Abstract
Theiler’s murine encephalomyelitis virus infection of C57BL/6 mice results in acute behavioral seizures in 50% of the mice. Treatment of infected mice with minocycline or infection of interleukin (IL)-6-deficient chimeric mice results in a significant decrease in the number of mice developing seizures. However, in those mice that do develop seizures, the pathological changes [neuronal cell loss, inflammation (perivascular cuffing, gliosis, activated microglia/macrophages], and the numbers of virus infected cells in minocycline-treated or IL-6-deficient chimeric mice are very similar. Therefore, once seizures develop, the pathological changes are consistent no matter the treatment or genetic background.
Keywords: Theiler’s murine encephalomyelitis virus, Macrophages, Microglia
Our laboratory has described (Libbey et al. 2008) and characterized (Kirkman et al. 2010; Libbey et al. 2010; Libbey et al. 2011) a virus-induced mouse model for the development of acute seizures: the Theiler’s murine encephalomyelitis virus (TMEV)-induced seizure model. Infection of C57BL/6 mice with the Daniels (DA) strain of TMEV, a picornavirus, induced acute, afebrile, limbic seizures in 50% of the mice (Libbey et al. 2008). Typically, seizures were first observed on day 3 post infection (p.i.), the peak of seizure activity was day 6 p.i., no seizures were observed after day 10 p.i. and the majority of seizures had a Racine scale score of 3 or above (Libbey et al. 2008). There was mouse-to-mouse variation in both seizure score for any given day and the pattern of days on which seizures were observed. Also, both motor function and coordination were impaired in mice experiencing seizures (Libbey et al. 2008). Many neurons within the pyramidal layer of the hippocampus were infected and lost early after infection (Buenz et al. 2009; Kirkman et al. 2010; Libbey et al. 2008), likely due to apoptosis (Buenz et al. 2009; Tsunoda et al. 1997).
The proinflammatory cytokine interleukin (IL)-6, within the central nervous system (CNS), has been implicated in the development of seizures in this model (Kirkman et al. 2010; Libbey et al. 2011). Both resident cells within the CNS, such as microglia, and infiltrating cells from the periphery, such as macrophages, have been implicated as the source of the IL-6 within the CNS (Libbey et al. 2011).
The role for both resident CNS cells and infiltrating cells was detected by modulating seizure development through administration of minocycline, an antibiotic that has been shown to decrease monocyte/macrophage and microglial activation and monocyte/macrophage infiltration into the CNS (Bye et al. 2007; Campbell et al. 2009; Dutta et al. 2010; Mishra and Basu 2008). Minocycline was administered to TMEV-infected mice and the mice were observed for seizures as previously described (Libbey et al. 2011).
The production of IL-6 within the CNS by both resident CNS cells and infiltrating cells and its role in the development of seizures has been explored through the creation of radiation bone marrow chimeric mice (Libbey et al. 2011). Chimeric mice were generated and infected with TMEV and mice were observed for seizures as previously described (Libbey et al. 2011).
Mice were euthanized on days 7 and 14 p.i. Animals were perfused and brains were fixed and embedded as previously described (Libbey et al. 2011). Multiple tissue sections, containing five coronal slabs per brain, were cut, mounted on slides and stained. The tissue section of only one of the five coronal slabs per slide contained the hippocampal/dentate gyral regions of the brain. Cytoarchitecture of the hippocampus was referenced to Figs. 43-49 in The Mouse Brain in Stereotaxic Coordinates (Franklin and Paxinos 1997). All slides were blindly assessed using one slide per brain (N = 3 to 14 brains per group for minocycline-treated mice; N = 1 to 15 brains per group for chimeric mice).
Activated microglia/macrophages were identified by Ricinus communis agglutinin (RCA)-I lectin histochemistry as previously described (Kirkman et al. 2010). RCA-I+ cells were counted and summed in each of the two hippocampi and dentate gyri.
