Abstract
This study examined the effects of amblyopia on perceptual decision-making processes to determine the consequences of visual deprivation on development of higher-level cortical networks outside of visual cortex. A variant of the Eriksen flanker task was used to measure response time and accuracy for decisions made in the presence of response-selection conflict. Performance of adults with amblyopia was compared to that of neurotypical participants of the same age. Additionally, simple and choice reaction time tasks presented in the visual and the auditory modality were used to control for factors such as feature visibility, crowding, and motor execution speed. A selective deficit in response time for visual decisions was found when individuals with amblyopia used either the amblyopic or non-amblyopic (dominant) eye, and this deficit was independent of visual acuity, motor time, and performance accuracy. In trial conditions that provoked response-selection conflict, responses were significantly delayed in amblyopic relative to neurotypical participants, and were not subject to standard trial sequence effects. Our results indicate that, beyond the known effects of abnormal visual experience on visual cortex, suboptimal binocular input during a developmental critical period may also impact cortical connections to downstream areas of the brain, including parietal and frontal cortex, that are believed to underlie decision and response-selection processes.
Keywords: visual development, amblyopia, strabismus, decision, response conflict, cognitive control
INTRODUCTION
Visual deprivation during early life alters both the anatomical organization and the function of primary visual cortex (Hubel & Wiesel, 1977). Visual deprivation in humans that can then lead to amblyopia arises from ocular misalignment (strabismus), very large or unequal refractive errors between the two eyes (anisometropia), or a combination of the two conditions during a critical window of development. The effects of abnormal visual experience resulting from amblyopia have traditionally been tested by measuring accuracy on visual detection or discrimination tasks, such as contrast sensitivity and grating, letter or vernier acuity (Freeman & Thibos, 1975; Hess & Howell, 1977; Klein & Levi, 1985; McKee, Levi, & Movshon, 2003), and more recently on mid-level visual tasks involving local feature and contour integration (Chandna, Pennefather, Kovács, & Norcia, 2001; Hess, McIlhagga, & Field, 1997; Kovács, Polat, Pennefather, Chandna, & Norcia, 2000; Simmers & Bex, 2004) and motion perception (Ho, et al., 2005; Simmers, Ledgeway, Hess, & McGraw, 2003; Simmers, Ledgeway, Mansouri, Hutchinson, & Hess, 2006). The possible effects of visual deprivation on higher-level decision-making have yet to be studied.
Visual decision-making involves a set of processes through which a behavioral choice is made in the context of alternative interpretations of a sensory stimulus, and this choice is then translated into the selection and execution of a corresponding motor response (Gold & Shadlen, 2007; Heekeren, Marrett, & Ungerleider, 2008; Shadlen & Newsome, 2001; Usher & McClelland, 2001). A growing body of evidence from neurophysiological and neuroimaging studies has shown that decision-making processes are distributed over many cortical areas, engaging recurrent interactions between sensory, parietal, and frontal cortices (Gold & Shadlen, 2001, 2007; Heekeren, et al., 2008). During development, the amblyopic eye is habitually denied involvement in the control of visual decisions, either through the presence of chronic blur or through constant, suppressive binocular interactions that eliminate the representation from the amblyopic eye from conscious awareness. This suboptimal monocular input may have consequences for the structural and functional development of brain regions downstream from primary visual cortex. The amblyopic visual system thus presents an opportunity to examine the impact of abnormal visual input on the development of brain networks involved not only in sensory encoding, but also in decision-making.
To test visual decision-making abilities in adults with amblyopia, we used a version of the Eriksen flanker task (Eriksen & Eriksen, 1974), a classic paradigm that elicits decisions made in the presence of stimulus- and response-conflict. In the Eriksen task, participants are instructed to identify a central target stimulus in the presence of surrounding distractor stimuli that are the same as or different from the target. Each target identity is assigned to one of two motor responses, typically right- or left-hand button presses. Because the distractors map to responses that are either compatible or incompatible with the target, the task confronts the participant with different levels of stimulus and response conflict. Trials in which the flankers are identical to the target (congruent), and therefore associated with the same response button, present no conflict. However, trials in which the target and flankers do not match (incongruent), present a visual array containing sensory information that activates both responses, from which one response must be selected to correctly identify the target. Neutral trials involve flankers with no response assignments and therefore present conflict at the stimulus, but not at the response, level. Response times and error rates are typically greatest when the target and flankers are incongruent as a result of conflict between competing, concurrently active, and mutually incompatible response mappings, and are lowest for congruent trials (Botvinick, Braver, Barch, Carter, & Cohen, 2001). The difference in performance between congruent and incongruent trials is referred to as the flanker-congruency effect. Current behavioral models of the Eriksen task posit that translation of the sensory representation into execution of the appropriate motor response relies on attentional and top-down cognitive control processes, both of which are hypothesized to originate beyond the level of stimulus encoding (Botvinick, et al., 2001; Egner, 2008; Lamers & Roelofs, 2011; Yeung, Botvinick, & Cohen, 2004). The recruitment, engagement, and monitoring of cognitive control as a result of response conflict in the Eriksen task have been associated with increased activation of medial frontal brain areas such as the anterior cingulate cortex (Botvinick, Cohen, & Carter, 2004; Egner, 2008; van Veen & Carter, 2002).
The purpose of the present study was to investigate the effects of visual deprivation on perceptual decisions made under varying degrees of stimulus- and response-conflict. To that end, we measured accuracy and response time from individuals diagnosed with amblyopia tested monocularly on the Eriksen flanker task. In order to minimize the effects of reduced visibility in the amblyopic eye, stimuli presented to the amblyopic eye were scaled according to the individual’s full line letter acuity. Here we provide evidence for the presence of a delay in response selection for visual, but not auditory, decisions made by individuals with amblyopia. This deficit was found irrespective of whether the patient’s amblyopic or non-amblyopic (dominant) eye was used, and was present even when a single (unflanked) letter was presented. Further, the deficit was independent of visual acuity loss, motor response time, and performance accuracy. Our findings suggest that normal binocular visual experience may be necessary for proper development of higher-order cortical areas much like it is for development of visual cortex.
