Abstract
Previous findings point to the involvement of the dorsal raphe nucleus (DRN) and dorsal periaqueductal gray (dPAG) serotonergic receptors in the mediation of defensive responses that are associated with specific subtypes of anxiety disorders. These studies have mostly been conducted with rats tested in the elevated T-maze, an experimental model of anxiety that was developed to allow the measurement, in the same animal, of two behaviors mentioned: inhibitory avoidance and one-way escape. Such behavioral responses have been respectively related to generalized anxiety disorder (GAD) and panic disorder (PD). In order to assess the generality of these findings, in the current study we investigated the effects of the injection of 5-HT-related drugs into the DRN and dPAG of another rodent species, mouse, on the mouse defense test battery (MDTB), a test of a range of defensive behaviors to an unconditioned threat, a predator. Male CD-1 mice were tested in the MDTB after intra-DRN administration of the 5-HT1A receptor antagonist WAY-100635 or after intra-dPAG injection of two serotonergic agonists, the 5-HT1A receptor agonist 8-OH-DPAT and the 5-HT2A/2C receptor agonist DOI. Intra-DRN injection of WAY-100635 did not change behavioral responses of mice confronted with a rat in the MDTB. In the dPAG, both 8-OH-DPAT and DOI consistently impaired mouse escape behavior assessed in the MDTB. Intra-dPAG infusion of 8-OH-DPAT also decreased measures of mouse risk assessment in the rat exposure test. In conclusion, the current findings are in partial agreement with previous results obtained with rats tested in the elevated T-maze. Although there is a high level of similarity between the behavioral effects obtained in rats (elevated T-maze) and mice (MDTB and RET) with the infusion of 5-HT agonists into the dPAG, the same is not true regarding the effects of blockade of DRN 5-HT1A receptors in these rodent species. These data suggest that there may be differences between mice and rats regarding the involvement of the DRN in the mediation of defensive behaviors.
Keywords: serotonin, anxiety, dorsal raphe nucleus, dorsal periaqueductal gray, mouse defense test battery
1. Introduction
A wealth of evidence supports the involvement of serotonin (5-HT) in the physiopathology of anxiety disorders (Blier and de Montigny, 1999; Lowry et al., 2005; Maron and Shlik, 2006; Millan, 2003) and, indeed, many currently available anxiolytic drugs are known to act either by interfering with 5-HT reuptake or directly at 5-HT receptors (Argyropoulos et al., 2000; Hoyer et al., 2002; Millan, 2006). Nevertheless, despite intensive research, there are many controversies about the exact role 5-HT plays in the physiopathology of anxiety.
In an attempt to interpret the existing evidence on the role of 5-HT in anxiety, Deakin and Graeff (1991) have proposed that distinctive serotonergic pathways originating in the dorsal raphe nucleus (DRN) may differentially affect the expression of defensive behaviors that have been associated with two subtypes of anxiety pathologies, generalized anxiety disorder (GAD) and panic disorder (PD). According to this proposal, activation of the ascending DRN 5-HT pathway, which innervates the amygdala and frontal cortex, would facilitate defensive responses to potential or distal threat stimuli, such as inhibitory avoidance and risk assessment behaviors. Based on the effect of anxiolytic drugs, such behaviors have been related in clinical terms to GAD (Blanchard et al., 2003a; Graeff, 2002; McNaughton and Corr, 2004). On the other hand, activation of the DRN periventricular 5-HT pathway, which innervates the dorsal periaqueductal gray (dPAG), would inhibit escape or flight reactions in response to proximal danger, behavioral reactions that have been related to PD (Blanchard et al., 2001, 2003a; Graeff, 2004; McNaughton and Corr, 2004).
A series of experimental analysis have been carried out to investigate the involvement of the DRN and dPAG 5-HT neurotransmission system in the modulation of defensive behaviors potentially related to different subtypes of anxiety disorders (Graeff et al., 1996; Pobbe and Zangrossi, 2005; Sena et al., 2003; Soares and Zangrossi, 2004; Zanoveli et al., 2003). These studies have mostly been conducted with rats tested in the elevated T-maze, an experimental model of anxiety that was developed to allow the measurement, in the same animal, of two behaviors mentioned: inhibitory avoidance and one-way escape (Graeff et al., 1998; Viana et al., 1994; Zangrossi and Graeff, 1997). Reported drug effects on these tasks have largely supported the proposed relationship of these responses to GAD and PD, respectively (for a review see Graeff et al., 1998; Graeff and Zangrossi, 2002; Pinheiro et al., 2007).
Regarding the DRN, local administration of drugs that inhibit the firing rate of 5-HT neurons (e.g. the 5-HT1A receptor agonist 8-OH-DPAT or the GABAA receptor agonist muscimol) and consequently decrease 5-HT release in post-synaptic areas impaired inhibitory avoidance and facilitated escape behavior of rats tested in the elevated T-maze (Sena et al., 2003). The same result was also observed after the selective lesion of DRN 5-HT neurons by local infusion of the neurotoxin 5,7-DHT. On the other hand, intra-DRN injection of the glutamate receptor agonist kainic acid, which increases the firing rate of 5-HT neurons and 5-HT release in post-synaptic areas (Tao and Auerbach, 2000; Viana et al., 1997), facilitated inhibitory avoidance and impaired escape of rats in the elevated T-maze (Graeff et al., 1996). A similar effect was observed after local microinfusion of the 5-HT1A antagonist WAY-100635 (Pobbe and Zangrossi, 2005). This latter result concurs with previous evidence that 5-HT exerts a tonic regulation on rat DRN 5-HT1A somatodendritic auto-receptors (Haddjeri et al., 2004; Hajós et al., 2001; Liu et al., 2005).
In the dPAG, local infusion of the endogenous agonist 5-HT, as well as 8-OH-DPAT and the 5-HT2A/2C receptor agonist DOI consistently impaired escape behavior of rats tested in the elevated T-maze (Soares and Zangrossi, 2004; Zanoveli et al., 2003), corroborating Deakin and Graeff’s proposal. These agonists differentially interfered with inhibitory avoidance measured in the elevated T-maze. Intra-dPAG infusion of 5-HT facilitated this response while 8-OH-DPAT induced the opposite effect and DOI was without effect upon this behavioral task. Based on this evidence, the authors suggest that the dPAG serotonergic system is involved in the modulation of both escape and inhibitory avoidance behaviors.
In order to assess the generality of these behavioral findings, in the current study the effects of the injection of 5-HT-related drugs into the DRN and dPAG on defensive behaviors were investigated in another rodent species, mouse, utilizing the mouse defense test battery (MDTB). As with the elevated T-maze, this test allows the separation of defensive responses related to GAD and PD. More specifically, the MDTB is based on the direct confrontation of mouse subjects with a predatory stimulus, a hand-held anesthetized rat, that first approaches, then chases, and finally contacts the mouse. The behavioral responses elicited in the MDTB include flight, freezing, risk assessment and defensive threat and attack; pre-test activity and post-test defensiveness are also recorded (Blanchard et al., 2001). According to evidence obtained in a wealth of studies conducted for pharmacological validation of the MDTB (for a review see Blanchard et al., 2003a, 2003b), risk assessment and defensive threat/attack behaviors together with escape attempts expressed by mice after the predator is removed are consistently modified by the administration of compounds clinically effective in the treatment of GAD, such as buspirone and chlordiazepoxide. On the other hand, mouse flight behavior is significantly inhibited by the chronic injection of drugs that are clinically used for treating PD, such as fluoxetine and alprazolam.
In the first experiment, we investigated the effects of DRN infusions of the 5-HT1A antagonist WAY-100635 on behavioral responses of mice confronted with a rat in the MDTB. In experiment 2, we used this same battery of tests to verify the behavioral effects obtained after intra-dPAG administration of the 5-HT agonists 8-OH-DPAT and DOI. In this last experiment, we also investigated the effects of intra-dPAG infusions of 8-OH-DPAT and DOI on mouse defensive responses measured in the rat exposure test (RET). The RET was designed to afford a less intense threatening situation, in which a wire mesh prevents the predator (rat) from approaching or contacting the mouse, allowing the subject to seek or to avoid the predator stimulus and thus regulate its own exposure to the threat (for a review see Yang et al., 2004).
