Abstract
Conscious visual perception of the constantly changing environment is one of the brain’s most critical functions. In virtually every moment of every daily activity, the visual system is confronted with the task of accurately representing and interpreting scenes that change rapidly over time. Adults can judge the identity and order of changing images presented at a rate of up to 10 Hz (~50 ms per image); this limit reflects a finite temporal resolution of attention. In the research reported here, although 6- to 15-month-old infants could detect the presence of rapid flicker without difficulty, their ability to segment individual alternating states within the flicker was severely limited: Fifteen-month-old infants had a temporal resolution of attention approximately one order of magnitude lower than that of adults (~1 Hz). Coarse temporal resolution constrains how infants perceive and utilize dynamic visual information and may play a role in the visual processing deficits found in individuals with neurodevelopmental disorders.
Keywords: temporal individuation, Gestalt flicker fusion, contrast sensitivity
Given the highly dynamic nature of the visual world, the ability to derive a temporally continuous and accurate percept is critical for survival. From driving a car, to crossing a street, to catching a ball, most everyday experiences require temporal perception so that one can form precise representations, anticipate relevant events, and plan and execute corresponding actions. The temporal resolution of the adult human visual system (i.e., the temporal interval over which the system can integrate or segregate information) varies with the information being processed: The temporal limit for mechanisms involved in the detection of flicker and low-level (e.g., luminance-based) motion is higher (up to 60 Hz) than the temporal limit for the conscious individuation of changing visual states (up to 10 Hz; Battelli, Pascual-Leone, & Cavanagh, 2007; Battelli, Walsh, Pascual-Leone, & Cavanagh, 2008; Holcombe, 2009). For example, neurotypical adults can perceive the flicker of fluorescent lights at a frequency of up to nearly 60 Hz, but cannot isolate and identify the individual flashes that compose the flicker beyond frequencies of about 10 Hz. As a result, it is impossible to judge whether two fluorescent lights flickering at frequencies greater than 10 Hz are in or out of phase relative to each other; in such cases, adult observers lose the ability to individuate light and dark phases and perceive Gestalt flicker fusion, or continuous flickering light (van de Grind, Grusser, & Lunkenheimer, 1973).
It has been suggested that the limiting factor that determines people’s ability to consciously perceive the identity of events occurring closely in time is the temporal resolution of visual attention. Temporal attention is required for individuating the components in a changing sequence of events (Battelli et al., 2007, 2008). For instance, as people drive, visual input changes from moment to moment as a result of their constant eye movements and ongoing motion in their surroundings. Despite these constant changes, drivers need to be able to identify individual objects in the environment (e.g., a pedestrian stepping into the crosswalk) as belonging to a distinct moment in time. If a pedestrian were not individuated in time—if the pedestrian’s presence were not recognized as occurring in the appropriate moment—drivers might misperceive when and where they saw him or her. Accurate identification of sequential objects and events is clearly essential for people’s coordination of any action in their environment, and the temporal resolution of attention is thought to be the limiting factor for such identification.
How well can infants resolve the identity of changing visual events? The early development of temporal visual attention is not yet understood. Knowledge of infants’ sensitivity to temporal change comes primarily from research demonstrating that infants have a visual preference for dynamic over static stimuli; this preference has made it possible to measure infants’ ability to detect or discriminate flicker or motion (Braddick & Atkinson, 2009). The minimum rate at which infants no longer show a visual preference for a high-contrast flickering stimulus paired with a static stimulus, or the critical flicker frequency, reaches a level comparable to that observed in adults (~55 Hz) among infants as young as 2 months of age (Dobkins, Anderson, & Lia, 1999; Dobkins, Lia, & Teller, 1997; Regal, 1981). Although infants have a reduced sensitivity to contrast relative to that of adults, their temporal contrast sensitivity reveals that they are still able to detect temporally modulated luminance information (e.g., moving gratings) as well as adults can by the age of 3 months, exhibiting a peak sensitivity to stimuli presented at 5 to 10 Hz (Dobkins et al., 1999; Dobkins & Teller, 1996; Hartmann & Banks, 1992; Rasengane, Allen, & Manny, 1997; Swanson & Birch, 1990; Teller, Lindsey, Mar, Succop, & Mahal, 1992). Research has demonstrated that infants have a temporal limit for the detection of flicker that is similar to that of adults, but the temporal resolution of infant attention has yet to be measured. The limit of temporal attention is not necessarily challenged or revealed by experiments that measure detection of visual flicker or auditory gaps (Smith, Trainor, & Shore, 2006; Werner, Marean, Halpin, Spetner, & Gillenwater, 1992), visual changes (Fletcher-Watson, Collis, Findlay, & Leekam, 2009; Oakes, Ross-Sheehy, & Luck, 2006; Shore, Burack, Miller, Joseph, & Enns, 2006), or the causal order of events (Friedman, 2002), because the tasks in these experiments can be performed either without temporally individuating objects or by relying on low-level cues (e.g., luminance transients).
