Abstract
Background
A matched vaccine for the Pandemic (H1N1) 2009 virus will not be ready until autumn, 2009; decisions regarding timing of vaccination and percentage of population to vaccinate are complex.
Objective
To determine the effectiveness and cost-effectiveness of Pandemic (H1N1) vaccination in October or November, 2009.
Design
Compartmental epidemic model in conjunction with a Markov model of disease progression.
Data Sources
Literature and expert opinion.
Target Population
Residents of a major U.S. metropolitan city with a population of 8.3 million.
Time Horizon
Lifetime.
Perspective
Societal.
Interventions
Vaccination in mid-October or mid-November, 2009.
Outcome Measures
Infections and deaths averted, costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness.
Results of Base Case Analysis
At R0 of 1.5, vaccinating 20% of the population in October or November would be cost-saving. Vaccination in October would avert 1,067 deaths, gain 36,610 QALYs, and save $159 million; vaccination in November would avert 802 deaths, gain 27,416 QALYs and save $83 million relative to no vaccination. Vaccination of 37% of the population in October or 33% in November would slow widespread transmission of the pandemic.
Results of Sensitivity Analysis
If longer incubation periods, lower infectiousness, or increased implementation of non-pharmaceutical interventions delay time to the peak of the pandemic, vaccination in the autumn could be even more cost-saving. In contrast, if the epidemic peaks earlier, vaccination saves fewer lives and is less cost-effective.
Limitations
The model assumed homogenous mixing; heterogeneous mixing would result in more rapid initial spread, followed by slower spread to lower contact rates. Additional costs and savings not included in the model would make vaccination more cost-saving.
Conclusions
Absent additional harms, vaccination earlier in the epidemic prevents more deaths and saves more costs. Complete population coverage is not necessary to reduce viral reproductive rate sufficiently to help shorten the pandemic.
BACKGROUND
Pandemic (H1N1) 2009 has caused 182,166 confirmed infections and 1,799 deaths in over 150 countries to date (1). Both the World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention (CDC) have declared public health emergencies in response to global circulation of this virus, and the WHO has raised the influenza pandemic alert level from 3 to 6 (2). As a result of the strain’s novelty, most people lack innate immunity to Pandemic (H1N1) (4), currently available vaccines do not provide protection against the virus, and the time to manufacture, test, and distribute a matched vaccine is several months (56, 57).
In the absence of a matched vaccine, infections and deaths from Pandemic (H1N1) will continue globally until a sufficient proportion of the population has developed immunity through infection and recovery, inducing “herd immunity,” (population immunity that decreases the effective reproductive rate of the virus below one, ending the pandemic by epidemiologic definitions (58)). Public health officials were planning to begin vaccination campaigns in mid-October, 2009 (59); however, the National Biodefense Science Board, a group of advisors to the U.S. Department of Health and Human Services, recommended speeding large-scale vaccine administration to mid-September, 2009 (60). Decisions regarding vaccination timing and distribution are complicated: it is unclear how many individuals would require vaccination to substantially reduce transmission once vaccine is available (some scientists note that the first epidemic wave may in fact already be complete by this time (61)), and it could be expensive to manufacture and administer the vaccine, and to treat its side effects.
To help guide policymakers in advising vaccine manufacturers, we developed a model of progression of the 2009 (H1N1) Pandemic to determine how vaccination in October or November, 2009 would affect the course of the pandemic. We compared the effectiveness and cost-effectiveness of no vaccination, vaccination in mid-October, and vaccination in mid-November.
METHODS
Overview
We developed a compartmental epidemic model in conjunction with a Markov model of disease progression of the human spread of Pandemic (H1N1) to elucidate the dynamics of disease transmission and progression of the first pandemic wave. Following the recommendations of the Panel on Cost-Effectiveness in Health and Medicine (55), we adopted a societal perspective for costs and benefits, discounted at 3% annually. We analyzed outcomes for the remaining lifetime of each individual. We expressed these outcomes in infections and deaths, costs, quality-adjusted life years (QALYs) and incremental cost-effectiveness ratios. We developed the simulation model and performed analyses with Microsoft Excel (62).
Study Population and Disease Parameters
Susceptible population
We followed a hypothetical cohort of 8.3 million persons living in a large, U.S. city with a sex distribution (53% women), age range 0 to 100 years, and average remaining life expectancy similar to the population of New York City (3). The WHO officially declared the start of the pandemic on 11 June, 2009 (2). We assumed 10,000 individuals were infected at that time, based on a New York City telephone survey of influenza-like illness (ILI) (10) and CDC data of ILI cases testing positive for Pandemic (H1N1) (11–13) (calculations in Appendix). We varied this number from 1,000 to 50,000 in sensitivity analysis. Based on data showing some pre-existing population immunity to Pandemic (H1N1) (4), we assumed that 10% of the population entered the model immune, while 90% of individuals entered susceptible to infection. In sensitivity analysis, we examined scenarios in which up to 20% of individuals entered immune to the virus.
Infected population
Based on available evidence (5–7), we assumed that in the base-case, the R0 (number of secondary infections caused by each primary infection in susceptible population) of Pandemic (H1N1) is approximately 1.5. As new data on infectious spread emerges, this number may change. In sensitivity analysis, we varied R0 from 1.2 to 1.8.
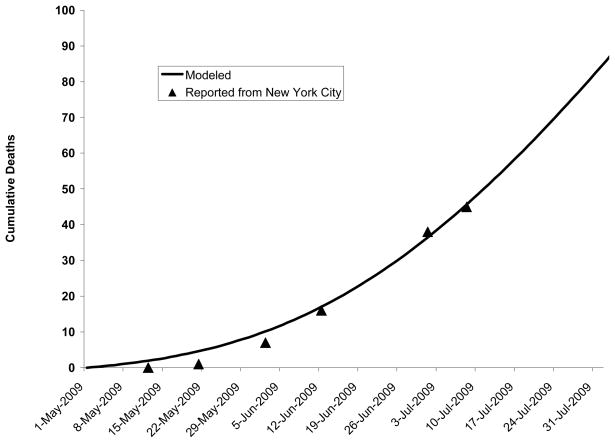
New York City death rates and other epidemiologic data suggest that viral transmission may be decreased over the warm summer months (10, 63–67). Because the timing of the peak of the epidemic is an important determinant of the effectiveness of vaccination, we modeled seasonal variation (described in Appendix) by calibrating to decreased deaths confirmed in New York City over the summer (Figure 1). We also evaluated the effectiveness of vaccination in scenarios in which the epidemic grew more slowly or more rapidly.
Figure 1. Deaths to date: Predicted by model versus confirmed in New York City.
Comparison of deaths predicted by model and confirmed by the New York City Department of Mental Health and Hygiene on 12 May, 21 May, 2 June, 12 June, 1 July, and 8 July, 2009.
Based on Influenza A virus infections (14–22, 68), we assumed that 67% of infected individuals developed symptoms. 50% of these individuals entered a state of isolation, either voluntarily, or because of physical limitation secondary to illness or admission to a hospital. We assumed that those who did not isolate (69) continued to infect contacts. Based on information to date on Pandemic (H1N1) 2009 and other influenza A viruses (26, 29), we assumed infected individuals had a mean incubation time of 3 days, had symptoms (if symptomatic) for 10 days, and were capable of transmitting the virus for 4 days. We evaluated infectivity of 7 days in sensitivity analyses. Based on studies of Influenza A infection and nasal viral shedding (23, 28), we assumed that incubating individuals transmitted influenza at half the rate of symptomatic individuals, and that asymptomatic individuals transmitted at one-quarter the rate of symptomatic individuals. Consistent with published CDC assumptions (30), we estimated that 3.3% of symptomatic individuals required 5 days of hospital care and 10% of hospitalized patients required 10 days of ICU care.
