Abstract
Previous research on functional hemispheric differences in visual processing has associated global perception with low spatial frequency (LSF) processing biases of the right hemisphere (RH) and local perception with high spatial frequency (HSF) processing biases of the left hemisphere (LH). The Double Filtering by Frequency (DFF) theory expanded this hypothesis by proposing that visual attention selects and is directed to relatively LSFs by the RH and relatively HSFs by the LH, suggesting a direct causal relationship between SF selection and global versus local perception. We tested this idea in the current experiment by comparing activity in the EEG recorded at posterior right and posterior left hemisphere sites while participants’ attention was directed to global or local levels of processing after selection of relatively LSFs versus HSFs in a previous stimulus. Hemispheric asymmetry in the alpha band (8–12 Hz) during preparation for global versus local processing was modulated by the selected SF. In contrast, preparatory activity associated with selection of SF was not modulated by the previously attended level (global/local). These results support the DFF theory that top-down attentional selection of SF mediates global and local processing.
Keywords: global versus local processing, hemispheric asymmetry, spatial frequency, alpha reduction
Introduction
Our environment is hierarchically organized in that scenes are composed of objects that exist at different levels of detail. Efficient visual perception requires the ability to represent such multiple levels so that attention can be directed to the level of the whole (“global processing”) or to the level of its components (“local processing”). This ability relies on neural mechanisms that allow for the concurrent segregation and integration of object features in order to represent objects that are embedded within one another rather than being located in distinct locations. These mechanisms have been the focus of ample research, but their characterization remains incomplete. For example, previous neuropsychological, cognitive, and imaging research has converged to establish functional hemispheric asymmetries in global versus local perception, with the right hemisphere (RH) biased toward global processing and the left hemisphere (LH) biased toward local processing (e.g., Delis, Robertson, & Effron, 1986; Fink et al., 1997; Han et al., 2002; Lamb, Robertson, & Knight, 1989; Martin, 1979, Martinez et al., 1997; Robertson & Delis, 1986; Robertson & Lamb, 1991; Robertson, Lamb, & Knight, 1988; Robertson, Lamb, & Zaidel, 1993; Sergent, 1982, 1983, Volberg & Hübner, 2004; Weissman & Woldorff, 2005). However, global and local are inherently relative (i.e., they only exist in relation to the other level(s)) and the cognitive and neural processes that yield these asymmetric hemispheric biases continue to be debated.
Several theories of global versus local perception have proposed selective processing of spatial frequency (SF) ranges to underlie the hemispheric asymmetry. These theories rely on evidence that the visual system filters incoming information into channels that are tuned for different SF bands (De Valois & De Valois, 1988). Other studies have shown that SFs are flexibly used to influence performance depending on task demands (e.g., Loftus & Harley, 2004; Morrison & Schyns, 2001; Schyns & Oliva, 1999).
Broadbent (1977) was among the first to suggest that the important functional distinction between attending to global and local levels of a hierarchical display is in their SF content; low spatial frequencies (LSFs) attract more attention to the global than local levels, and high spatial frequencies (HSFs) attract more attention to the local than global levels. The importance of SFs in global and local processing has more recently been supported by several behavioral (e.g., Goffaux, Hault, Michel, Vuong, & Rossion, 2005; Goffaux & Rossion, 2006) and ERP studies of face processing (e.g., Boeschoten, Kemnera, Kenemansc, & van Engeland, 2005; Flevaris, Robertson, & Bentin, 2008).
Other studies have found an analogous hemispheric asymmetry in processing SFs, with the RH biased in discriminating LSFs and the LH biased in discriminating HSFs (Christman, Kitterle, & Hellige, 1991; Kitterle, Christman, & Hellige, 1990; Kitterle, Hellige, & Christman, 1992), as well as a direct link between LSF versus HSF discrimination and attending to global versus local objects, respectively (Flevaris, Bentin, & Robertson, 2011; Shulman, Sullivan, Gish, & Sakoda, 1986; Shulman & Wilson, 1987). Furthermore, the asymmetry occurs whether the stimuli are small or large (Robertson & Lamb, 1991), making the role of SFs more complex than a simple bottom-up analysis. To account for these and other data, Ivry and Robertson (1998; Robertson & Ivry, 2000) developed the Double Filtering by Frequency (DFF) theory of hemispheric specialization for SF processing. According to this theory, there are two stages of SF filtering, and the two hemispheres differ in how they process SF information after the initial visual stage. Specifically, attention first selects an SF range from the incoming spectrum that is most suited for the current task (e.g., the range of spatial scales that is most likely to contain the target; see Watt, 1988 for a model of how this might work). This SF range is then fed forward in both cerebral hemispheres, but it is at this second stage that the hemispheres differ, with the LH acting as a relatively high-pass filter, emphasizing information from the higher SFs within the initially selected range, and the RH as a relatively low-pass filter, emphasizing the lower SFs within the range.
Despite evidence associating SF processing with global ersus local perception, a number of alternative proposals have also been made to account for these hemispheric asymmetries. Among those are theories proposing hemispheric differences in sensitivity to the saliency of the stimulus, with the right hemisphere biased toward more salient objects and the left hemisphere biased toward less salient objects (Mevorach, Humphreys, & Shalev, 2005, 2006), or in shape-level integration, with the right hemisphere involved in binding shapes to the global level and the left hemisphere involved in binding shapes to the local level (Hubner & Volberg, 2005). Moreover, there is some disagreement even among theories positing SF processing differences. In contrast to the flexible mechanism posited by DFF theory, some researchers have assumed that the use of SF scales reflects a lower level mechanism by which the two hemispheres receive different input (or asymmetrically emphasize different input) during lower level visual processing and, thus, differ in absolute SF biases (e.g., Jacobs & Kosslyn, 1994; Sasaki et al., 2001; Sergent, 1982).
We recently reported evidence in support of a flexible mechanism, based on relative SF, to underlie hemispheric asymmetry in global versus local perception (Flevaris et al., 2011). In that study, we found that allocating attention to global or local levels of hierarchical displays (Navon letters) biased selection of LSFs or HSFs, respectively, in subsequently presented compound gratings. Importantly, this bias was determined by the relationship between the two SFs in the compound grating (i.e., their relative frequency) rather than the absolute SF values. That is, global attention biased selection of a 1.8 cycles/degree grating when it was paired with a higher SF (5.3 cycles/ degree), but local attention biased selection of the same 1.8 cycles/degree grating when it was paired with a lower SF (0.9 cycle/degree) in the compound. Although these data are consistent with DFF theory in the sense that global/local selection is based on relative SF, neuro-biological measures of hemispheric asymmetries were not directly assessed in that study. In a different study examining hemispheric differences in integrating shapes with hierarchical level, we found evidence that attention to LSFs facilitates binding of shapes to the global level by the RH and that attention to HSFs facilitates binding of shapes to the local level by the LH (Flevaris, Bentin, & Robertson, 2010). These data suggest that attentional selection of relative SF scales could provide the mechanism for shape-to-level integration proposed by Hubner and Volberg (2005), thereby integrating the DFF and shape-to-level integration theories of global versus local processing in a unified framework.