Therefore, the question remains: What is the temporal resolution of infants’ conscious perception of dynamic events? Identifying this resolution is essential for a basic understanding of how infants use visual information to make sense of and act in the world—for example, for purposes of scene segmentation, motion perception, and motor coordination. In the study reported in this article, we determined the temporal resolution of visual attention in infants between the ages of 6 and 15 months, using eye tracking to psychophysically measure thresholds for individuating the phase of a flickering stimulus.
Experiment 1: Phase Individuation
Method
Subjects
Ninety-six healthy, full-term infants participated in this experiment. They included twenty-two 6-month-olds (mean age = 6 months 14 days; 15 boys and 7 girls), twenty-three 9-month-olds (mean age = 9 months 15 days; 13 boys and 10 girls), thirty-one 12-month-olds (mean age = 12 months 14 days; 20 boys and 11 girls), and twenty 15-month-olds (mean age = 15 months 19 days; 15 boys and 5 girls). An additional 7 infants were tested but excluded from the final analysis because their gaze data were not recorded on at least half the trials (4 subjects) or because their data could not be fitted to a psychometric function (3 subjects). Infants were recruited through flyers, letters to parents, and word of mouth in Davis, California. In addition, 4 adult undergraduate students (mean age = 21 years 3 months; 2 males, 2 females) participated for course credit. The institutional review board at the University of California, Davis, approved the experimental protocol, and informed consent was obtained for each subject.
Apparatus
The experiment was conducted in a darkened testing room. Stimuli were presented on a 17-in. LCD binocular eye-tracking monitor (Tobii Technology, Danderyd, Sweden; 1024 × 768 pixels, 50-Hz data capture rate, 60-Hz refresh rate). The luminance of the LCD display was gamma- corrected to minimize luminance nonlinearities. The task was programmed and presented using Presentation Version 11.3 (Neurobehavioral Systems, Albany, CA), and eye-tracking data were recorded using Clear View Version 2.7.1 (Tobii Technology).
Procedure
Infants were seated on a parent’s or caregiver’s lap, approximately 60 cm from the monitor. A five-point calibration routine was used to accurately estimate each infant’s gaze position. The task was a four-alternative forced-choice (4-AFC) preferential-looking paradigm that used the method of constant stimuli. Trials began with a fixation video of a dynamic toy, paired with a synchronized sound, that was presented at the center of the screen for 1 s. Immediately after the conclusion of this video (a 0-ms delay), four squares subtending 2.5° by 2.5° of visual angle were presented 5° to the left and right of, and above and below, the center, against a gray background (77.24 cd/m2). All four squares underwent square-wave flicker between white (133.8 cd/m2) and black (0.26 cd/m2) states. One of the squares was the target, chosen randomly from the four locations, and it flickered 180° out of phase with the three distractor squares (Fig. 1). For example, the target was always black when the distractors were white, and vice versa. Flickering occurred at one of four temporal frequencies: 0.2, 0.5, 1, or 2 Hz. At slower rates of flicker, the target square in the display can be more easily identified because individuation of the alternating black and white states is possible, but at frequencies above the threshold for phase individuation, all squares appear to be flickering identically (i.e., their phase cannot be isolated). Therefore, we predicted that infants would be able to perceive the target square and would show a visual preference for it only if they could individuate the phase of the squares. Trial duration was 5 s, and eight trials were presented at each temporal frequency, in random order.
Fig. 1.
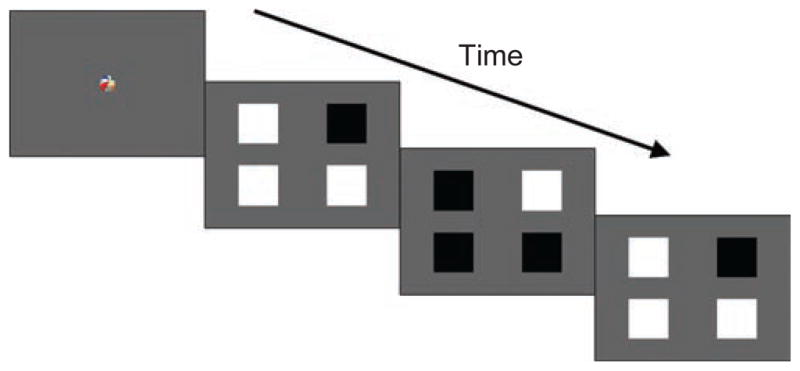
Schematic illustration of a trial in Experiment 1. From left to right, the figure shows a frame from the fixation video and three frames from the phase-individuation paradigm, in which four flickering squares, one of which flickered 180° out of phase with the others, were presented.