Recovered population
Estimates of re-infection with antigenically drifted influenza A viruses range from 2 – 25% (33–36) throughout the course of epidemics. Because most re-infected individuals are either asymptomatic or have mild symptoms, with a shorter duration of illness and less viral shedding, we assumed that 5% of the recovered population was once again susceptible to infection at an average of 5 months following recovery. We examined a range of re-infection from 2 to 25% in sensitivity analysis.
Death from influenza
We calculated a case-fatality proportion of 0.1%, based on the percent of cases of ILI in the U.S. which have tested positive for Pandemic (H1N1) (11–13, 70–80) This number is lower than estimated and documented case-fatality proportions (6, 27), secondary to less frequent testing of individuals with ILI as the pandemic progresses (81, 82). In sensitivity analysis, we modeled a more severe pandemic, with a 1.0% clinical case-fatality proportion (consistent with Pandemic (H1N1) global case-fatality proportions (37)), and a less severe pandemic, with a 0.01% clinical case-fatality proportion. We modeled age-specific mortality with increases in deaths in newborns, young adults, and individuals over 65 years, consistent with current Pandemic (H1N1) mortality (27, 80).
Interventions
Non-pharmaceutical interventions
Current CDC recommended non-pharmaceutical interventions for Pandemic (H1N1) include closures of school and childcare facilities, home isolation, cough etiquette, hand washing, use of alcohol-based hand gels, and use of personal protective equipment such as masks (83). Incorporating the results of a complex network model of pandemic spread through communities (8), we assumed that these non-pharmaceutical interventions are reducing contacts by 15%. A recent randomized trial of facemasks and hand washing found that under optimal circumstances, these measures reduced transmission among households by 66% (9); therefore, we evaluated reduction in contacts from 10% to 70% in sensitivity analysis (84).
Vaccination
One-dose vaccine schedules routinely used for seasonal influenza have had limited success in eliciting human antibodies to novel influenza A viruses, but two-dose (primer and booster) adjuvanted vaccines have been much more successful; not only do they more frequently elicit antibody responses, but they also protect against different influenza clades, an important advantage in light of the virus’s ability to mutate (39, 40, 85, 86). Based on these properties, vaccine manufacturers are designing two-dose adjuvanted vaccines for Pandemic (H1N1) (87). Pending final results of “mix-and-match” antigen plus adjuvant studies (48), we assumed a 15μg adjuvant-to-antigen concentration, and examined lower (3.8μg (40)) concentrations in sensitivity analyses. We assumed this vaccination sequence was 80% effective, comparable to a well-matched seasonal influenza vaccine (38). In sensitivity analyses, we examined ranges of vaccine effectiveness from 50% to 90%.
The U.S. government expects to have 120 million doses of vaccine available in the autumn, a quantity sufficient to vaccinate 20% of the U.S. population with the two-dose vaccination sequence (59, 88). Based on historical precedent (New York City, 1976 (89)) and modern mass vaccination exercises (90), we estimated that a rapid influenza vaccination campaign, using published emergency response logistic plans, could inoculate approximately 250 people per vaccination center per hour, providing coverage for all 8.3 million individuals over a 10 day period (91). Following the results of studies of two-dose adjuvanted vaccination (39), we assumed that a second dose administered 21 days later would be administered and provide full immunity.
We assumed 45% of Pandemic (H1N1) vaccinated individuals experienced mild to moderate adverse reactions such as pain, redness, swelling, induration, ecchymosis, low-grade fevers, arthralgias, fatigues, headaches, myalgias, shivering, or sweating for up to seven days, based on adjuvanted A (H5N1) vaccination data (39, 40). We assumed 0.001% of the population experienced severe adverse reactions such as angioedema, anaphylaxis, or Guillain-Barré Syndrome, consistent with 1976 vaccination data (41).
Costs and Utilities
We expressed all costs in 2009 U.S. dollars using the GDP deflator. Intervention costs included the cost of a vaccine, administration, the value of an individual’s time receiving it and the costs of treating individuals with severe side effects (Table 1). We estimated treatment costs at a hospital from the average cost of general medical hospitalization for influenza (52) or medical intensive care unit hospitalization (51). We based utility estimates on EuroQol and Time Trade-Off ratings and included the remaining lifetime of individuals alive at the end of the year. We calculated remaining life-years from the New York census, then adjusted life expectancy for quality of life by using age-and sex-specific utilities from the Beaver Dam Health Outcomes Study (45).
Table 1.
Variables and Sources
| Variable | Base Case (Range) | Source |
|---|---|---|
| Susceptible | ||
| Population | 8,300,000 | New York Vital Statistics (3) |
| Age (range, years) | 0–100 | New York Vital Statistics (3) |
| Percent Female | 53 | New York Vital Statistics (3) |
| Pre-existing population immunity | 10% (0–20%) | MMWR (4) |
| Infected | ||
| R0 | 1.5 (1.2–1.8) | CDC(5), Fraser et al. (6), Pourbohloul et al. (7) |
| Impact of season on transmission | 0.2 (0–0.5) | Assumed |
| Non-pharmaceutical interventions reduction in contacts | 15% (0–70%) | Assumed, Davey et al. (8), Cowling et al.(9) |
| Number of infected individuals at start of pandemic | 10,000 (1,000–50,000) | NYC Department of Health and Mental Hygiene (10), CDC(11–13) |
| Probability of symptomatic infection | 67% (50–90%) | Ferguson et al. (14), Longini et al. (15), Katz et al. (16), Dinh et al (17)., Vong et al. (18), Buxton Bridges et al. (19), Aparnthanarak et al (20)., Liem et al (21)., Wang et al. (22) |
| Reduced infectiousness by incubating | 50% (10–62.5%) | Hayden et a (23)., Wein et al. (24) |
| Reduced infectiousness by asymptomatic | 25% (10–50%) | Hayden et al (23)., Wein et al. (24) |
| Probability of isolating given symptomatic infection | 50% (37.5–62.5%) | Longini et al.(25) |
| Mean incubation time (days) | 3 (1–7) | Novel Swine-Origin Virus Investigation Team (26), CDC (27) |
| Mean duration of infectiousness (days) | 4 (3–7) | Hayden et al. (23), Leekha et al. (28) |