2. Experimental procedures
2.1. Subjects
Male CD-1 mice between the ages of 10–12 weeks, obtained from Charles River Laboratories (Wilmington, MA) were used for this study. Subjects weighed between 30 and 40 g at the time of surgery. Mice were individually housed in standard polypropylene cages in a temperature controlled room (20 ± 2°C). All subjects were maintained on a 12-h light/ dark cycle (lights on at 06:00 a.m.), with free access to food and water in their home cages. All procedures were conducted in accordance with protocols approved by the University of Hawaii Institutional Animal Care and Use Committee.
2.2. Drugs
The following drugs were used: (N-{2-[4-(2-methoxyphenyl)-1-piperazinyl] ethyl}-N-2-pyridinyl-cyclohexanecarboxamide maleate (WAY-100635; Sigma, USA), (±)8-hydroxy-2-(di-n-propylamino) tetralin hydrobromide (8-OH-DPAT; Sigma, USA) and (±)2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI; Sigma, USA). All drugs were dissolved in sterile saline.
2.3. Apparatus
2.3.1. Mouse defense test battery
The MDTB test was conducted in an oval runway, 0.40 m wide, 0.30 m high and 4.8 m in total length, consisting of two 2-m straight segments joined by two 0.4-m curved segments and separated by a median wall (2.0 m long × 0.30 m high). The apparatus is elevated 0.8 m from the floor to enable the experimenter to hold the rat and move with ease, while minimizing the subjects’ ability to view the experimenter. All parts of the apparatus were made of black Plexiglas. The floor of the apparatus was marked every 20 cm with white lines to facilitate measurement of locomotion distances. Two ceiling-mounted video cameras were used to record the test and the room was illuminated with one 100-W red light.
2.3.2. Rat exposure test
Testing procedures in the RET were conducted in a 46 × 24 × 21 cm clear polycarbonate cage (exposure cage) covered with a metal lid. The exposure cage was divided into two equal sized compartments by a wire mesh screen. The home chamber was a 7 × 7 × 12 cm box made of black Plexiglas on three sides and clear Plexiglas on one side to facilitate videotaping. The home chamber was connected to the exposure cage by a clear Plexiglas tube tunnel (4.4 cm in diameter, 13 cm in length, 1.5 cm elevated from the floor of the two chambers). One vertically mounted camera linked to a video monitor and DVD was used to record the experiment.
2.4. Surgery
Mice were anesthetized with a systemic injection of sodium pentobarbital (90 mg/kg, i.p.) and placed in a stereotaxic apparatus (David-Kopf Instruments, USA). A guide cannula made of stainless steel (length 8 mm, inner diameter 0.3 mm) was implanted 1 mm above the DRN or the dPAG, according to the following stereotaxic coordinates from the atlas by Paxinos and Franklin (2001): DRN = 4.36 mm posterior to bregma, 1.40 mm lateral to the midline and 2.60 ventral to the skull surface; dPAG = 4.16 mm posterior to bregma, 1.32 mm lateral to the midline and 2.25 ventral to the skull surface. The guide cannulae were inserted into the right-side brain hemisphere at an angle of 26° with the vertical plane to avoid the sagittal sinus. Each cannula was fixed to the skull by means of acrylic resin and one small screw (Small Parts Inc.) that was inserted anterolaterally to the site of cannulation. At the end of the surgery, the guide cannula was sealed with a stainless wire to protect it from obstruction. Upon removal from the stereotaxic apparatus, mice were administered 1 mL 0.9% saline s.c. in order to prevent dehydration.
2.5. Intra-encephalic drug administration
The subjects were randomly assigned to each experimental group before drug administration. For drug infusion, each mouse was gently restrained and an injection needle (length 9.0 mm, outer diameter 0.2 mm) was inserted into the guide cannula. The injection needle was connected via a polyethylene tubing (PE-10) to a 10 µL Hamilton microsyringe. The solution administration was controlled by an infusion pump (Harvard Apparatus Inc., USA) programmed to deliver a volume of 0.1 µL over a period of 60 s. The needle was removed 1 min after the injection was finished. Confirmation of successful infusion was obtained by monitoring the movement of a small air bubble in the PE-10 tubing.
2.6. Behavioral test schedule
In experiment 1, mice were infused into the DRN with WAY-100635 (0.185, 0.37 or 0.74 nmol; i.e., 0.1, 0.2 or 0.4 µg) or saline, 10 min before being tested in the MDTB. In experiment 2, mice were injected into the dPAG with 8-OH-DPAT (0, 1.6 or 3.2 nmoles; i.e., 0, 0.5 or 1.0 µg) or DOI (0, 8 or 16 nmoles; i.e., 0, 2.85 or 5.7 µg), 10 (8-OH-DPAT) or 20 min (DOI) before being tested in the MDTB. The doses used and the time interval between drug injection and testing were chosen based on previously published studies (Pobbe and Zangrossi, 2005; Soares and Zangrossi, 2004; Zanoveli et al., 2003). All tests were conducted during the light phase of the light/dark cycle. Experimenters and scorers were blind to treatment conditions.
2.7. Behavioral tests
2.7.1. Mouse defense test battery
Seven days after surgery, mice from experiments 1 and 2 were transported to the experimental room and left undisturbed for 60 min prior to testing. The apparatus was cleaned with 20% alcohol and dried with paper towels in between trials. Ten min before the test session began, the Long-Evans rat (average weight of 450 g) used as the predator stimulus was deeply anesthetized with sodium pentobarbital (80 mg/kg, i.p.) in order to minimize its discomfort.
The MDTB consists of the following subtests:
Pre-test: subjects were placed into the MDTB apparatus for a 3-min familiarization period during which total line crossings and wall rears were recorded.
Predator avoidance test: avoidance and escape distances were measured when a predator stimulus (a hand-held rat) was brought up to the subject at a speed of approximately 0.5 m/s. Approach was terminated when contact with the subject was made or if the subject ran away from the approaching rat. This was repeated five times.
Chase/flight test: the hand-held rat was brought up to the subject at a speed of approximately 2.0 m/s. Chase was initiated only when the subject was standstill with its head oriented toward the rat, and completed when the subject had traveled a distance of 14.4 m (three laps in the runway). The time spent by the mouse to travel this distance was recorded. With this latency, mean flight speed (m/s) was calculated. In addition, the number of stops (pauses in locomotion), orientations (subject orients its head toward the oncoming rat), reversals (subject turned and ran in the rat direction), jump attacks and jump escapes were also recorded.
Straight alley test: the runway is then converted into a straight alley, 80 cm long, by the closing of a door at one end and the placement of a removable barrier at the other. The rat is placed at one end while the mouse begins the test at the other. During a 30-s period, the number of approach-withdrawals (subject moves toward the rat more than 20 cm and then returns), as well as voluntary contacts with the rat stimulus were recorded. Another measures scored included immobility time (freezing) and the frequencies of jump attacks and jump escapes.
Forced contact test: in this situation, the straight alley length was reduced to 40 cm. The rat was brought up by the experimenter in five sudden contacts directed toward the subject. For each such contact, the number of vocalizations, defensive uprights, jump attacks and jump escapes were recorded. This procedure was repeated three times.
Post-test: upon completion of the forced contact test, the alley doors were opened allowing the subject locomotion around the oval runway. The subject exploratory activity was recorded for another 3-min period during which the experimenter and the rat stimulus were out of sight. Line crossings, wall rears and escape attempts were recorded.