On the basis of previous research, we selected the following temporal frequencies for the flicker presented to the adult subjects: 0.1, 5, 7, and 10 Hz. Adult subjects were instructed to identify the quadrant of the screen containing the target square by pressing a key.
Data coding and threshold estimation
A trained observer coded the data off-line using Noldus Observer Version 5.0 (Noldus Information Technology, Leesburg, VA). A second observer coded a randomly chosen 25% of the test sessions, and the mean interobserver reliability was high (r = .97). Both coders were blind to the location of the target. A fixation was defined as a series of gaze points that occurred within a 0.76° radius for a minimum duration of 100 ms, and fixation position was coded by splitting the screen into four quadrants. Total fixation duration was used as the measure of time spent looking at each quadrant of the screen. For each trial, a target-preference score was calculated by dividing the time spent looking at the quadrant containing the target by the total time spent looking at the four quadrants. Target-preference scores ranged from 0 (never looked at the target) to 1 (looked only at the target), with .25 considered the chance level. For each infant, an average target-preference score was computed for each temporal frequency. To obtain individual phase- individuation thresholds, we fit a logistic function to the target-preference scores as a function of temporal frequency using the psignifit toolbox Version 2.5.6 (Wichmann & Hill, 2001) for MATLAB, applying maximum likelihood as the estimation procedure. For adults, an upper asymptote of 1 was employed. For infants, because the peak target-preference score was .58 at the slowest temporal frequency (0.2 Hz), the upper asymptote was fixed at .70, the value corresponding to twice the standard deviation of the mean target-preference score at 0.2 Hz, in order to improve the fit to the data (Dobkins et al., 1999). Threshold was defined as the temporal frequency yielding half the asymptotic performance: accuracy of .625 in adults and a target-preference score of .475 in infants. (Note that using this threshold level is equivalent to using the .75 threshold level for a typical 2-AFC task.) A bootstrapping technique that included 5,000 replications for each fitted function was used.
The distributions of thresholds in these replications were used to generate 95% confidence intervals for the threshold estimates. Individual threshold values were averaged across infants to calculate the temporal phase-individuation threshold for each age group.
Results and discussion
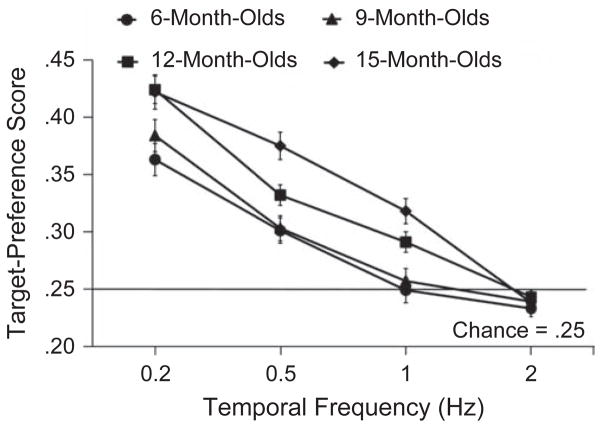
Figure 2 presents the average target-preference score as a function of temporal frequency for infants in each age group. A 4 (temporal frequency) × 4 (age group) repeated measures analysis of variance (ANOVA) revealed a significant main effect of temporal frequency, F(3, 90) = 148.27, p = .0001, η2 = 0.832, and a main effect of age group, F(3, 92) = 8.922, p = .0001, η2 = .225. Infants had significantly higher target-preference scores at lower temporal frequencies, and 12- and 15-month-old infants had significantly higher target-preference scores than did 6- and 9-month-old infants. In addition, the analysis revealed an interaction between temporal frequency and age group, F(9, 276) = 3.535, p = .0001, η2 = .103; temporal individuation improved with age. Two-tailed t tests (p < .05, Bonferroni corrected for multiple comparisons) were conducted to compare observed performance with chance-level performance (target-preference score of .25) at each temporal frequency and for each age group. Results confirmed that infants in all age groups exhibited a significant preference for the target square at flicker rates of 0.2 Hz—6-month-olds: t(21) = 8.03, p = .0001; 9-month-olds: t(22) = 8.58, p = .0001; 12-month-olds: t(30) = 17.21, p = .0001; 15-month-olds: t(19) = 11.003, p = .0001—and of 0.5 Hz—6-month-olds: t(21) = 3.87, p = .001; 9-month-olds: t(22) = 6.55, p = .0001; 12-month-olds: t(30) = 11.89, p = .0001; 15-month-olds: t(19) = 7.89, p = .0001. However, only 12-month-olds, t(30) = 3.19, p = .003, and 15-month-olds, t(19) = 5.08, p = .0001, showed a preference for the target when the flicker rate was 1 Hz. None of the age groups shows a preference for the target square when it flickered at a rate of 2 Hz—6-month-olds: t(21) = −0.127, p = .900; 9-month-olds: t(22) = −0.193, p = .690; 12-month-olds: t(30) = 0.853, p = .401; 15-month-olds: t(19) = 0.989, p = .61. In other words, younger infants were able to perceive and consciously individuate the alternating states of the squares only up to a flicker rate of 0.5 Hz, whereas older infants could do so up to a flicker rate of 1 Hz.