METHODS
Participants
Two groups of participants completed the experimental tasks: ten individuals with amblyopia (2 with anisometropia, 2 with strabismus, and 6 with both strabismus and anisometropia; 5 males) between the ages of 43–72 years (M = 51.6, SD = 10.1) and ten neurotypical (NT) controls (5 males) between the ages of 47–59 years (M = 54.5, SD = 3.6). Individuals in the amblyopia group underwent standard visual assessment, including best corrected visual acuity using the Bailey-Lovie LogMAR chart (Bailey & Lovie, 1976), horizontal and vertical angles of deviation using the prism-cover test, measurement of refractive errors, and stereoacuity using the RANDOT stereotests (Stereo Optical CO., Inc.). Clinical characteristics of individuals with amblyopia are listed in Table 1. Control participants had normal or corrected-to-normal acuity (20/20 or better) in both eyes. Sighting dominance was established by asking each participant to raise a card with a small hole in it (1 cm) with both hands, so they could see a distant target (6 m) through the hole. One eye was then occluded and the eye that retained sight of the target was designated as the dominant eye.
Table 1.
Clinical characteristics of individuals with amblyopia.
| Participant | Sex | Age | Type | Visual Acuity (LogMAR) |
Stereopsis (Arcsec) |
Ocular Deviation (ΔDiopters) |
Refractive Error | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Distance | Near | Left | Right | |||||
| 1 | M | 47.5 | S+A | 1.4 | −0.2 | None | ET 4 R/L 16 |
R/L 8 | −13.00 | Plano |
| 2 | M | 43.2 | S | 0.8 | 0.1 | >400 | ET 10 R/L 2 |
ET 12 R/L 4 |
Plano-0.50×70 | Plano |
| 3 | F | 44.1 | A | 0.7 | −0.2 | >400 | Ortho | Ortho | +3.00-0.50×90 | Plano |
| 4 | F | 60.4 | S+A | 1.62 | 0 | >400 | ET 12 | Ortho | −1.25-3.00×110 | Plano |
| 5 | M | 54.9 | S+A | 0.7 | 0 | >400 | ET 4 | ET 4 | −1.50-1.50×10 | 0.50-1.00×5 |
| 6 | M | 58.2 | S+A | 1.3 | 0.1 | >400 | R/L 20 | XT 4 R/L 24 |
+5.00-1.25×130 | +1.00-0.5×130 |
| 7 | M | 72.5 | S+A | 0.8 | −0.19 | None | XT 14 | XT 30 XT 25 |
+1.00+1.00×110 | Plano |
| 8 | F | 51.4 | A | 0.1 | 0.40 | >400 | Ortho | Ortho | −2.00-2.50×170 | −5.00-1.50×170 |
| 9 | F | 40.6 | S+A | −0.19 | 0.6 | None | XT 8 R/L 12 |
Ortho | Plano | −1.00 |
| 10 | F | 42.8 | S | 0.50 | 0.1 | >400 | ET 4 | Ortho | Plano | Plano |
Note. Age in years; S, strabismus; A, anisometropia; S+A, strabismus and anisometropia; ET, esotropia; XT, expotropia; R/L; higher vertical deviation in Right eye, Ortho, orthotropic; Plano, no refractive error.
Visual stimuli were scaled for viewing by the amblyopic eye according to the patient’s full line letter acuity in order to minimize differences in performance resulting from limited visibility. For individuals with LogMAR acuity between 0.5 and 1.0, letters were scaled by a factor of three, and for acuities greater than or equal to 1.0 letters were scaled by a factor of four. Individuals with amblyopia were tested while wearing optical correction. All testing was done monocularly by patching the untested eye and each eye was tested in random order. Task order was also randomly chosen for each participant. The study was approved by the local institutional review board and adhered to guidelines of the Declaration of Helsinki. Informed consent was obtained from each participant.
Experimental Tasks and Procedures
Landolt C Flanker Task
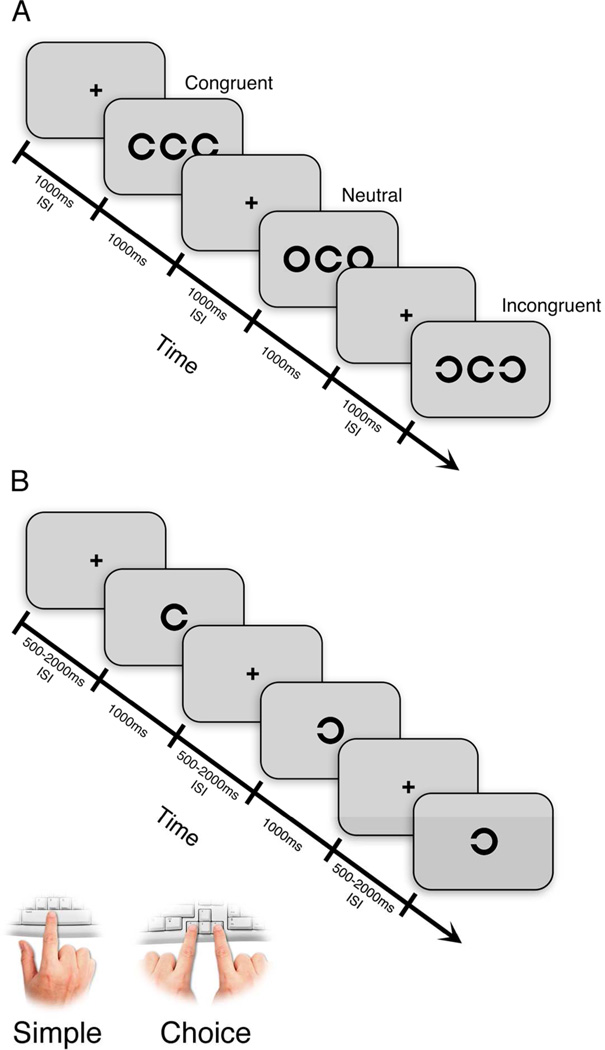
Tasks were generated using Presentation software version 14.9 (Neurobehavioral Systems, Inc.) and were displayed on a 19-inch gamma corrected CRT monitor (Mitsubishi Diamond Pro 2040U) at a resolution of 800 by 600 pixels and a refresh rate of 75 Hz. Participants performed the tasks under monocular viewing from a distance of 70 cm. Each trial began with a black fixation cross (0.8 deg by 0.8 deg, luminance of 2.4 cd/m3) in the center of the screen presented against a mid-gray background (40.9 cd/m3) for 1000 ms. Fixation was immediately followed by a three element array comprised of a central target flanked by two distractors. The target was a black (2.4 cd/m3; Weber contrast of 94%) Landolt C (1.47 deg by 1.47 deg, stroke width of 0.29 deg) with a 0.29 deg by 0.29 deg gap positioned on the left or right side of the C. The target was flanked by identical Landolt C distractors spaced 2.05 deg to the right and to the left of the target (center-to-center horizontal distance) (Figure 1A). Flankers were always the same size as the target and flanker spacing was scaled in equal proportion to the size-scaling factor. The three-character array remained on the screen until the participant responded, or for a maximum duration of 1000 ms. Trials in which the participant did not respond within 1000 ms were counted as missed trials.