2.7.2. Rat exposure test
One week after the MDTB, animals from experiment 2 were once again randomized and injected into the dPAG with 8-OH-DPAT (0, 1.6 or 3.2 nmoles) or DOI (0, 8 or 16 nmoles). The bedding material from each subject’s home cage was placed in the home chamber and exposure cage compartments of the RET, prior to the start of each trial. The RET apparatus was cleaned and wiped down with 20% alcohol between trials. Adult male Long-Evans rats from breeding colonies maintained at the University of Hawaii were used as predator stimuli. D-Amphetamine sulfate (Research Biochemicals, MA) was dissolved in sterile saline and administered i.p. to rats at a single dose of 5.0 mg/kg 15 min prior to placement into the rat exposure chamber. This procedure was used to facilitate the maintenance of motor activity throughout the course of the 10-min trial duration. A new rat was used after every five trials or if cessation of movement or stereotypy was observed. The testing procedure consists of the following phases:
Phase 1: Habituation. Each subject was allowed one daily habituation session during three consecutive days in the apparatus. The mouse was placed in the center of the surface and was allowed to freely explore the apparatus during 10 min with no rat present.
Phase 2: Exposure test. On the fourth day, an amphetamine treated male Long-Evans rat was introduced behind the wire mesh. Ten (8-OH-DPAT) or twenty (DOI) min after receiving the intra-dPAG infusion of the drug, the mouse was placed in the center of the surface area.
The behavioral parameters comprised spatiotemporal and ethological measures. The spatiotemporal measures were frequency and time spent in the home chamber, tunnel and on the surface area. Total contact time included time spent in contact with the wire mesh. The ethological measures were frequency and duration of stretch attended postures (SAP, a posture in which the body is stretched forward oriented toward the threat stimulus), freezing (complete cessation of movement except breathing), grooming and defensive burying (sawdust pushed from the home cage into the tunnel opening).
2.8. Histology
Upon completion of the experiments, the animals were deeply anesthetized with pentobarbital (100 mg/kg; Sigma, USA) and received an infusion with 0.1 µL of methylene blue into the DRN or the dPAG. Brains were then intra-cardially perfused with 0.9% saline followed by 4% formalin solution and removed from the cranial cavity. Following this procedure, brains were immersed in 4% formalin for 48h and then transferred into a 30% sucrose-formaline solution. Serial 50 µm brain sections were cut, and methylene blue staining was used to locate the positions of the cannulae tip sites in the DRN or dPAG, following the atlas by Paxinos and Franklin (2001). Only animals with injection sites located inside the DRN and dPAG were included in the statistical analysis.
2.9. Data analysis
The behavioral data from the MDTB and RET were analyzed by one-way analysis of variance (ANOVA), with drug infusion into the DRN or the dPAG as the independent factor. Post hoc Dunnet’s t tests were conducted for subsequent comparisons between treatment and control groups. A p value ≤ 0.05 was considered significant.
3. Results
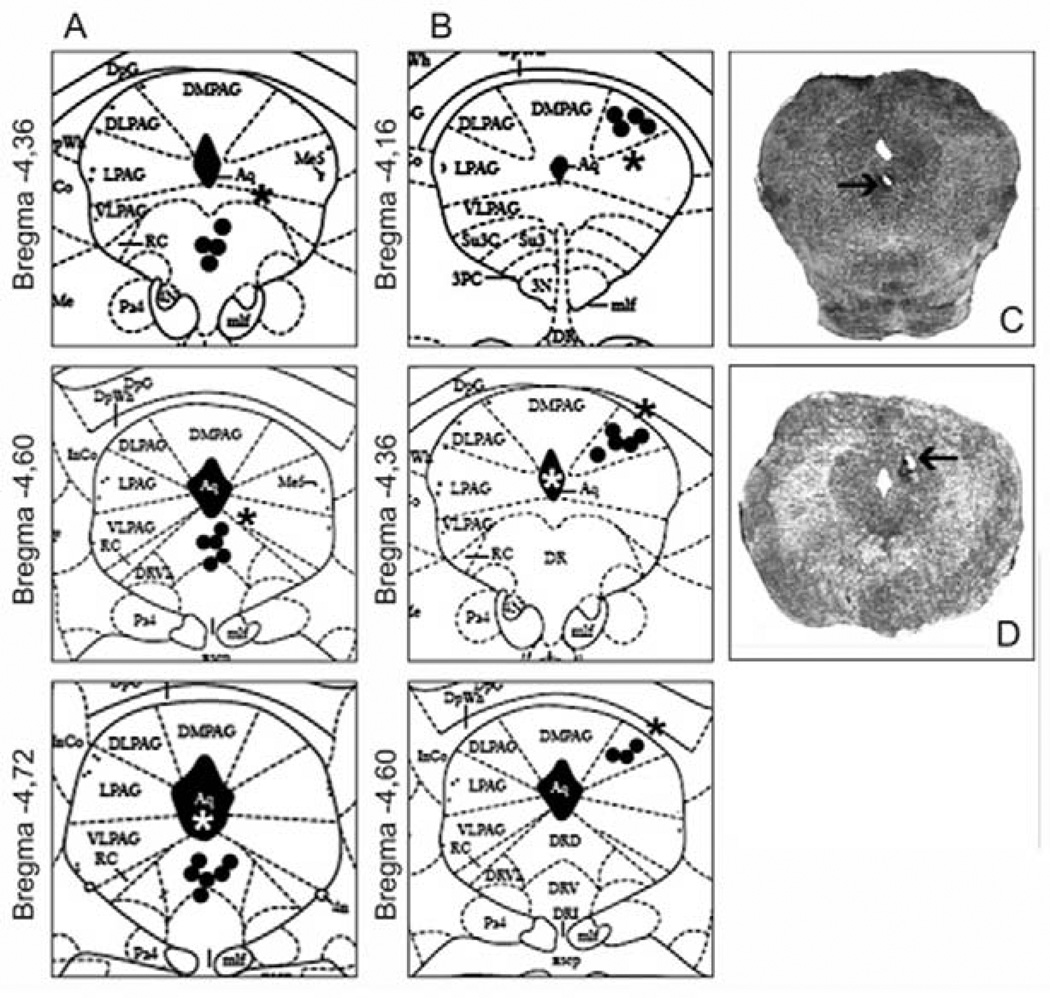
Figure 1 depicts the sites of drug injections into the DRN and dPAG of animals tested in the present study.
Figure 1.
Diagrammatic representation of coronal sections through the mouse brain (Atlas of Paxinos and Franklin, 2001) showing the location of injection sites inside (circles) and outside (asterisks) the DRN (A) and the dPAG (B); Photomicrographs showing a typical injection site into the DRN (C) and dPAG (D). The number of points shown in A/B is fewer than the total number of mice used because of several overlaps.
Experiment 1: effect of intra-DRN injection of WAY-100635 on mice defensive responses measured in the MDTB
All behavioral data (means ± SEM and F value) obtained in the MDTB after intra-DRN administration of WAY-100635 are listed in Table 1. The injection of this 5-HT1A antagonist into the DRN did not produce significant differences between control and drug dose groups in any of the MDTB subtests.
Table 1.
