Abstract
The etiology of radiation-induced cerebrovascular rare-faction remains unknown. In the present study, we examined the effect of whole-brain irradiation on endothelial cell (EC) proliferation/apoptosis and expression of various angiogenic factors in rat brain. F344×BN rats received either whole-brain irradiation (a single dose of 10 Gy γ rays) or sham irradiation and were maintained for 4, 8 and 24 h after irradiation. Double immunofluorescence staining was employed to visualize EC proliferation/apoptosis in brain. The mRNA and protein expression levels of vascular endothelial growth factor (VEGF), angiopoietin-1 (Ang-1), endothelial-specific receptor tyrosine kinase (Tie-2), and Ang-2 in brain were determined by real-time RT-PCR and immunofluores-cence staining. A significant reduction in CD31-immunore-active cells was detected in irradiated rat brains compared with sham-irradiated controls. Whole-brain irradiation significantly suppressed EC proliferation and increased EC apoptosis. In addition, a significant decrease in mRNA and protein expression of VEGF, Ang-1 and Tie-2 was observed in irradiated rat brains. In contrast, whole-brain irradiation significantly upregulated Ang-2 expression in rat brains. The present study provides novel evidence that whole-brain irradiation differentially affects mRNA and protein expression of VEGF, Ang-1, Tie-2 and Ang-2. These changes are closely associated with decreased EC proliferation and increased EC apoptosis in brain.
INTRODUCTION
Radiation therapy is an important intervention in controlling tumor growth and increasing survival rate of brain tumor patients (1). According to the Central Brain Tumor Registry of the United States (CBTRUS), approximately 200,000 patients with brain tumors are treated with either partial large-field or whole-brain irradiation each year (2). Whole-brain radiation therapy may, however, result in significant reductions in learning and memory in brain tumor patients as long-term consequences of treatment (1, 3, 4). At present, there are no known successful treatments or effective preventive strategies for mitigating radiation-induced brain injury. Although a number of in vitro and in vivo studies have demonstrated the pathogenesis of radiation-mediated brain injury (5-10), the cellular and molecular mechanisms by which radiation induces damage to normal tissue in brain remain largely unknown.
Vessel rarefaction, defined as a decrease in vascular density, has been implicated in the onset and progression of various pathological processes (11-13). Previous studies have shown that radiation induces both acute and late changes in the vasculature (14-16). For example, Ljubimova et al. (14) found a decrease in EC density in rat brain within 24 h after large single doses of radiation. In addition, an initial marked decline and a subsequent slow loss of EC numbers were observed after 24 h and between 26 and 52 weeks after a single dose of radiation to the rat brain (17). A substantial decrease in vessel density and length was also detected in irradiated rat brains 10 weeks after fractionated whole-brain irradiation (5). These results suggest that radiation-induced early and persistent damage to the microvasculature may be responsible for vessel rarefaction leading to late-onset brain injury. The molecular mechanisms of radiation-induced vessel rarefaction in brain, however, remain to be further investigated.
The vascular system is characterized by a dynamic temporally and spatially coordinated interaction among EC, angiogenic factors and surrounding extracellular matrix proteins (18). A controlled balance between VEGF, a prototypical angiogenesis factor, and a new class of angiogenic regulators, including Ang-1, Tie-2 and Ang-2, is essential for vessel rarefaction and growth (19). However, the potential contribution of these angiogenic factors to radiation-induced vessel rarefaction in brain has not yet been explored.
In the present study, we demonstrate for the first time that differential expression of VEGF, Ang-1, Tie-2 and Ang-2 is associated with a decrease in EC proliferation and an increase in EC apoptosis in irradiated brain that ultimately contributed to cerebrovascular rarefaction.
MATERIALS AND METHODS
Animals
Four-month-old Fischer 344-Brown Norway (F344×BN) male rats were purchased from Harlan Laboratories (Indianapolis, IN). Animals were housed on a 12/12-h light-dark cycle with food and water provided ad libitum. Animal care was conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, and the study was approved by the Institutional Animal Care and Use Committee.
Whole-Brain Irradiation and Tissue Sample Preparation
After an acclimatization period of 1 week, the animals received either whole-brain irradiation or sham irradiation. Rats were anesthetized by intraperitoneal injection of a 350-μl mixture of ketamine/xylazine (80/12 mg/kg body weight). Whole-brain irradiation was performed in a 12,000 Ci (444 TBq) self-shielded 137Cs γ irradiator (Gammacell 40 Exactor, Nordion International Inc., Kanata, Ontario, Canada) using lead and Cerrobend shielding devices to collimate the beam so that the whole rat brain including the brain stem was irradiated. Dosimetry was performed using thermoluminescence dosimeters placed in the skull of dead rats and confirmed with ionization chambers in tissue-equivalent phantoms. Rats received 10 Gy at an average dose rate of 4.23 Gy/min. To ensure that each rat received the same midline brain irradiation, half the dose (5 Gy) was delivered to each side of the head. Control rats were anesthetized but not irradiated. The animals were returned to their home cages and euthanized by rapid decapitation 4, 8 and 24 h postirradiation. For real-time RT-PCR, the brains were rapidly removed, immediately frozen in liquid nitrogen, and stored at −80°C until analysis. For immunofluorescence staining, rats were given ketamine/xylazine (80/12 mg/kg body weight) immediately prior to perfusion. Animals were then transcardially perfused with ice-cold phosphate-buffered saline (PBS) containing 6 U/ml heparin and the brains were rapidly removed, immediately frozen in liquid nitrogen, and stored at −80°C until analysis.
