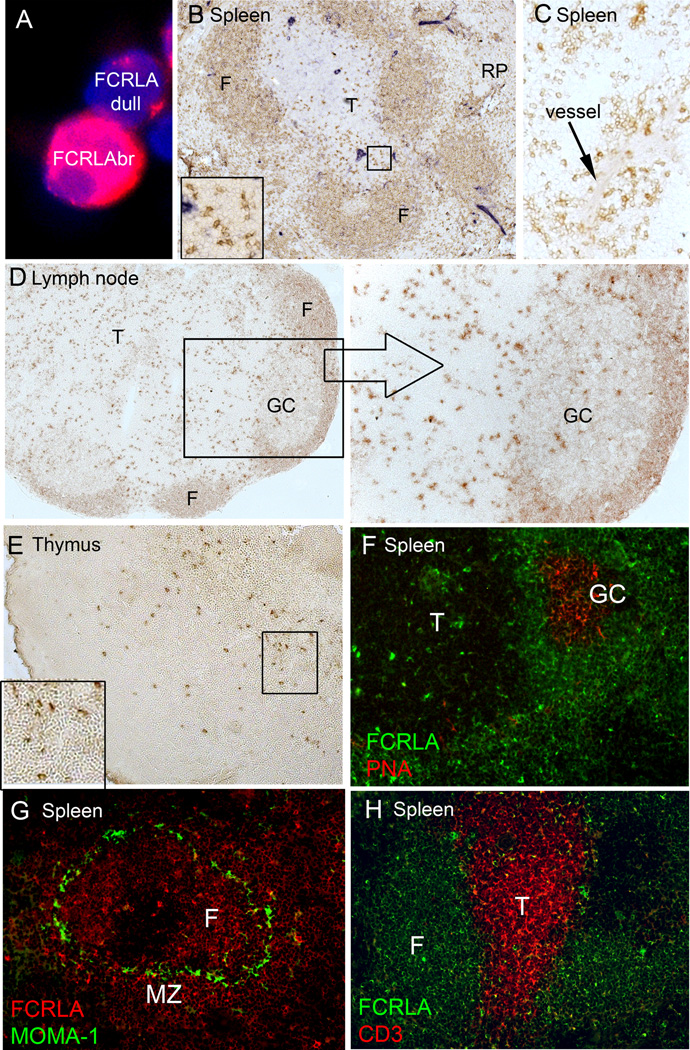
Fig. 4.
Immunostaining of lymphoid tissues of conventional mice for FCRLA. FCRLAbr and FCRLAdl cells had a quite different distribution in tissue compartments. (A) Immunofluorescent staining for FCRLA and DAPI of mouse splenocytes is shown to illustrate the difference in the intensity of staining between FCRLAdl and FCRLAbr cells. (B) Immunoperoxidase staining of the spleen shows that most of the cells in lymphoid follicles are weakly stained FCRLAdl cells and rare scattered cells in T-cell zone and red pulp are strongly stained FCRLAbr cells. (Inset) Higher power view of the part of T-cell zone showing strongly positive staining cells with asteroid morphology. (C) In the red pulp, clusters of FCRLAbr cells are often located close to blood vessels. (D) Immunoperoxidase staining of the lymph node shows FCRLAdl cells in primary B cell follicles and in the mantle zone of secondary follicles, and FCRLAbr cells localized preferentially to paracortex and medullar cords, and rarely to the GCs. The right section shows the GC at a higher magnification. (E) Rare FCRLAbr cells are located in the thymic medulla. The inset shows that these cells have an asteroid morphology. (F) Double immunofluorescent staining of the spleen confirms the location of FCRLAbr cells outside the GCs, in the T-cell zone. (G) In the spleen, FCRLAbr cells are present in both the follicles and the marginal zone, marked with MOMA-1. (H) Double immunofluorescent labeling of the spleen confirms the location of rare FCRLAbr cells in the T-cell zone and shows that CD3-positive T-cells do not coexpress FCRLA. F, B-cell follicle; T, T-cell zone; RP, red pulp; GC, germinal center; MZ, marginal zone.
Cell counts on splenocyte smears showed that in BALB/c mice housed under conventional conditions, 9–15% of the cells were FCRLAdl and only 0.5%–1% were FCRLAbr. The two subpopulations had a quite different distribution in tissue compartments. The FCRLAdl cells in spleen and lymph nodes demonstrated typical B cell localization (Fig. 4 B–D). Weak staining for the protein was observed in the majority of cells of primary follicles and the mantle zone of secondary follicles. FCRLA expression in marginal zone B cells, identified by staining with a MOMA1-specific Ab (Fig. 4G), was similar to that in follicular B cells. While the FCRLAbr cells could be found in follicular areas, they were mainly localized outside the B cell zone (Fig. 4D, F). In spleen, strongly stained cells were often present in the red pulp, close to blood vessels (Fig. 4C). Strong labeling for FCRLA was also found in large cells scattered in the T-cell zone (Fig. 4B, F). In lymph nodes, FCRLAbr cells were predominantly observed in the T cell zone and medullary cords (Fig. 4D). In thymus, FCRLAbr cells were found at low frequency in the thymic medulla but not in the cortex (Fig. 4E). The FCRLAbr cells located in T cell zones of spleen and lymph nodes, as well as those in thymus, frequently showed asteroid morphology indicating their close contact with surrounding cells (Fig. 4B, E).