Effect (mean ± SEM) of WAY-100635 infusions into the DRN on behavioral responses of mice confronted with a rat in the MDTB
| Saline | WAY (0.185 nmol) |
WAY (0.37 nmol) |
WAY (0.74 nmol) |
F (3,36) = | |
|---|---|---|---|---|---|
| Pre-test activity | |||||
| Line crossings | 148.60 ± 13.51 | 133.56 ± 14.69 | 147.44 ± 13.31 | 158.44 ± 9.20 | 0.61 |
| Rears | 12.40 ± 2.12 | 12.56 ± 1.40 | 10.44 ± 1.84 | 15.44 ± 3.79 | 0.69 |
| Predator avoidance test | |||||
| Avoidance distance (cm) | 48.00 ± 9.98 | 63.89 ± 17.11 | 96.67 ± 19.51 | 62.96 ± 13.71 | 1.82 |
| Avoidance frequency | 1.30 ± 0.37 | 1.56 ± 0.41 | 1.44 ± 0.24 | 2.22 ± 0.36 | 1.33 |
| Escape distance (cm) | 96.40 ± 11.54 | 94.81 ± 21.40 | 92.78 ± 9.36 | 82.30 ± 8.99 | 0.21 |
| Escape frequency | 3.60 ± 0.60 | 2.89 ± 0.56 | 3.89 ± 0.48 | 4.00 ± 0.37 | 0.90 |
| Chase/flight test | |||||
| Flight speed (m/s) | 0.28 ± 0.03 | 0.26 ± 0.03 | 0.30 ± 0.03 | 0.34 ± 0.03 | 1.27 |
| Stops | 6.10 ± 0.81 | 7.11 ± 0.65 | 4.67 ± 1.04 | 3.78 ± 0.97 | 2.79 |
| Orientations | 3.60 ± 0.65 | 5.11 ± 1.03 | 3.67 ± 1.15 | 3.56 ± 1.24 | 0.53 |
| Reversals | 3.60 ± 1.16 | 4.11 ± 0.81 | 2.00 ± 0.67 | 2.67 ± 0.88 | 1.04 |
| Jump attacks | 1.60 ± 0.69 | 2.67 ± 1.08 | 2.22 ± 0.78 | 2.56 ± 1.14 | 0.27 |
| Jump escapes | 0.80 ± 0.42 | 0.56 ± 0.56 | 0.33 ± 0.24 | 2.22 ± 0.81 | 2.41 |
| Straight alley test | |||||
| Approaches/withdrawals | 1.30 ± 0.37 | 1.67 ± 0.41 | 2.22 ± 0.40 | 2.67 ± 0.37 | 2.46 |
| Contacts | 0.90 ± 0.28 | 0.56 ± 0.18 | 0.56 ± 0.18 | 0.67 ± 0.24 | 0.54 |
| Jump escapes | 0.50 ± 0.50 | 0.89 ± 0.65 | 0.44 ± 0.34 | 1.22 ± 0.52 | 0.49 |
| Freezing (s) | 5.30 ± 2.16 | 4.78 ± 1.75 | 2.22 ± 0.98 | 2.56 ± 1.23 | 0.90 |
| Forced contact test | |||||
| Uprights | 6.60 ± 0.91 | 6.11 ± 0.72 | 6.56 ± 1.03 | 6.00 ± 0.69 | 0.13 |
| Vocalizations | 7.60 ± 1.30 | 4.11 ± 1.50 | 7.44 ± 1.45 | 6.89 ± 1.27 | 1.37 |
| Jump attacks | 2.70 ± 0.75 | 3.11 ± 0.89 | 3.78 ± 0.81 | 3.11 ± 0.70 | 0.32 |
| Jump escapes | 0.90 ± 0.41 | 1.78 ± 0.76 | 2.11 ± 0.59 | 3.00 ± 0.76 | 1.90 |
| Post-test | |||||
| Line crossings | 144.50 ± 14.20 | 146.11 ± 18.77 | 168.44 ± 20.45 | 131.89 ± 14.48 | 0.77 |
| Rears | 20.70 ± 1.49 | 17.11 ± 1.88 | 19.00 ± 3.23 | 22.89 ± 1.77 | 1.26 |
| Escape attempts | 0.50 ± 0.22 | 1.67 ± 1.19 | 2.89 ± 2.16 | 4.67 ± 1.17 | 1.81 |
n=9/10 for each group
Experiment 2: effect of intra-dPAG injection of 8-OH-DPAT and DOI on mice defensive responses measured in the MDTB and RET
Table 2 depicts all behavioral data (means ± SEM and F value) obtained in the MDTB after intra-dPAG injection of 8-OH-DPAT. One-way ANOVA followed by Dunnet’s t test revealed that intra-dPAG infusion of both doses of this agonist decreased two measures related to flight response: escape distance assessed in the predator avoidance test and flight speed measured in the chase/flight test. Injection of 8-OH-DPAT into the dPAG also increased the occurrence of mouse contacts with the predator and decreased the number of line crossings in the post-test.
Table 2.
Effect (mean ± SEM) of 8-OH-DPAT infusions into the dPAG on behavioral responses of mice confronted with a rat in the MDTB
| Saline | 8-OH-DPAT (1.6 nmol) |
8-OH-DPAT (3.2 nmol) |
F (2,27) = | |
|---|---|---|---|---|
| Pre-test activity | ||||
| Line crossings | 157.89 ± 4.93 | 144.33 ± 16.58 | 135.40 ± 11.10 | 0.92 |