Based on previous studies from our laboratories and others (7-9, 20-26), a rat model of whole-brain irradiation with a single dose of 10 Gy was chosen for three reasons: (1) it is known as the lowest dose to have a biological effect, (2) it is well below the threshold for vascular changes, demyelination or radionecrosis, and (3) it is close to a clinically relevant dose in humans since the rat brain is more resistant to radiation injury than the human brain.
Double Immunofluorescence Staining
For assessment of EC proliferation, sections were stained for rabbit anti-Ki67 monoclonal antibody (Abcam, Cambridge, MA) and mouse anti-CD31 (PECAM-1) monoclonal antibody (BD Pharmingen, San Jose, CA) followed by Alexa Fluor 555-conjugated goat anti-rabbit IgG and Alexa Fluor 488-conjugated donkey anti-mouse IgG. For detection of EC apoptosis, sections were incubated with rabbit anti-cleaved caspase-3 polyclonal antibody (Cell Signaling Technology, Inc., Danvers, MA) and mouse anti-CD31 monoclonal antibody before staining with Alexa Fluor 555-conjugated goat anti-rabbit IgG and Alexa Fluor 488-conjugated donkey anti-mouse IgG. After washing with PBS, sections were mounted in Vectashield mounting medium (Vector Laboratories Inc., Burlingame, CA) and examined on a Zeiss AXIO Imager A1m fluorescence microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY). Images were acquired with a 100× objective by AxioCam MRc5 Digital Imaging System. The fluorescence intensity of acquired digital images was quantified by ImageJ software (NIH, Bethesda, MD).
Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Quantitative real-time RT-PCR using fluorogenic 5′nuclease assay technology with TaqMan® probes and primers (Applied Biosystems, Foster City, CA) was employed for gene expression analyses. Rat brains were homogenized with 1 ml of TRI Reagent (Sigma-Aldrich, St. Louis, MO) in a tissue homogenizer, and total RNA was isolated from tissue homogenates as described previously (27). Then 1 μg of total RNA was reverse transcribed at 25°C for 15 min, 42°C for 45 min, and 99°C for 5 min in 20 μl of 5 mM MgCl2, 10 mM Tris-HCl, pH 9.0, 50 mM KCl, 0.1% Triton X-100, 1 mM dNTP, 1 U/μl of recombinant RNasin, 15 U/μg of Avian Myeloblastosis Virus (AMV) reverse transcriptase, and 0.5 μg of random hexamers. Amplification of individual genes was performed on the Applied Biosystems 7300 Real-Time PCR System using TaqMan® Universal PCR Master Mix and a standard thermal cycler protocol (50°C for 2 min before the first cycle, 95°C for 15 s and 60°C for 1 min, repeated 45 times). TaqMan® Gene Expression Assay Reagents for rat VEGF, Ang-1, Tie-2, Ang-2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used for specific probes and primers of PCR amplifications. The threshold cycle (CT), which indicates the fractional cycle number at which the amount of amplified target gene reaches a fixed threshold, was determined for each well using the Applied Biosystems Sequence Detection Software v1.2.3, and relative quantification was calculated by the comparative CT method as described previously (28, 29). The data were analyzed using the equation 2−ΔΔCT, where ΔΔCT = [CT of target gene − CT of housekeeping gene]treated group − [CT of target gene − CT of housekeeping gene]untreated control group. For the treated samples, evaluation of 2−ΔΔCT indicates the relative change in gene expression, normalized to a housekeeping gene (GAPDH), compared to the untreated control.
Immunofluorescence Staining
Frozen brain tissues were cut into 20-μm sections using a Microm HM 550 cryostat (MICROM International GmbH, Walldorf, Germany) and mounted on Superfrost/Plus microscope slides (Fisher Scientific, Pittsburgh, PA). Sections were fixed in 4% paraformaldehyde for 15 min at room temperature, rinsed with PBS, and incubated in 0.5% Triton X-100 for 15 min. After washing with PBS, sections were incubated with 3% bovine serum albumin (BSA) in PBS for 1 h at room temperature, followed by incubation with primary antibody, e.g., mouse anti-VEGF monoclonal antibody, goat anti-Ang-1 polyclonal antibody, rabbit anti-Tie-2 polyclonal antibody, or goat anti-Ang-2 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), diluted in 1.5% BSA, overnight at 4°C. Sections were then washed with PBS and incubated with secondary antibody, including donkey anti-mouse IgG conjugated with Alexa Fluor 488, donkey anti-goat IgG conjugated with Alexa Fluor 488, or goat anti-rabbit IgG conjugated with Alexa Fluor 555, diluted 1/400 in PBS, in the dark for 1 h. After washing with PBS, sections were mounted in Vectashield mounting medium and examined on a Zeiss AXIO Imager A1m fluorescence microscope. Images were acquired with a 100×objective by AxioCam MRc5 Digital Imaging System. The fluorescence intensity of acquired digital images was quantified by ImageJ software.
Statistical Analysis
Statistical analysis of data was completed using SigmaStat 3.5 (SPSS Inc., Chicago, IL). One-way analysis of variance (ANOVA) was used to compare mean responses among the treatments. For each end point, the treatment means were compared using the Bonferroni least significant difference procedure. A statistical probability of P < 0.05 was considered significant.