Figure 1.
(A) Schematic illustration of three trials from the Landolt C flanker task. Participants responded to the gap location of the central letter using the left and right arrow keys. (B) Schematic illustration of three trials from the simple detection and choice tasks in the visual modality. Participants responded using the spacebar (simple detection) or the left and right arrow keys (choice).
Participants were instructed to discriminate as quickly and accurately as possible the gap location of the central Landolt C target by pressing the left or right arrow key when the gap was positioned on the left or right, respectively. For all discrimination tasks, participants used their left index finger to press the left arrow key and their right index finger to press the right arrow key. Trials were defined by one of three levels of target-flanker congruence. For the congruent condition, flankers consisted of two Landolt Cs with gaps in the same position as the target C, thus signaling the same key-press response as the target. For the incongruent condition, flanker Cs had gaps in the opposite position as the target C and signaled a conflicting response. In the neutral condition, flankers consisted of two O characters (no gap present) that did not correspond to a directional response key. The letter array condition (congruent, incongruent, neutral) was determined randomly and occurred with equal probability within a block of trials. Following a block of 30 practice trials completed binocularly, each participant completed five blocks of 100 trials per block, in each eye.
Simple and Choice Visual Tasks
In these tasks, the target was presented in isolation (uncrowded). The fixation cross was presented for a variable duration of 500, 1000, or 2000 ms (random inter-trial interval), immediately followed by a single Landolt C target (1.47 deg by 1.47 deg for NTs, scaled to a maximum size of 5.96 deg by 5.96 deg for amblyopic eyes with LogMAR acuity ≥ to 1). The target remained on the screen until the participant responded or for a maximum duration of 1000 ms (Figure 1B). For the choice response task, participants were instructed to discriminate as quickly and accurately as possible the location of the gap in the Landolt C by pressing the left or right arrow key when the gap was on the left or right, respectively. For the simple response task, participants were asked to press the spacebar as soon as they detected the appearance of the character on the screen. Each task consisted of five blocks of fifty trials per block presented to each eye.
Simple and Choice Auditory Tasks
Trial structure was identical to that of the uncrowded visual tasks, except that following presentation of the fixation cross, a 300 Hz (low frequency) or 600 Hz (high frequency) tone was presented binaurally through speakers (Dell, Altec Lansing A215) located on either side of the monitor. For the choice response task, participants were instructed to discriminate as quickly and accurately as possible the frequency of the tone by pressing the left or right arrow key when the tone was low or high, respectively. For the simple response task, participants were asked to press the spacebar as soon as they detected a tone. Five blocks of fifty trials per block were presented in each task.