| Rears | 17.67 ± 3.11 | 12.33 ± 2.13 | 18.70 ± 3.01 | 1.46 |
| Predator avoidance test | ||||
| Avoidance distance (cm) | 48.74 ± 9.51 | 36.67 ± 5.27 | 29.73 ± 7.78 | 1.55 |
| Avoidance frequency | 2.22 ± 0.55 | 2.33 ± 0.29 | 2.10 ± 0.62 | 0.05 |
| Escape distance (cm) | 115.85 ± 10.25 | 72.11 ± 5.42** | 65.33 ± 6.00** | 13.43 |
| Escape frequency | 4.56 ± 0.24 | 4.44 ± 0.34 | 4.30 ± 0.42 | 0.13 |
| Chase/flight test | ||||
| Flight speed (m/s) | 0.42 ± 0.04 | 0.28 ± 0.03* | 0.28 ± 0.03* | 5.51 |
| Stops | 6.44 ± 1.39 | 10.67 ± 1.60 | 10.50 ± 1.26 | 2.81 |
| Orientations | 1.56 ± 0.58 | 2.67 ± 0.67 | 3.70 ± 0.72 | 2.66 |
| Reversals | 2.11 ± 0.51 | 3.11 ± 0.56 | 2.80 ± 0.42 | 1.03 |
| Jump attacks | 0.22 ± 0.15 | 0.56 ± 0.44 | 0.40 ± 0.16 | 0.34 |
| Jump escapes | 1.00 ± 0.44 | 1.67 ± 0.53 | 0.90 ± 0.28 | 0.98 |
| Straight alley test | ||||
| Approaches/withdrawals | 3.33 ± 0.50 | 2.22 ± 0.52 | 2.40 ± 0.69 | 1.01 |
| Contacts | 0.56 ± 0.18 | 0.89 ± 0.31 | 1.90 ± 0.53* | 3.46 |
| Jump escapes | 1.00 ± 0.41 | 0.44 ± 0.24 | 0.10 ± 0.10 | 2.83 |
| Freezing (s) | 1.33 ± 0.75 | 2.22 ± 0.60 | 2.50 ± 1.09 | 0.50 |
| Forced contact test | ||||
| Uprights | 2.33 ± 0.73 | 1.78 ± 0.55 | 2.60 ± 0.96 | 0.29 |
| Vocalizations | 4.22 ± 0.97 | 4.33 ± 1.21 | 6.90 ± 0.74 | 2.48 |
| Jump attacks | 1.89 ± 0.59 | 1.56 ± 0.82 | 1.20 ± 0.49 | 0.29 |
| Jump escapes | 5.22 ± 1.12 | 2.89 ± 1.10 | 3.70 ± 1.25 | 0.99 |
| Post-test | ||||
| Line crossings | 157.11 ± 7.41 | 135.78 ± 8.75 | 125.70 ± 8.80* | 3.66 |
| Rears | 28.56 ± 3.31 | 35.78 ± 3.00 | 30.10 ± 2.55 | 1.62 |
| Escape attempts | 2.44 ± 1.25 | 1.00 ± 0.50 | 0.40 ± 0.22 | 1.91 |
n=9/10 for each group;
p<0.01 and
p<0.05 compared to control by Dunnet’s t test
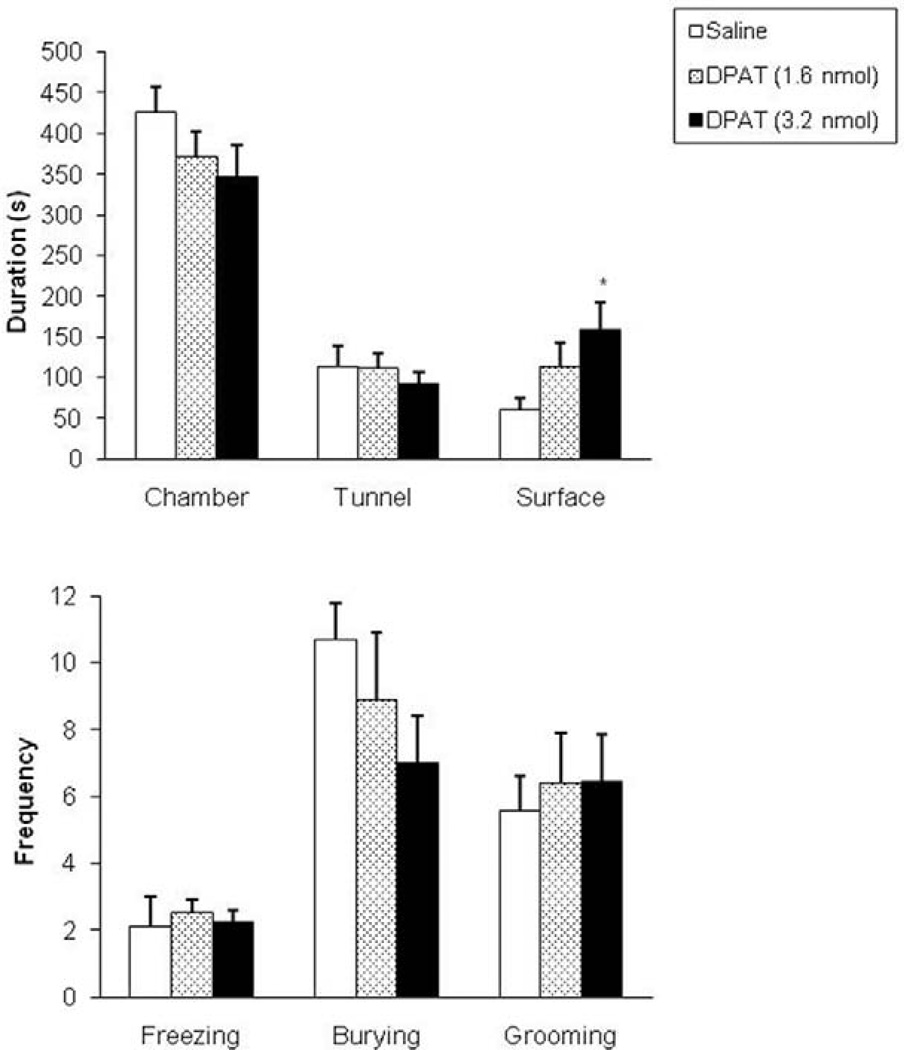
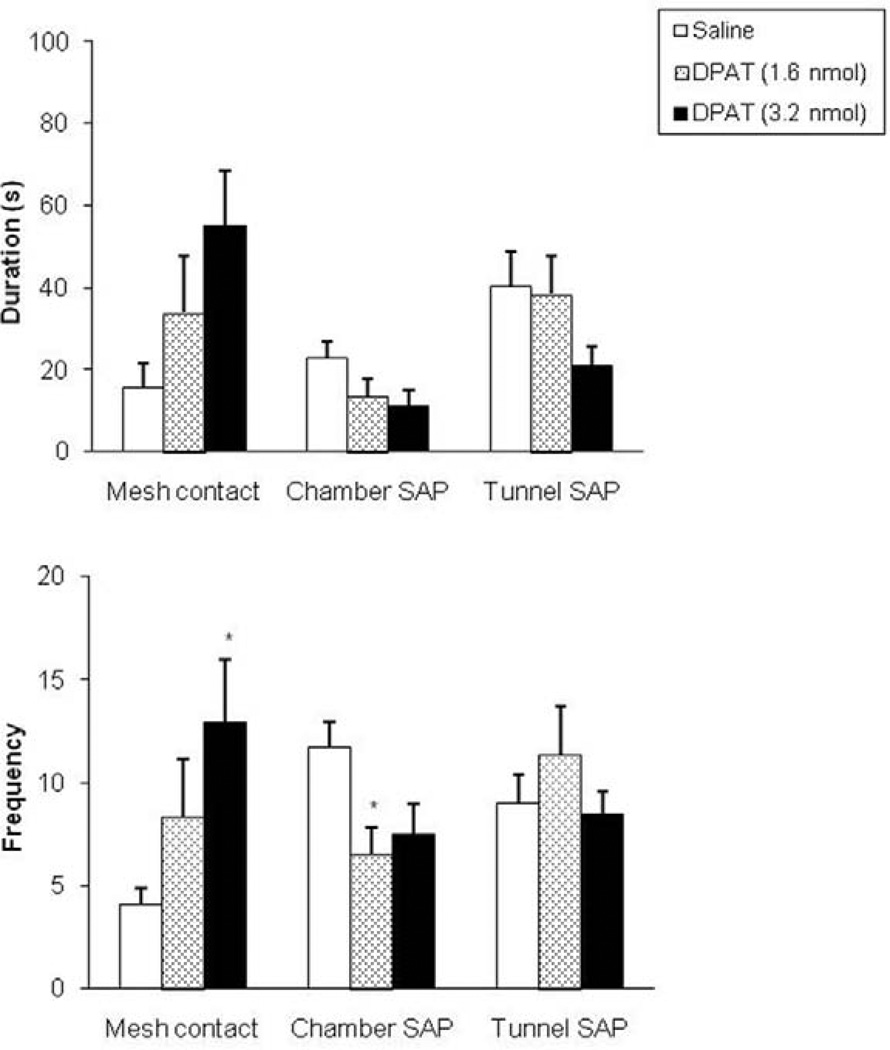
In the RET, intra-dPAG injection of 8-OH-DPAT enhanced the time spent in the surface compartment (F(2,25) = 3.43, p<0.05, see Fig. 2, upper panel). Such manipulation also significantly increased mesh contact frequency (F(2,25) = 3.44, p<0.05, see Fig. 3, lower panel) and decreased the occurrence of stretched attend postures in the chamber compartment (F(2,25) = 3.95, p<0.05, Fig. 3, lower panel).
Figure 2.
Effects (mean±SEM) of intra-dPAG injection of 8-OH-DPAT on the duration of time spent in the chamber, tunnel and in the surface area (upper panel) and on the frequency of selected behaviors (lower panel) in the RET. n=8/9 for each group; *p<0.05 compared to control group by Dunnet’s t test.
Figure 3.
Effects (mean±SEM) of intra-dPAG injection of 8-OH-DPAT on the duration (upper panel) and frequency (lower panel) of mesh contact, chamber and tunnel SAP. n=8/9 for each group; *p<0.05 compared to control group by Dunnet’s t test.
The behavioral data (means ± SEM and F value) obtained in the MDTB after intra-dPAG administration of DOI are listed in Table 3. One-way ANOVA followed by Dunnet’s t test revealed that intra-dPAG infusion of DOI significantly reduced escape distance measured in the predator avoidance test, and flight speed assessed in the chase/flight test. Intra-dPAG injection of DOI also enhanced the frequency of stops in the chase/flight test and the occurrence of mouse contacts with the rat in the straight alley test.
Table 3.