Notably, most mouse GC cells were FCRLA-negative and only rare GC cells were FCRLAbr. This is in stark contrast to the human protein, which has been shown to be preferentially expressed in GC cells [10, 11, 16]. The results of immunoperoxidase staining were confirmed by double fluorescence labeling of GCs with anti-FCRLA and PNA (Fig. 4F). Examination of numerous spleens and lymph nodes from conventional and SPF mice at different time post immunization showed that the frequency of FCRLA-positive cells in GCs was independent of the age of the GCs (not shown).
To further characterize FCRLA-positive cells, we performed two-color immunofluorescence analysis with anti-FCRLA and Abs against IgM, IgD, IgG, B220, CD19, CD138, CD3, and CD11b on spleen cryosections and splenocyte smears. The results of these analyses supported the subdivision of FCRLA-positive cells into two subpopulations and their differential localization in tissue compartments. In agreement with the results of immunoperoxidase staining, FCRLAdl cells were found primarily in the B cell follicles and the mantle zone. As expected from their localization, FCRLAdl cells generally co-expressed IgD, CD19, B220, and low level IgM (Fig. 5A, B, E).
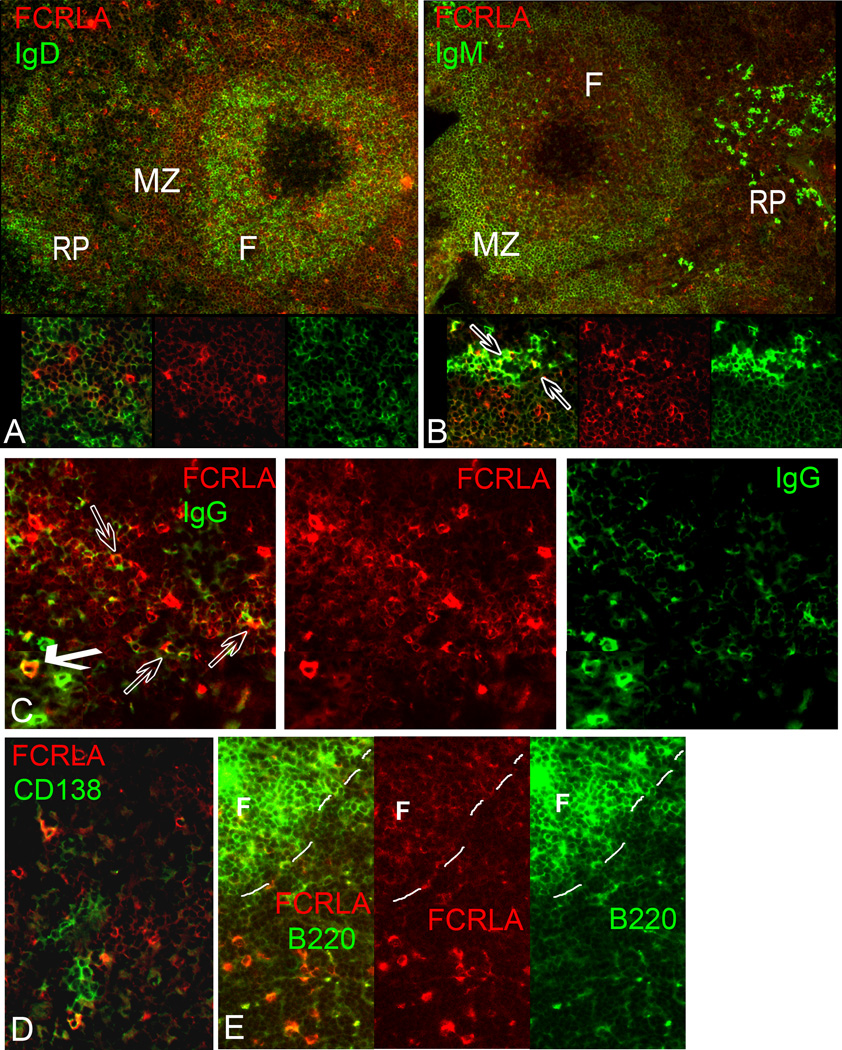
Fig. 5.
Double immunofluorescent staining of spleen cryosections for FCRLA and various lymphocyte markers. (A) IgD/FCRLA: Many of the FCRLAdl cells located in the mantle zone of the follicle coexpress IgD; by contrast, FCRLAbr cells localized outside B-cell follicles are IgD-negative (inset). (B) IgM/FCRLA: FCRLAdl cells of the B-cell follicle coexpress low levels of IgM. Some of the FCRLAbr cells located in the red pulp are strongly stained for IgM (indicated by arrows in inset), while others are weakly stained for IgM or negative. (C) IgG/FCRLA: Some FCRLAbr cells in the red pulp are brightly stained for cytoplasmic IgG (bold arrow), other FCRLAbr cells are weakly stained for IgG (transparent arrows) or negative. (D) CD138/FCRLA: In the red pulp, FCRLAbr cells are found in a close proximity to clusters of CD138-postive plasma cells. Only few of these cells show costaining for both markers. (E) B220/FCRLA: Both FCRLAdl and FCRLAbr cells coexpress B220. However, expression of B220 in T-cell zone FCRLAbr cells is much weaker than that in follicular FCRLAdl cells (the dashed line outlines the border of the follicle). Colors of FCRLA and other markers are as indicated on the figure. F, B-cell follicle; MZ, marginal zone; RP, red pulp.