RESULTS
Landolt C Flanker Task
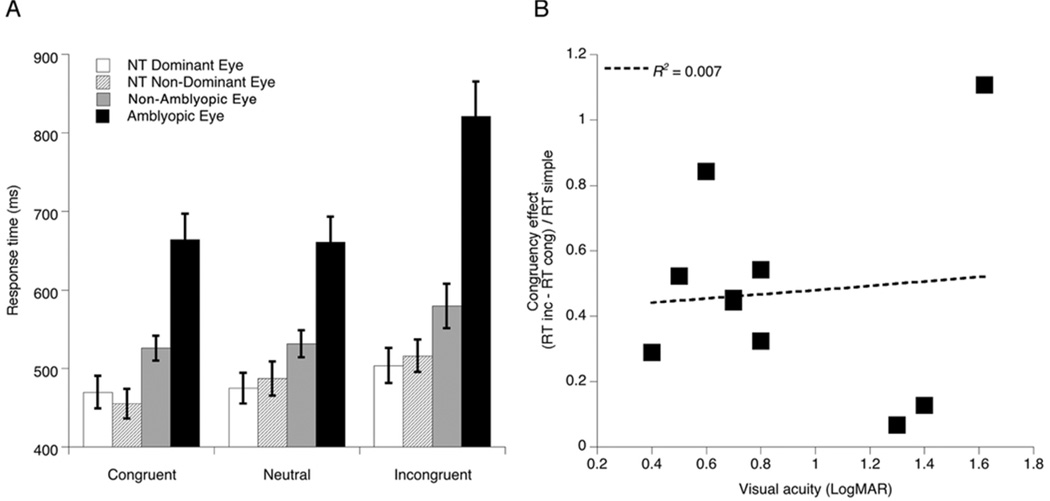
Response time analysis was based on correct responses and only responses greater than 200 ms were included. Groups did not differ on total number of trials included in the analysis (F(1, 19) = 0.28, p = 0.868). Response time data from the flanker task are shown in Figure 2A. A 3 (trial condition: neutral, congruent, incongruent) by 2 (eye: dominant or non-dominant) by 2 (group: NT or amblyopic) repeated measures analysis of variance (ANOVA) with mean response time as the dependent measure was conducted. The standard flanker-congruency effect was present across participants (main effect of trial condition: F(2, 17) = 19.99, p = 0.0001, ηp2 = 0.702), as indicated by significantly longer responses on incongruent trials (M = 605.1 ms, SEM = 17.3) compared to neutral (M = 538.3 ms, SEM = 13.8) or congruent trials (M = 528.5 ms, SEM = 12.4). We also found a main effect of eye (F(1, 18) = 22.72, p = 0.0001, ηp2 = 0.558) resulting from longer response times in the non-dominant (M = 600.1 ms, SEM = 19.8) compared to the dominant eye (M = 513.9 ms, SEM = 11.2), and this was driven by the non-dominant (amblyopic) eye of individuals with amblyopia, which also produced a significant interaction between eye and group (F(1, 18) = 20.99, p = 0.0001, ηp2 = 0.538). Individuals with amblyopia demonstrated significantly longer response times compared to NTs (main effect of group: F(1, 18) = 30.37, p = 0.0001, ηp2 = 0.628), and critically, this delay was exaggerated on incongruent trials, as qualified by a significant group by trial condition interaction effect (F(2, 17) = 4.91, p = 0.021, ηp2 = 0.366).
Figure 2.
(A) Results from Landolt C flanker task: mean response times for Congruent, Neutral, and Incongruent trials for Neurotypical (NT) and Amblyopic participants, by eye. Error bars indicate standard errors of the mean. (B) Congruency effect for Amblyopic participants (calculated as (RT incongruent - RT congruent) / RT simple) versus LogMAR visual acuity of the amblyopic eye. Dashed line represents best fitting linear regression.
To determine whether the greater congruency effect in the amblyopic group could be related to loss of acuity in the amblyopic eye, a correlation analysis was carried out between visual acuity (logMAR) of the amblyopic eye and an index of the congruency effect, calculated as response time on incongruent trials minus response time on congruent trials divided by the simple response time. As shown in Figure 2B, there was not a significant relationship between acuity of the amblyopic eye and impact of the incongruent flankers on response time (r(10) = 0.085, p = 0.813).
Accuracy on the flanker task was high in both groups, with all participants achieving over 95% accuracy across trial conditions. A 3 (trial condition: neutral, congruent, incongruent) by 2 (eye: dominant or non-dominant) by 2 (group: NT or amblyopic) repeated measures ANOVA with mean accuracy as the dependent measure showed that the presence of incongruent flankers significantly reduced accuracy regardless of group status or eye (main effect of trial condition: F(2, 17) = 4.08, p = 0.025, ηp2 = 0.185). Participants were less accurate on incongruent trials (M = 96.7%, SEM = 1.2) compared to neutral (M = 98.9%, SEM = 0.26) or congruent trials (M = 99.1%, SEM = 0.33). No other significant effects emerged from the analysis. Individuals with amblyopia were as accurate (M = 97.6%, SEM = 0.97) as NTs (M = 98.9%, SEM = 0.71) on all trial conditions, suggesting that the delayed responses cannot be accounted for solely in terms of greater task difficulty or reduced task performance. Equivalent levels of accuracy also rule out a substitution effect in which amblyopic participants may have erroneously reported the identity of the flanker instead of the target, which would have resulted in a greater proportion of errors on incongruent trials. Instead, these results indicate that the amblyopic participants were able to process the stimuli to the point of correct target identification, but that the interference from incongruent flankers had a greater impact on their response-selection times than it did for NTs.
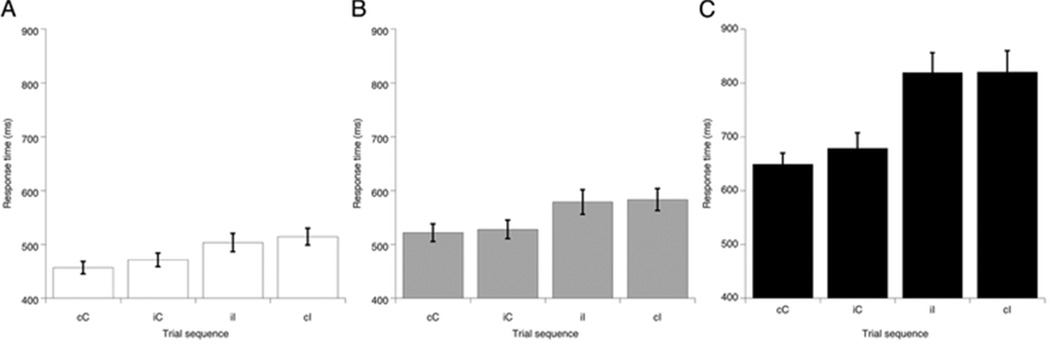
As a further assessment of the effects of visual deprivation on higher-level cognitive processes, we tested for the presence of a well-known pattern of behavioral adjustments that manifest as trial sequence effects on response time. Sequence effects observed using the Eriksen task, also termed the Gratton effect (Gratton, Coles, & Donchin, 1992), include faster response times on incongruent trials that are preceded by an incongruent trial (iI) compared to those preceded by a congruent trial (cI), and slower response times on congruent trials that are preceded by an incongruent trial (iC) than by a congruent trial (cC). This pattern yields an interaction between response time on the previous trial condition and the current trial condition. The dominant view in the literature considers these sequence effects to reflect a conflict-monitoring strategy that gives rise to increased recruitment of top-down attentional and cognitive control in order to optimize performance (Botvinick, et al., 2001; Botvinick, et al., 2004; Carter, et al., 1998; Lamers & Roelofs, 2011; Mayr, Awh, & Laurey, 2003; Yeung, et al., 2004). In other words, the amount of response competition, or conflict, is monitored to adjust the amount of cognitive control accordingly. Importantly, these effects have been shown to involve the operation of cognitive mechanisms in frontal cortex that function beyond the level of the visual representation in visual cortex (Botvinick, et al., 2001; Botvinick, et al., 2004; Kerns, et al., 2004).