Fig. 2.
Results for infants in Experiment 1: mean target-preference score as a function of temporal frequency for each age group. Error bars indicate standard errors of the mean.
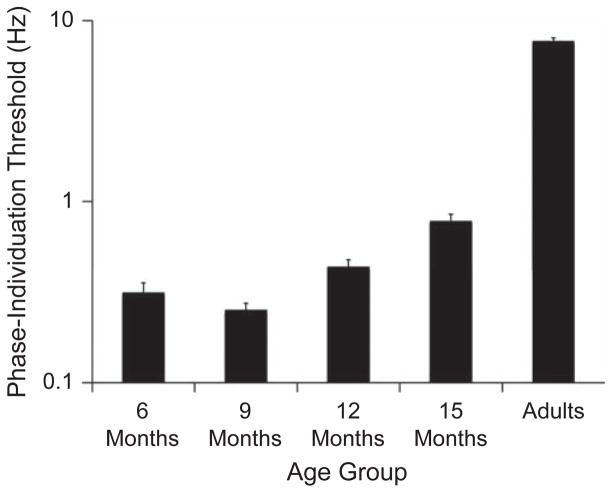
To more precisely evaluate the temporal limit of attention in infants in the four age groups, we examined the infants’ phase-individuation thresholds in a one-way ANOVA with age group as the independent factor. This analysis revealed a significant effect of age group, F(3, 93) = 21.00, p = .0001, η2 = .409, which reflected higher temporal-frequency thresholds (better performance) in the older infants (Fig. 3). Independent- samples t tests revealed significantly lower thresholds in 6-month-olds than in 12-month-olds, t(49) = −1.82, p = .056, and 15-month-olds, t(39) = −5.29, p = .0001, and significantly lower thresholds in 9-month-olds than in 12-month-olds, t(51) = −3.66, p = .001, and 15-month-olds, t(41) = −7.03, p = .0001. The thresholds for 6- and 9-month-olds did not differ significantly. The Spearman rank order correlation between age (in months) and phase-individuation threshold was positive (rs = .573, p = .0001), a result that further demonstrates the developmental trend and confirms that categorizing infants into age groups had no effect on the experimental results.
Fig. 3.
Results from Experiment 1: mean phase-individuation threshold on a log scale as a function of age group. Threshold was defined as the temporal frequency yielding accuracy of .625 in adults and a target-preference score of .475 in infants. A lower threshold signifies a lower resolution of temporal attention. Error bars indicate standard errors of the mean.
Results for the adults were consistent with previous reports of temporal limits of attention in adults. The phase-individuation thresholds obtained from the 4 adult subjects ranged from 7.07 to 8.42 Hz (M = 7.67 Hz, SD = 0.69). Thus, the stimuli used in our experiment tapped the same mechanisms of temporal attention that experiments described in previous publications did (Battelli, Cavanagh, Martini, & Barton, 2003; Battelli et al., 2008; van de Grind et al., 1973; Verstraten, Cavanagh, & Labianca, 2000).
The failure of younger infants to show a visual preference for the target square in displays with faster flicker cannot be explained by general inattention or difficulty perceiving the squares because overall looking time did not differ significantly across temporal frequencies, F(3, 90) = 1.371, p = .201, η2 = .024, or age groups, F(3, 92) = 1.781, p = .157, η2 = .097.
The results from this experiment demonstrate that the rate of alternation at which events can be individuated is dramatically reduced in infants, compared with adults; this reduced rate reflects a coarser temporal resolution of visual attention. Six- and 9-month-olds could individuate dynamic events only if they were presented at a rate of 0.5 Hz or slower, whereas infants older than the age of 9 months demonstrated a robust developmental improvement, exhibiting an ability to individuate that began to approach the level of adults (Fig. 3). This pattern is consistent with the developmental timeline of significant neuroanatomical and metabolic changes, such as increased myelination in cortical areas, that have been shown to increase the efficiency of neural processing (Kinney, Brody, Kloman, & Gilles, 1988).
