Abstract
Somatosensory evoked potential (SSEP) monitoring is commonly used to detect changes in nerve conduction and prevent impending nerve injury. We present a case series of 2 patients who had SSEP monitoring for their surgical craniotomy procedure, and who, upon positioning supine with their head tilted 30–45 degrees, developed unilateral upper extremity SSEP changes. These SSEP changes were reversed when the patients were repositioned. These cases indicate the clinical usefulness of monitoring SSEPs while positioning the patient and adjusting position accordingly to prevent injury.
Introduction
Neurologic injury secondary to positioning is a significant perioperative problem and a common cause of patient injury in the practice of anesthesiology. Somatosensory evoked potential (SSEP) monitoring is reproducible, reliable and commonly used during surgical procedures to detect changes in electrophysiological conduction in peripheral nerves and central nerve pathways and thus, to prevent nervous system damage.1 A significant change in the SSEP responses is indicated by a decrease in amplitude and/or an increase in latency. Changes in SSEP responses may be due to spinal instrumentation, hypoperfusion, hypothermia, anesthetic drugs, and positioning.
SSEP monitoring has also been noted to be useful in evaluating upper extremity conduction changes related to positioning. Changes in nerve conduction are expected to occur before permanent nerve injury, thus, repositioning that reverses SSEP conduction changes should prevent perioperative peripheral nerve injury.2–4
The following case series is the first, to our knowledge, to describe 2 cases of asymmetric SSEP changes in the brachial plexus after positioning for craniotomy that resolved after repositioning. It underlines the clinical usefulness of monitoring SSEPs while positioning the patient and adjusting the position accordingly to prevent peripheral neurological injury, a common cause of patient injury and malpractice claims.
Case Description
Case 1
A 73-year-old female with a history of progressive loss of coordination and left-sided hearing presented for a suboccipital craniectomy for acoustic neuroma. Her medical history was significant for hypertension and diabetes. The magnetic resonance imaging showed an acoustic neuroma with enlarged ventricles and chronic displacement of the cerebellum and brainstem.
After induction of general anesthesia, the patient was positioned supine. Her head was turned approximately 45° towards the right, and fixed with a Mayfield neurosurgical head holder. A “sandbag” was placed under the mattress on the left side of the operating table to extend the neck and left shoulder.
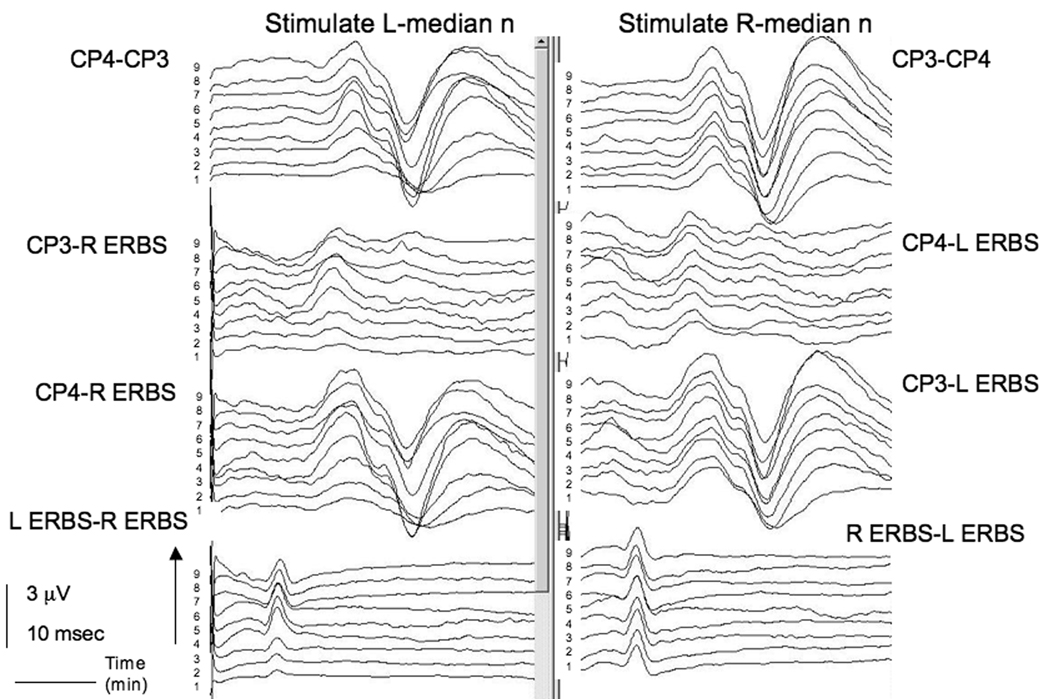
Electrophysiologic monitoring was performed bilaterally for SSEPs in upper (median nerve) and lower (posterior tibial nerve) extremities, brainstem auditory evoked potentials and motor evoked potentials. SSEPs from the median nerves were recorded using needle electrodes placed at Erb's point over the brachial plexus for assessing peripheral conduction and on the scalp at CP3 and CP4 locations, for assessing central conduction.