However, the behavioral priming effects in the previous studies may have been observed due to a bidirectional additive model, in which the right hemisphere is biased toward relative LSFs and global objects whereas the left hemisphere is biased toward relative HSFs and local objects. This is in contrast to the DFF theory proposal that selection of SF induces global/local processing. To this end, in the current study, we adapted the behavioral experiment described in Flevaris et al. (2011) to an EEG study in order to examine the direction of modulation of responses in the two cerebral hemispheres while participants were preparing to respond to relative SF and hierarchical level. We investigated preparatory neural activity by examining oscillatory brain responses in the scalp EEG in alpha band (8–12 Hz).
Modulation of the alpha band has been previously established as a measure of brain activity and used as a direct index of attention (Bastiaansen, Böcker, Brunia, de Munck, & Spekreijse, 2001; Thut, Nietzel, Brandt, & Pascal-Leone, 2006; Volberg, Kliegl, Hansimayr, & Greenlee, 2009; Ward, 2003; Worden, Foxe, Wang, & Simpson, 2000). Specifically, since the alpha band has been considered as “idling rhythms” of the brain (Pfurtscheller, Stancak, & Neuper, 1996), reduction in alpha amplitude (Event-Related Desynchronization—ERD) is assumed to reflect elevated brain activation, whereas augmentation of alpha amplitude (Event-Related Synchronization—ERS) is assumed to reflect inhibitory processes (Klimesch, Doppelmayr, & Hanslmayr, 2007). In the case of directing attention to global versus local objects, Volberg et al. (2009) showed that, at least for trials in which behavioral performance was optimal (i.e., trials in which response times were fastest), alpha amplitudes at centro-parietal sites were greater over the right hemisphere (i.e., higher left than right hemisphere activation) before participants reported local targets and greater over the left hemisphere (i.e., higher right than left hemisphere activation) before they reported global targets. These data confirmed that the hemispheric asymmetry in global versus local processing occurs not only during perceptual processing but also during attentional orienting in preparing for one level or the other and that EEG oscillations in the alpha band can index relative attentional orienting across the two cerebral hemispheres. In the current study, we examined the modulation of this orienting response after selection of an SF in a complex grating and before the global or the local stimulus actually appeared. In this way, we could examine how HSF versus LSF selection influenced the preparatory response to the global or local level. We also looked at alpha modulation at the LH and RH sites prior to the processing of gratings following a global or local response and prior to global or local processing following the selection of either LSF or HSF in the gratings. This allowed us to determine the directionality or symmetry of the SF level of processing relationship.
Participants performed two tasks in alternating sequence: an SF grating orientation task, in which they were instructed to report the orientation of either the relatively LSF or HSF stripes in a compound SF grating, and a hierarchical level same/different task, in which they reported whether the global or local letters in two hierarchical Navon displays were the same or different (see Figure 1). There were approximately 2 s in between each stimulus (grating/Navon). The long inter-stimulus interval allowed enough time to sufficiently measure amplitudes in the alpha band. In our previous behavioral study (Flevaris et al., 2011), the SF grating appeared immediately after response to the Navon display, and as mentioned above, we found priming effects such that attention to the global level facilitated responses to relatively LSFs and attention to the local level facilitated responses to relatively HSFs. Since, in the present study, we extended the interval between the Navon and grating stimuli, we did not necessarily expect a priming effect on reaction times (RTs); instead, we focused on preparatory activity as reflected in modulations of alpha amplitude during the inter-task intervals relative to a baseline period. Specifically, we compared the alpha ERD over the left and right hemispheres directly prior to the onset of the Navon letters as a function of the relative SF that was previously attended, as well as the alpha ERD directly prior to the compound SF grating as a function of the level that was previously attended in the Navon task. If the behavioral priming effects associating relative LSF processing with global perception and relative HSF processing with local perception were due to the bidirectional additive model described previously, then we would expect a similar pattern of alpha ERD in preparation for the response to the gratings as in preparation for response to the Navon. Conversely, if the DFF hypothesis is correct, and SF selection is the medium by which hierarchical perception occurs, then we would expect attended SF to modulate preparatory alpha activity unidirectionally. Moreover, in line with our hypothesis that LSF selection mediates global perception by the right hemisphere and HSF selection mediates local perception by the left hemisphere, we predicted a second-order interaction between task (global/local), SF, and hemisphere (which was observed).
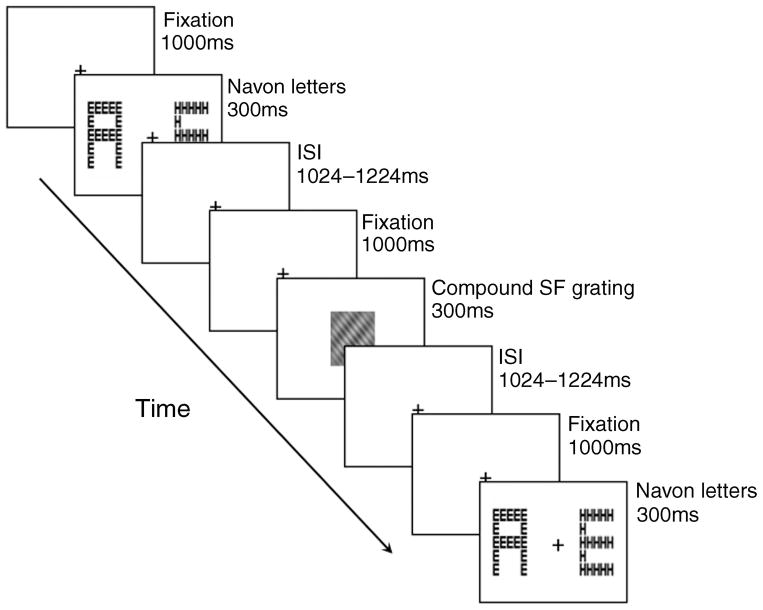
Figure 1.