RESULTS
Effect of Whole-Brain Irradiation on EC Proliferation and EC Apoptosis in Rat Brain
To investigate whether whole-brain irradiation affects EC density in brain, immunofluorescence staining for CD31, a specific marker for EC, was performed. As shown in Fig. 1 and Fig. 2, significantly reduced levels of CD31-immunoreactive cells were detected in irradiated rat brains compared with sham-irradiated controls, indicating that whole-brain irradiation decreases EC density in brain. Double immunofluorescence staining for CD31 and Ki67 (cell proliferation marker) was performed to identify proliferating EC in brain. Large numbers of double-immunoreactive cells (white arrows; proliferating EC) were observed in sham-irradiated control brains (Fig. 1C). The number of proliferating EC, however, dramatically decreased in rat brains 4 and 8 h after irradiation (Fig. 1F and I) and barely proliferating EC were detectable in rat brains 24 h postirradiation (Fig. 1L). In addition, double immunofluorescence staining for CD31 and cleaved caspase-3 (an apoptotic cell marker) was conducted to measure apoptotic EC in brain. In sham-irradiated control brains, the colocalization of CD31 and cleaved caspase-3 (white arrows; apoptotic EC) was sparse (Fig. 2C). In contrast, more apoptotic EC were observed in rat brains 4 h after irradiation (Fig. 2F), and this level of activity was maintained at 8 and 24 h postirradiation (Fig. 2I and L). Quantitative analysis demonstrated that whole-brain irradiation significantly decreased EC proliferation (Fig. 1N) and increased EC apoptosis (Fig. 2N) in rat brains. These results suggest that the radiation-induced decrease in EC proliferation and increase in EC apoptosis may be responsible for a reduction of EC density in irradiated rat brains.
FIG. 1.
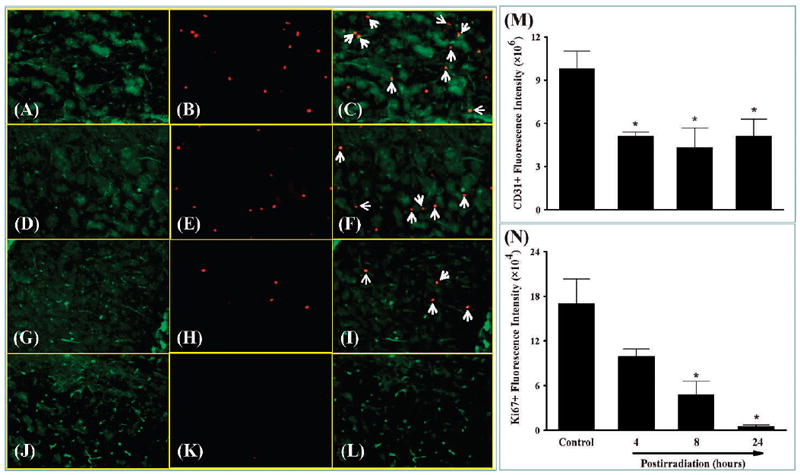
Effect of whole-brain irradiation on endothelial cell proliferation in rat brain. Representative images show double immunofluorescence staining for CD31 in green and Ki67 in red (panels A–L). Whole-brain irradiation resulted in a marked decrease in the fluorescence intensity of CD31 labeling of endothelial cells (panels A, D, G and J). Ki67 labeling of proliferating cells was dramatically reduced in irradiated rats (panels B, E, H and K). CD31+/Ki67+ proliferating endothelial cells (white arrows) were more abundant in sham-irradiated controls compared with irradiated rats (panels C, F, I and L). Quantitative analysis indicated that radiation significantly reduced proliferating endothelial cells in rat brains compared with sham-irradiated controls (panels M and N). Data shown are means ± SEM for each group (n = 4). *Statistically significant difference from control (P < 0.05). Panels A–C: Sham irradiation (Control); panels D–F: 4 h postirradiation; panels G–I: 8 h postirradiation; panels J–L: 24 h postirradiation. Panels A, D, G and J: green staining of CD31-positive immunoreactivity; panels B, E, H and K: red staining of Ki67-positive immunoreactivity; panels C, F, I and L: merged images of the green and red fluorescence staining. Original magnification of the images in panels A–L is 100×.
FIG. 2.
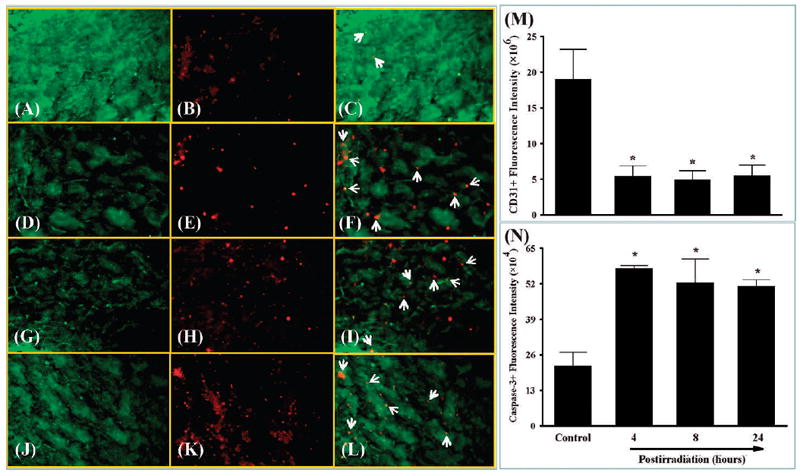
Effect of whole-brain irradiation on endothelial cell apoptosis in rat brain. Representative images show double immunofluorescence staining for CD31 in green and cleaved caspase-3 in red (panels A–L). Whole-brain irradiation resulted in a marked decrease in the fluorescence intensity of CD31 labeling of endothelial cells (panels A, D, G and J). Cleaved caspase-3 labeling of apoptotic cells was dramatically increased in irradiated rats (panels B, E, H and K). CD31+/cleaved caspase-3+ apoptotic endothelial cells (white arrows) were more abundant in irradiated rats than in sham-irradiated controls (panels C, F, I and L). Quantitative analysis indicated that radiation significantly increased endothelial cell apoptosis in rat brains compared with sham-irradiated controls (panels M and N). Data shown are means ± SEM for each group (n = 4). *Statistically significant difference from control (P < 0.05). Panels A–C: sham irradiation (Control); panels D–F: 4 h postirradiation; panels G–I: 8 h postirradiation; panels J–L: 24 h postirradiation. Panels A, D, G and J: green staining of CD31-positive immunoreactivity; panels B, E, H and K: red staining of cleaved caspase-3-positive immunoreactivity; panels C, F, I and L: merged images of the green and red fluorescence staining. Original magnification of the images in panels A–L is 100×.