SSEP needle electrodes were placed on the patient before positioning. Positioning required 8 minutes, after which, SSEPs were recorded from both upper and lower extremities. Standard filter settings for these SSEPs are 30–1500 Hz and standard stimulation parameters were used including: rate of 4.7 per second, 20 mamps, 0.3 msec duration. At the start of recording, after a one minute interval of acquiring a steady signal, the SSEP amplitude from stimulation of the left median nerve was noted to be reduced at Erb’s point on all recording channels in comparison to contralateral recordings. When the patient's left shoulder was raised to reduce extension of the neck and shoulder, the SSEPs from stimulation of the left median nerve were improved at recordings from Erb’s point and subcortical and cortical electrodes and became equal in amplitude to the contralateral side in 4 minutes (Figure 1). Surgical exposure was not compromised significantly with this adjustment.
Figure 1.
SSEP changes in the left median nerve in Case 1. Somatosensory evoked potentials (SSEPs) from bilateral median nerves were recorded using needle electrodes placed at Erb's point over the brachial plexus, for assessing peripheral conduction and on the scalp at CP3 and CP4 locations, for assessing central conduction. Time in minutes is recorded on the y axis. From the start of the recordings (time = 1 minute), there is a unilateral decrease in the amplitude of the left median nerve recordings compared to the right median nerve. This difference exists at both peripheral (Erb’s) and central (CP3 and CP4) recordings, suggesting a peripheral mechanism. With repositioning of the shoulder, the amplitude increases on the left side with time in central and peripheral recordings, approaching the amplitude on the right side.
Case 2
A 62-year-old male with a history of transient diffuse extremity weakness presented for left temporal and suboccipital craniotomy for sub-total resection of a skull base meningioma. His medical history was significant for hypertension, gout, and diverticulitis. A magnetic resonance imaging showed the presence of a left-sided mass consistent with a meningioma, at the level of the clivus and petrous bone, with compression of the pons and ventricular dilation.
After inducing general anesthesia, the patient was positioned supine. His head was turned towards the right by 30°, slightly extended and fixed with a Mayfield neurosurgical head holder. A “sandbag” was placed under the mattress on the left side of the operating table to extend the neck and left shoulder.
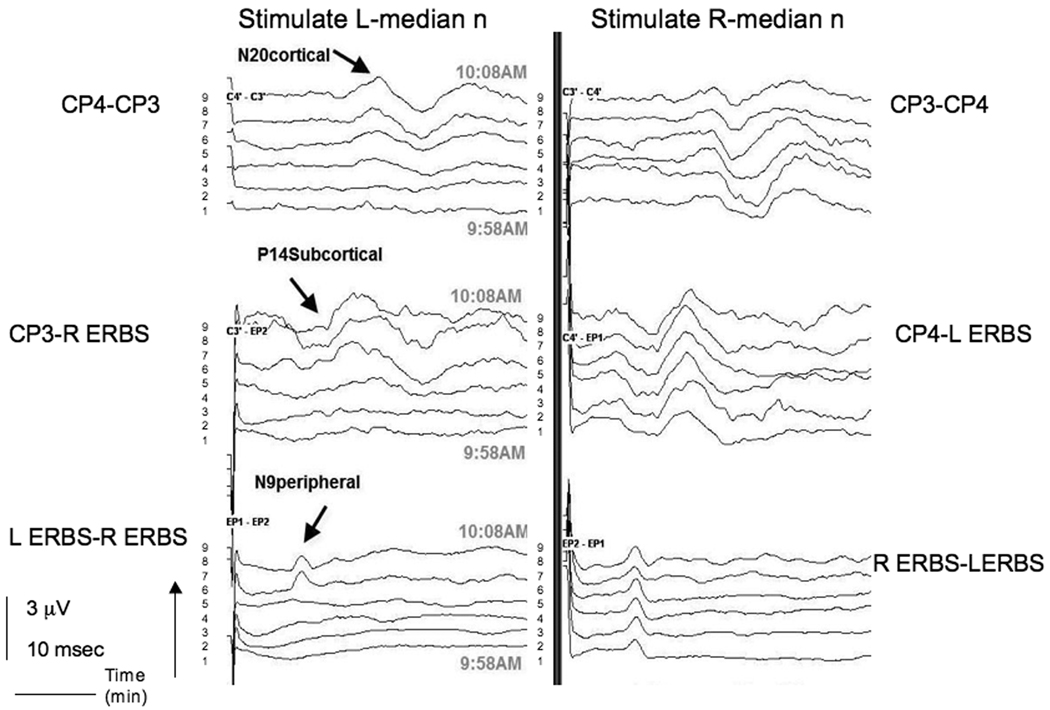
Electrophysiologic monitoring was performed bilaterally for SSEPs in upper (median nerve) and lower (posterior tibial nerve) extremities, and brainstem auditory evoked potentials. Motor evoked potentials were not obtained because most of the surgery was performed under microscope guidance. SSEPs from the median nerves were recorded using needle electrodes placed at Erb's point over the brachial plexus for assessing peripheral conduction and on the scalp at CP3 and CP4 locations, for assessing central conduction.