The FCRLAbr cells differed from FCRLAdl cells not only in tissue localization but also in phenotype. They were B220-positive but the level of B220 staining was lower than that in FCRLAdl cells (Fig. 5E). The level of CD19 expression varied from low to undetectable on the FCRLAbr cells. Importantly, the FCRLAbr cells were IgD-negative and IgM or IgG positive. Moreover, some of them expressed high levels of cytoplasmic IgM or IgG, suggesting that they may represent plasmablasts/PCs (Fig. 5B, C). Double immunostaining for FCRLA and CD138, a marker of PCs, showed that in the red pulp, FCRLAbr cells were often located near or within clusters of CD138-positive PCs. However, co-expression of FCRLA with CD138 was observed only rarely (Fig. 5D). There was no co-staining of FCRLA with CD11b (not shown) or CD3 (Fig. 4J), confirming its B cell-specific expression.
Both FCRLAdl and FCRLAbr populations were found in bone marrow of conventional mice where they comprised roughly 15% and 0.5% of total bone marrow leukocytes, respectively. As in the spleen and lymph nodes, bone marrow FCRLAdl cells were uniformly positive for IgM and IgD. The FCRLAbr cells in the bone marrow were IgD-negative and 4%–25% of them were IgMbr; in general, they had a phenotype similar to their spleen and lymph node counterparts. The results of immunohistochemical and double immunofluorescent staining demonstrate that FCRLAdl cells are IgM+/IgD+/CD19+/B220+ naïve follicular and marginal zone B cells. FCRLAbr cells are uniformly IgD-negative, express B220 and CD19 at low levels, and are scattered along the migratory pathways in the secondary lymphoid organs. These cells appear to represent a small proportion of activated B cells. One group of FCRLAbr cells expresses Ig at barely detectable level, whereas the rest contain high levels of cytoplasmic IgM or IgG. FCRLAbr cells are mainly CD138-negative.
3.4. FCRLAbr cells are generated during the course of an immune response
To assess the role of immunization in generation of FCRLAbr cells, we examined cryosections of spleen and lymph nodes, and bone marrow cell smears from SPF BALB/c mice before and 3, 7, and 14 days after immunization with RRBC. In non-immunized SPF mice, FCRLAbr cells could be found in red pulp of the spleen and in medullary cords of the lymph nodes, but they were roughly 5-fold less frequent than in conventional mice. In striking contrast to conventional mice, FCRLAbr cells were absent from the T cell zones of the lymph nodes and spleen of naïve SPF mice (Fig. 6A, B, left). Immunization caused an increase in the frequency of FCRLAbr cells as well as a change in their tissue distribution. In T cell zones of spleen and lymph nodes FCRLAbr cells were readily detectable by day 7, and their number substantially increased by day 14 (Fig. 6A, B, right). The response to immunization was even more striking in the bone marrow. Only rare FCRLAbr cells per slide could be found on bone marrow cell smears of non-immunized SPF mice, but by day 14 after immunization the percentage of FCRLAbr cells increased to reach 1% (Fig. 6C). About 10% of these cells expressed high levels of cytoplasmic immunoglobulin, and the others were Iglow or negative. Interestingly, approximately a half of the IgGbr and IgMbr cells found in the bone marrow of immunized SPF mice by day 14 were FCRLAbr. These findings support the idea that FCRLA expression is up-regulated in antigen-stimulated B cells and show that the emergence of FCRLAbr cells in the T cell zones and in bone marrow is a consequence of the immune response.