Effect of Whole-Brain Irradiation on Expression of Angiogenic Factors in Rat Brain
To examine whether whole-brain irradiation affects expression of angiogenic factors in brain, mRNA expression levels of several angiogenic factors, including VEGF, Ang-1, Tie-2 and Ang-2, were analyzed by quantitative real-time RT-PCR. As shown in Fig. 3A, VEGF mRNA expression was significantly suppressed in rat brains 8 h (0.69-fold) and 24 h (0.58-fold) postirradiation compared with sham-irradiated controls. In addition, whole-brain irradiation resulted in a significant reduction in Ang-1 mRNA expression in rat brains 8 h (0.66-fold) after irradiation (Fig. 3B). A significant downregulation of Tie-2 mRNA expression was also observed in rat brains 4 h (0.62-fold) and 8 h (0.39-fold) postirradiation (Fig. 3C). In contrast, a significant and dramatic upregulation of Ang-2 mRNA expression was detected in rat brains 4 h postirradiation (2.5-fold), reached a maximum at 8 h postirradiation (10.4-fold), and was maintained at significantly higher levels at 24 h postirradiation (3.5-fold) compared with sham-irradiated controls (Fig. 3D). The mRNA expression levels of GAPDH (a housekeeping gene) were not affected by whole-brain irradiation (data not shown).
FIG. 3.
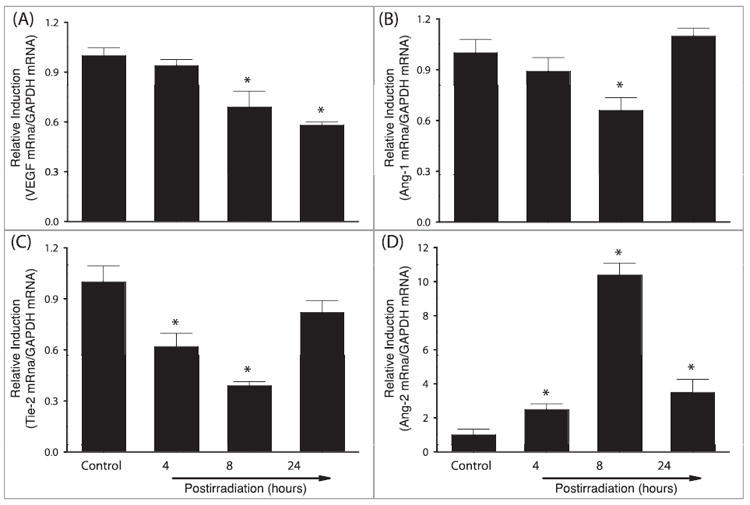
Effect of whole-brain irradiation on mRNA expression of VEGF, Ang-1, Tie-2 and Ang-2 in rat brain. Compared with sham-irradiated controls, radiation significantly downregulated mRNA expression levels of VEGF (panel A), Ang-1 (panel B) and Tie-2 (panel C) in rat brains. In contrast, mRNA expression levels of Ang-2 were upregulated by radiation (panel D). Data shown are means ± SEM for each group (n = 4). *Statistically significant difference from control (P < 0.05).
A series of immunofluorescence staining was conducted to determine whether radiation-mediated changes in mRNA expression levels of angiogenic factors were associated with protein expression in rat brains. Strong VEGF-positive immunoreactivity was detected in sham-irradiated control rat brains, whereas significantly reduced VEGF protein levels were found in brains 4, 8 and 24 h postirradiation. Quantitative analysis demonstrated a significant reduction in VEGF protein expression in rat brains 4, 8 and 24 h after irradiation compared with sham-irradiated controls (Fig. 4). In addition, whole-brain irradiation resulted in a significant decrease in protein expression levels of Ang-1 in rat brains 8 and 24 h postirradiation (Fig. 5) and Tie-2 in rat brains 4, 8 and 24 h postirradiation (Fig. 6) compared with sham-irradiated controls. In contrast, a marked increase in Ang-2-positive immunoreactivity was observed in rat brains 8 and 24 h after irradiation compared with sham-irradiated controls (Fig. 7). These results demonstrate that whole-brain irradiation differentially affects mRNA and protein expression of angiogenic factors in brain.
FIG. 4.