| Mean duration of symptomatic illness (days) | 10 (7.5–12.5) | CDC (29) |
| Proportion of symptomatic patients requiring inpatient care | 3.3% (1–10%) | CDC (30), HHS (31), MMWR (32) |
| Mean duration of non-ICU hospital stay (days) | 5 (3.75–6.25) | CDC (30) |
| Proportion of hospitalized patients requiring ICU care | 10% (7.5–12.5%) | CDC (30) |
| Mean duration of ICU stay (days) | 10 (7.5–12.5 ) | CDC (30) |
| Recovered | ||
| Susceptibility to re-infection following recovery | 5% (2–25%) | Smith et al. (33) Monto et al. (34) Sonoguchi et al (35). Davies et al.(36) |
| Timing of waning immunity (months) | 5 (2–8) | Smith et al. (33) Monto et al. (34) Sonoguchi et al (35). Davies et al. (36) |
| Dead | ||
| Case-fatality proportion | 0.1% (0.01%–1.0%) | Assumed, Pandemic (H1N1) case fatalities (27, 37) |
| Intervention Effectiveness | ||
| Adjuvanted two-dose vaccine | 80% (50–90%) | Assumed, Bridges et al. (38) |
| Vaccination side effects | ||
| Mild-moderate side-effects | 45% (5–75%) | Treanor et al. (39), Leroux-Roels et al. (40) |
| Severe side effects | 0.001% (0–0.01%) | Neustadt and Fineberg (41) |
| Risk of death from severe side effects | 5% (1–10%) | Chio et al. (42) |
| Risk of long-term care from severe side effects | 5% (1–10%) | Kissel et al. (43) |
| Vaccination side effects reduction in quality of life* | ||
| Mild-moderate side-effects | 0.05 (0–0.1) | Treanor et al. (39), Leroux-Roels et al. (40), CDC (44) |
| Severe side effects | 0.5 (0–1) | Neustadt and Fineberg (41) |
| Duration of mild-moderate side effects (days) | 2 (1–7) | Treanor et al. (39), Leroux-Roels et al. (40) |
| Duration of hospitalization for severe side effects (days) | 14 (7–28) | Chio et al. (42) |
| Influenza-related quality of life | ||
| Uninfected/Asymptomatic | 0.96 (0.92–1.00) | New York Census (3), Beaver Dam Health Outcomes (45) |
| Symptomatic Influenza | 0.8 (0.7–0.9) | Turner et al. (46) |
| Post-influenza disabled state for patients requiring ICU care | 0.9 (0.85–0.95) | Assumed |
| Costs | ||
| Vaccine | ||
| Antigen per μg ($) | 0.45 (0.15–0.70) | HHS (47) |
| Adjuvant ($) | 7.00 (5.25–8.75) | BARDA (personal communication – Michael Perdue) |
| μg adjuvant per vaccine | 15 (3.8–90) | HHS (48) |
| Administration | 8.73 (6.54–10.91) | Calculated: 10 minutes of nurse wages (49) |
| Patient Time | 10.55 (5.28–21.10) | U.S. Bureau of Labor Statistics (50) |
| Daily health care costs ($) | ||
| Patient with severe side effects (treated in ICU) | 3,739.05 (2,804.29 – 4,673.82) | Desta et al. (51) |
| General medical hospitalized patient | 1,830.46 (1429.37–1870.54) | Talbird et al. (52) |
| ICU hospitalized patient | 3,739.05 (2,804.29 – 4,673.82) | Desta et al. (51) |
| Long-term treatment facility costs | 313.05 (234.79–391.31) | Metlife Survey (53) |
| Normal health care expenditures | 19.56 (14.67–24.45) | Statistical Abstract of the United States (54) |
| Other variables | ||
| Discount Rate (annual %) | 3 (0–5%) | Weinstein et al. (55) |
Quality of life variables represent a person’s preference for a given state of health and are scaled form 0 to 1, with 1 equivalent to perfect health.
Sensitivity Analysis
We used sensitivity analysis to identify important model uncertainties. When available, we based variable ranges on reported 95% confidence intervals from the data sources. Otherwise, we determined ranges by adding or subtracting 25% from the baseline estimate.
Model Validation
We had previously validated our model by comparing its clinical attack rate and first pandemic wave duration to other models of community influenza A epidemics (8, 92) (Appendix). We performed additional validation by comparing the number of deaths in our city at six time points to confirmed deaths from Pandemic (H1N1) in New York City (10, 63–67).
RESULTS
Model Validation
Our model predicted similar deaths to those which had occurred in New York City at six time points between May and July, 2009 (Figure 1 and Appendix).
Base-Case Analysis
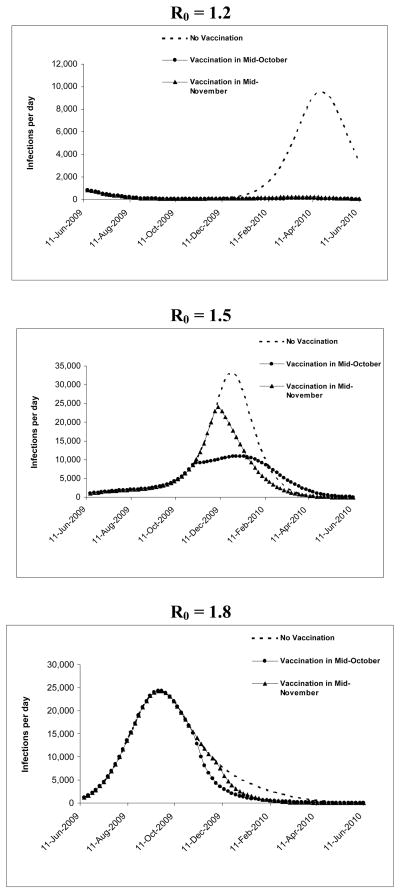
Under no vaccination at R0 1.5, in mid-October, 285,566 of the city’s 8.3 million individuals would have been symptomatically infected and 286 would have died (Table 2 and Figure 2). In November, 541,865 would have been infected and 542 would have died. Due to the development of immunity in individuals in the population who had been infected and recovered, 85% of individuals would still be susceptible to infection in October, and 80% would be susceptible to infection in November.
Table 2.
Health outcomes assuming no vaccination for a city of 8.3 million individuals
| VACCINATION IN OCTOBER | VACCINATION IN NOVEMBER | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R0 | Symptomatic infections by October 15, 2009* | Deaths to date* | % vaccination to decrease widespread transmission† | % still susceptible to infection | Deaths averted following vaccination‡ | Symptomatic infections by November 15, 2009 | Deaths to date | % vaccination to decrease widespread transmission | % still susceptible to infection | Deaths averted following vaccination |
| 1.2 | 38,304 | 38 | 20% | 89% | 755 | 40,416 | 40 | 20% | 89% | 736 |
| 1.5 | 285,566 | 286 | 37% | 85% | 1,067 | 541,865 | 542 | 33% | 80% | 802 |
| 1.8 | 1,742,525 | 1,743 | 24% | 59% | 421 | 2,246,591 | 2,247 | 8% | 50% | 235 |
From a total population of 8,300,000 in target city
R0 ≤ 1 (by epidemiologic definitions, end of pandemic)
At 12 months, compared to continuing with no vaccination
Figure 2. Progression of pandemic with no vaccination under different R0s to time of vaccine availability.
The effective viral reproductive rate would be 1.42 in October, and 1.35 in November, due to the development of immunity in individuals in the population who had been infected and recovered.
Varying R0 from 1.2 to 1.8 (Table 2 and Figure 3), symptomatic infections would range from 38,304 to 1.74 million in October and 40,416 to 2.25 million in November; deaths would range from 38 to 1,743 in October and 40 to 2,247 in November. At R0 of 1.2, fewer individuals would become infected, so less immunity would develop, and 89% of individuals would still be susceptible to infection in October and November. At R0 of 1.8, a significant number of infections would increase population immunity, with 59% of individuals susceptible to infection in October, and 50% susceptible to infection in November.
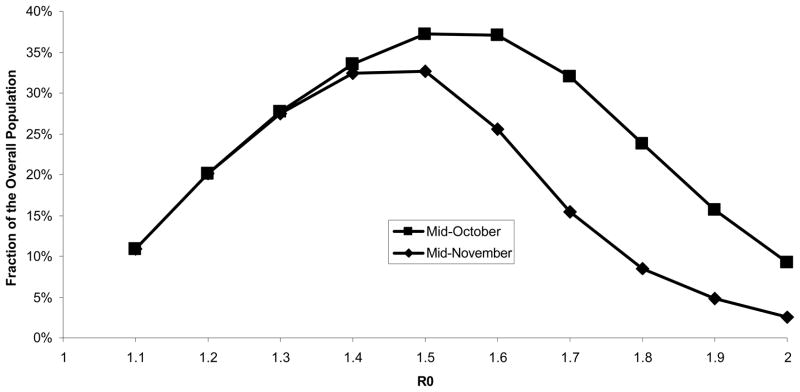
Figure 3. Percent vaccination required to decrease widespread transmission in October and November.