Effects (mean ± SEM) of DOI infusions into the dPAG on behavioral responses of mice confronted with a rat in the MDTB
| Saline | DOI (8 nmoles) |
DOI (16 nmoles) |
F (2,26) = | |
|---|---|---|---|---|
| Pre-test activity | ||||
| Line crossings | 150.78 ± 15.28 | 139.78 ± 10.28 | 124.33 ± 14.77 | 0.94 |
| Rears | 19.33 ± 3.54 | 17.33 ± 2.67 | 17.67 ± 3.55 | 0.10 |
| Predator avoidance test | ||||
| Avoidance distance (cm) | 80.37 ± 14.91 | 63.33 ± 19.44 | 57.41 ± 16.54 | 0.48 |
| Avoidance frequency | 2.11 ± 0.31 | 1.11 ± 0.39 | 1.78 ± 0.57 | 1.35 |
| Escape distance (cm) | 104.00 ± 10.75 | 70.67 ± 8.47 | 57.04 ± 13.07** | 4.89 |
| Escape frequency | 4.78 ± 0.15 | 3.22 ± 0.57 | 3.22 ± 0.76 | 2.61 |
| Chase/flight test | ||||
| Flight speed (m/s) | 0.27 ± 0.02 | 0.16 ± 0.01** | 0.21 ± 0.03 | 6.40 |
| Stops | 5.78 ± 0.80 | 9.00 ± 0.62* | 8.44 ± 1.12 | 3.91 |
| Orientations | 3.89 ± 0.70 | 5.00 ± 0.78 | 4.78 ± 0.72 | 0.64 |
| Reversals | 2.00 ± 0.41 | 3.11 ± 0.89 | 3.00 ± 0.47 | 0.95 |
| Jump attacks | 0.33 ± 0.33 | 0.22 ± 0.22 | 0.22 ± 0.15 | 0.06 |
| Jump escapes | 0.56 ± 0.56 | 0.89 ± 0.77 | 0.56 ± 0.29 | 0.11 |
| Straight alley test | ||||
| Approaches/withdrawals | 1.89 ± 0.35 | 2.78 ± 0.55 | 2.11 ± 0.31 | 1.23 |
| Contacts | 0.11 ± 0.11 | 1.11 ± 0.39 | 1.33 ± 0.41* | 3.85 |
| Jump escapes | 0.11 ± 0.11 | 0.44 ± 0.34 | 0.11 ± 0.11 | 0.80 |
| Freezing (s) | 1.89 ± 0.99 | 3.11 ± 1.84 | 1.44 ± 0.77 | 0.45 |
| Forced contact test | ||||
| Uprights | 3.78 ± 0.92 | 3.56 ± 0.88 | 4.00 ± 1.14 | 0.05 |
| Vocalizations | 6.00 ± 1.03 | 3.56 ± 1.07 | 5.89 ± 1.59 | 1.20 |
| Jump attacks | 1.89 ± 0.86 | 1.78 ± 0.80 | 1.22 ± 0.55 | 0.23 |
| Jump escapes | 3.33 ± 1.20 | 3.11 ± 1.02 | 2.78 ± 1.13 | 0.06 |
| Post-test | ||||
| Line crossings | 141.56 ± 12.20 | 123.22 ± 9.31 | 120.78 ± 11.19 | 1.07 |
| Rears | 28.89 ± 2.79 | 25.33 ± 2.38 | 25.78 ± 3.34 | 0.45 |
| Escape attempts | 2.78 ± 1.38 | 2.22 ± 1.09 | 0.33 ± 0.24 | 1.56 |
n=9 for each group;
p<0.01 and
p<0.05 compared to control by Dunnet’s t test
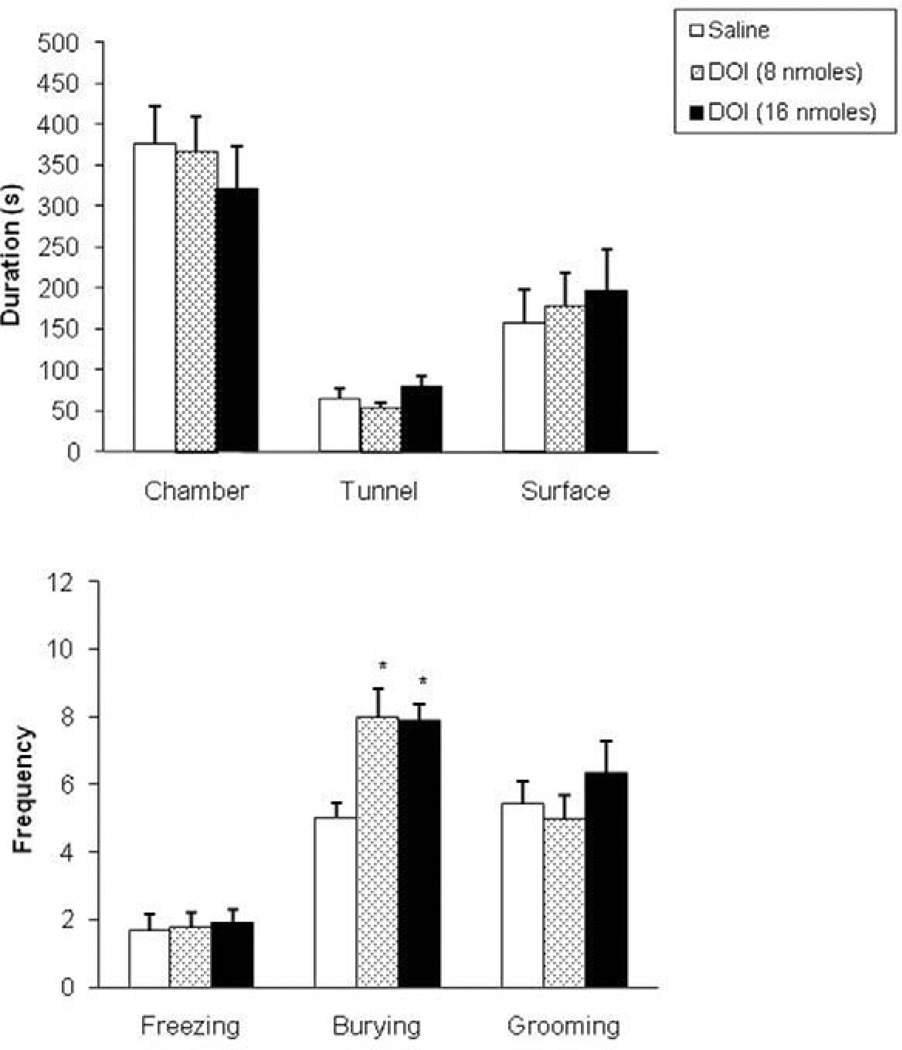
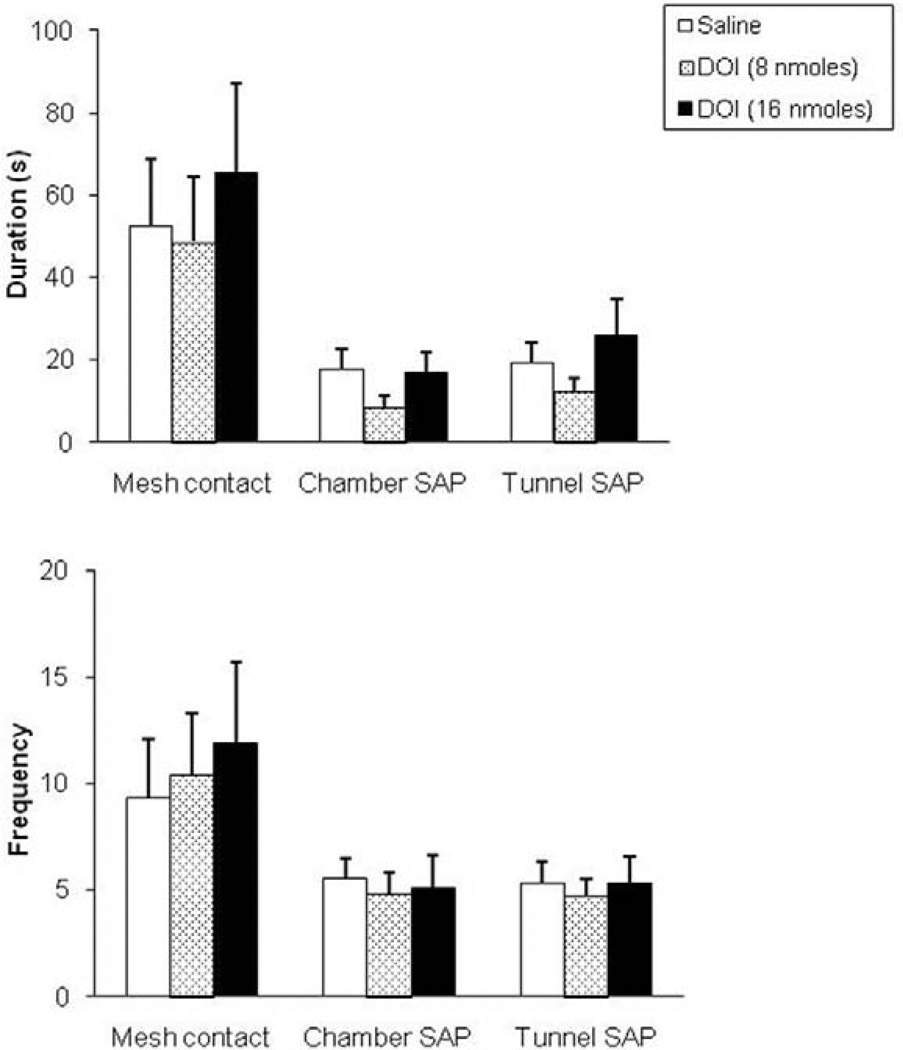
In the RET, one-way ANOVA followed by Dunnet’s t test revealed no significant effects of DOI infusion on the spatiotemporal or ethological measures assessed in this test (Fig. 4 and Fig. 5). The only behavioral effect observed within this test was an increase in the frequency of defensive burying (F(2,26) = 7.36, p<0.05, see Fig. 4, lower panel).