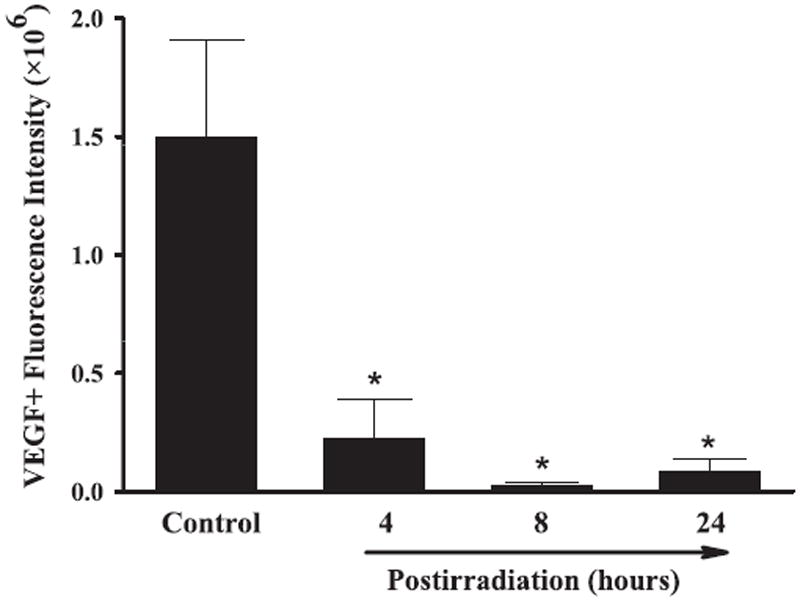
Effect of whole-brain irradiation on VEGF protein expression in rat brain. Quantitative analysis indicated that radiation significantly reduced protein expression levels of VEGF in rat brains compared with sham-irradiated controls. Data shown are means ± SEM for each group (n = 4). *Statistically significant difference from control (P < 0.05).
FIG. 5.
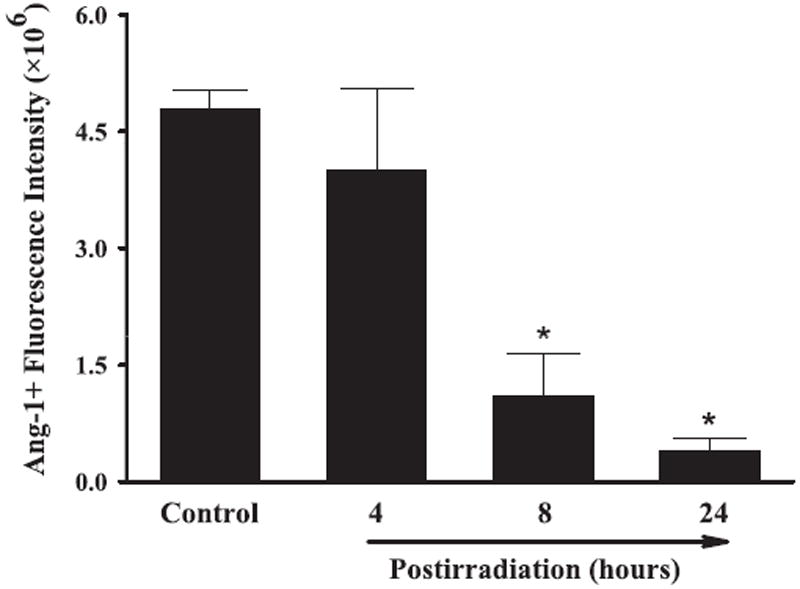
Effect of whole-brain irradiation on Ang-1 protein expression in rat brain. Quantitative analysis indicated that radiation significantly reduced protein expression levels of Ang-1 in rat brains compared with sham-irradiated controls. Data shown are means ± SEM for each group (n = 4). *Statistically significant difference from control (P < 0.05).
FIG. 6.
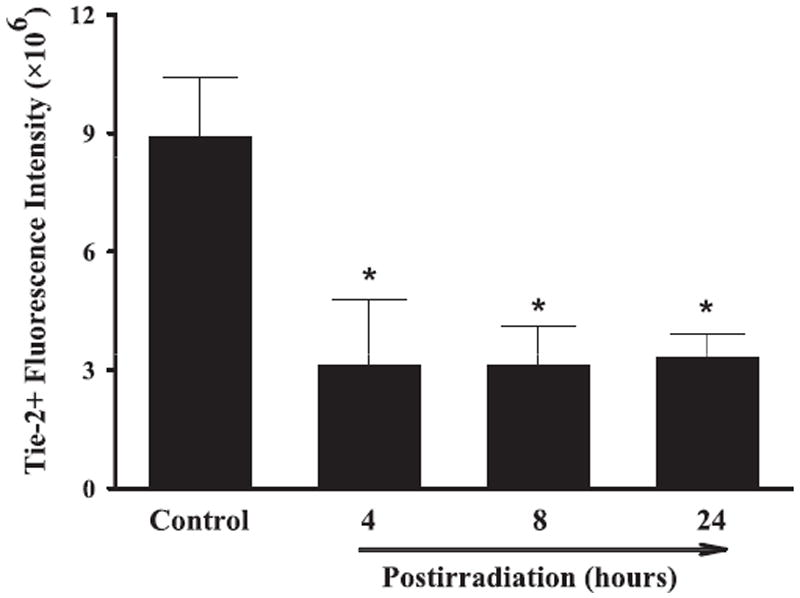
Effect of whole-brain irradiation on Tie-2 protein expression in rat brain. Quantitative analysis indicated that radiation significantly reduced protein expression levels of Tie-2 in rat brains compared with sham-irradiated controls. Data shown are means ± SEM for each group (n = 4). *Statistically significant difference from control (P < 0.05).