Error trials and trials immediately following errors were excluded from the sequence analysis. The remaining trials (NT Dominant = 198, NT Non-Dominant = 224, Non-Amblyopic = 217, Amblyopic = 220) were used to calculate mean response times on the four trial sequence combinations. A 2 (previous trial: congruent or incongruent) by 2 (current trial: congruent or incongruent) by 2 (eye: dominant or non-dominant) repeated measures ANOVA with mean response time as the dependent measure was conducted for each group. Post-conflict sequence effects were found in the NT group (Figure 3A), such that overall response times on iI trials (M = 503.7 sec, SEM = 16.9) were on average 11 ms faster than responses on cI trials (M = 514.6 sec, SEM = 15.5), and response times on cC trials (M = 456.4 sec, SEM = 11.4) were on average 15 ms faster than responses on iC trials (M = 471.1 sec, SEM = 12.9), generating a significant interaction effect between previous trial and current trial: F(1, 9) = 6.98, p = 0.027, ηp2 = 0.437). Pairwise comparisons confirmed that NT response times on cC trials were significantly faster than on iC trials (t(9) = 2.231, p = 0.053). In the group of amblyopic participants, virtually no sequence dependency was present in either eye, yielding a non-significant interaction between previous and current trial conditions (F(1, 9) = 2.25, p = 0.168, ηp2 = 0.2) (Figure 3B and 3C). This pattern of results suggests that post-conflict modulation of attentional and cognitive control processes may be impaired in individuals with amblyopia.
Figure 3.
Sequence effects from Landolt C flanker task: mean response times by trial sequence, for the (A) Neurotypical (NT; average of dominant and non-dominant eyes), (B) Non-amblyopic, and (C) Amblyopic eyes. Error bars indicate standard errors of the mean. Trial sequence is labeled as follows: first letter indicates the congruency of the previous trial and the second letter indicates the congruency of the current trial. The basic pattern typically consists of faster response times for cC trials relative to iC trials and faster response times for iI trials relative to cI trials.
Crowding --- the reduced ability to discriminate objects and their features when surrounded by other objects --- is exhibited to a greater extent in the central visual field in individuals with amblyopia compared to NTs who experience crowding primarily in the peripheral visual field (Klein & Levi, 1985; Levi & Carney, 2011; Levi, Hariharan, & Klein, 2002). Therefore, the longer reaction times observed on all trial conditions in the amblyopic eye may be due in part to crowding between letters in the stimulus array. However, crowding is unlikely to be the full explanation for the deficit found in the flanker task because each trial condition had the same level of crowding between letters and yet we found a significant effect of flanker congruency. This excess impact of the incongruent flankers in the amblyopic group is inconsistent with conventional crowding because crowding is dominated by the spectral power of the flankers (Tjan & Dang, 2005) and crowding is known to increase with target-flanker similarity (Bernard & Chung, 2011; Kooi, Toet, Tripathy, & Levi, 1994). Nonetheless, to confirm that the effects found in the first experimental task were not exclusively the consequence of crowding by the flankers, we tested participants monocularly on discriminating the location of the gap in a Landolt C when it was presented in isolation. In addition, a simple stimulus detection task was carried out to exclude non-decision related delays possibly arising from slower motor responses in the amblyopic participants.
Simple and Choice Visual Tasks
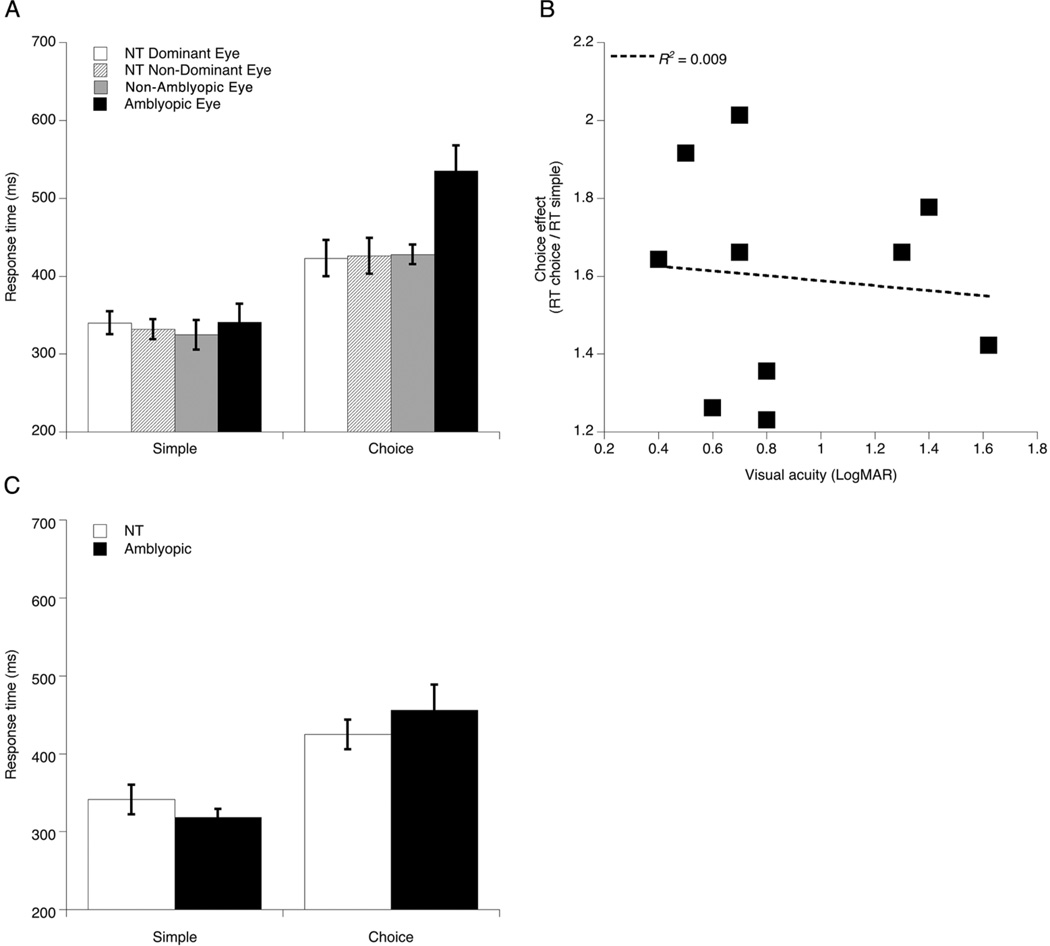