Experiment 2: Flicker Contrast Detection
Detection of luminance transients generated during the contrast reversal of the flickering squares in Experiment 1 was a prerequisite for identifying the out-of-phase square. The findings of Experiment 1 might therefore be interpreted as reflecting young infants’ inability to perceive rapid luminance changes, perhaps because of immaturities within lower-level visual areas responsible for simple contrast detection. In Experiment 2, using a procedure similar to that of the control experiment Battelli et al. (2003) conducted with adults, we psychophysically measured infants’ contrast sensitivity for flicker at a temporal frequency of 10 Hz. On the basis of previous research, we expected our infant subjects to be able to detect flicker of a relatively high temporal frequency (Atkinson, Braddick, & Moar, 1977; Bosworth & Dobkins, 2009; Dobkins et al., 1999); confirming that they could do so at a flicker rate of 10 Hz in Experiment 2 would demonstrate that low-level temporal contrast sensitivity was not the limiting factor in Experiment 1.
Method
Subjects
Of the 96 infants who participated in Experiment 1, 61 also participated in Experiment 2. They included fifteen 6-month-olds (mean age = 6 months 5 days; 10 boys and 5 girls), sixteen 9-month-olds (mean age = 9 months 9 days; 8 boys and 8 girls), twenty 12-month-olds (mean age = 12 months 19 days; 13 boys and 7 girls), and ten 15-month-olds (mean age = 15 months 15 days; 8 boys and 2 girls). Eleven additional infants were tested but excluded because insufficient gaze data were recorded for threshold estimation. Experiments 1 and 2 were presented in a counterbalanced order and were completed in a single testing session.
Apparatus
The apparatus in this experiment was identical to that of Experiment 1. Stimuli were generated using the Vision Shell PPC graphics libraries (Comtois, 2003), and stimulus presentation was controlled by an Apple G4 Power Macintosh.
Procedure
Infants’ contrast sensitivity was determined using a 2-AFC preferential-looking paradigm that used the method of constant stimuli (for a description, see Farzin et al., 2008). The stimulus consisted of a vertically oriented Gabor patch with a single luminance-defined sinusoid; the Gabor patch subtended half of the monitor (12.8° × 9.6°). Spatial frequency of the sine wave was 0.2 cycles/°, and the phase of each Gabor reversed sinusoidally at a rate of 10 Hz (see Fig. S1a in the Supplemental Material available online). Four levels of Michelson contrast (the difference between the maximum and minimum luminance of the grating, divided by their sum) were presented (0.14, 0.19, 0.28, and 0.42); each level was presented on 10 trials, and trials were ordered randomly. The stimulus appeared on either the left or the right half of the screen (side was counterbalanced across trials) and was presented within a 3-s Gaussian window, fading in and out of view. The nonstimulus half of the screen was equiluminant gray. Between trials, a 1° attention-getter was presented to reorient infants’ fixation to the center of the screen.
Data coding and threshold estimation
Coding was the same as in Experiment 1, with the following exceptions. Fixation position was coded as left or right of center, and total fixation duration was used as the measure of time spent looking at the left and right sides of the screen. A visual-preference score for each trial was calculated by dividing the time spent looking at the side of the screen with the stimulus by the total time spent looking at both sides. Visual-preference scores ranged from 0 to 1, with .5 considered performance at the chance level. Trials in which no fixations occurred (on average, 6% of trials per infant) were considered missing trials and were not given a preference score or included in the final analysis. For each infant, a mean visual-preference score was calculated for each contrast level.
Contrast sensitivity was obtained by fitting a logistic function to individual infants’ average visual-preference scores as a function of contrast, using the psignifit toolbox for MAT-LAB. Threshold was defined as the contrast yielding a score of .75, and sensitivity was computed as the inverse of the threshold. Sensitivity values were log-transformed to conform to normal distributions (Graham, 1989).
Results and discussion
Visual-preference scores from all infants indicated that at the highest contrast presented (0.42), the flickering gratings were reliably perceived well above the chance level (M = .94, SD = .14). This finding confirmed that infants were able to detect flicker with near-perfect accuracy when the contrast was high. Overall, temporal contrast sensitivity did not differ across the age range from 6 to 15 months, F(3, 60) = 0.565, p = .640, η2 = .022 (see Fig. S1b in the Supplemental Material). This finding is consistent with studies that have demonstrated that the rate at which contrast sensitivity develops slows by age 6 months (Dobkins, Bosworth, & McCleery, 2009), and that luminance contrast sensitivity, in particular, is more closely tied to preprogrammed mechanisms than to visual experience (Bosworth & Dobkins, 2009).
The flickering squares used in Experiment 1 were at nearly 100% contrast, a level of contrast substantially higher than the contrast-detection thresholds (M = 28%, SD = .09) found in Experiment 2; thus, the infants must have been able to perceive the flicker in the squares presented in Experiment 1, even in the case of the squares that flickered at the fastest rate. We intentionally employed different stimuli and paradigms in Experiments 1 and 2 because the two experiments were designed to independently measure different levels of processing: low-level temporal contrast sensitivity and high-level attentional resolution. Therefore, we attribute the results of Experiment 1 to infants’ inability to temporally individuate the alternating states of the flicker as a consequence of their reduced resolution of temporal attention, rather than to limited visibility of the flickering stimuli.