FIG. 7.
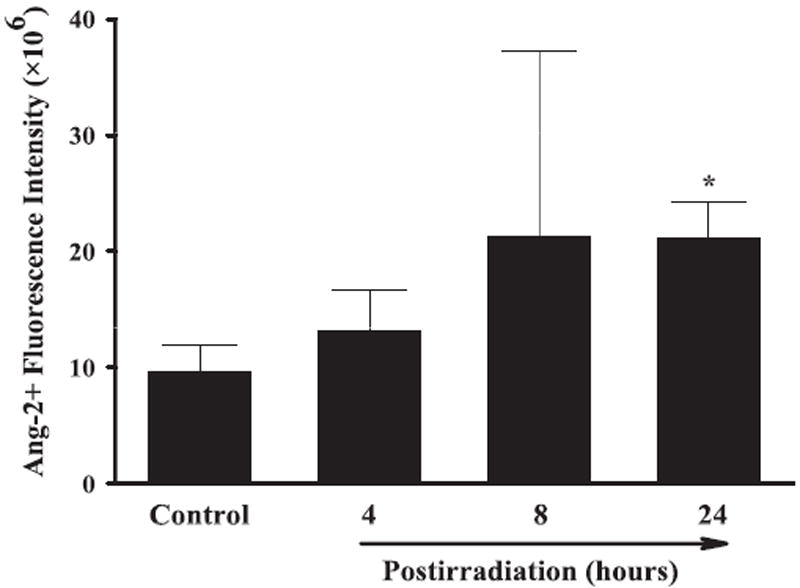
Effect of whole-brain irradiation on Ang-2 protein expression in rat brain. Quantitative analysis indicated that radiation significantly increased protein expression levels of Ang-2 in rat brains compared with sham-irradiated controls. Data shown are means ± SEM for each group (n = 4). *Statistically significant difference from control (P < 0.05).
DISCUSSION
The present study provides compelling evidence that whole-brain irradiation decreases EC proliferation, increases EC apoptosis, and differentially affects the expression of various angiogenic factors including VEGF, Ang-1, Tie-2 and Ang-2 in rat brains. Radiation-induced brain damage is primarily a consequence of injury to the vascular endothelium (16). However, it is well documented that nonvascular effects of radiation on normal brain tissue are correlated with cognitive deficits. For example, damaging effects of radiation of nonvascular consequence include parenchymal loss, demyelination, depletion of neural precursor cells, impairment of neurogenesis, induction of neuroinflammation, and degradation of extracellular matrix (7-9, 21, 24, 26, 30-33). Thus it is likely that functional changes in the brain induced by radiation result from both vascular and nonvascular effects.
Previous evidence suggested that EC apoptosis may be responsible for the radiation-induced decrease in EC density. Pena et al. (34) showed that EC apoptosis occurred in mouse brain and spinal cord between 4 and 24 h after a single high dose of radiation. In addition, increased numbers of apoptotic EC at 24 h postirradiation were associated with a decrease in vessel density in rat spinal cord (35). However, the present results should be distinguished from the aforementioned studies because of the different experimental procedures (tissues irradiated and radiation dose). Pena et al. demonstrated radiation-induced EC apoptosis in mouse brain. However, animals received whole-body irradiation with a single dose of 50 Gy, which may not be clinically relevant. In the study of Li et al., animals received cervical spinal cord irradiation and radiation-induced EC apoptosis was not determined in brain. Therefore, we believe that the present study provides new evidence that whole-brain irradiation results in a significant increase in EC apoptosis in rat brain. In addition to EC apoptosis, we examined EC proliferation and found that whole-brain irradiation significantly decreased the number of proliferating EC in rat brain. These data suggest that the radiation-mediated increase in EC apoptosis and decrease in EC proliferation contribute to vascular rarefaction in irradiated brain.
Angiogenesis has a critical role not only in physiological processes such as embryonic development and wound healing but also in the development of a number of pathological conditions, including inflammation and tumor progression (36, 37). One of the most important angiogenic factors is VEGF, which has a potent and specific activity for the vascular endothelium (38). VEGF and its receptors serve to initiate EC proliferation, EC migration and production of new capillary sprouts, which subsequently promote vasculogenesis and angiogenesis (39, 40). VEGF is also considered as a survival factor for EC and protects cells from apoptotic death (38, 41). In addition to VEGF, angiopoietins are a second family of vascular regulatory molecules that are specific for the vascular endothelium involved in both physiological and pathological blood vessel generation (42). Although Ang-1 does not directly affect proliferation of cultured EC, it has a major role in mediating interactions between the endothelium and the surrounding matrix, stimulating EC migration (43), sprouting (44) and tubule formation (45). Under physiological conditions, Ang-1 is necessary for subsequent vascular remodeling as well as vessel maturation and stabilization by binding to the endothelial receptor Tie-2 (46). Members of the angiopoietin family bind to Tie-2 (47) and the balance of Ang-1/Tie-2 system has been known to be crucial for maintenance of vessel integrity and development of mature vessels (48). Ang-2, a functional antagonist of Ang-1, competitively inhibits interaction between Ang-1 and Tie-2. By blocking cell signaling pathways initiated by the Ang-1/Tie-2 system, Ang-2 leads to destabilization of vascular structure, which allows EC to become more sensitive and responsive to other angiogenic factors, including VEGF (48). In the presence of high levels of VEGF, Ang-2 facilitates the angiogenic process by increasing EC proliferation, EC migration and vessel sprouting. In the absence of the activating signal from VEGF, Ang-2 promotes EC apoptosis and subsequently leads to vessel rarefaction (48). These studies suggest that the dynamic interplay among VEGF, Ang-1, Tie-2 and Ang-2 is necessary for physiological angiogenesis. In the present study, significantly lower levels of mRNA and protein expression of VEGF, Ang-1 and Tie-2 were detected in brains from irradiated animals. In contrast, whole-brain irradiation significantly upregulated Ang-2 mRNA and protein expression in rat brains. It is well known that mRNA expression positively correlates with protein expression. Since the translational process from mRNA to protein expression is dependent on time, it is common to observe either concurrent or modest delay in expression levels between mRNA and protein. Data from the present study clearly demonstrated a positive relationship between mRNA expression and protein expression levels for VEGF (8 and 24 h), Ang-1, Tie-2 and Ang-2. Although further research is necessary, there is a possibility that radiation suppresses translational process of VEGF protein expression without affecting mRNA expression at 4 h postirradiation. These results suggest that whole-brain irradiation may decrease vessel integrity and maturation by causing an imbalance in the relative ratio of Ang-1 to Ang-2 levels in brain. Additionally, alterations in the balance of Ang-2/VEGF in irradiated rat brain may initiate vessel rarefaction by decreasing EC proliferation and increasing EC apoptosis. However, the causal relationship between EC proliferation/apoptosis and differential expression of angiogenic factors in the irradiated brain remains unclear and needs to be investigated further.