Response time data from the simple and choice visual tasks were entered into a 3 (task: simple or choice) by 2 (eye: dominant or non-dominant) by 2 (group: NT or amblyopic) repeated measures ANOVA (Figure 4A). As expected, choice response times for gap position discrimination of the single (unflanked) Landolt C were significantly longer (M = 452.0 ms, SEM = 15.6) than simple response times (M = 334.4 ms, SEM = 10.6), generating a main effect of task (F(1, 18) = 78.42, p = 0.0001, ηp2 = 0.813). Individuals with amblyopia were slower relative to NTs (main effect of group: F(1, 18) = 4.49, p = 0.048, ηp2 = 0.200), which was qualified by significant interactions between task and group (F(1, 18) = 6.26, p = 0.022, ηp2 = 0.258) and between task and eye (F(1, 18) = 21.66, p = 0.0001, ηp2 = 0.546). Importantly, the amblyopic participants were slower only on the choice task (NTs: M = 425.6 ms, SEM = 23.2; amblyopic: M = 484.4 ms, SEM = 18.1; t(18) = −2.033, p = 0.057). We also found a main effect of eye (F(1, 18) = 5.79, p = 0.027, ηp2 = 0.244) driven by longer response times in the non-dominant (M = 408.5 ms, SEM = 15.2) compared to the dominant (M = 377.9 ms, SEM = 10.8) eye, and this was moderated by a significant interaction between eye and group (F(1, 18) = 6.81, p = 0.018, ηp2 = 0.274). Finally, the three-way interaction between task, group, and eye was found to be significant (F(1, 18) = 13.43, p = 0.002, ηp2 = 0.427), representing slower responses on the choice task made by individuals with amblyopia when using their non-dominant (amblyopic) eye (dominant: M = 423.7 ms, SEM = 12.6; non-dominant (amblyopic): M = 535.2 ms, SEM = 32.9; t(9) = −4.108, p = 0.003). Therefore, even in the absence of flankers (uncrowded), a deficit in decision response time was present in the amblyopic eyes of participants with amblyopia. Critically, no group difference emerged for response times in the simple detection task for either eye (p = 0.879). Also, as was the case with the flanker task, we found that visual acuity (logMAR) of the amblyopic eye did not predict magnitude of the choice effect, calculated as the difference in response times between the choice and simple tasks divided by the simple response time (r(10) = −0.096, p = 0.791) (Figure 4B).
Figure 4.
(A) Results from the simple and choice response tasks in the visual modality: mean response times for Neurotypical (NT) and Amblyopic participants, by eye. Error bars indicate standard errors of the mean. (B) Choice effect for Amblyopic participants (RT choice / RT simple) versus LogMAR visual acuity of the amblyopic eye. Dashed line represents best fitting linear regression. (C) Results from the simple and choice response tasks in the auditory modality: mean response times for each task, for Neurotypical (NT) and Amblyopic participants. Error bars indicate standard errors of the mean.
As in the flanker task, individuals with amblyopia performed just as well (M = 98.9%, SEM = 0.58) as NTs (M = 98.5% ms, SEM = 0.69) on the unflanked Landolt C gap position discrimination task when using either their amblyopic (M = 99.3% ms, SEM = 0.73) or non-amblyopic (M = 98.4% ms, SEM = 0.74) eye. No group difference in accuracy was observed (p = 0.523).
Taken together, the results from the first two tasks demonstrate that decision response time is significantly longer in individuals with amblyopia compared to NTs. But, it is not clear whether this response selection deficit is specific to the demands being placed on the visual system or whether some unknown bias unrelated to decision processes exists in the groups we tested. To determine whether differences in general cognitive ability that could affect decision latencies were present in the amblyopic group, we also tested the participants using simple and choice tasks presented in the auditory modality.
Simple and Choice Auditory Tasks
As was the case with the visual tasks, tone discrimination responses (NTs: M = 424.8 ms, SEM = 19.0; amblyopic: M = 455.6 ms, SEM = 32.7) were significantly longer than simple tone detection responses (NTs: M = 341.3 ms, SEM = 18.9; amblyopic: M = 318.2 ms, SEM = 10.8 ms), which manifested as a main effect of task (F(1, 18) = 27.17, p = 0.0001, ηp2 = 0.602). No difference in choice response times was found between the two groups (p = 0.431). Also, no difference in tone discrimination accuracies was found between groups (NTs: M = 97.8%, SEM = 0.56; amblyopic: M = 95.1%, SEM = 1.96; p = 0.203). Response time data from the auditory detection and discrimination tasks are shown in Figure 4C. This key control experiment confirmed that the response time impairments observed in the visual tasks are indeed restricted to the visual modality. Equivalent auditory choice response times between participant groups also implies that stimulus-response mapping, per se, was not differentially more difficult for the individuals with amblyopia.