At R0 of 1.2, fewer individuals would become infected, so less immunity would develop and a greater number of individuals would require vaccination to decrease widespread transmission. However, at R0 of 1.8, a significant number of infections would occur, increasing population immunity and decreasing the number of individuals who would require vaccination to decrease widespread transmission.
Health Outcomes
The number of individuals requiring vaccination to reduce R0 below 1 and thus help end widespread transmission is related to the initial R0 for the pandemic (Table 2 and Figure 3). At our base case R0 of 1.5, 37% of the population would require vaccination in October to slow widespread transmission, and 33% would require vaccination in November. At a lower R0 of 1.2, 20% of the population would require vaccination in October or November. At a higher R0 of 1.8, 24% of the population would require vaccination in October, and 8% would require vaccination to decrease widespread transmission in November.
The relationship of deaths averted following vaccination in October or November is also related to R0. In our base case analysis, at R0 of 1.5, 802 deaths would be averted with vaccination in November compared to no vaccination; an additional 265 deaths would be averted if vaccination were performed in October. At R0 of 1.2, infectious spread would be slow enough that vaccination would avert 755 deaths in October and 736 deaths in November. At R0 of 1.8, the pandemic would spread quickly, such that vaccination in October would avert 421 deaths and vaccination in November would avert 235 deaths.
Cost-Effectiveness
Vaccinating 20% of the population in October would be cost-saving, adding 36,610 QALYS and saving $159 million. Vaccination in November would add 27,416 QALYs and save $83 million relative to no vaccination (Table 3). Vaccinating 37% of the population in October would slow widespread transmission, and add 66,391 QALYs, saving $283 million relative to no vaccination. Vaccinating 33% of the population in November would slow widespread transmission, and add 40,813 QALYs, saving $101 million relative to no vaccination.
Table 3.
Economic outcomes* following vaccination for a city of 8.3 million individuals
| VACCINATION IN OCTOBER | VACCINATION IN NOVEMBER | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R0 | Vaccination Costs (millions of $) | Treatment Costs (millions of $) | Normal Healthcare Costs† (millions of $) | Total Costs (millions of $) | QALYs‡ | ICER§ ($/QALY) | Vaccination Costs (millions of $) | Treatment Costs (millions of $) | Normal Healthcare Costs (millions of $) | Total Costs, (millions of $) | QALYs | ICER ($/QALY) |
| 1.2 | 145 | (352) | 133 | (70) | 26,078 | Cost-Saving | 145 | (343) | 130 | (65) | 25,410 | Cost-Saving |
| 1.5 | 145 | (500) | 190 | (159) | 36,610 | Cost-Saving | 145 | (375) | 143 | (83) | 27,416 | Cost-Saving |
| 1.8 | 145 | (197) | 75 | 25 | 14,163 | 1,776 | 145 | (110) | 42 | 78 | 7,712 | 10,140 |
All outcomes are relative to no vaccination
Accounting for normal healthcare expenditures, total costs will increase in situations where deaths are averted.
QALY = quality-adjust life year
ICER = incremental cost-effectiveness ratio
When the reproductive rate of the virus is low, the pandemic spreads more slowly, and fewer treatment costs and deaths are averted with vaccination (Figure 2 and Table 3). At R0 of 1.2, vaccination in October would add 26,078 QALYs and save $70 million, and vaccination in November would add 25,410 QALYs and save $65 million relative to no vaccination. When the reproductive rate of the virus is higher, widespread transmission of the virus is already decreasing by November, leading to fewer lives saved with vaccination at that time. At R0 of 1.8, vaccination in October would add 14,163 QALYs at $25 million, for a cost of $1,776 per QALY, and vaccination in November would add 7,712 QALYs at $78 million, for a cost of $10,140 per QALY relative to no vaccination.
In considering short-term local budgetary implications, at R0 of 1.5, federal costs for vaccination in November for a city of 8.3 million individuals would be $46 million in federal costs for vaccine antigen and adjuvant; city costs would be $58 million to administer the vaccines; city and individual costs would be $35 million in vaccine recipient time and $6.4 million treating short-term severe side effects. Savings to the city and individuals would be $375 million in influenza treatment costs (Table 3).
Results under varied growth scenarios
In light of significant local and regional variations in the growth of the pandemic in the United States (11–13, 70–79), we examined a slower growth scenario, based on confirmed deaths to date throughout the country on average (11–13, 70–79). In this scenario, with slower spread of the virus (Appendix Figure B1), more individuals would be susceptible to infection in the autumn: 41% of individuals would require vaccination to decrease widespread transmission in October and 40% would require vaccination in November. Vaccinating 20% of the population would be cost-saving, adding 40,345 QALYS and saving $189 million relative to no vaccination in October, and adding 36,290 QALYs and saving $155 million relative to no vaccination in November. Vaccinating 41% of the population in October would slow widespread transmission, add 92,342 QALYs, and save $465 million relative to no vaccination. Vaccinating 40% of the population in November would slow widespread transmission, add 71,188 QALYs, and save $299 million relative to no vaccination.
We also examined a scenario with no reduction in viral transmission over the summer months. In this case, with more rapid spread of the pandemic (Appendix Figure B2), the peak of the epidemic would have passed, and widespread transmission would be decreasing without vaccination by October. Assuming non-pharmaceutical interventions reducing infectious contacts by 15% remained in effect, vaccinating 20% of the population in October would gain 1,867 QALYs at a cost of $67,441 per QALY, and vaccinating in November would gain 212 QALYs at a cost of $658,568 per QALY.
Results assuming lesser vaccine availability
Recent announcements by public health officials suggest that the originally anticipated 120 million vaccine doses may not be ready for distribution in October, but that at least 45 million doses, a quantity sufficient to vaccinate 7.5% of the population, will be available (88, 93). Vaccinating 7.5% of the population in mid-October would avert 372,925 infections and 373 deaths, saving $52 million versus no vaccination.
The costs and feasibility of expediting from 7.5% to 20% population vaccination to mid-October are unknown. At a willingness to pay threshold of $50,000 per QALY, over the range of R0 from 1.2 to 1.8, the additional acceptable costs would range from $261 million to $1.3 billion for a city of 8.3 million individuals.
Sensitivity Analyses
We conducted univariate sensitivity analysis on all variables (Appendix Table B1). The parameters most likely to affect the number of deaths averted in November were reduction in contacts secondary to non-pharmaceutical interventions, vaccine efficacy, duration of incubation, and duration of infectiousness.
If more effective non-pharmaceutical interventions were implemented, fewer individuals would become infected, and the peak of the pandemic would be delayed while the interventions were in effect. If 70% reduction in contacts were achieved through non-pharmaceutical interventions, the pandemic would be contained (R0<1), and vaccinating 20% of the population in November would allow reductions in implementation of non-pharmaceutical interventions.
If the incubation period increased, the pandemic would have a later peak at a given R0. At mean incubation times of seven days, with R0 of 1.5, 1,324 deaths would be averted with vaccination in November. At mean incubation times of one day, 433 deaths would be averted with vaccination in November.
If infected individuals transmitted virus for longer durations, the pandemic would spread more rapidly because the reproductive rate would increase, and fewer individuals would require vaccination to end widespread transmission in the autumn.