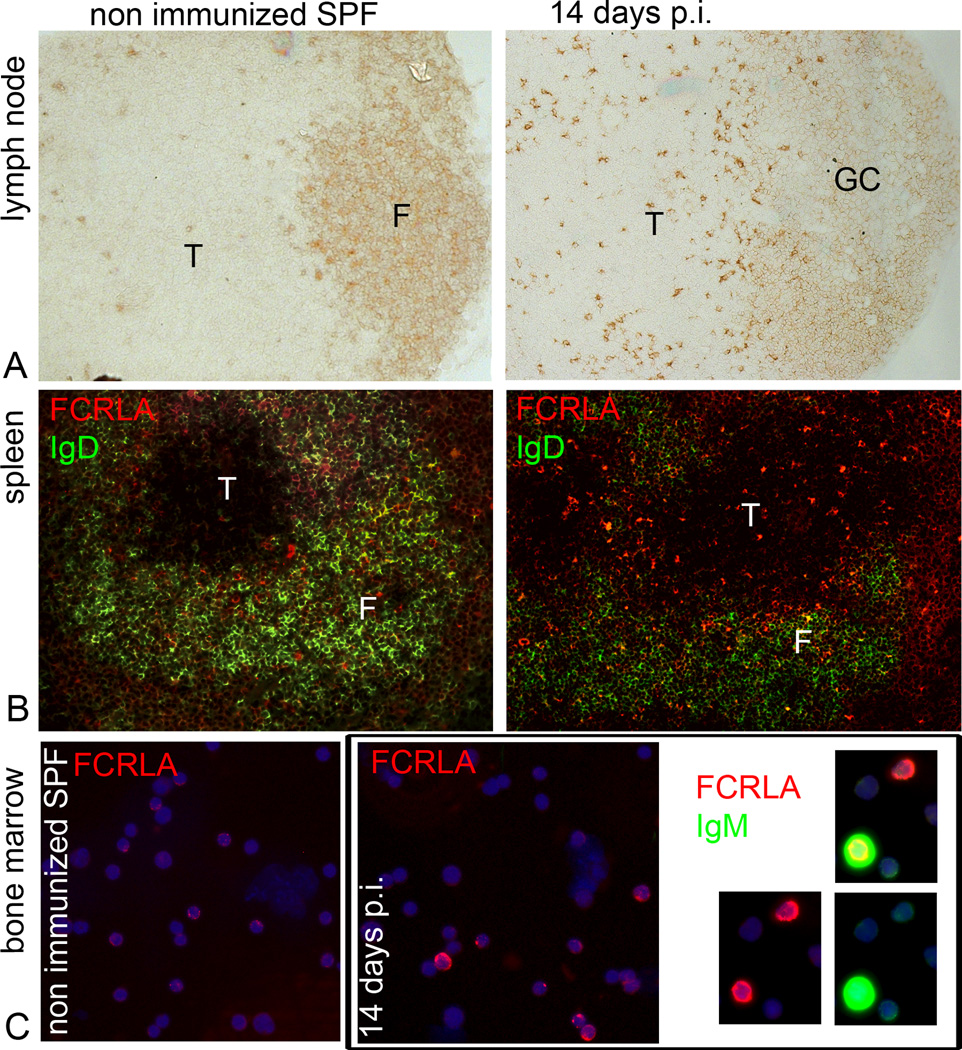
Fig. 6.
Appearance of FCRLAbr cells during the course of immune response in vivo. Imunohistochemical and immunofluorescent staining for FCRLA performed on cryosections of (A) mesenteric lymph nodes, (B) spleens, and (C) on smears of bone marrow cells of SPF BALB/c mice before and after immunization with RRBC. (Left, A–C) FCRLAbr cells are absent from the T-cell zones of lymph nodes and spleen and from the bone marrow of the SPF mice before immunization. (Right, A–C) Number of FCRLAbr cells significantly increases in the T-cell zones of lymph node and spleen and in bone marrow on day 14 post immunization (p.i.). (C, far right) Of note are the FCRLAbr cells co-expressing high levels of cytoplasmic IgM in the bone marrow on day 14 p.i. The data shown are representative of 4 mice. F, B-cell follicle; T, T-cell zone; GC, germinal center.
3.5. FCRLA is differentially expressed during the course of T-independent and T-dependent responses in vitro
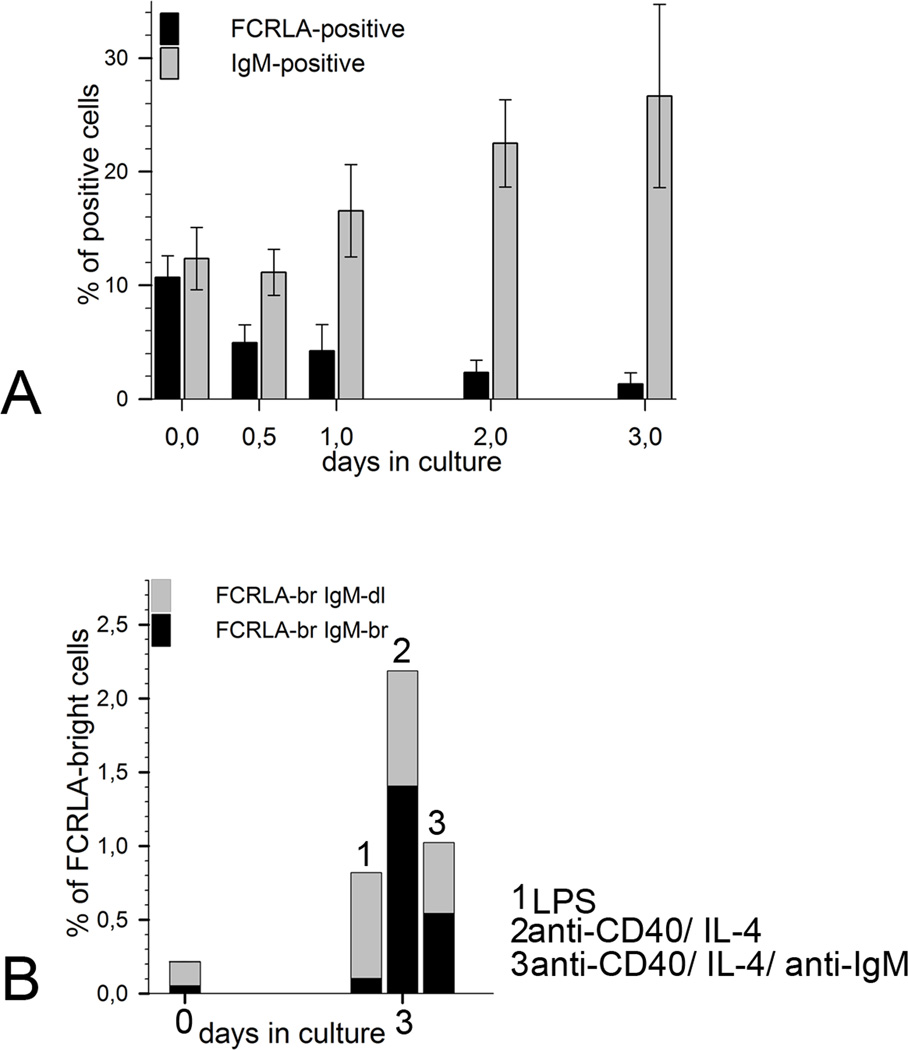
To gain a deeper insight into developmental regulation of FCRLA expression, we stimulated splenocytes of 12 wk old SPF CBA/J mice in vitro either with LPS or with a combination of anti-CD40, IL4 and anti-IgM to model T-independent and T-dependent immune responses, respectively. Cells and supernatants were harvested for analysis at 12 and 24 h and then every 24 h until day five. Before stimulation 8–14% of splenocytes were FCRLAdl and only about 0.2% were FCRLAbr (Fig. 7A).
Fig. 7.
FCRLA expression by in vitro-activated mouse splenocytes. (A) Time course showing the percentage of FCRLA- and IgM-positive cells during in vitro stimulation of mouse splenocytes with LPS (20µg/ml). Each column represents the mean of 4 mice, bars show standard deviation. (B) Ratio of FCRLAbrIgMdl and FCRLAbrIgMbr cells in the cultures of mouse splenocytes after stimulation with different mitogens. Each column represents the mean of 4 mice.
Stimulation of the splenocytes with LPS resulted in prominent proliferation and differentiation of B cells into IgM secreting cells as indicated by a substantial increase in the IgM concentration in conditioned media from day 2 till day 5 of the experiment. Starting from day 2 after stimulation, the generation of antibody-secreting cells became evident by morphological changes of the cultured cells, such as the appearance of blasts, and elevated expression of cytoplasmic IgM. Differentiation of B lymphocytes into IgM-secreting cells was accompanied by a dramatic change in FCRLA expression. By day 3 after activation, the population of FCRLAdl splenocytes decreased approximately 7 times and reached 2%, whereas the percentage of FCRLAbr cells increased to about 