CONCLUSION
The present study demonstrates for the first time that differential expression of VEGF, Ang-1, Tie-2 and Ang-2 may be responsible for cerebral microvascular rarefaction and may contribute to decreased EC proliferation and increased EC apoptosis in irradiated rat brains. These findings provide potential cellular and molecular mechanisms by which radiation alters physiological angiogenesis in brain that will lead to new opportunities for preventive and therapeutic interventions for patients with brain tumors who receive radiation therapy.
Acknowledgments
The project described was supported by Grant Number R01NS056218 from the National Institute of Neurological Disorders and Stroke (NINDS).
References
- 1.Sheline GE, Wara WM, Smith M. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6:1215–28. doi: 10.1016/0360-3016(80)90175-3. [DOI] [PubMed] [Google Scholar]
- 2.Central Brain Tumor Registry of the United States (CBTRUS) Primary brain and central nervous system tumors diagnosed in the United States. [updated 2010 Mar]. Available from: http://www.abta.org.
- 3.Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound’. Radiother Oncol. 2002;63:129–45. doi: 10.1016/s0167-8140(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 4.Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–44. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 5.Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT. Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res. 2005;164:662–8. doi: 10.1667/rr3453.1. [DOI] [PubMed] [Google Scholar]
- 6.Lamproglou I, Chen QM, Boisserie G, Mazeron JJ, Poisson M, Baillet F, et al. Radiation-induced cognitive dysfunction: an experimental model in the old rat. Int J Radiat Oncol Biol Phys. 1995;31:65–70. doi: 10.1016/0360-3016(94)00332-F. [DOI] [PubMed] [Google Scholar]
- 7.Lee WH, Sonntag WE, Mitschelen M, Yan H, Lee YW. Irradiation induces regionally specific alterations in pro-inflammatory environments in rat brain. Int J Radiat Biol. 2010;86:132–44. doi: 10.3109/09553000903419346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee WH, Sonntag WE, Lee YW. Aging attenuates radiation-induced expression of pro-inflammatory mediators in rat brain. Neurosci Lett. 2010;476:89–93. doi: 10.1016/j.neulet.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee WH, Warrington JP, Sonntag WE, Lee YW. Irradiation alters MMP-2/TIMP-2 system and collagen type IV degradation in brain. Int J Radiat Oncol Biol Phys Forthcoming. 2011 doi: 10.1016/j.ijrobp.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warrington JP, Csiszar A, Johnson DA, Herman TS, Ahmad S, Lee YW, et al. Cerebral microvascular rarefaction induced by whole brain radiation is reversible by systemic hypoxia in mice. Am J Physiol Heart Circ Physiol. 2011;300:H736–44. doi: 10.1152/ajpheart.01024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goligorsky MS. Microvascular rarefaction: The decline and fall of blood vessels. Organogenesis. 2010;6:1–10. doi: 10.4161/org.6.1.10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasad A, Dunnill GS, Mortimer PS, MacGregor GA. Capillary rarefaction in the forearm skin in essential hypertension. J Hypertens. 1995;13:265–8. [PubMed] [Google Scholar]
- 13.Rakusan K, Moravec J, Hatt PY. Regional capillary supply in the normal and hypertrophied rat heart. Microvasc Res. 1980;20:319–26. doi: 10.1016/0026-2862(80)90032-1. [DOI] [PubMed] [Google Scholar]
- 14.Ljubimova NV, Levitman MK, Plotnikova ED, Eidus LKh. Endothelial cell population dynamics in rat brain after local irradiation. Br J Radiol. 1991;64:934–40. doi: 10.1259/0007-1285-64-766-934. [DOI] [PubMed] [Google Scholar]
- 15.Dimitrievich GS, Fischer-Dzoga K, Griem ML. Radiosensitivity of vascular tissue. I. Differential radiosensitivity of capillaries: a quantitative in vivo study. Radiat Res. 1984;99:511–35. [PubMed] [Google Scholar]
- 16.Roth NM, Sontag MR, Kiani MF. Early effects of ionizing radiation on the microvascular networks in normal tissue. Radiat Res. 1999;151:270–7. [PubMed] [Google Scholar]
- 17.Lyubimova N, Hopewell JW. Experimental evidence to support the hypothesis that damage to vascular endothelium plays the primary role in the development of late radiation-induced CNS injury. Br J Radiol. 2004;77:488–92. doi: 10.1259/bjr/15169876. [DOI] [PubMed] [Google Scholar]
- 18.Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18:5356–62. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- 19.Clark RAF. Wound repair: overview and general considerations. In: Clark RAF, editor. The molecular and cellular biology of wound repair. 2. New York: Plenum Press; 1996. pp. 3–50. [Google Scholar]