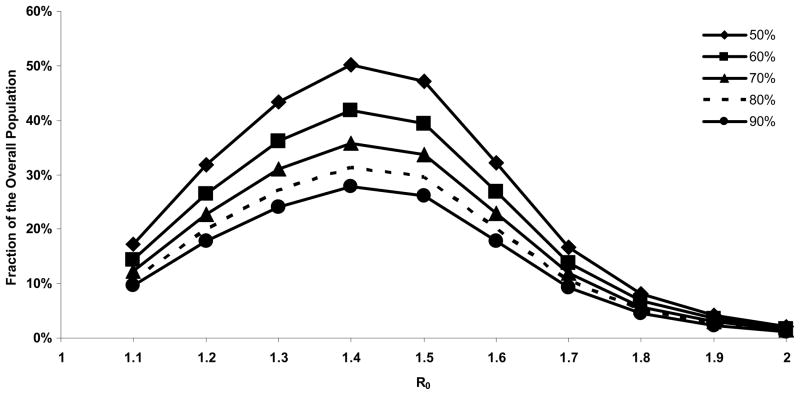
Decreases in vaccine efficacy would increase the percent requiring vaccination to decrease widespread transmission in November (Figure 4.).
Figure 4. Percent vaccination to decrease widespread transmission in November with varying vaccine efficacy.
The percent of population requiring vaccination to reduce widespread transmission increases with decreases in vaccine efficacy
Sensitivity to Severe Vaccine Side Effects
Under our base case assumption of severe side effects from vaccination occurring in 1 in 100,000 vaccinated individuals, vaccinating 20% of the city’s population would cause approximately 2 deaths from severe vaccine side effects, approximately 0.2% of the lives saved from vaccination in November. If severe side effects from adjuvanted vaccination occur in 1 in 400 individuals, 415 deaths would occur due to severe side effects, approximately half the number of lives saved from vaccination in November.
Monte Carlo Probabilistic Sensitivity Analysis
In 31% of Monte Carlo probabilistic sensitivity analysis simulations (Appendix Figure B3), vaccinating 20% of the population in November is cost-saving versus no vaccination; in 63% of simulations, vaccinating 20% of the population in November has an estimated incremental cost less than $50,000 per QALY saved; and in 67% of simulations, an estimated incremental cost less than $100,000 per QALY saved. In 23% of simulations, no vaccination is more cost-effective than vaccinating 20% of the population in November.
DISCUSSION
We examined the costs and benefits of vaccination in the autumn for the ongoing 2009 (H1N1) pandemic. Our analysis suggests that absent additional harms, earlier vaccination, as advised by the National Biodefense Science Board (60), would save more costs and avert a greater number of deaths than vaccination later in the autumn. Because accelerating large-scale vaccination efforts in this time frame may be costly, we have provided a range of acceptable costs of vaccination, given different reproductive rates, to guide policymakers in situations in which they might consider speeding vaccine production and administration. We defined the number of individuals requiring vaccination to reduce widespread transmission in a metropolitan city under a broad range of possible reproductive rates and note that regardless of the timing of vaccination, complete population coverage is not necessary to reduce the viral reproductive rate sufficiently to help shorten the pandemic. These results have important ramifications both for vaccine production goals and preparations for a potentially unprecedented fall vaccination campaign.
We found that the effectiveness and cost-effectiveness of vaccination are most dependent on the speed at which the pandemic grows. Our finding that earlier vaccination saves more costs and averts more deaths may be most important for those areas in which there is more rapid growth of the pandemic; it is important to note that the virus is not spreading at the same rate throughout the United States, but appears to be evolving as different regional and local epidemics (11–13, 70–79). Several factors may delay the peak of the pandemic, leaving a greater proportion of the population susceptible to infection, and increasing the effectiveness and cost-effectiveness of vaccination later in the autumn. Viral characteristics that would delay the peak include a lower reproductive rate, a longer incubation period, and a shorter duration of infectiousness. Importantly, non-pharmaceutical interventions could also have a marked effect on the speed at which the pandemic grows: our analysis shows that increased implementation of highly effective non-pharmaceutical interventions, such as early use of hand hygiene and surgical masks (84) can significantly delay the peak of the pandemic, increasing the effectiveness and cost-effectiveness of delayed vaccination. In contrast, if the epidemic grows rapidly and peaks in October, vaccination becomes substantially less effective and less cost-effective.
While greater than 50% population coverage with an effective vaccine for Pandemic (H1N1) may be desirable (94), this goal does not appear to be logistically feasible for the autumn vaccination campaign. Our analysis suggests that vaccinating even 20% of the population can be effective and cost-effective. We also note that over a wide range of viral reproductive rates and pandemic growth scenarios, vaccinating up to 41% of the population can be sufficient to slow widespread viral transmission by inducing herd immunity within the population, shortening the pandemic.
We assumed that severe Pandemic (H1N1) vaccine side effects could occur in 1 in 100,000 vaccinated individuals (41). Under these assumptions, vaccinating 20% of the city’s population in November would cause approximately 1 death secondary to severe vaccine side effects for every 437 lives saved from vaccination. We emphasize that our analysis assumes that vaccination would not cause additional harms, and we encourage thorough testing and evaluation of vaccines prior to large-scale vaccination campaigns (95).
Key limitations of the analysis include an assumption that disease transmission occurs with homogenous mixing; all individuals, regardless of age and occupation, have the same frequency of contacts, and our model is not designed to make recommendations about the impacts of prioritizing vaccination for different groups. In the 1918 and 1957 pandemics, influenza was transmitted more readily in children in close proximity, such as schools (96). If this pattern occurs in the 2009 (H1N1) Pandemic, heterogeneous mixing would result in a more rapid initial spread of the pandemic, followed by slowing as it spreads to lower contact rates (97). Our analysis provides insights into the magnitude of the pandemic and the response to vaccination (98); however, policymakers may wish to prioritize vaccination based on differing patterns of transmission in specific age groups, as well as groups noted to have higher morbidity and mortality from Pandemic (H1N1) infection.
We did not account for all costs to uninfected individuals in the setting of the 2009 (H1N1) pandemic; costs incurred by uninfected individuals from school and workplace closures, decreases in tourism and group recreation, and loss of firm-specific knowledge may be greater than costs to sick individuals (99). We did not include potential savings of effective vaccination, such as limiting displacement of hospitalized patients, or decreasing school and workplace closures. However, including these costs and savings would make vaccination even more cost-effective or cost-saving. Additionally, we account for normal health care expenditures, which significantly increase total costs for each life saved through vaccination; not including costs of long-term normal health care in our analysis would also make Pandemic (H1N1) vaccination more cost-saving.
Covering the majority of the population with an effective vaccine for Pandemic (H1N1) would prevent the most morbidity and mortality from influenza, but will not be achievable within the short time frame for vaccine development and with projected supplies (88, 93). Our analysis suggests that vaccination can be a valuable and effective intervention even it is reaches less than half the population. Many uncertainties remain about the transmissibility and mortality of Pandemic (H1N1) 2009; however, absent serious vaccine side effects, vaccination earlier in the autumn is likely to be cost-saving and avert a greater number of deaths than later vaccination, which highlights the urgency of vaccine development, with attention to safety. On 24 June, 2009, President Obama signed into law an emergency spending bill devoting $2 billion in additional funding to 2009 (H1N1) Pandemic mitigation efforts (100); our analyses suggest that vaccination strategies could be a valuable component of such efforts.
Acknowledgments
Funding/Support and Role of Sponsor
This research was supported by the Agency for Healthcare Research and Quality (1 F32 HS018003-01A1, Dr. Khazeni), the National Institute on Drug Abuse (2 R01 DA15612-016, Dr. Owens), a Stanford Graduate Fellowship (Mr. Hutton), and the Department of Veterans Affairs (Drs. Owens and Garber). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; and preparation, review, or approval of the manuscript.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
References
- 1.World Health Organization. [Accessed: 18 August, 2009];Pandemic (H1N1) 2009 - update 62. 2009 August 13; http://www.who.int/csr/don/2009_08_19/en/index.html.