DISCUSSION
In the present study we used accuracy and response time measures and a conceptual framework motivated by the perceptual decision-making literature to study the effects of visual deprivation on response-selection and decision-making. By examining decision-making processes over and above factors such as feature visibility, crowding, and motor execution, we tested the hypothesis that abnormal visual input produces changes in visual functions that are supported by higher-level brain networks, including frontal cortex. Here we provide the first evidence that decisions relying on visual input, particularly in the presence of stimulus-response conflict, are delayed in adult individuals with amblyopia.
Visual decision response times were delayed in both the amblyopic and nonamblyopic eyes of individuals with amblyopia, and were distinct from group differences in stimulus discriminability and performance level, as assayed by simple response time and accuracy measures. Furthermore, the differential choice response time between amblyopic participants and NTs was not present for auditory stimuli, confirming a vision-specific response-selection deficit. Finally, abnormal conflict-monitoring in the amblyopic eye of participants, as evidenced by a lack of sequence effects, further implicates a specific deficit in control mechanisms that operate well beyond the level of stimulus representation.
The fact that the response selection impairment is present in both the amblyopic and non-amblyopic eye of individuals with amblyopia suggests that normal development of not only visual cortex, but also the cortical network underlying decision processing and response selection depends on binocular visual experience during early life. Normal binocularity confers the visual system signal-to-noise ratio benefits through binocular summation (Campbell & Green, 1965) as well as access to the primary cue for depth --- binocular disparity --- that provides important cues to scene segmentation (Julesz, 1960; Parker, 2007). It has already been established that sensitivity (accuracy) in a variety of visual detection and discrimination tasks is reduced during monocular viewing with either the amblyopic or the habitually fixating, non-amblyopic eye (Giaschi, Regan, Kraft, & Hong, 1992; Leguire, Rogers, & Bremer, 1990). In addition to abnormalities in contrast sensitivity and visual acuity (Hess & Howell, 1977; Levi & Klein, 1982), performance is impaired on tasks involving higher-level visual processing, including those engaging “cognitive” factors such as attention (Chandna, et al., 2001; Ho, et al., 2006; Popple & Levi, 2008; Sharma, Levi, & Klein, 2000; Simmers & Bex, 2004; Simmers, et al., 2003; Simmers, et al., 2006; Tripathy & Levi, 2008). However, these studies have focused solely on deficits in visual representation, and have not assessed the consequences of visual deprivation on the decision and cognitive control processes that underlie response selection. The absence of binocular visual input may have downstream consequences for decision processes that depend on early visual experience for their complete maturation. Our results suggest that abnormal binocular experience may interfere with the development of visual decision-making.
Perceptual decision-making involves a collection of integrative processes, including accumulation of sensory evidence, deployment of attentional resources, motor planning/response selection and performance monitoring (Gold & Shadlen, 2007; Heekeren, et al., 2008; Shadlen & Newsome, 2001). Decision-making in the presence of conflict effectively engages these processes and we demonstrate here that these processes exhibit experience-dependent plasticity. Current models of the Eriksen task posit that prefrontal cortex provides the interface between sensory evidence and response selection and that ACC performs a conflict monitoring function that regulates cognitive control (Botvinick, et al., 2004; Gehring & Fencsik, 2001; Hazeltine, Poldrack, & Gabrieli, 2000; Kerns, et al., 2004). From our data we propose that functional connections between visual cortex and prefrontal and medial frontal cortex that are used to program and execute the response-selection stage of the decision may be degraded both through disuse during a developmental critical period (in the amblyopic eye) and through loss of fully binocular input (in the non-amblyopic eye). Further studies, in which parameters such as decision threshold and speed-accuracy tradeoff are manipulated, will help to localize more precisely the critical bottlenecks in perceptual decision-making associated with abnormal visual experience during early development.
Acknowledgements
Authors thank Chuan Hou, M.D., Ph.D. for conducting the ophthalmic examinations. This work was supported by National Institutes of Health grants EY06579 (AMN) and F32EY021389 (FF) and by a Walt and Lilly Disney Amblyopia Research Award from Research to Prevent Blindness (AMN).