Sample trial. Participants performed two tasks in alternating sequence: a Navon task and a grating task. For the Navon task, two Navon letters appeared on the screen side by side and participants indicated if they were the same or different at the global or local level (in separate blocks of trials). For the grating task, a single compound grating appeared at fixation and participants reported the orientation of the lower spatial frequency (LSF) or higher spatial frequency (HSF) grating (in separate blocks of trials). A 1000-ms fixation cross preceded each stimulus. Additionally, the fixation cross appeared on the screen after each stimulus for a variable interval between 1024 and 1224 ms (resulting in 2024–2224 ms between each stimulus).
Methods
Participants
Sixteen undergraduates (ten women) from the University of California, Berkeley participated in the experiment for monetary compensation. All were right-handed and had normal or corrected-to-normal vision. All gave informed consent as approved by the Committee for the Protection of Human Subjects at the University of California, Berkeley.
Stimuli
The Navon patterns were created using Adobe Photoshop by arranging a series of (local) letters in black Helvetica bold font to form a larger (global) letter. The letters used were A, C, D, E, F, and H, in all their global and local combinations, with the exception of congruent combinations (e.g., a global A made up of local A’s). This resulted in 30 distinct Navon displays. Seen from a distance of 1 m, each local letter subtended 0.7° of visual angle and each global letter was 4.0° wide by 5.7° high.
The compound gratings were generated in Matlab (Mathworks, Natick, MA) with a sinusoid function for each SF. Each compound grating subtended 5.4° of visual angle overall and was composed of a 0.7 cycle/degree (4 cycles/image) grating (the relatively LSF component) and a 2.4 cycle/degree (13 cycles/image) grating (the relatively HSF component). Both SF gratings were at 100% contrast. The relatively LSF component was oriented either at 45° (tilted to the right) or at 135° (tilted to the left) and the relatively HSF component was oriented in the opposite direction.
Procedure
The stimuli were shown on a Dell 17-inch color CRT monitor with a vertical refresh rate of 60 Hz and a resolution of 1024 × 768 pixels. Participants were seated 1 m from the screen in a dark room. Trial timing was controlled by Presentation (Neurobehavioral Systems, Albany, CA) and is depicted in Figure 1. Each trial began with a central fixation cross presented for 1000 ms. Two Navon patterns were then presented simultaneously for 300 ms, one in the left and one in the right visual field, with the medial edge 1° from fixation. A variable inter-trial interval (ITI) between 1024 ms and 1224 ms followed the Navon patterns, during which time participants indicated via button press if the two patterns were the same or different at the global level (during half of the blocks) or local level (during the other half of the blocks). A 1000-ms fixation period followed the ITI, after which a compound SF grating was presented at fixation for 300 ms. A variable ITI between 1024 ms and 1224 ms followed the grating, during which time participants indicated via button press whether the relatively LSF component was tilted to the left or right (during half of the blocks) or whether the relatively HSF component was tilted to the left or right (during the other half of the blocks). At the end of this ITI, a new trial began. Hence, the Navon letters were separated from the grating by a random interval between 2024 and 2224 ms and a similar interval separated the grating from the Navon letters. This ensured that the two tasks were perceived as alternating rather than one preceding the other. The response mappings for the four response buttons (i.e., two for the Navon task and two for the grating task) were counterbalanced across participants. Participants performed four blocks of 120 trials each, in counterbalanced order: attention to the global level in the Navon patterns/LSFs in the grating, global/HSF, local/ LSF, and local/HSF. This block design was used to eliminate confusion about the dual task as well as keep the experiment as close as possible to our previous behavioral version (Flevaris et al., 2011). A 20-trial practice block preceded each experimental block. Additionally, EEG was recorded while participants closed their eyes for 2 min prior to the experiment and 2 min following the experiment. Alpha activity during this period was averaged and used as a baseline for calculating the ERD.
EEG recording and analysis
The EEG analog signals were recorded continuously by 64 Ag–AgCl pin-type active electrodes mounted on an elastic cap (ECI) according to the extended 10–20 system and from two additional electrodes placed at the right and left mastoids, all referenced to a common-mode signal (CMS) electrode between POz and PO3 and were subsequently re-referenced digitally to the average reference. Eye movements and blinks were monitored using bipolar horizontal and vertical EOG derivations via two pairs of electrodes: one pair attached to the external canthi and the other to the infraorbital and supraorbital regions of the right eye. Both EEG and EOG were sampled at 256 Hz using a Biosemi Active II digital 24-bit amplification system with an active input range of −262 μV to +262 μV per bit without any filter at input. The digitized EEG was saved and processed offline.
Data were analyzed using Matlab (Mathworks, Natick, MA). To remove EOG and EEG artifacts, a change in voltage of more than 100 μV during a 100-ms epoch in any of the channels was considered an artifact and the EEG recorded in the interval 200 ms before and after the artifact was removed. Following this artifact removal procedure, more than 90% of the trials were preserved. To analyze oscillatory cortical activity associated with preparing for the global/local and SF tasks, the single trial EEG signal on each channel was convolved with 6-cycle Morlet wavelets over a 2950-ms window beginning 2700 ms prior to stimulus onset. To examine oscillatory cortical activity during and following each stimulus, the EEG signal on each channel was also convolved as over a 2950-ms window beginning 250 ms prior to stimulus onset. Instantaneous power and phase were extracted at each time point (at 256-Hz sampling rate) over frequency scales from 0.7 to 60 Hz incremented logarithmically (Lakatos et al., 2005). The square roots of the power values (the sum of the squares of the real and imaginary Morlet components) were then averaged over single trials to yield the total averaged spectral amplitudes (in μV) for each condition. The averaged spectral amplitude at each time point and frequency was baseline-corrected by dividing the mean spectral amplitude found during the baseline period (Tallon-Baudry, Bertrand, Peronnet, & Pernier, 1998). The ratio was used to reduce the impact of the variability in absolute EEG power caused by individual differences such as scalp thickness and electrode impedance (Pineda & Oberman, 2006). Further, since ratio data are inherently not normally distributed as a result of lower bounding, a log transform (of that ratio) was used for analysis. Hence, a log ratio of less than zero indicates ERD, a value of zero indicates no change, and values greater than zero indicate ERS.
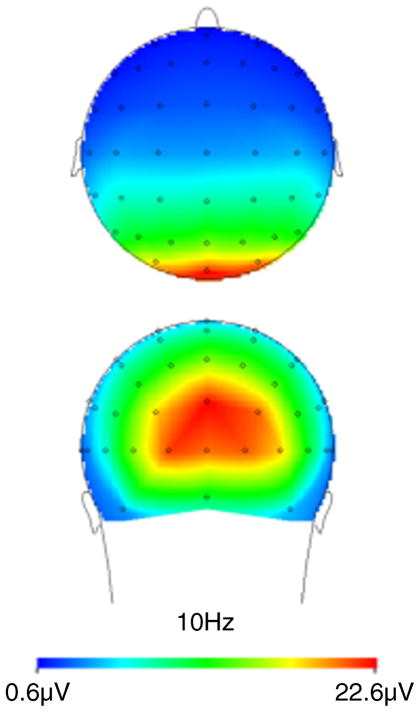
Analyses of variance (ANOVAs) with repeated measures were performed on the mean signal change in the EEG alpha band amplitudes (8–12 Hz). Following inspection of the alpha distribution, we focused on the parieto-occipital sites (see below), in which the alpha power was greatest (see Figure 2).