- 2.World Health Organization. [Accessed: July 28, 2009];World now at the start of the 2009 influenza pandemic. 2009 June 11; http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html.
- 3.Bureau of Vital Statistics - New York City Department of Health and Mental Hygiene. [Accessed: 17 July, 2009];SUMMARY OF VITAL STATISTICS, THE CITY OF NEW YORK. 2007 http://www.nyc.gov/html/doh/downloads/pdf/vs/2007sum.pdf.
- 4.Centers for Disease Control and Prevention. Serum Cross-Reactive Antibody Response to a Novel Influenza A (H1N1) Virus After Vaccination with Seasonal Influenza Vaccine. Morb Mortal Wkly Rep. 2009;58(19):521–24. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Briefing on Public Health Investigation of Human Cases of H1N1 Flu (Swine Flu) 2009 http://www.cdc.gov/media/transcripts/2009/t090501.htm Accessed.
- 6.Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, Hollingsworth TD, et al. Pandemic Potential of a Strain of Influenza A (H1N1): Early Findings. Science. 2009;324(5934):1557–1561. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pourbohloul B, Ahued A, Davoudi B, Meza R, Meyers LA, Skowronski DM, et al. Initial human transmission dynamics of the pandemic (H1N1) 2009 virus in North America. Influenza and Other Respiratory Viruses. 2009;3(5):215–222. doi: 10.1111/j.1750-2659.2009.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davey VJ, Glass RJ, Min HJ, Beyeler WE, Glass LM. Effective, Robust Design of Community Mitigation for Pandemic Influenza: A Systematic Examination of Proposed US Guidance. PLoS ONE. 2008;3(7):e2606. doi: 10.1371/journal.pone.0002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowling BJ, Chan K-H, Fang VJ, Cheng CKY, Fung ROP, Wai W, et al. Facemasks and Hand Hygiene to Prevent Influenza Transmission in Households: A Randomized Trial. Ann Intern Med. 2009 doi: 10.7326/0003-4819-151-7-200910060-00142. 0000605–200910060–00142. [DOI] [PubMed] [Google Scholar]
- 10.New York City Department of Health and Mental Hygiene. [Accessed: 29 July, 2009];Health Alert #22: Pandemic (H1N1) 2009 Influenza Update, Revised Reporting Requirements and Testing Procedures. 2009 June 12; http://www.nyc.gov/html/doh/downloads/pdf/cd/2009/09md22.pdf.
- 11.Centers for Disease Control and Prevention. [Accessed: 29 July, 2009.];2008–2009 Influenza Season Week 21 ending. 2009 May 30; http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/weekly21.htm.
- 12.Centers for Disease Control and Prevention. [Accessed: 29 July, 2009.];2008–2009 Influenza Season Week 19 ending. 2009 May 16; http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/weekly19.htm.
- 13.Centers for Disease Control and Prevention. [Accessed: 29 July, 2009.];2008–2009 Influenza Season Week 20 ending. 2009 May 23; http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/weekly20.htm.
- 14.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442(7101):448–52. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longini IM, Jr, Nizam A, Xu S, Ungchusak K, Hanshaoworakul W, Cummings DA, et al. Containing pandemic influenza at the source. Science. 2005;309(5737):1083–7. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 16.Katz JM, Lim W, Bridges CB, Rowe T, Hu-Primmer J, Lu X, et al. Antibody response in individuals infected with avian influenza A (H5N1) viruses and detection of anti-H5 antibody among household and social contacts. J Infect Dis. 1999;180(6):1763–70. doi: 10.1086/315137. [DOI] [PubMed] [Google Scholar]
- 17.Dinh PN, Long HT, Tien NTK, Hien NT, Mai LTQ, Phong LH, et al. Risk factors for human infection with avian influenza A H5N1, Vietnam, 2004. Emerg Infect Dis. 2006;12(12):1841–1847. doi: 10.3201/eid1212.060829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vong S, Coghlan B, Mardy S, Holl D, Seng H, Ly S, et al. Low frequency of poultry-to-human H5NI virus transmission, southern Cambodia, 2005. Emerg Infect Dis. 2006;12(10):1542–7. doi: 10.3201/eid1210.060424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buxton Bridges C, Katz JM, Seto WH, Chan PK, Tsang D, Ho W, et al. Risk of influenza A (H5N1) infection among health care workers exposed to patients with influenza A (H5N1), Hong Kong. J Infect Dis. 2000;181(1):344–8. doi: 10.1086/315213. [DOI] [PubMed] [Google Scholar]
- 20.Apisarnthanarak A, Erb S, Stephenson I, Katz JM, Chittaganpitch M, Sangkitporn S, et al. Seroprevalence of anti-H5 antibody among Thai health care workers after exposure to avian influenza (H5N1) in a tertiary care center. Clin Infect Dis. 2005;40(2):e16–8. doi: 10.1086/427034. [DOI] [PubMed] [Google Scholar]
- 21.Liem NT, Lim W. Lack of H5N1 avian influenza transmission to hospital employees, Hanoi, 2004. Emerg Infect Dis. 2005;11(2):210–5. doi: 10.3201/eid1102.041075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M, Di B, Zhou DH, Zheng BJ, Jing H, Lin YP, et al. Food markets with live birds as source of avian influenza. Emerg Infect Dis. 2006;12(11):1773–5. doi: 10.3201/eid1211.060675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayden FG, Fritz R, Lobo MC, Alvord W, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest. 1998;101(3):643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atkinson MP, Wein LM. Quantifying the routes of transmission for pandemic influenza. Bull Math Biol. 2008;70(3):820–67. doi: 10.1007/s11538-007-9281-2. [DOI] [PubMed] [Google Scholar]
- 25.Longini IM, Jr, Halloran ME, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am J Epidemiol. 2004;159(7):623–33. doi: 10.1093/aje/kwh092. [DOI] [PubMed] [Google Scholar]
- 26.Novel Swine-Origin Influenza A Virus Investigation Team. Emergence of a Novel Swine-Origin Influenza A (H1N1) Virus in Humans. N Engl J Med. 2009;360(25):2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. [Accessed: June 22, 2009.];Swine Influenza (Flu) 2009 http://www.cdc.gov/swineflu/
- 28.Leekha S, Zitterkopf N, Espy M, Smith T, Thompson R, Sampathkumar P. Duration of Influenza A Virus Shedding in Hospitalized Patients and Implications for Infection Control. Infection Control and Hospital Epidemiology. 2007;28(9):1071–1076. doi: 10.1086/520101. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. [Accessed: June 17, 2009.];Influenza: The Disease. 2007 http://www.cdc.gov/flu/about/disease.htm.
- 30.Coordinating Center for Infectious Diseases. FluSurge, Version 2.0. Atlanta, Georgia: 2006. [Google Scholar]
- 31.U.S. Department of Health and Human Services. [Accessed: June 17, 2009.];Pandemic Influenza Plan. http://www.hhs.gov/pandemicflu/plan/
- 32.Centers for Disease Control and Prevention. Hospitalized Patients with Novel Influenza A (H1N1) Virus Infection --- California, April--May, 2009. Morb Mortal Wkly Rep. 2009;58(19):536–41. [PubMed] [Google Scholar]
- 33.Smith CB, Cox NJ, Subbarao K, Taber LH, Glezen WP. Molecular epidemiology of influenza A (H3N2) virus reinfections. J Infect Dis. 2002;185(7):980–5. doi: 10.1086/339416. [DOI] [PubMed] [Google Scholar]
- 34.Monto AS, Davenport FM, Napier JA, Francis T. Modification of an outbreak of influenza in Tecumseh, Michigan by vaccination of schoolchildren. The Journal of Infectious Diseases. 1970;122(1):16–25. doi: 10.1093/infdis/122.1-2.16. [DOI] [PubMed] [Google Scholar]
- 35.Sonoguchi T, Sakoh M, Kunita N, Satsuta K, Noriki H, Fukumi H. Reinfection with influenza A (H2N2, H3N2, and H1N1) viruses in soldiers and students in Japan. The Journal of Infectious Diseases. 1986;153(1):33–40. doi: 10.1093/infdis/153.1.33. [DOI] [PubMed] [Google Scholar]
- 36.Davies JR, Grilli EA, Smith AJ. Influenza A: infection and reinfection. The Journal of hygiene. 1984;92(1):125–7. doi: 10.1017/s002217240006410x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. [Accessed: June 22, 2009.];Epidemic and Pandemic Alert and Response (EPR): Swine Influenza. http://www.who.int/csr/disease/swineflu/en/index.html.