- 20.Voges J, Treuer H, Sturm V, Büchner C, Lehrke R, Kocher M, et al. Risk analysis of linear accelerator radiosurgery. Int J Radiat Oncol Biol Phys. 1996;36:1055–63. doi: 10.1016/s0360-3016(96)00422-1. [DOI] [PubMed] [Google Scholar]
- 21.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–62. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 22.Hodges H, Katzung N, Sowinski P, Hopewell JW, Wilkinson JH, Bywaters T, et al. Late behavioural and neuropathological effects of local brain irradiation in the rat. Behav Brain Res. 1998;91:99–114. doi: 10.1016/s0166-4328(97)00108-3. [DOI] [PubMed] [Google Scholar]
- 23.Calvo W, Hopewell JW, Reinhold HS, Yeung TK. Time- and dose-related changes in the white matter of the rat brain after single doses of X rays. Br J Radiol. 1988;61:1043–52. doi: 10.1259/0007-1285-61-731-1043. [DOI] [PubMed] [Google Scholar]
- 24.Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16:129–34. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- 25.Kim SH, Lim DJ, Chung YG, Cho TH, Lim SJ, Kim WJ, et al. Expression of TNF-α and TGF-β1 in the rat brain after a single high-dose irradiation. J Korean Med Sci. 2002;17:242–8. doi: 10.3346/jkms.2002.17.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conner KR, Forbes ME, Lee WH, Lee YW, Riddle DR. AT1 receptor antagonism does not influence early radiation-induced changes in microglial activation or neurogenesis in the normal rat brain. Radiat Res. 2011;176:71–83. doi: 10.1667/rr2560.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toborek M, Lee YW, Kaiser S, Hennig B. Measurement of inflammatory properties of fatty acids in human endothelial cells. Methods Enzymol. 2002;352:198–219. doi: 10.1016/s0076-6879(02)52020-6. [DOI] [PubMed] [Google Scholar]
- 28.Deng X, Li H, Tang YW. Cytokine expression in respiratory syncytial virus-infected mice as measured by quantitative reverse-transcriptase PCR. J Virol Methods. 2003;107:141–6. doi: 10.1016/s0166-0934(02)00211-2. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Otsuka S, Coderre JA, Micca PL, Morris GM, Hopewell JW, Rola R, et al. Depletion of neural precursor cells after local brain irradiation is due to radiation dose to the parenchyma, not the vasculature. Radiat Res. 2006;165:582–91. doi: 10.1667/RR3539.1. [DOI] [PubMed] [Google Scholar]
- 31.Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–30. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 33.Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–42. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 34.Pena LA, Fuks Z, Kolesnick RN. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 2000;60:321–7. [PubMed] [Google Scholar]
- 35.Li YQ, Chen P, Jain V, Reilly RM, Wong CS. Early radiation-induced endothelial cell loss and blood-spinal cord barrier breakdown in the rat spinal cord. Radiat Res. 2004;161:143–52. doi: 10.1667/rr3117. [DOI] [PubMed] [Google Scholar]
- 36.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 37.Miller JW, Adamis AP, Shima DT, D’Amore PA, Moulton RS, O’Reilly MS, et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol. 1994;145:574–84. [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999;237:1–30. doi: 10.1007/978-3-642-59953-8_1. [DOI] [PubMed] [Google Scholar]
- 39.Plate KH. Mechanisms of angiogenesis in the brain. J Neuropathol Exp Neurol. 1999;58:313–20. doi: 10.1097/00005072-199904000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–32. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- 41.Alavi A, Hood JD, Frausto R, Stupack DG, Cheresh DA. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 2003;301:94–6. doi: 10.1126/science.1082015. [DOI] [PubMed] [Google Scholar]
- 42.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–9. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 43.Witzenbichler B, Maisonpierre PC, Jones P, Yancopoulos GD, Isner JM. Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J Biol Chem. 1998;273:18514–21. doi: 10.1074/jbc.273.29.18514. [DOI] [PubMed] [Google Scholar]
- 44.Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr Biol. 1998;8:529–32. doi: 10.1016/s0960-9822(98)70205-2. [DOI] [PubMed] [Google Scholar]
- 45.Hayes AJ, Huang WQ, Mallah J, Yang D, Lippman ME, Li LY. Angiopoietin-1 and its receptor Tie-2 participate in the regulation of capillary-like tubule formation and survival of endothelial cells. Microvasc Res. 1999;58:224–37. doi: 10.1006/mvre.1999.2179. [DOI] [PubMed] [Google Scholar]
- 46.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–4. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 47.Peters KG, Kontos CD, Lin PC, Wong AL, Rao P, Huang L, et al. Functional significance of Tie2 signaling in the adult vasculature. Recent Prog Horm Res. 2004;59:51–71. doi: 10.1210/rp.59.1.51. [DOI] [PubMed] [Google Scholar]
- 48.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]