Figure 2.
Scalp distribution of power at 10 Hz. Alpha power is greatest in the occipital sites (PO7/PO8, PO3/PO4, and O1/O2).
Results
Behavioral results
Accuracy was high for both the SF grating and the Navon tasks (96% and 92%, respectively). Reaction times (RTs) to accurate responses that were within 2 standard deviations on the mean were analyzed with separate 2 × 2 ANOVAs for the Navon task and the SF grating task. The factors were level (global versus local) and SF (LSF versus HSF). As expected, these analyses did not yield significant effects.
Global/local preparation
To assess the alpha activity associated with preparation for global versus local target discrimination, the mean alpha signal change (relative to baseline, see above) in the 1-s period preceding the Navon display was analyzed with a 2 × 2 × 2 × 3 ANOVA with repeated measures. The factors were to-be-attended level (global versus local), previously attended SF (LSF versus HSF), hemisphere (LH versus RH), and site (PO7/PO8, PO3/ PO4, O1/O2). As described above, these sites were selected because they yielded the greatest power in the alpha band when inspecting the distribution across the entire scalp.
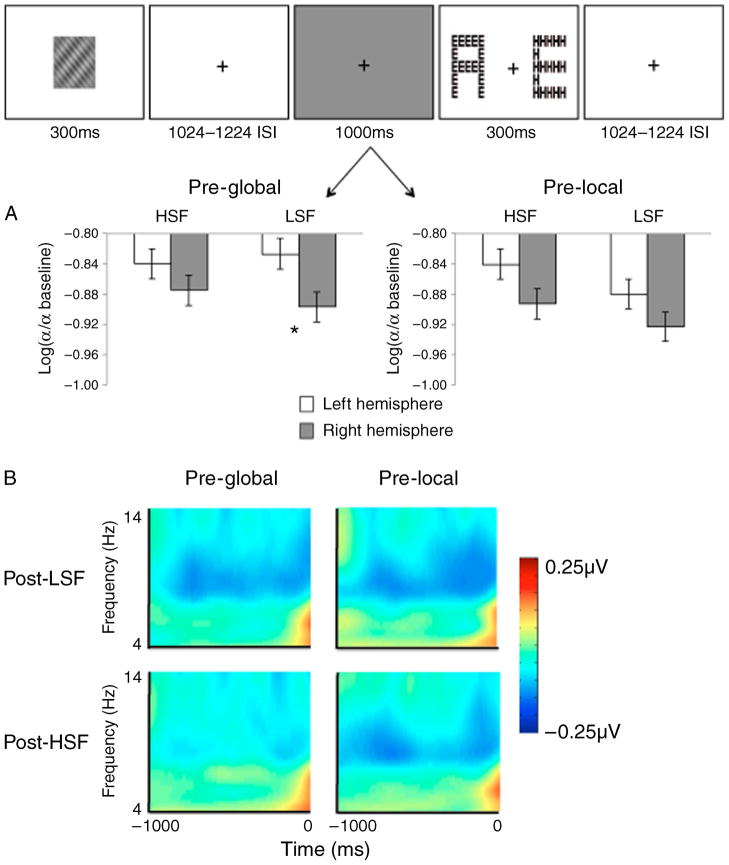
The overall ANOVA revealed a significant level × SF × hemisphere second-order interaction [F(1,15) = 6.29, p < 0.05, MSE = 0.002, partial η2 = 0.30], and no other effects were statistically significant. To examine this interaction, we conducted SF (LSF versus HSF) by Hemisphere (LH versus RH) ANOVAs for the global and local preparation conditions separately, and these data are depicted in Figure 3. When participants prepared for the global task, there was a significant SF × hemisphere interaction [F(1,15) = 11.84, p < 0.01, MSE = 0.001, partial η2 = 0.28]. Follow-up t-tests revealed significantly greater alpha reduction in the right hemisphere (−0.897) than in the left hemisphere (−0.827) when attention was previously allocated to LSFs [t(15) = 2.14, p < 0.05] and a smaller, non-significant hemispheric difference (−0.875 in the right hemisphere and −0.840 in the left hemisphere)when attention was previously allocated to HSFs [t(15) = 1.07, p = 0.30]. When participants prepared for the local task, the ANOVA did not yield significant results.
Figure 3.
(A) Alpha reduction (relative to baseline) during highlighted interval (shown in gray) for global versus local preparation, following LSF versus HSF selection, shown for occipital electrodes (PO7/8, PO3/4, O1/O2). In preparation for the global task, there is significantly greater alpha reduction in the right hemisphere than in the left hemisphere following LSF selection but no significant hemispheric difference following HSF selection. There were no significant hemispheric differences in preparation for the local task. (B) Spectral amplitudes in the right hemisphere (electrode PO8) minus amplitudes in the left hemisphere (electrode PO7) shown for each condition for the 1-s period preceding the Navon. The greater alpha reduction in the right hemisphere is absent for global preparation following HSF selection.
To provide evidence that the alpha ERD preceding the Navon display was indeed preparatory activity associated with global versus local processing, we also analyzed the mean alpha signal change during the 1-s period following the compound SF grating, prior to the 1-s window preceding the Navon display. This analysis did not yield significant results, suggesting that the SF modulation of alpha activity preceding the Navon stimuli was associated with preparing to respond to the global or local levels rather than residual activity from processing the grating (see Figure 4B).