- 38.Bridges CB, Thompson WW, Meltzer MI, Reeve GR, Talamonti WJ, Cox NJ, et al. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: A randomized controlled trial. Jama. 2000;284(13):1655–63. doi: 10.1001/jama.284.13.1655. [DOI] [PubMed] [Google Scholar]
- 39.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354(13):1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 40.Leroux-Roels I, Borkowski A, Vanwolleghem T, Drame M, Clement F, Hons E, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. The Lancet. 370(9587):580–589. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 41.Neustadt RE, Fineberg HV. The Swine Flu Affair: Decision Making on a Slippery Disease. www.nap.edu/catalog/12660.html National Academies Press Online; 1978. [PubMed]
- 42.Chio A, Cocito D, Leone M, Giordana MT, Mora G, Mutani R, et al. Guillain-Barre syndrome: A prospective, population-based incidence and outcome survey. Neurology. 2003;60(7):1146–1150. doi: 10.1212/01.wnl.0000055091.96905.d0. [DOI] [PubMed] [Google Scholar]
- 43.Kissel JT, Cornblath DR, JRM . Diagnosis and management of peripheral nerve disorders. New York, NY: Oxford University Press; 2001. Guillain-Barre Syndrome. [Google Scholar]
- 44.Centers for Disease Control and Prevention. [Accessed: June 25, 2009.];Key Facts About Flu Vaccination. 2006 http://www.cdc.gov/flu/protect/keyfacts.htm.
- 45.Fryback DG, Dasbach EJ, Klein R, Klein BEK, Dorn N, Peterson K, et al. The Beaver Dam Health Outcomes study: Initial Catalog of Health-state Quality Factors. Medical Decision Making. 1993;13(2):89–102. doi: 10.1177/0272989X9301300202. [DOI] [PubMed] [Google Scholar]
- 46.Turner DA, Wailoo AJ, Cooper NJ, Sutton AJ, Abrams KR, Nicholson KG. The cost-effectiveness of influenza vaccination of healthy adults 50–64 years of age. Vaccine. 2006;24(7):1035–43. doi: 10.1016/j.vaccine.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 47.Morgan R, King D. Influenza vaccination in the elderly. Postgrad Med J. 1996;72(848):339–42. doi: 10.1136/pgmj.72.848.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.U.S. Department of Health and Human Services. [Accessed: 29 July, 2009.];Pandemic Planning Update VI. 2009 www.pandemicflu.gov/plan/pdf/panflureport6.pdf.
- 49.County of Santa Clara. Santa Clara County Employee Earnings. San Jose Mercury News. 2007 June 17; http://www.bayareanewsgroup.com/multimedia/mn/news/scc_salaries_090507.pdf.
- 50.U.S. Department of Labor - Bureau of Labor Statistics. [Accessed: June 26, 2009.]; www.bls.gov.
- 51.Desta JF, Mclaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: The contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266–71. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 52.Talbird SE, Brogan AJ, Winiarski AP, Sander B. Cost-effectiveness of treating influenzalike illness with oseltamivir in the United States. Am J Health Syst Pharm. 2009;66(5):469–480. doi: 10.2146/ajhp080296. [DOI] [PubMed] [Google Scholar]
- 53.MetLife Survey of Nursing Homes and Home Care Costs and MetLife Market Survey of Assisted Living Costs. [Accessed: 17 June, 2009];What Does Long Term Care Cost in Your State. September, 2006 and October, 2006. http://www.aarp.org/families/caregiving/state_ltc_costs.html.
- 54.United States Census Bureau. [Accessed: 17 June, 2009];Statistical Abstract of the United States. 2009 http://www.census.gov/compendia/statab/2009edition.html.
- 55.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276(15):1253–1258. [PubMed] [Google Scholar]
- 56.World health Organization. [Accessed: 6 August, 2009];Pandemic influenza vaccine manufacturing process and timeline. 2009 August 6; http://www.who.int/csr/disease/swineflu/notes/h1n1_vaccine_20090806/en/index.html.
- 57.DeNoon D. [Accessed: 28 July, 2009];Swine Flu Vaccine Timeline: Key Decisions, Key Milestones. 2009 July 20; http://www.webmd.com/cold-and-flu/news/20090720/swine-flu-vaccine-when?page=4&print=true.
- 58.Anderson RM, May RM. Immunisation and herd immunity. The Lancet. 1990;335(8690):641–645. doi: 10.1016/0140-6736(90)90420-a. [DOI] [PubMed] [Google Scholar]
- 59.McKay B. [Accessed: 30 July, 2009];Pregnant Women, Kids, to Get Vaccine First. 2009 July 30; http://online.wsj.com/article/SB124887563173290207.html?mod=googlenews_wsj.
- 60.Roos R. HHS advisors urge full speed on H1N1 vaccine production. 2009 July 17; http://www.cidrap.umn.edu/cidrap/content/influenza/swineflu/news/jul1709board.html.
- 61.Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, et al. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proceedings of the National Academy of Sciences. 2009;106(19):7962–7967. doi: 10.1073/pnas.0903181106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cooper HM, Hedges LV. The Handbook of research synthesis. New York: Russell Sage Foundation; 1994. [Google Scholar]
- 63.New York City Department of Health and Mental Hygiene. [Accessed: 29 July, 2009];Health Alert #27: Pandemic (H1N1) 2009 Influenza Update, Revised Reporting Requirements and Testing Procedures. 2009 July 8; http://www.nyc.gov/html/doh/downloads/pdf/cd/2009/09md27.pdf.
- 64.New York City Department of Health and Mental Hygiene. [Accessed: 29 July, 2009];Health Alert #19: Pandemic (H1N1) 2009 Influenza Update, Revised Reporting Requirements and Testing Procedures. 2009 May 21; http://www.nyc.gov/html/doh/downloads/pdf/cd/2009/09md19.pdf.
- 65.New York City Department of Health and Mental Hygiene. [Accessed: 29 July, 2009];Health Alert #17: Pandemic (H1N1) 2009 Influenza Update, Revised Reporting Requirements and Testing Procedures. 2009 May 12; http://www.nyc.gov/html/doh/downloads/pdf/cd/2009/09md17.pdf.
- 66.New York City Department of Health and Mental Hygiene. [Accessed: 29 July, 2009];Health Alert #21: Pandemic (H1N1) 2009 Influenza Update, Revised Reporting Requirements and Testing Procedures. 2009 June 2; http://www.nyc.gov/html/doh/downloads/pdf/cd/2009/09md21.pdf.