Figure 4.
Effects (mean±SEM) of intra-dPAG injection of DOI on the duration of time spent in the chamber, tunnel and in the surface area (upper panel) and on the frequency of selected behaviors (lower panel) in the RET. n=9 for each group; *p<0.05 compared to control group by Dunnet’s t test.
Figure 5.
Effects (mean±SEM) of intra-dPAG injection of DOI on the duration (upper panel) and frequency (lower panel) of mesh contact, chamber and tunnel SAP. n=9 for each group.
4. Discussion
The present study evaluated the effects of the administration of 5-HT-related drugs into the DRN and dPAG of mice exposed to the MDTB. In experiment 1, intra-DRN infusion of the 5-HT1A antagonist WAY-100635 did not produce significant differences between control and drug dose groups in any of the MDTB subtests. This behavioral pattern differs from the effects obtained with similar manipulations on rats in the elevated T-maze, a test that generates defensive responses potentially related to GAD and PD (Graeff et al., 1998; Zangrossi and Graeff, 1997). In this test, intra-DRN injection of WAY-100635 facilitated inhibitory avoidance response and impaired escape from one of the open arms (Pobbe and Zangrossi, 2005). Therefore, the behavioral effects verified in the elevated T-maze with the infusion of WAY-100635 into the rat DRN were not corroborated in the current MDTB analysis.
Only one previous study has investigated the effect of WAY-100635 infusion into the DRN on mouse defensive behaviors. Canto-de-Souza and coworkers (2002) reported that such injection did not alter mouse behavior in the elevated plus-maze, in agreement with the results obtained in experiment 1. As noted above, there is a clear discrepancy between the behavioral effects induced by intra-DRN administration of WAY-100635 in rats tested in the elevated T-maze and the lack of effects observed with the infusion of this antagonist into the mouse DRN. These findings may indicate that in contrast to rats (Hajós et al., 2001; Haddjeri et al., 2004), 5-HT1A auto-receptors in the mouse DRN may not be under tonic inhibitory influence by endogenous 5-HT.
The reasons for this discrepancy are not clear. However, one specific factor, differences in the behavioral defensive repertoire between these two species, should be considered. In response to exposure to a potential predator and/or partial predator cues in a visible burrow system (VBS), the main strategy of mice involves approach/risk assessment while avoidance and freezing are the main defensive responses observed in rats (Blanchard and Blanchard, 1989, Blanchard et al., 1995). Finally, the range of drug doses used in the present mouse analysis and in our previous rat study (Pobbe and Zangrossi, 2005): the same doses of WAY-100635 (0.185–0.74 nmol) were used in both studies. Therefore, we can not exclude the possibility that higher doses of this 5-HT1A antagonist might be necessary to induce significant behavioral effects in the mouse.
In the dPAG, the 5-HT1A agonist 8-OH-DPAT consistently reduced the distances run during escape, and flight speed while escaping, suggesting a reduction of fear or panic-like behavior. Intra-dPAG infusion of 8-OH-DPAT also increased the number of mouse contacts with the threat stimulus and decreased the number of line crossings in the post-test. While the contacts data clearly indicates a reduction in avoidance, the post-test line crossings measure may be more equivocal. This measure does respond to drugs that influence anxiety (Blanchard et al, 2003a), but the specificity of an anxiety interpretation in the present study is reduced by two factors. First, there was a similar, albeit nonsignificant, difference between the groups in pre-test line crossings, and second, the line crossings data for the control group did not decline from pre- to post-test, suggesting that control subjects failed to show a change in activity, potentially associated with a conditioned anxiety response, following exposure to the threat stimulus.
The 5-HT2A/2C agonist DOI had a very similar effect profile, with reductions in escape distance run, and flight speed, along with enhancement of stops and increased contact with the predator in the straight alley test. In earlier MDTB studies, Griebel and coworkers (1995a, 1995b) found that chronic administration of drugs effective against PD, such as the benzodiazepine receptor full agonist alprazolam or chronic administration of the 5-HT reuptake inhibitors imipramine and fluoxetine, impaired mouse escape response in this model. Therefore, such findings support an interpretation of an anti-PD effect induced by the injection of either 8-OH-DPAT or DOI into the mouse dPAG. Similarly, the infusion of both 5-HT agonists into the mouse dPAG increased the frequency of contacts with the threat stimulus, indicating that such manipulation produced anxiolytic effects in the MDTB.
A more marked pattern of differences between effects of the two drugs was obtained in the RET. In this test, 8-OH-DPAT into the dPAG impaired the mouse avoidance response, increasing the duration of time spent in the surface area. While mesh contact frequency also increased, this may be nonspecific, as animals were in the surface adjacent to the mesh for longer durations. The mice that received the lower dose of 8-OH-DPAT into the dPAG also showed less risk assessment behavior in the chamber compartment, suggesting a consistent decrement in defensiveness with such manipulation. Intra-dPAG infusions of DOI did not result in significant behavioral changes, except for an increase in the frequency of defensive burying. Anxiolytic drugs reduce defensive burying (De Boer and Koolhaas, 2003), consonant with an anxiogenic interpretation of intra-dPAG DOI effects that contrasts with the apparently anxiolytic effect of this compound in the MDTB. However, the functional relationship between defensive burying and other measures of anxiety is not clear: De Boer and Koolhaas (2003) reported the absence of any correlation between defensive burying and percentage of open arm scores in an elevated plus maze, a very commonly used index of anxiety. These data, suggesting that specific behaviors that respond to anxiolytic drugs may show poor correlations with each other, add to a view that anxiety is a multifaceted phenomenon, such that different behaviors, or different combinations of behaviors, may be encompassed within a diagnosis such as GAD.
Injection of 8-OH-DPAT or DOI into the rat dPAG inhibited escape from one of the open arms in the elevated T-maze (Soares and Zangrossi, 2004; Zanoveli et al., 2003), which corroborates the anti-escape effect of these drugs in mice in the MDTB. Also in agreement with the current MDTB and RET results, the administration of 8-OH-DPAT into the rat dPAG impaired inhibitory avoidance in the elevated T-maze. The enhanced defensive burying by DOI-infused mice provides an exception to this pattern of general agreement with the earlier rat results. As a whole, however, these findings suggest the involvement of rat and mouse 5-HT1A and 5-HT2A/2C dPAG receptors in the modulation of escape responses, with the 5-HT1A receptors particularly involved in the regulation of avoidance and risk assessment behaviors.
The behavioral effect on escape response obtained with the infusion of 5-HT agonists into the mouse dPAG is in agreement with the proposed role for such area in the regulation of PD-related behaviors (Deakin and Graeff, 1991). However, the effects of such manipulation on risk assessment and avoidance behaviors are less so. This evidence seems to be compatible with the McNaughton and Corr (2004) proposal which suggests that the PAG and the medial hypothalamus, besides fundamentally controlling fear-like responses, are also involved in the modulation of anxiety-related defensive responses (e.g., avoidance and risk assessment).