General Discussion
The visual world is highly dynamic: Visual scenes are constantly changing as a result of the movement of objects and fast and frequent eye movements. Observers must therefore accurately and reliably assign identity to rapidly changing events. In adults, the ability to perceive and individuate changing events is limited to rates no faster than 7 to 10 Hz. This limit has been taken as a measure of the temporal resolution of visual attention (Battelli et al., 2001, 2007; Verstraten et al., 2000). The present study is the first to characterize the temporal resolution of visual attention in infants, as well as its developmental course.
We measured temporal frequency thresholds at which an out-of-phase flickering stimulus could be identified by infants ages 6 to 15 months. Our data establish that the resolution of temporal visual attention is strikingly poor in infants: Six- and 9-month-olds could individuate alternating states of flicker up to a rate of only 0.5 Hz, and the limit for 15-month-olds was a rate of 1 Hz. Thus, the temporal resolution of 15-month-olds was almost 8 times coarser than the resolution observed in adults presented with the same stimuli. Despite this reduced temporal resolution of attention, the infants were able to perceive the presence of rapid flicker at a rate of 10 Hz. These findings indicate that temporal attention develops more slowly than temporal vision, and that this protracted development is specific to the selection of individual event identity in time. Furthermore, the shape of the developmental function of infants’ temporal phase individuation was markedly different from that of the developmental function of contrast detection, showing a significant effect of age only for selection of the out-of-phase flicker; thus, individuation of temporal phase relies on a mechanism independent of low-level temporal resolution. We therefore conclude that the temporal resolution of visual attention is coarse in infancy and undergoes an extended period of development beyond the 1st year of life.
This coarse temporal resolution has implications for how infants interact with, and thereby learn from, their visual environment. Temporal segregation and integration of discrete events likely play a role in infants’ eye, head, and body movements during activities ranging from anticipating the trajectory of a moving object in order to plan and execute the timing of a reach, to perceptually binding synchronous temporal events (e.g., audible speech and mouth movements) across space. In the auditory modality, fine temporal resolution is known to be especially important during language development, as the rate of individual speech sounds needs to be processed so that one can detect and discriminate phonemes, words, and sentences (Jusczyk, Rosner, Reed, & Kennedy, 1989).
Converging findings from transcranial magnetic stimulation studies and studies of neurotypical adults and brain-lesion patients have shown that the temporal limit of visual attention is likely set at a high level in the visual system. These findings have led researchers to propose the existence of a “when” pathway that is localized in the right parietal lobe (Battelli et al., 2007, 2008). The “when” visual pathway has been characterized by its functional role in temporal processing of midrange timescales (50 ms–1 s). The perception of most immediate, ongoing visual events occurs over such midrange timescales (Battelli et al., 2007), which are considerably longer than the timescales for the localization of flicker (Holcombe, 2009) or sound (Mauk & Buonomano, 2004), for example, and considerably shorter than the timescales for cognitive temporal judgments, such as the experience of elapsed time.
Our findings concerning the development of temporal resolution of attention may bring researchers closer to understanding the functional development of the proposed “when” pathway and, more generally, the right parietal cortex. Little is known about when and how the parietal cortex develops, but some studies using positron emission tomography (Chiron et al., 1992; Chugani & Phelps, 1986) and MRI (Geidd et al., 1999) have provided evidence that parietal areas mature substantially between the ages of 3 and 6 months (Gilmore & Johnson, 1998), and that general changes in cortical thickness begin in the 1st year and extend into preadolescence (Greenough, Black, & Wallace, 1987). It is therefore possible that development of parietal cortex cytoarchitecture, including synaptic and axonal pruning and myelination, contributes to more stable connections between parietal cortex and other areas involved in temporal perception.
Our results may also advance the understanding of the possible consequences of delayed or atypical development of temporal visual attention. Abnormally coarse temporal resolution early in life likely has consequences for the development of visual functions that require precise temporal sensitivity, including motion perception, attention deployment, and tracking. These processes have been reported to be impaired in multiple neurodevelopmental disorders, including fragile X syndrome, Williams syndrome, and autism (Atkinson et al., 1997; Farzin et al., 2008; Kaiser & Shiffrar, 2009; Kogan et al., 2004). Further studies are needed to investigate the relationship between temporal visual attention and the atypical development of perceptual, cognitive, and motor skills characteristic of individuals with these and other disorders.
Supplementary Material
Acknowledgments
We are grateful to Patrick Cavanagh for valuable discussions, Jason Fischer for programming of Experiment 2, and Emilio Ferrer and Kami Koldewyn for helpful comments on the manuscript. Portions of these data were presented at the 2009 annual meeting of the Vision Sciences Society, held in May in Naples, Florida.