1% (Fig. 7B). Staining for IgM revealed that the newly generated FCRLAbr cells could be subdivided into IgMdl and IgMbr cells. The ratio of the FCLRAbrIgMdl to FCLRAbrIgMbr cells in the LPS-stimulated cultures was approximately 9:1 (Fig. 7B). The higher proportion of FCLRAbrIgMdl cells was not due the expression of other Ig isotypes because only rare FCLRAbr cells expressed IgG (not shown). The vast majority of in vitro generated IgMbr plasmablasts were FCRLA-negative.
The stimulation of the splenocytes with anti-CD40/IL4/anti-IgM and anti-CD40/IL4 resulted in a less vigorous differentiation compared to that seen with LPS. However, levels of IgM in the supernatants from both cultures increased in parallel with the emergence of cells with high levels of cytoplasmic IgM (not shown). Similarly to stimulation with LPS, we observed a profound decrease in the FCRLAdl population in these TD-cultures. At the same time the percentage of FCRLAbr cells increased 5- to 10-fold by day 3. The ratio of the FCRLAbrIgMdl to FCRLAbrIgMbr cells in T-dependent stimulation differed from that in LPS-stimulation. Anti-CD40/IL4/anti-IgM stimulated the generation of approximately equal proportions of these two cell types, whereas anti-CD40/IL4 gave rise to a higher proportion of FCRLAbr IgMbr cells (Fig. 7B). As in the response to LPS, the IgMbr and IgGbr plasmablasts generated in the TD cultures were predominantly FCRLA-negative.
In the absence of stimulation, the splenocytes died rapidly and the number of cells dropped significantly by day 3. In this case there were no appearance of the blasts, no accumulation of Ig in the medium of the cultured cells, and no change in the ratio of FCRLAdl and FCRLAbr cells.
4. Discussion
We have shown that all mouse B-cell subpopulations examined expressed FCRLA and, importantly, that the low and high levels of FCRLA expression define different stages of B cell differentiation, thus the corresponding cell populations should be considered separately. Weak FCRLA expression is characteristic of naïve follicular (IgD++/IgM+/CD19+/B220+) and marginal zone (IgD+/IgM++/CD19+/B220+) B cells but that expression is upregulated in a small proportion of cells generated during an immune response. These FCRLAbr cells constitute 0.1%–0.4% of total splenocytes in non-immunized SPF mice, and their frequency increases to 1%–3% by day 14 after immunization with RRBC. The FCRLAbr cells have the phenotype of activated B cells; they are IgD-negative and express low levels of B220 and CD19. With respect to Ig, they can be subdivided into two subpopulations: some FCRLAbr cells contain intracellular Ig at a level comparable to that seen in PCs, while others express Ig at barely detectable levels.