SSEP needle electrodes were placed on the patient before positioning. Positioning required 10 minutes, after which, SSEPs were recorded from both upper and lower extremities. Standard filter settings for these SSEPs were the same as the previous case. At the start of recording, after a one minute interval of acquiring a steady signal, the SSEP amplitude from stimulation of the left median nerve was reduced at Erb’s point on all recording channels in comparison to contralateral recordings. When the patient's left shoulder was raised to reduce extension of the neck and shoulder, the SSEPs from stimulation of the left median nerve were improved at recordings from Erb’s point and subcortical and cortical electrodes and became equal in amplitude to the contralateral side in 6 minutes (Figure 2). Surgical exposure was not compromised significantly in this position.
Figure 2.
Somatosensory evoked potential (SSEP)changes in left median nerve in Case 2. SSEPs from bilateral median nerves were recorded using needle electrodes placed at Erb's point over the brachial plexus, for assessing peripheral conduction and on the scalp at CP3 and CP4 locations, for assessing central conduction. Time in minutes is recorded on the y axis. Upon stimulation of the left median nerve, there is no SSEP response from the start of the recordings (time = 1 minute). Again, the difference exists at both peripheral (Erb’s) and central (CP3 and CP4) recordings, suggesting a peripheral mechanism. With repositioning of the shoulder, the amplitude increases on the left side with time in central and peripheral recordings, approaching the amplitude on the right side.
Both patients gave witnessed verbal informed consent to publish any collected data.
Discussion
The reversibility of upper extremity SSEP changes with repositioning in our patients establishes a cause and effect relationship between positioning and SSEP change. Although we cannot be certain that the observed SSEP changes would have been associated with a permanent deficit, if the changes indeed heralded ischemia of the nerves, clinical neuropathy would be likely after a lengthy procedure. In addition, although not routine in our center for cases not involving cervical instability, these cases identify the utility of pre-position baseline SSEPs, especially since the patients will be monitored during the case. This would clearly identify positioning changes by having pre- and post- recordings.
SSEP changes caused by positioning patients have been reported for prone2, 5, 6 and supine spine surgery,7–10 cardiac,2, 4 and orthopedic surgeries.10–12 Our case report shows a change in SSEPs of the brachial plexus during positioning for craniotomy.
The incidence of SSEP changes during positioning of the prone spine surgery patient ranges from 3.6–15%. Schwartz et al.2 found a 30 % decrease in ulnar nerve SSEP amplitude in 3.6% of pediatric patients having scoliosis correction. O'Brien et al.5 reported a 15% prevalence of brachial plexopathy during lumbar and spinal deformity surgery (≥ 60% decrease in amplitude or ≥ 10% increase in latency). Kamel et al.6 found the lateral decubitus position (7.5%) and prone "superman" position (7.0%) had a higher incidence of position-related upper extremity SSEP changes compared with other positions including supine, arms tucked and arms out. (1.8%–3.2%).
The incidence of positioning changes in SSEP recordings during anterior spine surgery is lower than that of prone spine surgery13. Schwartz et al.7 found that 1.8% of patients undergoing anterior cervical spine surgery showed intraoperative SSEP changes secondary to positioning. SSEP changes are more frequent in patients with myelopathy who were having anterior spine surgery8.
There has been only one study to describe positioning changes during SSEP monitoring for craniotomy surgery. Deinsberger et al.14 performed SSEP monitoring for positioning of patients for posterior fossa surgery in the semi-sitting position. Monitoring of the median and tibial nerve was recorded for 55 consecutive patients. SSEPs of the median nerve showed no changes while placing patients in the sitting position; however, tibial SSEP recordings were altered in 14 cases (25%).