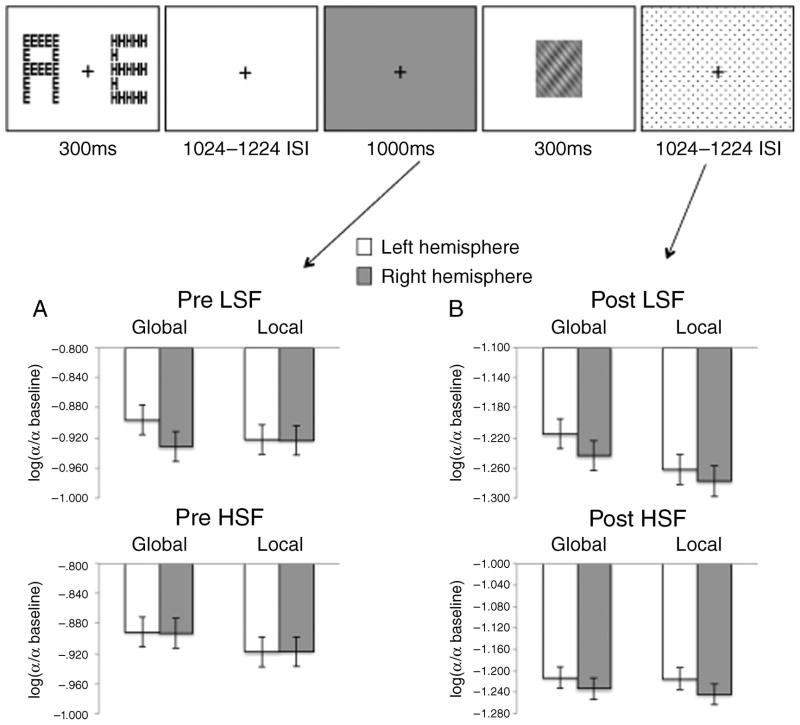
Figure 4.
Spectral amplitudes in the right hemisphere (electrode PO8) versus amplitudes in the left hemisphere (electrode PO7) shown for each condition for (A) the 1-s period preceding the SF grating (shown in gray) and (B) the 1-s period following the grating (shown as dotted). There were no significant hemispheric differences for any condition in either period.
LSF/HSF preparation
To assess the alpha activity associated with preparation for LSF versus HSF selection, we performed the same 2 × 2 × 2 × 3 repeated measures ANOVA on the mean alpha signal change in the 1-s period preceding the compound SF grating. This analysis did not yield any significant results. Importantly, there was no SF × level × hemisphere interaction in the analysis of the 1-s period preceding the grating [F(1,15) < 1], consistent with our hypothesis that SF mediates the hemispheric asymmetry in global versus local processing.
Statistical analysis of preparatory time window
We also conducted an overall ANOVA comparing both preparatory time windows (i.e., pre-Navon and pre-SF grating). The factors were Analysis window (pre-Navon vs. pre-grating, Stimulus 1 (HSF vs. LSF, or local vs. global), Stimulus 2 (local vs. global, or HSF vs. LSF), Hemisphere, and Site. This analysis revealed a significant 4-way interaction between Analysis window, Stimulus 1, Stimulus 2, and Hemisphere [F(1,15) = 5.4, p < 0.05, MSE = 0.004, η2 = 0.27], indicating that the modulation in preparation for global stimuli (that we found in the pre-Navon analysis) is in fact significantly different from modulation in preparation to LSF stimuli.
Theta band
Although we had no a priori predictions about preparatory oscillations in other frequency ranges, we conducted similar analyses for the mean signal change in the theta (4–7 Hz) band.1 As we did for the alpha band, we focused our analyses on electrode sites in which the theta power was the greatest (Figure 5). For the theta band, this included the same 3 sites as the alpha band analysis with the two additional sites P1/P2 and P3/P4.
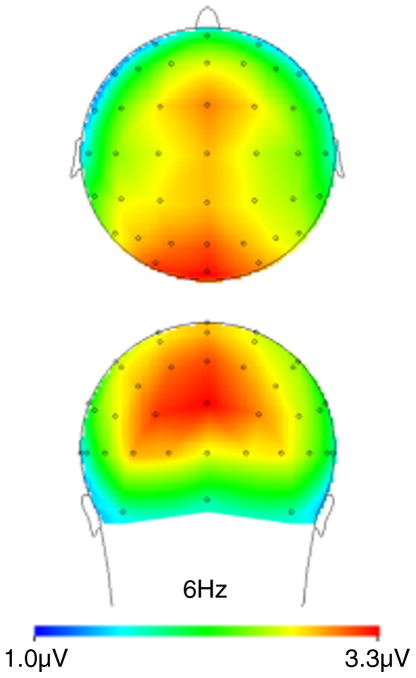
Figure 5.
Scalp distribution of power at 6 Hz. Theta power is greatest in the parieto-occipital sites (PO7/PO8, PO4/PO3, P3/P4, P1/P2, and O1/O2).
In the theta band, the to-be-attended level (Global versus Local) × previously attended SF (LSF versus HSF) × hemisphere (left versus right) × site (PO7/PO8, PO3/PO4, O1/O2, P3/P4, and P1/P2) ANOVA for the 1-s period preceding the Navon showed a significant main effect of hemisphere [F(1,15) = 4.9, p < 0.05, MSE = 0.022, partial η2 = 0.25], with greater theta reduction in the right hemisphere (−0.747) than in the left hemisphere (−0.721). There was also a significant level × hemisphere interaction [F(1,15) = 4.9, p < 0.05, MSE = 0.002, partial η2 = 0.25]. Follow-up t-tests revealed significantly greater theta reduction in the right hemisphere (−0.748) than in the left hemisphere (−0.715) preceding global attention [t(15) = 2.54, p < 0.05] and a smaller non-significant difference preceding local attention [t(15) = 1.65, p = 0.12]. This analysis also revealed a main effect of site [F(1,15) = 70.7, MSE = 0.043, p < 0.001, partial η2 = 0.84], demonstrating greatest theta reduction at the more medial sites P1/P2 (−0.849) and P3/P4 (−0.851), followed by more anterior medial sites PO3/PO4 (−0.730; t(15) = 4.7, p < 0.001), which in turn demonstrated greater alpha reduction than the more lateral posterior sites PO7/PO8 (−0.613) and O1/O2 (−0.627; t(15) = 4.56, p < 0.001).
The ANOVA for the 1-s period preceding the SF grating only revealed a main effect of site [F(1,15) = 70.7, p < 0.001], demonstrating a similar pattern as that found for the period preceding the Navon. The greatest theta reduction was seen at the more medial sites P1/P2 (−0.910) and P3/P4 (−0.908), followed by more anterior medial sites PO3/PO4 (−0.804; t(15) = 13.7, p < 0.001), which in turn demonstrated greater alpha reduction than the more lateral posterior sites PO7/PO8 (−0.693) and O1/O2 (−0.683; t(15) = 14.9, p < 0.001).