There has been some discrepancy in the reported expression pattern of FCRLA by human B cells, although all studies agree that it is highest in the GC B cells [10, 11, 15, 16].Facchetti et al. [11] using flow cytometry originally reported fairly restricted expression, i.e. blood B cells were FCRLA negative, but expression could be induced by mitogen/cytokine treatment. These authors did not examine FCRLA expression in tonsil B cells by flow cytometry, restricting their analysis to immunofluorescence of tissue sections. We recently performed an extensive analysis of human FCRLA expression by blood B cells and B cell subpopulations in the tonsil [15] using a directly conjugated FCRLA mAb and flow cytometry, and have also reexamined FCRLA-stained human tissue sections (not shown). These analyses clearly demonstrated that FCRLA is expressed by freshly isolated blood B cells and by all B lineage cells in the tonsil – naïve, pre-GC, GC, memory, and to a lesser extent PCs. In agreement with previous studies, the GC B cells in human express the highest levels of FCRLA. The recent study of FCRLA expression in mouse by Wilson et al. [14] has stated that FCRLA is expressed in all B cell subsets as determined by flow cytometry. Based on these findings and the ones reported here, we propose that FCRLA is expressed by all B cell subpopulations in both mouse and human. Moreover, our subdivision of FCRLA-positive cells into bright and dull populations is correct for human B cells as well in that the two original publications of Mechetina et al. [10] and Facchetti et al. [11] described primarily FCRLAbr cells. What is significantly different between man and mouse is that the FCRLAbr cells are rarely observed in the mouse GCs. Compared to humans, this meager expression of FCRLA by mouse GC cells is reminiscent of other differences between these two species, e.g., in the expression of Blimp-1 and Ig J-chain. Both of these proteins mark terminal differentiation of B cells into antibody-secreting cells. While cells expressing Blimp-1 and J-chain are rather abundant in human GCs, they are less frequent in mouse GCs [17, 18]. Thus, the scarcity of FCRLAbr cells in mouse spleen and lymph node GCs compared to the human secondary follicles may reflect more general differences in compartmentalization of the B cell maturation processes in primates and rodents.
Apart from this, human and mouse FCRLA expression patterns are very similar. In both species FCRLAbr cells are scattered in the spleen red pulp and are often embedded in PC clusters near blood vessels (Fig. 4, 5 and [16]). However, both mouse and human FCRLAbr cells generally lack plasma cell markers such as CD138 in mouse or CD38br in human. Our finding that the mouse FCRLAbr cells are either Igbr or Igdl is consistent with the observation that some of the human FCRLA-positive GC cells do not express cytoplasmic Ig or, more likely, produce it at a level undetectable in frozen tissue sections [11].
Instead of being in the GCs, mouse FCRLAbr cells are frequent in the spleen and lymph node T cell zones of conventional and immunized SPF mice. Interestingly, these cells are as a rule Igdl, and they often show asteroid morphology suggesting close contact with surrounding cells. The T cell zones of non-immunized SPF mice generally lack such cells. During the primary immune response against RRBC we observed the gradual accumulation of FCRLAbr cells in T cell zones from day 7 to 14. Their relatively late appearance suggests that FCRLAbr cells may be generated early in the GC reaction and that they may up-regulate FCRLA after their exit from GCs. In support of this suggestion is our observation of clustered FCRLAbr cells in close proximity to GCs in some of the lymph node sections from immunized mice. However, an extrafollicular antigen response may contribute to the generation of T cell zone-localized FCRLAbr cells as well. Antigen-specific stimulation of B lymphocytes produces a complex mixture of cells, among which are short- and long-lived PCs and memory cells [4–7]. In addition, terminology such as plasmablasts, pre-PCs or pre-memory cells are often but not always uniformly used for putative precursors of PCs and memory cells. Depending on the type of antigen, the subset of B cells that has encountered the antigen, and the tissue compartment where the activation occurs, B cell differentiation may lead to the generation of cells that vary both in phenotype and functional capacities. What niche might FCRLAbr cells occupy within this landscape? It appears that FCRLAbr Igdl and FCRLAbr Igbr cells represent separate differentiation pathways rather than sequential stages: both populations are generated with comparable kinetics during the course of an in vitro response and coexist in bone marrow in vivo. The accumulation of FCRLAbr cells in the bone marrow after antigenic challenge is itself important evidence indicating that both populations of FCRLAbr cells are functionally mature lineages [19,20].
It is generally agreed that bone marrow provides an important niche for long-term survival of PCs and memory cells [20]. FCRLAbrIgbr cells may represent a fraction of PCs. Rare expression of CD138 in FCRLAbr cells does not completely invalidate this assumption since the existence of CD138-negative PCs in bone marrow has been documented in several studies [21–23].