DA-viral-antigen positive cells (virus infected cells) were identified using TMEV hyperimmune serum as described (Kirkman et al. 2010). In all mice, DA-viral-antigen positive cells were counted and summed in the following brain regions: frontal lobe, septum, caudoputamen, hippocampus, thalamus, midbrain and cortex (with the exception of the thalamus for the minocycline-treated mice).
Luxol fast blue stain was used to examine perivascular cuffing (PVC) and neuronal cell loss. PVC were counted and summed in each of the two hippocampi and dentate gyri (Kirkman et al. 2010). Neuronal cell loss in the pyramidal cell layer of the hippocampus (CA1 to CA3) was scored as we have described (Kirkman et al. 2010). A score was given for each of the two hippocampi and the scores were summed.
Gliosis was semi-quantified by scoring glial fibrillary acidic protein (GFAP)+ activated astrocytes as previously described (Kirkman et al. 2010). A score was given for each of the two hippocampi and dentate gyri and the scores were summed.
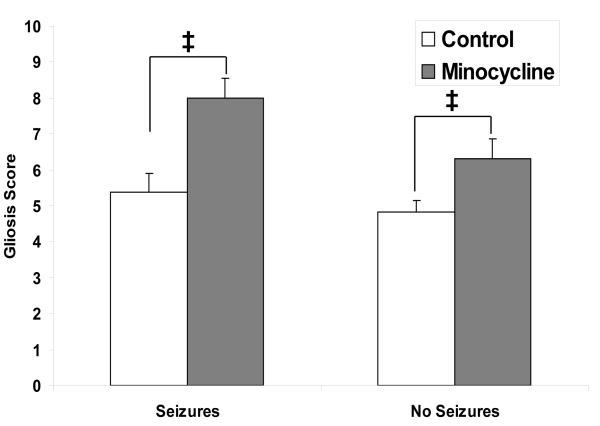
In our TMEV-induced seizure model, there were no significant differences in neuronal cell loss, PVC, microglia/macrophage activation or numbers of virus infected cells between the minocycline-treated mice and the wild-type control mice, sacrificed on day 14 p.i., for those mice that did or did not have seizures (data not shown), although significantly fewer minocycline-treated mice developed seizures [(Libbey et al. 2011) & Table 1]. Differences in gliosis were seen (Fig. 1). A reduction in monocyte/macrophage and microglial activation and monocyte/macrophage infiltration into the CNS due to minocycline treatment resulted in an increase in activated astrocytes irrespective of the presence or absence of seizures.
Table 1.
Seizure (Racine scale stage 3-5) frequency in minocycline-treated mice.
| Treatment | Number mice with seizures / total number mice (percent) |
Chi-Square (compared to) |
|---|---|---|
| Control (No treatment) | 17 / 28 (60.7) | |
| Minocycline | 6 / 20 (30) | p < 0.05 (control) |
Fig. 1. Gliosis within the hippocampus and dentate gyrus of C57BL/6 mice treated with minocycline.
One measure of inflammation is the presence of activated astrocytes (gliosis), detected through GFAP immunohistochemistry. Gliosis was scored as described in the text for mice sacrificed on day 14 p.i. Mice treated with minocycline that did or did not have seizures had a significantly higher gliosis score than the wild-type control mice. ‡, p < 0.05 (Mann-Whitney U test). Results are mean + standard error of the mean (SEM) of groups with three to fourteen mice per group.
The number of TMEV-infected mice experiencing seizures was significantly reduced in chimeric mice generated from irradiated IL-6-deficient mice (IL-6-deficient CNS) reconstituted with cells from wild-type donors (IL-6-normal periphery) (WT-to-IL-6−/−) and in chimeric mice generated from irradiated wild-type mice (IL-6-normal CNS) reconstituted with cells from IL-6-deficient mice (IL-6-deficient periphery) (IL-6−/−-to-WT). Also, the seizure frequency of chimeric mice generated via transfer of IL-6-deficient bone marrow into irradiated IL-6-deficient recipients (IL-6−/−-to-IL-6−/−) was comparable to both WT-to-IL-6−/− and IL-6−/−-to-WT chimeric mice, although the number of mice in the IL-6−/−-to-IL-6−/− group was too few for any statistical comparison [(Libbey et al. 2011) & Table 2].