- 67.NY Politics. [Accessed: 29 July, 2009];New York Confirms Six More H1N1-Related Deaths. 2009 July 1; http://www.nypolitics.com/2009/07/01/new-york-city-confirms-six-more-h1n1-related-deaths/
- 68.Bridges CB, Lim W, Hu-Primmer J, Sims L, Fukuda K, Mak KH, et al. Risk of influenza A (H5N1) infection among poultry workers, Hong Kong, 1997–1998. J Infect Dis. 2002;185(8):1005–10. doi: 10.1086/340044. [DOI] [PubMed] [Google Scholar]
- 69.Thorson A, Petzold M, Nguyen TK, Ekdahl K. Is exposure to sick or dead poultry associated with flulike illness?: a population-based study from a rural area in Vietnam with outbreaks of highly pathogenic avian influenza. Arch Intern Med. 2006;166(1):119–23. doi: 10.1001/archinte.166.1.119. [DOI] [PubMed] [Google Scholar]
- 70.Centers for Disease Control and Prevention. [Accessed: 29 July, 2009.];2008–2009 Influenza Season Week 18 ending. 2009 May 9; http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/weekly18.htm.
- 71.Centers for Disease Control and Prevention. [Accessed: 29 July, 2009.];2008–2009 Influenza Season Week 22 ending. 2009 June 6; http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/weekly22.htm.
- 72.Centers for Disease Control and Prevention. [Accessed: 29 July, 2009.];2008–2009 Influenza Season Week 23 ending. 2009 June 13; http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/weekly23.htm.
- 73.Centers for Disease Control and Prevention. [Accessed: 29 July, 2009.];2008–2009 Influenza Season Week 24 ending. 2009 June 20; http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/weekly24.htm.
- 74.Centers for Disease Control and Prevention. [Accessed: 29 July, 2009.];2008–2009 Influenza Season Week 25 ending. 2009 June 27; http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/weekly25.htm.
- 75.Centers for Disease Control and Prevention. [Accessed: 29 July, 2009.];2008–2009 Influenza Season Week 26 ending. 2009 July 4; http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/weekly26.htm.
- 76.Centers for Disease Control and Prevention. [Accessed: 29 July, 2009.];2008–2009 Influenza Season Week 27 ending. 2009 July 11; http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/weekly27.htm.
- 77.Centers for Disease Control and Prevention. [Accessed: 29 July, 2009.];2008–2009 Influenza Season Week 28 ending. 2009 July 18; http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/weekly28.htm.
- 78.Centers for Disease Control and Prevention. [Accessed: 29 July, 2009.];2008–2009 Influenza Season Week 29 ending. 2009 July 25; http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/weekly29.htm.
- 79.Centers for Disease Control and Prevention. [Accessed: 29 July, 2009.];2008–2009 Influenza Season Week 17 ending. 2009 May 2; http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/weekly17.htm.
- 80. [Accessed: May 6, 2009.];New York City Department of Health and Mental Hygeine. 2009 http://www.nyc.gov/html/doh/html/home/home.shtml.
- 81.Centers for Disease Control and Prevention. [Accessed: 31 July, 2009.];H1N1 Monitoring Questions & Answers. 2009 http://www.cdc.gov/h1n1flu/reportingqa.htm.
- 82.Ghani AC, Donnelly CA, Cox DR, Griffin JT, Fraser C, Lam TH, et al. Methods for Estimating the Case Fatality Ratio for a Novel, Emerging Infectious Disease. Am J Epidemiol. 2005;162(5):479–486. doi: 10.1093/aje/kwi230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Centers for Disease Control and Prevention. Interim CDC Guidance for Nonpharmaceutical Community Mitigation in Response to Human Infections with Swine Influenza (H1N1) Virus. 2009 April 26; http://www.iaam.org/CVMS/Non-Pharmaceutical.pdf.
- 84.Cowling B, Kwok-Hung C, Fang V, Cheng C, Fung R, Wai W, et al. Facemasks and Hand Hygiene to Prevent Influenza Transmission in Households. A Randomized Trial. Annals of Internal Medicine. 2009 doi: 10.7326/0003-4819-151-7-200910060-00142. in press. [DOI] [PubMed] [Google Scholar]
- 85.Baras Bt, Stittelaar KJ, Simon JH, Thoolen RJMM, Mossman SP, Pistoor FHM, et al. Cross-Protection against Lethal H5N1 Challenge in Ferrets with an Adjuvanted Pandemic Influenza Vaccine. PLoS ONE. 2008;3(1):e1401. doi: 10.1371/journal.pone.0001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leroux-Roels I, Bernhard R, Gerard P, Drame M, Hanon E, Leroux-Roels G. Broad Clade 2 Cross-Reactive Immunity Induced by an Adjuvanted Clade 1 rH5N1 Pandemic Influenza Vaccine. PLoS ONE. 2008;3(2):e1665. doi: 10.1371/journal.pone.0001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.World Health Organization. [Accessed: 17 June, 2009];Summary of: WHO Virtual Consultation on the Safety of Adjuvanted Influenza Vaccines. 2009 June 3; http://www.who.int/vaccine_research/documents/Report_on_consultation_on_adjuvant_safety_2.pdf.
- 88.Schnirring L. [Accessed: 14 August, 2009];Officials lower expectations for size of first novel flu vaccine deliveries. 2009 August 14; http://www.cidrap.umn.edu/cidrap/content/influenza/swineflu/news/aug1409vaccine.html.
- 89.Thorpe LE, Mostashari F, Karpati AM, Schwartz SP, Manning SE, Marx MA, et al. Mass smallpox vaccination and cardiac deaths, New York City, 1947. Emerg Infect Dis. 2004;10(5):917–920. doi: 10.3201/eid1005.040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pine AE. Vaccination Ventures: Explanation and Outcomes of a Mass Smallpox Vaccination Clinic Exercise. San Francisco: Department of Public Health Communicable Disease Prevention Unit; Jun 17, 2003. 2005. [Google Scholar]
- 91.Centers for Disease Control and Prevention. Smallpox Vaccination Clinic Guide. 2002 http://www.bt.cdc.gov/agent/smallpox/vaccination/pdf/smallpox-vax-clinic-guide.pdf.
- 92.Eichner M, Schwehm M. InfluSim. 2009 http://www.influsim.info.
- 93.Schnirring L. [Accessed: 18 August, 2009];FDA clears CSL’s vaccine-finishing facility in US. 2009 August 18; http://www.cidrap.umn.edu/cidrap/content/influenza/swineflu/news/aug1809fill-br.html.
- 94.Landau E. [Accessed: 29 July, 2008];CDC: US may need 600 million swine flu vaccine doses. 2009 July 1; http://www.cnn.com/2009/HEALTH/07/01/swine.flu.h1n1.vaccine/index.html.
- 95.World health Organization. [Accessed: 6 August, 2009];Safety of pandemic vaccines. 2009 August 6; http://www.who.int/csr/disease/swineflu/notes/h1n1_safety_vaccines_20090805/en/index.html.
- 96.Glass R, Glass L, Beyeler W, Min H. Targeted social distancing design for pandemic influenza. Emerg Infect Dis. 2006;12(11):1671–81. doi: 10.3201/eid1211.060255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Larson RC. Simple Models of Influenza Progression Within a Heterogeneous Population. Operations Research. 2007;55(3):399–412. [Google Scholar]
- 98.Arino J, Brauer F, van den Driessche P, Watmough J, Wu J. Simple models for containment of a pandemic. J R Soc Interface. 2006;3(8):453–7. doi: 10.1098/rsif.2006.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McKibbin WJ, Sidorenko A. Global Macroeconomic Consequences of Pandemic Influenza. 2006 February 1; http://www.brookings.edu/papers/2006/02development_mckibbin.aspx.
- 100.U.S. Congress. [Accessed: 28 July, 2009.];Supplemental Appropriations Act. 2009 http://www.govtrack.us/congress/bill.xpd?bill=h111-2346.