Funding
This work was supported by National Institutes of Health Grants F31MH083386 (to F. Farzin) and EY018216 (to D. Whitney) and by National Science Foundation Grant 0748689 (to D. Whitney).
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Additional supporting information may be found at http://pss.sagepub.com/content/by/supplemental-data
References
- Atkinson J, Braddick O, Moar K. Contrast sensitivity of the human infant for moving and static patterns. Vision Research. 1977;17:1045–1047. doi: 10.1016/0042-6989(77)90008-6. [DOI] [PubMed] [Google Scholar]
- Atkinson J, King J, Braddick O, Nokes L, Anker S, Braddick F. A specific deficit of dorsal stream function in Williams’ syndrome. Neuro Report. 1997;8:1919–1922. doi: 10.1097/00001756-199705260-00025. [DOI] [PubMed] [Google Scholar]
- Battelli L, Cavanagh P, Intriligator J, Tramo MJ, Hénaff MA, Michèl F, Barton JJ. Unilateral right parietal damage leads to bilateral deficit for high-level motion. Neuron. 2001;32:985–995. doi: 10.1016/s0896-6273(01)00536-0. [DOI] [PubMed] [Google Scholar]
- Battelli L, Cavanagh P, Martini P, Barton JJ. Bilateral deficits of transient visual attention in right parietal patients. Brain. 2003;12:2164–2174. doi: 10.1093/brain/awg221. [DOI] [PubMed] [Google Scholar]
- Battelli L, Pascual-Leone A, Cavanagh P. The ‘when’ pathway of the right parietal lobe. Trends in Cognitive Sciences. 2007;11:204–210. doi: 10.1016/j.tics.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelli L, Walsh V, Pascual-Leone A, Cavanagh P. The ‘when’ parietal pathway explored by lesion studies. Current Opinion in Neurobiology. 2008;18:120–126. doi: 10.1016/j.conb.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosworth RG, Dobkins KR. Chromatic and luminance contrast sensitivity in fullterm and preterm infants. Journal of Vision. 2009;9(13):Article 15. doi: 10.1167/9.13.15. http://www.journalofvision.org/content/9/13/15. [DOI] [PMC free article] [PubMed]
- Braddick OJ, Atkinson J. Infants’ sensitivity to motion and temporal change. Optometry and Vision Science. 2009;86:577–582. doi: 10.1097/OPX.0b013e3181a76e84. [DOI] [PubMed] [Google Scholar]
- Chiron C, Raynaud C, Mazière B, Zilbovicius M, Laflamme L, Masure M, Syrota A. Changes in regional cerebral blood flow during brain maturation in children and adolescents. Journal of Nuclear Medicine. 1992;33:696–703. [PubMed] [Google Scholar]
- Chugani HT, Phelps ME. Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science. 1986;231:840–843. doi: 10.1126/science.3945811. [DOI] [PubMed] [Google Scholar]
- Comtois R. Vision Shell PPC [Software libraries] Water-town, MA: Author; 2003. [Google Scholar]
- Dobkins KR, Anderson CM, Lia B. Infant temporal contrast sensitivity functions (tCSFs) mature earlier for luminance than for chromatic stimuli: Evidence for precocious magnocellular development? Vision Research. 1999;39:3223–3229. doi: 10.1016/s0042-6989(99)00020-6. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Bosworth RG, McCleery JP. Effects of gestational length, gender, postnatal age, and birth order on visual contrast sensitivity. Journal of Vision. 2009;9(10):Article 19. doi: 10.1167/9.10.19. Retrieved from http://calendar.arvo.org/9/10/19/article.aspx. [DOI] [PMC free article] [PubMed]
- Dobkins KR, Lia B, Teller DY. Infant color vision: Temporal contrast sensitivity functions for chromatic (red/green) stimuli in 3-month-olds. Vision Research. 1997;37:2699–2716. doi: 10.1016/s0042-6989(97)81180-7. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Teller DY. Infant contrast detectors are selective for direction of motion. Vision Research. 1996;36:281–294. doi: 10.1016/0042-6989(95)00094-g. [DOI] [PubMed] [Google Scholar]
- Farzin F, Whitney D, Hagerman RJ, Rivera SM. Contrast detection in infants with fragile X syndrome. Vision Research. 2008;48:1471–1478. doi: 10.1016/j.visres.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher-Watson S, Collis JM, Findlay JM, Leekam SR. The development of change blindness: Children’s attentional priorities whilst viewing naturalistic scenes. Developmental Science. 2009;12:438–445. doi: 10.1111/j.1467-7687.2008.00784.x. [DOI] [PubMed] [Google Scholar]
- Friedman WJ. Arrows of time in infancy: The representation of temporal-causal invariances. Cognitive Psychology. 2002;44:252–296. doi: 10.1006/cogp.2001.0768. [DOI] [PubMed] [Google Scholar]