Table 2.
Seizure (Racine scale stage 3-5) frequency in chimeric mice.
| Mice | Number mice with seizures / total number mice (percent) |
Chi-Square (compared to) |
|---|---|---|
| 3-month-old control | 26 / 40 (65) | |
| WT to WT chimera | 14 / 28 (50) | |
| WT to IL-6−/− chimera | 4 / 16 (25) | p < 0.01 (3-month-old control) |
| IL-6−/− to IL-6−/− chimera | 1 / 4 (25) | |
| IL-6−/− to WT chimera | 3 / 18 (16.7) | p < 0.001 (3-month-old control) |
| p < 0.05 (WT to WT chimera) |
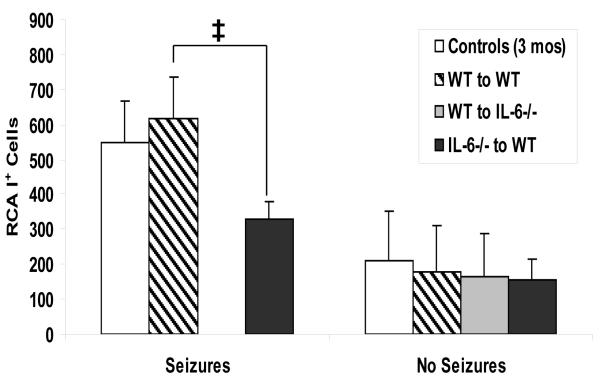
In our TMEV-induced seizure model, there were no significant differences in neuronal cell loss, PVC, gliosis or numbers of virus infected cells between the chimeric mice and the 3-month-old control mice, sacrificed on days 7 and 14 p.i., for those mice that did or did not have seizures (data not shown). Differences in the number of RCA-I+ cells between chimeric mice and 3-month-old control mice were seen at day 14 p.i. in those mice that had seizures (Fig. 2). There was a marked reduction in the number of activated microglia/macrophages in the CNS of chimeric mice that were IL-6-normal in the CNS and IL-6-deficient in the periphery and that had seizures.
Fig. 2. Activated microglia/macrophages within the hippocampus and dentate gyrus of chimeric mice.
One measure of inflammation is the presence of activated microglia/macrophages, detected through RCA-I lectin histochemistry. The numbers of RCA-I+ cells were enumerated for mice sacrificed on day 14 p.i. There were no irradiated IL-6-deficient chimeric mice reconstituted with IL-6-deficient donor cells (IL-6−/−-to-IL-6−/−) with or without seizures or irradiated IL-6-deficient chimeric mice reconstituted with wild-type donor cells (WT-to-IL-6−/−) with seizures for this time point. The irradiated 3-month-old wild-type chimeric mice reconstituted with wild-type donor cells (WT-to-WT) had a significantly higher number of RCA-I+ cells than the irradiated 3-month-old wild-type chimeric mice reconstituted with IL-6-deficient donor cells (IL-6−/−-to-WT). ‡, p < 0.05 (ANOVA, Fisher’s PLSD post hoc test). Results are mean + SEM of groups with one to fifteen mice per group.
The goal of the current study was to examine the pathological changes in both TMEV-infected minocycline-treated and IL-6 chimeric mice. Although TMEV infection of both minocycline-treated and IL-6-deficient chimeric mice resulted in significantly fewer mice experiencing seizures [(Libbey et al. 2011) & Tables 1 & 2], in those mice with pathological changes the pathology was very similar (Figs. 1 & 2). It therefore appears that once these virally-induced seizures develop then the pathological changes are consistent no matter the treatment or background. Further, others have examined various animal models of seizures and consistency in the neuropathology of seizures was found in four different models of complex partial (limbic) nonconvulsive status epilepticus (Hosford 1999). The two pharmacologically-induced models (pilocarpine and kainic acid) and the two electrically-induced models (prolonged kindling and self-sustained limbic status epilepticus) all demonstrated common neuropathologic features, including hippocampal neuronal cell loss, despite the different mechanisms of induction. This suggested that the damage in these models was due to the occurrence of seizures rather than the means of seizure induction. The common mechanism for seizure-induced damage in these models has been suggested to be excess activation of glutamate receptors, such as the N-methyl-D-aspartate (NMDA) subtype of glutamate receptor, resulting in excitotoxic effects (Hosford 1999). Our TMEV-induced seizure model has also been proposed to be an animal model for complex partial (limbic) seizures (Kirkman et al. 2010; Stewart et al. 2010); we are currently examining the role of NMDA receptors in this model.