Discussion
This experiment provides direct evidence in support of DFF theory by demonstrating that attentional selection of SF modulates preparatory activity in global versus local perception. Consistent with previous findings (Volberg et al., 2009), we found greater alpha reduction in the right hemisphere than in the left hemisphere in preparation for global targets. This hemispheric asymmetry was found following attentional selection of LSF but not following attentional selection of HSF, supporting the theory that LSF selection in the right hemisphere mediates global processing. We did not find similar SF modulation during the interval preceding the compound SF grating or immediately following it. If attentional selection of SF induces a bias for a particular SF range, one might wonder why we did not see significant alpha modulation in the 1-s period following the grating. Interestingly, when comparing the alpha reduction in the period immediately following the SF grating (Figure 4B) with that in the period immediately preceding the Navon (Figure 3A), there is far greater alpha reduction overall in the period immediately following the grating. As time passed, the alpha reduction dissipated across both the left and right hemispheres. However, when participants were preparing to attend to the global level, the reduction dissipated in the left hemisphere to a significantly greater degree than it did in the right hemisphere in the post-LSF condition. In the post-HSF condition, on the other hand, the reduction dissipated equally across the two hemispheres.
The unidirectional pattern of hemispheric modulation by SF suggests that the alpha modulation was not induced purely by processing low and high SFs but rather as an interaction between the relative frequency range selected in the grating and the preparation for the global or local task. This was further corroborated in the analysis comparing the preparatory interval before the Navon and the interval before the grating, in which we found a significant Analysis window (pre-Navon versus pre-grating) × Stimulus 1 (global versus local/LSF versus HSF) × Stimulus 2 (LSF versus HSF/global versus local) × hemisphere interaction. Thus, these data support a causal relationship between attentional selection of SF by the right hemisphere and the perception of hierarchical objects at the global level.
We did not find a significant opposite pattern, that is, alpha suppression was not larger over left hemisphere sites in preparation for the local level (e.g., Yamaguchi, Yamagata, & Kobayashi, 2000). Rather, although the main effect of hemisphere did not reach statistical significance in the analyses of preparatory alpha activity, there was greater alpha reduction in the right hemisphere than in left hemisphere in all conditions. Right hemisphere constrained modulation of alpha seems to be a robust phenomenon as it has been recently replicated in new studies in which attending to global or local levels in Navon stimuli modulated the congruency effect in the composite face illusion (Gao, Flevaris, Robertson, & Bentin, 2011). As in the present study, regardless of whether the Navon stimuli were processed at the local or the global level, the alpha reduction while preparing for the composite-face-matching task was larger over the right hemisphere than the left hemisphere. Note that this asymmetrical distribution of neural activity is in line with evidence suggesting right hemisphere dominance in visuospatial attention (e.g., studies of patients with unilateral lesions show more prolonged and severe attentional deficits following right hemisphere than left hemisphere damage; Becker & Karnath, 2007; Bowen, McKenna, & Tallis, 1999; Heilman, Watson, & Valenstein, 1985; Mesulam, 1999; Ringman et al., 2004). Further corroborating this idea, a number of neuroimaging studies have found greater right hemisphere activity associated with shifts of spatial attention and target detection (e.g., Gitelman et al., 1999; Nobre et al., 1997; Shulman et al., 2010). Finally, we should note that although not statistically significant in the alpha range, theta reduction was significantly greater over the right than the left hemisphere sites during the preparatory period preceding the Navon in the current experiment.
Although we did not find significantly greater alpha reduction in the left hemisphere than in the right hemisphere while preparing for local targets, the important point is that we observed a significant SF × level × hemisphere interaction. This is actually consistent with findings across the literature in global versus local processing as a whole, in which the simple effects are often not found to be significant for either the global or local task or both (e.g., Robertson et al., 1988; Weissman & Banich, 1999; Weissman & Woldorff, 2005). Instead, task × hemisphere interactions such as the ones found here have become the standard measures of hemispheric asymmetry (Hellige, 1983; Robertson et al., 1993; Weissman & Banich, 1999). In addition, it is consistent with proposals that posit a small bias one way or the other in terms of relative SFs, which appears in turn to operate in terms of a balance of activity between the two hemispheres (see Sergent, 1982).
Preparing for SF selection did not modulate theta activity. Akin to the alpha analyses, the only significant effects in the theta band occurred for the time interval preceding the Navon. We found greater overall theta reduction in the right than the left hemisphere, especially in the global preparation condition, as evidenced by the significant level × hemisphere interaction. This is consistent with findings associating theta oscillations with perceiving a global object in the context of an ambiguous image and, specifically, with the integration of LSF visual features in the experience of that global percept (Smith, Gosselin, & Schyns, 2006). However, that study did not examine preparatory EEG activity, and future research is needed to clarify the role of preparatory theta oscillations as they relate to global versus local processing.
In conclusion, the data from the current experiment are consistent with previous research showing that the hemispheric asymmetry in global versus local processing occurs not only during “bottom-up” perceptual processing of global versus local objects but also during “top-down” preparation for selecting those global/local objects (e.g., Volberg et al., 2009; Yamaguchi et al., 2000). The present data extend previous work by suggesting that attentional selection of spatial frequency modulates the relative neural activity in right and left hemispheres when involved in preparing for global or local processing.
Acknowledgments
This work was supported by an NIMH Grant to Lynn C. Robertson and Shlomo Bentin (Ro1 MH 64458). Lynn C. Robertson is a Senior Research Career Scientist in VA Clinical Sciences Research Service, Department of Veterans Affairs Medical Center, Martinez, CA. Anastasia V. Flevaris is now at Department of Neurosciences, UCSD.
Footnotes
Commercial relationships: none.
Given that our primary focus was on preparatory neural activity, it did not make sense to analyze oscillations in the beta (14–30 Hz) and gamma (30–60 Hz) bands, as activity in both of these frequency ranges generally occurs after stimulus onset and has been used as an index of visual perception (in the case of beta activity; Piantoni, Kline, & Eagleman, 2010; Smith et al., 2006) and is associated with higher level cognitive processes, including the integration of features into a coherent percept (in the case of gamma activity; Rodriguez et al., 1999; Tallon-Baudry & Bertrand, 1999; Zion-Golumbic & Bentin, 2007). Indeed, visual inspection of the spectral plots in the beta and gamma ranges did not show very much power in these ranges.
Contributor Information
Anastasia V. Flevaris, Department of Psychology, University of California at Berkeley, USA, & Veterans Administration Center, Martinez, CA, USA
Shlomo Bentin, Department of Psychology and the Center of Neural Computation, Hebrew University of Jerusalem, Jerusalem, Israel.