In summary, the present behavioral data is in partial agreement with previous findings obtained with rats tested in the elevated T-maze. Although there is a high level of similarity between the behavioral effects obtained in rats (elevated T-maze) and mice (MDTB and RET) with the infusion of 5-HT agonists into the dPAG, the same is not true regarding the effects of blockade of DRN 5-HT1A receptors in these rodent species. These data suggest that there may be differences between mice and rats regarding the involvement of the DRN in the mediation of defensive behaviors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argyropoulos SV, Sandford JJ, Nutt DJ. The psychobiology of anxiolytic drug. Part 2: Pharmacological treatments of anxiety. Pharmacol. Ther. 2000;88:213–227. doi: 10.1016/s0163-7258(00)00083-8. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci. Biobehav. Rev. 2001;25(3):205–218. doi: 10.1016/s0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. The Mouse Defense Test Battery: pharmacological and behavioral assays for anxiety and panic. Eur. J. Pharmacol. 2003a;463:97–116. doi: 10.1016/s0014-2999(03)01276-7. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. Conditioning and residual emotionality effects of predator stimuli: some reflections on stress and emotion. Prog. Neuro-Psychopharm. Biol. Psych. 2003b;27:1117–1185. doi: 10.1016/j.pnpbp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. J. Comp. Psychol. 1989;103:70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Parmigiani S, Bjornson C, Masuda C, Weiss SM, Blanchard DC. Antipredator behavior of Swiss-Webster mice in a visible burrow system. Agress. Behav. 1995;21:123–136. [Google Scholar]
- Blier P, de Montigny C. Serotonin and drug-induced therapeutic responses in major depression, obsessive-compulsive and panic disorders. Neuropsychopharmacology. 1999;21:91–98. doi: 10.1016/S0893-133X(99)00036-6. [DOI] [PubMed] [Google Scholar]
- Canto-de-Souza A, Nunes-de-Souza RL, Rodgers RJ. Anxiolytic-like effect of WAY-100635 microinfusions into the median (but not dorsal) raphe nucleus in mice exposed to the plus-maze: influence of prior test experience. Brain Res. 2002;928:50–59. doi: 10.1016/s0006-8993(01)03354-6. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur. J. Pharmacol. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- Deakin JFW, Graeff FG. 5-HT and mechanisms of defense. Psychopharmacology. 1991;5:305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- Graeff FG. On serotonin and experimental anxiety. Psychopharmacology (Berl) 2002;163:467–476. doi: 10.1007/s00213-002-1112-4. [DOI] [PubMed] [Google Scholar]
- Graeff FG. Serotonin, the periaqueductal gray and panic. Neurosci. Biobehav. Rev. 2004;28:239–259. doi: 10.1016/j.neubiorev.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Ferreira Netto C, Zangrossi H., Jr. The elevated T-maze as an experimental model of anxiety. Neurosci. Biobehav. Rev. 1998;23(2):237–246. doi: 10.1016/s0149-7634(98)00024-4. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Viana MB, Mora PO. Opposed regulation by dorsal raphe nucleus 5-HT pathways of two types of fear in the elevated T-maze. Pharmacol. Biochem. Behav. 1996;53:171–177. doi: 10.1016/0091-3057(95)02012-8. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Zangrossi H., Jr. Animal models of anxiety disorders. In: D’Haenen H, den Boer JA, Westenberg H, Willner P, editors. Textbook of Biological Psychiatry. London: Wiley; 2002. pp. 879–893. [Google Scholar]
- Griebel G, Blanchard DC, Agnes RS, Blanchard RJ. Differential modulation of antipredator defensive behavior in Swiss-Webster mice following acute and chronic administration of imipramine and fluoxetine. Psychopharmacology (Berl) 1995b;120:57–66. doi: 10.1007/BF02246145. [DOI] [PubMed] [Google Scholar]
- Griebel G, Blanchard DC, Jung A, Lee JC, Masuda CK, Blanchard RJ. Further evidence that the mouse defense test battery is useful for screening anxiolytic and panicolytic drugs: effects of acute and chronic treatment with alprazolam. Neuropharmacology. 1995a;34:1625–1633. doi: 10.1016/0028-3908(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Lavoie N, Blier P. Electrophysiological evidence for the tonic activation of 5-HT1A autoreceptors in the rat dorsal raphe nucleus. Neuropsycopharmacology. 2004;29:1800–1806. doi: 10.1038/sj.npp.1300489. [DOI] [PubMed] [Google Scholar]
- Hajós M, Hoffmann WE, Tetko IV, Hyland B, Sharp T, Villa AEP. Different tonic regulation of neuronal activity in the rat dorsal raphe and medial prefrontal cortex via 5-HT1A receptors. Neurosci. Lett. 2001;304:129–132. doi: 10.1016/s0304-3940(01)01751-7. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Liu RJ, Lambe EK, Aghajanian GK. Somatodendritic autoreceptor regulation of serotonergic neurons: dependence on L-tryptophan and tryptophan hydroxylase-activating kinases. Eur. J. Neurosci. 2005;21:945–958. doi: 10.1111/j.1460-9568.2005.03930.x. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Maron E, Shlik J. Serotonin function in panic disorder: important, but why? Neuropsychopharmacology. 2006;31:1–11. doi: 10.1038/sj.npp.1300880. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci. Biobehav. Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Prog. Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Serotonin 5-HT2c receptors as a target for the treatment of depressive and anxiety states: focus on novel therapeutic strategies. Therapie. 2006;60:441–460. doi: 10.2515/therapie:2005065. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Second edition. San Diego: Academic Press; 2001. [Google Scholar]
- Pinheiro SH, Zangrossi H, Jr., Del-Ben CM, Graeff FG. Elevated mazes as animal models of anxiety: effects of serotonergic agents. An. Acad. Bras. Cienc. 2007;79:71–85. doi: 10.1590/s0001-37652007000100010. [DOI] [PubMed] [Google Scholar]
- Pobbe RLH, Zangrossi H., Jr. 5-HT1A and 5-HT2A receptors in the rat dorsal periaqueductal gray mediate the antipanic-like effect induced by the stimulation of serotonergic neurons in the dorsal raphe nucleus. Psychopharmacology (Berl) 2005;183:314–321. doi: 10.1007/s00213-005-0196-z. [DOI] [PubMed] [Google Scholar]
- Sena LM, Bueno C, Pobbe RLH, Andrade TGCS, Zangrossi H, Jr., Viana MB. The dorsal raphe nucleus exerts opposed control on generalized anxiety and panic-related defensive responses in rats. Behav. Brain Res. 2003;142:125–133. doi: 10.1016/s0166-4328(02)00399-6. [DOI] [PubMed] [Google Scholar]
- Soares VP, Zangrossi H., Jr. Involvement of 5-HT1A and 5-HT2 receptors of the dorsal periaqueductal gray in the regulation of the defensive behaviors generated by the elevated T-maze. Brain Res. Bull. 2004;64:181–188. doi: 10.1016/j.brainresbull.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Regulation of serotonin release by GABA and excitatory amino acids. J. Psychopharmacology. 2000;14:100–113. doi: 10.1177/026988110001400201. [DOI] [PubMed] [Google Scholar]
- Viana MB, Graeff FG, Löschmann PA. Kainate microinjection into the dorsal raphe nucleus induces 5-HT release in the amygdala and periaqueductal gray. Pharmacol. Biochem. Behav. 1997;58:167–172. doi: 10.1016/s0091-3057(96)00451-0. [DOI] [PubMed] [Google Scholar]
- Viana MB, Tomaz C, Graeff FG. The elevated T-maze: a new animal model of anxiety and memory. Pharmacol Biochem Behav. 1994;49:549–554. doi: 10.1016/0091-3057(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Yang M, Augustsson H, Markham CM, Hubbard DT, Webster D, Wall PM, Blanchard RJ, Blanchard DC. The rat exposure test: a model of mouse defensive behaviors. Physiol. Behav. 2004;81:465–473. doi: 10.1016/j.physbeh.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Zangrossi H, Jr., Graeff FG. Behavioral validation of the elevated T-maze, a new animal model of anxiety. Brain Res. Bull. 1997;44:1–5. doi: 10.1016/s0361-9230(96)00381-4. [DOI] [PubMed] [Google Scholar]
- Zanoveli JM, Nogueira RL, Zangrossi H., Jr. Serotonin in the dorsal periaqueductal gray modulates inhibitory avoidance and one-way escape behaviors in the elevated T-maze. Eur. J. Pharmacol. 2003;473:153–161. doi: 10.1016/s0014-2999(03)01970-8. [DOI] [PubMed] [Google Scholar]