The IgD-negative phenotype of FCRLbrIgdl cells, their accumulation in the bone marrow after immunization, together with the low level of Ig-expression, are consistent with the features of mouse memory cells. However, the fact that both B220 and CD19 are down-regulated in FCRLAbr cells does not agree with generally accepted view on the phenotype of mouse memory B cells. While the existence of a CD19−B220− subset of mouse memory B cells has been reported [24, 25], these findings have been heavily debated [26–28]. It should be stressed, however, that FCRLAbr cells are B220-low rather than negative and may be thus similar to the subpopulation of mouse memory cells described by Rice et al [29]. Nevertheless, to prove the attribution of FCRLAbr Igdl cells to the memory pool, it would be necessary to investigate their maintenance in long-term immunity to specific antigens.
The recent finding that Fcrla gene deficiency in mice has no obvious effect on primary and secondary immune responses [14] is unexpected in view of broad expression of this protein in B cells. It has been also shown that FCRLA is a resident ER protein that can bind to multiple Ig heavy chain isotypes [14, 15]. It seems likely that FCRLA may participate directly or indirectly in the assembly or transport of Ig within the ER, although experimental evidence for this function is lacking. Antigen-stimulated B cells are known to vary greatly in the level of expression of both surface and secreted Ig isoforms. For instance, it has been shown that the CD138-negative bone marrow PCs secrete Ig at lower rates compared to the CD138-positive cells [22, 23]. Whether FCRLA may be implicated in such regulation remains to be determined. What is clear is that further studies of this unusual member of the FcR family could be helpful for better understanding of B cell differentiation.
Highlights.
We characterize FCRLA expression pattern in mouse.
We show that FCRLA is differentially expressed during mouse B cell differentiation.
Weak FCRLA expression is characteristic of naïve follicular and MZ B cells.
FCRLA expression is upregulated in small subset(s) of activated B cells.
Acknowledgement
We would like to thank Dr. Sergey Baiborodin (Institute of Cytology and Genetics SB RAS, Novosibirsk, Russia) for assistance with confocal microscopy.
Funding:
This work was supported by the RAS Program #21.24 “Basic Sciences to Medicine”, the RAS Program #22.18 “Molecular and Cellular Biology”, the SB RAS Integration Program №35C, the Russian Foundation for Basic Research (grant numbers 08-04-00522,10-04-01398), the SB RAS Program For Young Scientists (grant number 6.12), and by NIH AI069365 and AI39816 (P.D.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J. Exp. Med. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sze DM, Toellner KM, García de Vinuesa C, Taylor DR, MacLennan IC. Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J. Exp. Med. 2000;192:813–821. doi: 10.1084/jem.192.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zúñiga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol. Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 4.Oracki SA, Walker JA, Hibbs ML, Corcoran LM, Tarlinton DM. Plasma cell development and survival. Immunol. Rev. 2010;237:140–159. doi: 10.1111/j.1600-065X.2010.00940.x. [DOI] [PubMed] [Google Scholar]
- 5.Fairfax KA, Kallies A, Nutt SL, Tarlinton DM. Plasma cell development: from B-cell subsets to long-term survival niches. Semin. Immunol. 2008;20:49–58. doi: 10.1016/j.smim.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Dogan I, Bertocci B, Vilmont V, Delbos F, Mégret J, Storck S, Reynaud CA, Weill JC. Multiple layers of B cell memory with different effector functions. Nat. Immunol. 2009;10:1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 7.Tangye SG, Tarlinton DM. Memory B cells: effectors of long-lived immune responses. Eur. J. Immunol. 2009;39:2065–2075. doi: 10.1002/eji.200939531. [DOI] [PubMed] [Google Scholar]
- 8.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J. Exp. Med. 2007;204:2103–2114. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrhardt GR, Hsu JT, Gartland L, Leu CM, Zhang S, Davis RS, Cooper MD. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J. Exp. Med. 2005;202:783–791. doi: 10.1084/jem.20050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mechetina LV, Najakshin AM, Volkova OY, Guselnikov SV, Faizulin RZ, Alabyev BY, Chikaev NA, Vinogradova MS, Taranin AV. FCRL, a novel member of the leukocyte Fc receptor family possesses unique structural features. Eur. J. Immunol. 2002;32:87–96. doi: 10.1002/1521-4141(200201)32:1<87::AID-IMMU87>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Facchetti F, Cella M, Festa S, Fremont DH, Colonna M. An unusual Fc receptor-related protein expressed in human centroblasts. Proc. Natl. Acad. Sci. U S A. 2002;99:3776–3781. doi: 10.1073/pnas.022042699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis RS, Li H, Chen CC, Wang YH, Cooper MD, Burrows PD. Definition of an Fc receptor-related gene (FcRX) expressed in human and mouse B cells. Int. Immunol. 2002;4:1075–1083. doi: 10.1093/intimm/dxf074. [DOI] [PubMed] [Google Scholar]