Lynn C. Robertson, Department of Psychology, University of California at Berkeley, USA, & Veterans Administration Center, Martinez, CA, USA
References
- Bastiaansen MC, Böcker KB, Brunia CH, de Munck JC, Spekreijse H. Event-related desynchronization during anticipatory attention for an upcoming stimulus: A comparative EEG/MEG study. Clinical Neurophysiology. 2001;112:393–403. doi: 10.1016/s1388-2457(00)00537-x. [DOI] [PubMed] [Google Scholar]
- Becker E, Karnath HO. Incidence of visual extinction after left versus right hemisphere stroke. Stroke. 2007;38:3172–3174. doi: 10.1161/STROKEAHA.107.489096. [DOI] [PubMed] [Google Scholar]
- Boeschoten MA, Kemner C, Kenemans JL, van Engeland H. The relationship between local and global processing and the processing of high and low spatial frequencies studied by event-related potentials and source modeling. Cognitive Brain Research. 2005;24:228–236. doi: 10.1016/j.cogbrainres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Bowen A, McKenna K, Tallis RC. Reasons for variability in the reported rate of occurrence of unilateral spatial neglect after stroke. Stroke. 1999;30:1196–1202. doi: 10.1161/01.str.30.6.1196. [DOI] [PubMed] [Google Scholar]
- Broadbent DE. The hidden preattentive processes. American Psychologist. 1977;32:109–118. doi: 10.1037//0003-066x.32.2.109. [DOI] [PubMed] [Google Scholar]
- Christman S, Kitterle FL, Hellige J. Hemispheric asymmetry in the processing of absolute versus relative spatial frequency. Brain and Cognition. 1991;16:62–73. doi: 10.1016/0278-2626(91)90085-m. [DOI] [PubMed] [Google Scholar]
- Delis DC, Robertson LC, Efron R. Hemispheric specialization of memory for visual hierarchical stimuli. Neuropsychologia. 1986;24:205–214. doi: 10.1016/0028-3932(86)90053-9. [DOI] [PubMed] [Google Scholar]
- De Valois RL, De Valois K. Spatial vision. New York: Oxford University Press; 1988. [Google Scholar]
- Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Neural mechanisms involved in the processing of global and local aspects of hierarchically organized visual stimuli. Brain. 1997;120:1779–1791. doi: 10.1093/brain/120.10.1779. [DOI] [PubMed] [Google Scholar]
- Flevaris AF, Bentin S, Robertson LC. Local or global? Attentional selection of spatial frequencies binds shapes to hierarchical levels. Psychological Science. 2010;21:424–431. doi: 10.1177/0956797609359909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flevaris AF, Bentin S, Robertson LC. Attention to hierarchical level influences attentional selection of spatial scale. Journal of Experimental Psychology: Human Perception and Performance. 2011;37:12–22. doi: 10.1037/a0019251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flevaris AV, Robertson LC, Bentin S. Using spatial frequency scales for processing face features and face configuration: An ERP analysis. Brain Research. 2008;1194:100–109. doi: 10.1016/j.brainres.2007.11.071. [DOI] [PubMed] [Google Scholar]
- Gao Z, Flevaris AV, Robertson LC, Bentin S. Revisiting processing-bias effects on the composite-face illusion: EEG evidence for predominant right-hemisphere involvement. Paper presented at the Visual Science Society 11th Annual Meeting; Naples, Florida, USA. 2011. [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, et al. A large-scale distributed network for covert spatial attention. Brain. 1999;122:1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Goffaux V, Hault B, Michel C, Vuong QC, Rossion B. The respective role of low and high spatial frequencies in supporting configural and featural processing of faces. Perception. 2005;34:77–86. doi: 10.1068/p5370. [DOI] [PubMed] [Google Scholar]
- Goffaux V, Rossion B. Faces are “spatial”—Holistic face perception is supported by low spatial frequencies. Journal of Experimental Psychology: Human Perception and Performance. 2006;32:1023–1039. doi: 10.1037/0096-1523.32.4.1023. [DOI] [PubMed] [Google Scholar]
- Han S, Weaver JA, Murray SO, Kang X, Yund EW, Woods DL. Hemispheric asymmetry in global/local processing: Effects of stimulus position and spatial frequency. Neuroimage. 2002;17:1290–1299. doi: 10.1006/nimg.2002.1255. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical neuropsychology. New York: Oxford; 1985. pp. 243–293. [Google Scholar]
- Hellige JB. Feature similarity and laterality effects in visual masking. Neuropsychologia. 1983;21:633–639. doi: 10.1016/0028-3932(83)90061-1. [DOI] [PubMed] [Google Scholar]
- Hübner R, Volberg G. The integration of object levels and their content: A theory of global/ local processing and related hemispheric differences. Journal of Experimental Psychology: Human Perception and Performance. 2005;31:520–541. doi: 10.1037/0096-1523.31.3.520. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Robertson LC. The two sides of perception. Cambridge, MA: The MIT Press; 1998. [Google Scholar]
- Jacobs RA, Kosslyn SM. Encoding shape and spatial relations: The role of receptive field size in coordinating complementary representations. Cognitive Science. 1994;18:361–386. [Google Scholar]
- Kitterle FL, Christman S, Hellige JB. Hemispheric differences are found in the identification, but not the detection, of low versus high spatial frequencies. Perception & Psychophysics. 1990;48:297–306. doi: 10.3758/bf03206680. [DOI] [PubMed] [Google Scholar]
- Kitterle FL, Hellige JB, Christman S. Visual hemispheric asymmetries depend on which spatial frequencies are task relevant. Brain & Cognition. 1992;20:308–314. doi: 10.1016/0278-2626(92)90023-f. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Hanslmayr S. EEG alpha oscillations: The inhibition-timing hypothesis. Progress in Brain Research. 2007;159:151–165. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. Journal of Neurophysiology. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- Lamb MR, Robertson LC, Knight RT. Attention and interference in the processing of global and local information: Effects of unilateral temporal–parietal junction lesions. Neuropsychologia. 1989;27:471–483. doi: 10.1016/0028-3932(89)90052-3. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Harley EM. How different spatial-frequency components contribute to visual information acquisition. Journal of Experimental Psychology: Human Perception and Performance. 2004;30:104–118. doi: 10.1037/0096-1523.30.1.104. [DOI] [PubMed] [Google Scholar]
- Martin M. Hemispheric specialization for local and global processing. Neuropsychologia. 1979;17:33–40. doi: 10.1016/0028-3932(79)90019-8. [DOI] [PubMed] [Google Scholar]
- Martinez A, Moses P, Frank L, Buxton R, Wong E, Stiles J. Hemispheric asymmetries in global and local processing: Evidence from fMRI. Neuroreport. 1997;8:1685–1689. doi: 10.1097/00001756-199705060-00025. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Spatial attention and neglect: Parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philosophical Transactions of the Royal Society B: Biological Sciences. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevorach C, Humphreys GW, Shalev L. Effects of saliency, not global dominance, in patients with left parietal damage. Neuropsychologia. 2005;44:307–319. doi: 10.1016/j.neuropsychologia.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Mevorach C, Humphreys GW, Shalev L. Opposite biases in salience-based selection for the left and right posterior parietal cortex. Nature Neuroscience. 2006;9:740–742. doi: 10.1038/nn1709. [DOI] [PubMed] [Google Scholar]