Acknowledgement
We thank Daniel J. Doty, Braden T. McElreath and Jordan T. Sim for technical assistance. We acknowledge Kathleen Borick for manuscript preparation. This work was supported by NIH 1R01NS065714.
References
- Buenz EJ, Sauer BM, Lafrance-Corey RG, Deb C, Denic A, German CL, Howe CL. Apoptosis of hippocampal pyramidal neurons is virus independent in a mouse model of acute neurovirulent picornavirus infection. Am J Pathol. 2009;175:668–684. doi: 10.2353/ajpath.2009.081126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bye N, Habgood MD, Callaway JK, Malakooti N, Potter A, Kossmann T, Morganti-Kossmann MC. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp Neurol. 2007;204:220–233. doi: 10.1016/j.expneurol.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Campbell JH, Burdo TH, Autissier P, E-M Ratai, Bombardier JP, Westmoreland SV, Soulas C, Bonzáz RG, Williams KC. The tetracycline antibiotic minocycline prevents the activation of CD14/CD16 monocytes in blood and accumulation in the brain, as well as the development of SIV encephalitis in rhesus macaques. J NeuroVirol. 2009;15(Suppl.1):14. [Google Scholar]
- Dutta K, Kumawat KL, Nazmi A, Mishra MK, Basu A. Minocycline differentially modulates viral infection and persistence in an experimental model of Japanese encephalitis. J Neuroimmune Pharmacol. 2010;5:553–565. doi: 10.1007/s11481-010-9233-8. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Hosford DA. Animal models of nonconvulsive status epilepticus. J Clin Neurophysiol. 1999;16:306–313. doi: 10.1097/00004691-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Kirkman NJ, Libbey JE, Wilcox KS, White HS, Fujinami RS. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia. 2010;51:454–464. doi: 10.1111/j.1528-1167.2009.02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Interleukin-6, produced by resident cells of the central nervous system and infiltrating cells, contributes to the development of seizures following viral infection. J Virol. 2011 doi: 10.1128/JVI.00458-11. published online ahead of print on 4 May 2011:doi:10.1128/JVI.00458-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kirkman NJ, Smith MCP, Tanaka T, Wilcox KS, White HS, Fujinami RS. Seizures following picornavirus infection. Epilepsia. 2008;49:1066–1074. doi: 10.1111/j.1528-1167.2008.01535.x. [DOI] [PubMed] [Google Scholar]
- Libbey JE, Kirkman NJ, Wilcox KS, White HS, Fujinami RS. Role for complement in the development of seizures following acute viral infection. J Virol. 2010;84:6452–6460. doi: 10.1128/JVI.00422-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra MK, Basu A. Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis. J Neurochem. 2008;105:1582–1595. doi: 10.1111/j.1471-4159.2008.05238.x. [DOI] [PubMed] [Google Scholar]
- Stewart K-AA, Wilcox KS, Fujinami RS, White HS. Development of postinfection epilepsy after Theiler’s virus infection of C57BL/6 mice. J Neuropathol Exp Neurol. 2010;69:1210–1219. doi: 10.1097/NEN.0b013e3181ffc420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Kurtz CIB, Fujinami RS. Apoptosis in acute and chronic central nervous system disease induced by Theiler’s murine encephalomyelitis virus. Virology. 1997;228:388–393. doi: 10.1006/viro.1996.8382. [DOI] [PubMed] [Google Scholar]