We reviewed all cases of craniotomy for skull base tumors and acoustic neuromas in a 2 year period to derive an incidence of SSEP changes during this category of neurosurgical procedure. At our institution, all craniotomy cases for acoustic neuromas and skull base tumors receive SSEP neuromonitoring. We found 83 cases performed in the 2 year period between January 2007–December 2008. Of these cases, we reviewed the neuromonitoring records and identified 2 cases with SSEP changes upon positioning, which are described in this case series: an incidence of 2.4%. This is approximately the incidence for SSEP changes for anterior cervical spine surgery (1.8%), but less than that found in prone and lateral decubitus positioning.
The clinical correlation of SSEPs and clinical findings has been the subject of several investigations. Prielipp et al.15 studied the onset of clinical symptoms and SSEP changes in the ulnar nerve in 50 volunteers with their arm in 30–90° of abduction, as well as in supination, neutral orientation, and pronation. Half of the patients who developed SSEP changes did not develop clinical symptoms, 2 of which manifested severe SSEP changes (≥60% decrease in amplitude). In contrast, Lorenzini and Poterack16 monitored SSEPs at the median and ulnar nerves in awake volunteers placed in a prone position as their arms were moved in a step-wise cephalad position. Three of 7 patients developed upper extremity symptoms described as tingling, numbness, or aching in the hand, forearm, or upper arm without changes of their SSEPs, suggesting that SSEP monitoring is imperfect in detecting positioning injury. However, volunteer studies do not apply directly to anesthetized patients, who are often placed in positions that awake patients could not tolerate.
These data demonstrate the false positive and negative predictive values of clinical peripheral neuropathic changes based on SSEP monitoring. There may, indeed, be instances in which SSEP changes, or the lack thereof, may be misleading in predicting postoperative clinical neuropathy. However, many studies that have evaluated SSEP monitoring with postoperative peripheral neurological changes studied SSEP changes over a short interval. In the study by Lorenzini and Poterack16, recordings for SSEP signals were made 10–15 minutes after positioning the arm. The study by Prielipp et al. 15 was performed for a maximum of 60 minutes, and on average, patients experienced clinical symptoms after 37 minutes, with a range of 20–59 minutes. In contrast, our first case took 6 hours and 50 minutes, and the second case took 11 hours and 33 minutes. It is unclear, but seems likely, that changes observed by SSEP monitoring would eventually lead to clinical neuropathy during these lengthy procedures.
To determine if we had clinical peripheral neuropathy changes that were not predicted by SSEPs, we reviewed the case records of our department obtained from resident postoperative visits, consults, and patient phone calls after hospital discharge. From January 2007–December 2008, 65,041 cases were reviewed. Of all types of surgical cases, 10 cases were identified in which postoperative peripheral neuropathy was reported: an incidence of 0.02%. None of these cases were for craniotomy. The major limitation of this type of review is that it is retrospective, thus under-reporting of a complication can under-estimate the incidence of peripheral neuropathy. However, with SSEP monitoring for cases, we may be decreasing the incidence of peripheral neuropathy due to positioning.
Although the incidence of peripheral nerve injury is low, it does remain a common cause of professional liability in the practice of anesthesiology. Lee et al. 17 reviewed the ASA closed-claims analysis and found that peripheral nerve injury was the complication recorded in 21% of neurosurgical closed claims. This finding emphasizes the importance of taking measures to prevent peripheral nerve damage, including vigilance of neuromonitoring changes.
These cases demonstrate that changes in SSEPs of the brachial plexus can occur during positioning for skull base craniotomy and acoustic neuromas. This case series, in addition to the literature on this topic, underlines the clinical usefulness of monitoring SSEPs during and after positioning the patient and adjusting position accordingly to prevent neurological injury.
Acknowledgments
Financial Support:
E.J.H. was supported in part by a grant from the NIA (RO1 AG17604).
Footnotes
Disclaimers:
None.
Conflict of Interest:The authors have no conflicts of interest.