- 13.Maltais LJ, Lovering RC, Taranin AV, Colonna M, Ravetch JV, Dalla-Favera R, Burrows PD, Cooper MD, Davis RS. New nomenclature for Fc receptor-like molecules. Nat. Immunol. 2006;7:431–432. doi: 10.1038/ni0506-431. [DOI] [PubMed] [Google Scholar]
- 14.Wilson TJ, Gilfillan S, Colonna M. Fc receptor-like A associates with intracellular IgG and IgM but is dispensable for antigen-specific immune responses. J. Immunol. 2010;185:2960–2967. doi: 10.4049/jimmunol.1001428. [DOI] [PubMed] [Google Scholar]
- 15.Santiago T, Kulemzin SV, Reshetnikova ES, Chikaev NA, Volkova OY, Mechetina LV, Zhao M, Davis RS, Taranin AV, Najakshin AM, Hendershot LM, Burrows PD. FCRLA is a resident endoplasmic reticulum protein that associates with intracellular Igs, IgM, IgG and IgA. Int. Immunol. 2011;23:43–53. doi: 10.1093/intimm/dxq456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masir N, Jones M, Pozzobon M, Marafioti T, Volkova OY, Mechetina LV, Hansmann ML, Natkunam Y, Taranin AV, Mason DY. Expression pattern of FCRL (FREB, FcRX) in normal and neoplastic human B cells. Br. J. Haematol. 2004;127:335–343. doi: 10.1111/j.1365-2141.2004.05193.x. [DOI] [PubMed] [Google Scholar]
- 17.Korsrud FR, Brandtzaeg P. Immunohistochemical evaluation of J-chain expression by intra- and extra-follicular immunoglobulin-producing human tonsillar cells. Scand. J. Immunol. 1981;13:271–280. doi: 10.1111/j.1365-3083.1981.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 18.Angelin-Duclos C, Cattoretti G, Lin KI, Calame K. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J. Immunol. 2000;165:5462–5471. doi: 10.4049/jimmunol.165.10.5462. [DOI] [PubMed] [Google Scholar]
- 19.Blink EJ, Light A, Kallies A, Nutt SL, Hodgkin PD, Tarlinton DM. Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J. Exp. Med. 2005;201:545–554. doi: 10.1084/jem.20042060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moser K, Tokoyoda K, Radbruch A, MacLennan I, Manz RA. Stromal niches, plasma cell differentiation and survival. Curr. Opin. Immunol. 2006;18:265–270. doi: 10.1016/j.coi.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Underhill GH, Kolli KP, Kansas GS. Complexity within the plasma cell compartment of mice deficient in both E- and P-selectin: implications for plasma cell differentiation. Blood. 2003;102:4076–4083. doi: 10.1182/blood-2003-03-0947. [DOI] [PubMed] [Google Scholar]
- 22.Kallies A, Hasbold J, Tarlinton DM, Dietrich W, Corcoran LM, Hodgkin PD, Nutt SL. Plasma cell ontogeny defined by quantitative changes in blimp-1 expression. J. Exp. Med. 2004;200:967–977. doi: 10.1084/jem.20040973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kallies A, Hasbold J, Fairfax K, Pridans C, Emslie D, McKenzie BS, Lew AM, Corcoran LM, Hodgkin PD, Tarlinton DM, Nutt SL. Initiation of plasma-cell differentiation is independent of the transcription factor Blimp-1. Immunity. 2007;26:555–566. doi: 10.1016/j.immuni.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 24.McHeyzer-Williams LJ, Cool M, McHeyzer-Williams MG. Antigen-specific B cell memory: expression and replenishment of a novel b220-memory B cell compartment. J. Exp. Med. 2000;191:1149–1166. doi: 10.1084/jem.191.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cascalho M, Wong J, Brown J, Jack HM, Steinberg C, Walb M. A B220(−), CD19(−) population of B cells in the peripheral blood of quasimonoclonal mice. Int. Immunol. 2000;12:29–35. doi: 10.1093/intimm/12.1.29. [DOI] [PubMed] [Google Scholar]
- 26.Bell J, Gray D. Antigen-capturing cells can masquerade as memory B cells. J. Exp. Med. 2003;197:1233–1244. doi: 10.1084/jem.20020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mack M, Schneider MA, Moll C, Cihak J, Brühl H, Ellwart JW, Hogarth MP, Stangassinger M, Schlöndorff D. Identification of antigen-capturing cells as basophils. J. Immunol. 2005;174:735–741. doi: 10.4049/jimmunol.174.2.735. [DOI] [PubMed] [Google Scholar]
- 28.Wolniak KL, Noelle RJ, Waldschmidt TJ. Characterization of (4-hydroxy-3-nitrophenyl)acetyl (NP)-specific germinal center B cells and antigen-binding B220- cells after primary NP challenge in mice. J. Immunol. 2006;177:2072–2079. doi: 10.4049/jimmunol.177.4.2072. [DOI] [PubMed] [Google Scholar]
- 29.Rice JS, Newman J, Wang C, Michael DJ, Diamond B. Receptor editing in peripheral B cell tolerance. Proc. Natl. Acad. Sci. U S A. 2005;102:1608–1613. doi: 10.1073/pnas.0409217102. [DOI] [PMC free article] [PubMed] [Google Scholar]