- Geidd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nature. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gilmore RO, Johnson MH. Learning what is where: Oculomotor contributions to the development of spatial cognition. In: Simion F, Butterworth G, editors. The development of sensory, motor and cognitive capacities in early infancy: From perception to cognition. Hove, England: Psychology Press; 1998. pp. 25–48. [Google Scholar]
- Graham NVS. Visual pattern analyzers. New York, NY: Oxford University Press; 1989. [Google Scholar]
- Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Development. 1987;58:539–559. [PubMed] [Google Scholar]
- Hartmann EE, Banks MS. Temporal contrast sensitivity in human infants. Vision Research. 1992;32:1163–1168. doi: 10.1016/0042-6989(92)90018-e. [DOI] [PubMed] [Google Scholar]
- Holcombe AO. Seeing slow and seeing fast: Two limits on perception. Trends in Cognitive Sciences. 2009;13:216–221. doi: 10.1016/j.tics.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Jusczyk PW, Rosner BS, Reed MA, Kennedy LJ. Could temporal order differences underlie 2-month-olds’ discrimination of English voicing contrasts? Journal of the Acoustic Society of America. 1989;85:1741–1749. doi: 10.1121/1.397963. [DOI] [PubMed] [Google Scholar]
- Kaiser MD, Shiffrar M. The visual perception of motion by observers with autism spectrum disorders: A review and synthesis. Psychonomic Bulletin & Review. 2009;16:761–777. doi: 10.3758/PBR.16.5.761. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Brody A, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. Journal of Neuropathology & Experimental Neurology. 1988;47:217. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- Kogan CS, Bertone A, Cornish K, Boutet I, Der Kaloustian VM, Andermann E, Chaudhuri A. Integrative cortical dysfunction and pervasive motion perception deficit in fragile X syndrome. Neurology. 2004;63:1634–1639. doi: 10.1212/01.wnl.0000142987.44035.3b. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Buonomano DV. The neural basis of temporal processing. Annual Review of Neuroscience. 2004;27:307–340. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- Oakes LM, Sheehy S, Ross-Luck SJ. Rapid development of feature binding in visual short-term memory. Psychological Science. 2006;17:781–787. doi: 10.1111/j.1467-9280.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Rasengane TA, Allen D, Manny RE. Development of temporal contrast sensitivity in human infants. Vision Research. 1997;37:1747–1754. doi: 10.1016/s0042-6989(96)00300-8. [DOI] [PubMed] [Google Scholar]
- Regal DM. Development of critical flicker frequency in human infants. Vision Research. 1981;21:549–555. doi: 10.1016/0042-6989(81)90100-0. [DOI] [PubMed] [Google Scholar]
- Shore DI, Burack JA, Miller D, Joseph S, Enns JT. The development of change detection. Developmental Science. 2006;9:490–497. doi: 10.1111/j.1467-7687.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- Smith AN, Trainor LJ, Shore DI. The development of temporal resolution: Between-channel gap detection in infants and adults. Journal of Speech, Language, and Hearing Research. 2006;49:1104–1113. doi: 10.1044/1092-4388(2006/079). [DOI] [PubMed] [Google Scholar]
- Swanson WH, Birch EE. Infant spatiotemporal vision: Dependence of spatial contrast sensitivity on temporal frequency. Vision Research. 1990;30:1033–1048. doi: 10.1016/0042-6989(90)90113-y. [DOI] [PubMed] [Google Scholar]
- Teller DY, Lindsey DT, Mar CM, Succop A, Mahal MR. Infant temporal contrast sensitivity at low temporal frequencies. Vision Research. 1992;32:1157–1162. doi: 10.1016/0042-6989(92)90017-d. [DOI] [PubMed] [Google Scholar]
- van de Grind WA, Grusser OJ, Lunkenheimer HU. Temporal transfer properties of the afferent visual system: Psychophysical, neurophysiological and theoretical investigations. In: Jung R, editor. Handbook of sensory physiology. Berlin, Germany: Springer; 1973. pp. 431–573. [Google Scholar]
- Verstraten FAJ, Cavanagh P, Labianca A. Limits of attentive tracking reveal temporal properties of attention. Vision Research. 2000;40:3651–3664. doi: 10.1016/s0042-6989(00)00213-3. [DOI] [PubMed] [Google Scholar]
- Werner LA, Marean GC, Halpin CF, Spetner NB, Gillen-water JM. Infant auditory temporal acuity: Gap detection. Child Development. 1992;63:260–272. [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: II. Bootstrap-based confidence intervals and sampling. Perception & Psychophysics. 2001;63:1314–1329. doi: 10.3758/bf03194545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.