- Morrison DJ, Schyns PG. Usage of spatial scales for the categorization of faces, objects, and scenes. Psychonomic Bulletin & Review. 2001;8:434–469. doi: 10.3758/bf03196180. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiak RSJ, Frith CD. Functional localization of the system for visuospatial attention using positron emission tomography. Brain. 1997;120:515–553. doi: 10.1093/brain/120.3.515. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Neuper C. Event-related synchronization (ERS) in the alpha band—An electrophysiological correlate of cortical idling: A review. International Journal of Psychophysiology. 1996;24:39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- Piantoni G, Kline KA, Eagleman DM. Beta oscillations correlate with the probability of perceiving rivalrous visual stimuli. Journal of Vision. 2010;10(13):18, 1–11. doi: 10.1167/10.13.18. http://www.journalofvision.org/content/10/13/18. [DOI] [PubMed]
- Pineda JO, Oberman LM. What goads cigarette smokers to smoke? Neural adaptation and the mirror neuron system. Brain Research. 2006;1121:128–135. doi: 10.1016/j.brainres.2006.08.128. [DOI] [PubMed] [Google Scholar]
- Ringman JM, Manly R, Beschin N, Daini R, Haeske-Dewick H, Homberg V, et al. Frequency, risk factors, anatomy, and course of unilateral neglect in an acute stroke cohort. Neurology. 2004;63:468–474. doi: 10.1212/01.wnl.0000133011.10689.ce. [DOI] [PubMed] [Google Scholar]
- Robertson LC, Delis DC. ‘Part–whole’ processing in unilateral brain-damaged patients: Dysfunction of hierarchical organization. Neuropsychologia. 1986;24:363–370. doi: 10.1016/0028-3932(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Robertson LC, Ivry R. Hemispheric asymmetries: Attention to visual and auditory primitives. Current Directions in Psychological Science. 2000;9:59–63. [Google Scholar]
- Robertson LC, Lamb MR. Neuropsychological contributions to theories of part/whole organization. Cognitive Psychology. 1991;23:299–330. doi: 10.1016/0010-0285(91)90012-d. [DOI] [PubMed] [Google Scholar]
- Robertson LC, Lamb MR, Knight RT. Effects of lesions of temporal–parietal junction on perceptual and attentional processing in humans. Journal of Neuroscience. 1988;8:3757–3769. doi: 10.1523/JNEUROSCI.08-10-03757.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LC, Lamb MR, Zaidel E. Interhemispheric relations in processing hierarchical patterns: Evidence from normal and commissurotomized subjects. Neuropsychology. 1993;7:325–342. [Google Scholar]
- Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela F. Perception’s shadow: Long-distance synchronization of human brain activity. Nature. 1999;397:430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Hadjikhani N, Fischl B, Liu AK, Marrett S, Dale AM, et al. Local and global attention are mapped retinotopically in human occipital cortex. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2077–2082. doi: 10.1073/pnas.98.4.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schyns PG, Oliva A. Dr. Angry and Mr. Smile: When categorization flexibly modifies the perception of faces in rapid visual presentation. Cognition. 1999;69:243–265. doi: 10.1016/s0010-0277(98)00069-9. [DOI] [PubMed] [Google Scholar]
- Sergent J. The cerebral balance of power: Confrontation or cooperation? Journal of Experimental Psychology: Human Perception & Performance. 1982;8:253–272. doi: 10.1037//0096-1523.8.2.253. [DOI] [PubMed] [Google Scholar]
- Sergent J. Role of input in visual hemispheric asymmetries. Psychological Bulletin. 1983;93:481–512. [PubMed] [Google Scholar]
- Shulman GL, Pope DLW, Astafiev SV, McAvoiy MP, Snyder AZ, Corbetta M. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. Journal of Neuroscience. 2010;30:3640–3651. doi: 10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Sullivan MA, Gish K, Sakoda WJ. The role of spatial-frequency channels in the perception of local and global structure. Perception. 1986;15:259–273. doi: 10.1068/p150259. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Wilson J. SF and selective attention to local and global information. Perception. 1987;16:89–101. doi: 10.1068/p160089. [DOI] [PubMed] [Google Scholar]
- Smith ML, Gosselin F, Schyns PG. Perceptual moments of conscious visual experience inferred from oscillatory brain activity. Proceedings of the National Academy of Sciences. 2006;103:5626–5631. doi: 10.1073/pnas.0508972103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends in Cognitive Sciences. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. Journal of Neuroscience. 1998;18:4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascal-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. Journal of Neuroscience. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volberg G, Hübner R. On the role of response conflicts and stimulus position for hemispheric differences in global/local processing: An ERP study. Neurophsychologia. 2004;42:1805–1813. doi: 10.1016/j.neuropsychologia.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Volberg G, Kliegl K, Hansimayr S, Greenlee MW. EEG alpha oscillations in the preparation for global and local processing predicts behavioral performance. Human Brain Mapping. 2009;30:2173–2183. doi: 10.1002/hbm.20659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward LM. Synchronous neural oscillations and cognitive processes. Trends in Cognitive Sciences. 2003;7:553–559. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Watt RJ. Visual processing: Computational, psychophysical, and cognitive research. London: Erlbaum; 1988. [Google Scholar]
- Weissman DH, Banich MT. Global-local interference modulated by communication between the hemispheres. Journal of Experimental Psychology: General. 1999;128:283–308. doi: 10.1037//0096-3445.128.3.283. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Woldorff MG. Hemispheric asymmetries for different components of global/local attention occur in distinct temporo-parietal loci. Cerebral Cortex. 2005;15:870–876. doi: 10.1093/cercor/bhh187. [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. Journal of Neuroscience. 2000;20:RC63. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Yamagata S, Kobayashi S. Cerebral asymmetry of the “top-down” allocation of attention to global and local features. Journal of Neuroscience. 2000;20:RC72. doi: 10.1523/JNEUROSCI.20-09-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zion-Golumbic E, Bentin S. Dissociated neural mechanisms for face detection and configural encoding: Evidence from N170 and induced gamma-band oscillation effects. Cerebral Cortex. 2007;17:1741–1749. doi: 10.1093/cercor/bhl100. [DOI] [PubMed] [Google Scholar]