References
- 1.Epstein NE, Danto J, Nardi D. Evaluation of intraoperative somatosensory-evoked potential monitoring during 100 cervical operations. Spine. 1993;18:737–747. doi: 10.1097/00007632-199305000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz DM, Drummond DS, Hahn M, Ecker ML, Dormans JP. Prevention of positional brachial plexopathy during surgical correction of scoliosis. J Spinal Disord. 2000;13:178–182. doi: 10.1097/00002517-200004000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Jellish WS, Martucci J, Blakeman B, Hudson E. Somatosensory evoked potential monitoring of the brachial plexus to predict nerve injury during internal mammary artery harvest: Intraoperative comparisons of the rultract and pittman sternal retractors. J Cardiothorac Vasc Anesth. 1994;8:398–403. doi: 10.1016/1053-0770(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 4.Hickey C, Gugino LD, Aglio LS, Mark JB, Son SL, Maddi R. Intraoperative somatosensory evoked potential monitoring predicts peripheral nerve injury during cardiac surgery. Anesthesiology. 1993;78:29–35. doi: 10.1097/00000542-199301000-00006. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien MF, Lenke LG, Bridwell KH, Padberg A, Stokes M. Evoked potential monitoring of the upper extremities during thoracic and lumbar spinal deformity surgery: A prospective study. J Spinal Disord. 1994;7:277–284. [PubMed] [Google Scholar]
- 6.Kamel IR, Drum ET, Koch SA, Whitten JA, Gaughan JP, Barnette RE, Wendling WW. The use of somatosensory evoked potentials to determine the relationship between patient positioning and impending upper extremity nerve injury during spine surgery: A retrospective analysis. Anesth Analg. 2006;102:1538–1542. doi: 10.1213/01.ane.0000198666.11523.d6. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz DM, Sestokas AK, Hilibrand AS, Vaccaro AR, Bose B, Li M, Albert TJ. Neurophysiological identification of position-induced neurologic injury during anterior cervical spine surgery. J Clin Monit Comput. 2006;20:437–444. doi: 10.1007/s10877-006-9032-1. [DOI] [PubMed] [Google Scholar]
- 8.Kombos T, Suess O, Da Silva C, Ciklatekerlio O, Nobis V, Brock M. Impact of somatosensory evoked potential monitoring on cervical surgery. J Clin Neurophysiol. 2003;20:122–128. doi: 10.1097/00004691-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Jones SC, Fernau R, Woeltjen BL. Use of somatosensory evoked potentials to detect peripheral ischemia and potential injury resulting from positioning of the surgical patient: Case reports and discussion. Spine J. 2004;4:360–362. doi: 10.1016/j.spinee.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Swenson JD, Hutchinson DT, Bromberg M, Pace NL. Rapid onset of ulnar nerve dysfunction during transient occlusion of the brachial artery. Anesth Analg. 1998;87:677–680. doi: 10.1097/00000539-199809000-00035. [DOI] [PubMed] [Google Scholar]
- 11.Mills WJ, Chapman JR, Robinson LR, Slimp JC. Somatosensory evoked potential monitoring during closed humeral nailing: A preliminary report. J Orthop Trauma. 2000;14:167–170. doi: 10.1097/00005131-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Porter SS, Black DL, Reckling FW, Mason J. Intraoperative cortical somatosensory evoked potentials for detection of sciatic neuropathy during total hip arthroplasty. J Clin Anesth. 1989;1:170–176. doi: 10.1016/0952-8180(89)90037-8. [DOI] [PubMed] [Google Scholar]
- 13.Labrom RD, Hoskins M, Reilly CW, Tredwell SJ, Wong PK. Clinical usefulness of somatosensory evoked potentials for detection of brachial plexopathy secondary to malpositioning in scoliosis surgery. Spine. 2005;30:2089–2093. doi: 10.1097/01.brs.0000179305.89193.46. [DOI] [PubMed] [Google Scholar]
- 14.Deinsberger W, Christophis P, Jodicke A, Heesen M, Boker DK. Somatosensory evoked potential monitoring during positioning of the patient for posterior fossa surgery in the semisitting position. Neurosurgery. 1998;43:36–40. doi: 10.1097/00006123-199807000-00023. discussion 40-32. [DOI] [PubMed] [Google Scholar]
- 15.Prielipp RC, Morell RC, Walker FO, Santos CC, Bennett J, Butterworth J. Ulnar nerve pressure: Influence of arm position and relationship to somatosensory evoked potentials. Anesthesiology. 1999;91:345–354. doi: 10.1097/00000542-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Lorenzini NA, Poterack KA. Somatosensory evoked potentials are not a sensitive indicator of potential positioning injury in the prone patient. J Clin Monit. 1996;12:171–176. doi: 10.1007/BF02078139. [DOI] [PubMed] [Google Scholar]
- 17.Lee LA, Posner KL, Cheney FW, Domino KB. ASA Closed Claims Project: An Analysis of Claims Associated with Neurosurgical Anesthesia. Anesthesiology. 2003;99:A362. [Google Scholar]