References
- Bailey IL, Lovie JE. New design principles for visual acuity letter charts. American Journal of Optometry and Physiological Opthalmology. 1976;53:740–745. doi: 10.1097/00006324-197611000-00006. [DOI] [PubMed] [Google Scholar]
- Bernard JB, Chung ST. The dependence of crowding on flanker complexity and target-flanker similarity. Journal of Vision. 2011;11(8):1–16. doi: 10.1167/11.8.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Science. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Campbell FW, Green DG. Monocular versus binocular visual acuity. Nature. 1965;208:191–192. doi: 10.1038/208191a0. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinivk M, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the onlinemonitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Chandna A, Pennefather PM, Kovács I, Norcia AM. Contour integration deficits in anisometropic amblyopia. Investigative Opthalmology and Visual Science. 2001;42(3):875–878. [PubMed] [Google Scholar]
- Egner T. Multiple conflict-driven control mechanisms in the human brain. Trends in Cognitive Sciences. 2008;12(10):374–380. doi: 10.1016/j.tics.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a non-search task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Freeman RD, Thibos LN. Contrast sensitivity in humans with abnormal visual experience. Journal of Physiology. 1975;247:687–710. doi: 10.1113/jphysiol.1975.sp010952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. Journal of Neuroscience. 2001;21:9430–9437. doi: 10.1523/JNEUROSCI.21-23-09430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaschi DE, Regan D, Kraft SP, Hong XH. Defective processing of motion-defined form in the fellow eye of patients with unilateral amblyopia. Investigative Opthalmology and Visual Science. 1992;33(8):2483–2489. [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. Neural computation that underlie decisions about sensory stimuli. Trends in Cognitive Science. 2001;5:10–16. doi: 10.1016/s1364-6613(00)01567-9. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annual Review of Neuroscience. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. Optimizing the use of information: Strategic control of activation of responses. Journal of Experimental Psychology: General. 1992;121:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Poldrack R, Gabrieli JDE. Neural activation during response competition. Journal of Cognitive Neuroscience. 2000;12:118–129. doi: 10.1162/089892900563984. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Ungerleider LG. The neural systems that mediate human perceptual decision making. Nature Reviews Neuroscience. 2008;9(6):467–479. doi: 10.1038/nrn2374. [DOI] [PubMed] [Google Scholar]
- Hess RF, Howell ER. The threshold contrast sensitivity function in strabismic amblyopia: evidence for a two type classification. Vision Research. 1977;17:1049–1055. doi: 10.1016/0042-6989(77)90009-8. [DOI] [PubMed] [Google Scholar]
- Hess RF, McIlhagga W, Field DJ. Contour integration in strabismic amblyopia: the sufficiency of an explanation based on positional uncertainty. Vision Research. 1997;37:3145–3161. doi: 10.1016/s0042-6989(96)00281-7. [DOI] [PubMed] [Google Scholar]
- Ho CS, Giaschi DE, Boden C, Dougherty R, Cline R, Lyons C. Deficient motion perception in the fellow eye of amblyopic children. Vision Research. 2005;45:1615–1627. doi: 10.1016/j.visres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Ho CS, Paul PS, Asirvatham A, Cavanagh P, Cline R, Giaschi DE. Abnormal spatial selection and tracking in children with amblyopia. Vision Research. 2006;46(19):3274–3283. doi: 10.1016/j.visres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977;198(1130):1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Julesz B. Binocular depth perception of computer-generated patterns. Bell System Technical Journal. 1960;39:1125–1162. [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Klein SA, Levi DM. Vernier acuity, crowding and amblyopia. Vision Research. 1985;25:979–991. doi: 10.1016/0042-6989(85)90208-1. [DOI] [PubMed] [Google Scholar]
- Kooi FL, Toet A, Tripathy SP, Levi DM. The effect of similarity and duration on spatial interaction in peripheral vision. Spatial Vision. 1994;8:255–279. doi: 10.1163/156856894x00350. [DOI] [PubMed] [Google Scholar]
- Kovács I, Polat U, Pennefather PM, Chandna A, Norcia AM. A new test of contour integration deficits in patients with a history of disrupted binocular experience during visual development. Vision Research. 2000;40(13):1775–1783. doi: 10.1016/s0042-6989(00)00008-0. [DOI] [PubMed] [Google Scholar]
- Lamers MJM, Roelofs A. Attentional control adjustments in Eriksen and Stroop task performance can be independent of response conflict. The Quarterly Journal of Experimental Psychology. 2011;64:1056–1081. doi: 10.1080/17470218.2010.523792. [DOI] [PubMed] [Google Scholar]
- Leguire LE, Rogers GL, Bremer DL. Amblyopia: the normal eye is not normal. Journal of Pediatric Opthalmology and Strabismus. 1990;27:32–38. doi: 10.3928/0191-3913-19900101-10. [DOI] [PubMed] [Google Scholar]
- Levi DM, Carney T. The effect of flankers on three tasks in central, peripheral, and amblyopic vision. Journal of Vision. 2011;11 doi: 10.1167/11.1.10. [DOI] [PubMed] [Google Scholar]
- Levi DM, Hariharan S, Klein SA. Suppressive and facilitatory interactions in amblyopic vision. Vision Research. 2002;42:1379–1394. doi: 10.1016/s0042-6989(02)00061-5. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA. Hyperacuity and amblyopia. Nature. 1982;298:268–270. doi: 10.1038/298268a0. [DOI] [PubMed] [Google Scholar]
- Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nature Neuroscience. 2003;6:450–452. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. Journal of Vision. 2003;3:380–405. doi: 10.1167/3.5.5. [DOI] [PubMed] [Google Scholar]
- Parker AJ. Binocular depth perception and the cerebral cortex. Nature Reviews Neuroscience. 2007;8:379–391. doi: 10.1038/nrn2131. [DOI] [PubMed] [Google Scholar]
- Popple AV, Levi DM. The attentional blink in amblyopia. Journal of Vision. 2008;8(13) doi: 10.1167/8.13.12. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. Journal of Neurophysiology. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- Sharma V, Levi DM, Klein SA. Undercounting features and missing features: evidence for a high-level deficit in strabismic amblyopia. Nature Neuroscience. 2000;3(5):496–501. doi: 10.1038/74872. [DOI] [PubMed] [Google Scholar]
- Simmers AJ, Bex PJ. The representation of global spatial structure in amblyopia. Vision Research. 2004;44:523–533. doi: 10.1016/j.visres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Simmers AJ, Ledgeway T, Hess RF, McGraw PV. Deficits to global motion processing in human amblyopia. Vision Research. 2003;43:729–738. doi: 10.1016/s0042-6989(02)00684-3. [DOI] [PubMed] [Google Scholar]
- Simmers AJ, Ledgeway T, Mansouri B, Hutchinson CV, Hess RF. The extent of the dorsal extra-striate deficit in amblyopia. Vision Research. 2006;46:2571–2580. doi: 10.1016/j.visres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Tjan BS, Dang S. The spatial interaction zone of a shapeles noise flanker. Journal of Vision. 2005;10(5):227. [Google Scholar]
- Tripathy SP, Levi DM. On the effective number of tracked trajectories in amblyopic human vision. Journal of Vision. 2008;8(4):1–22. doi: 10.1167/8.4.8. [DOI] [PubMed] [Google Scholar]
- Usher M, McClelland JL. On the time course of perceptual choice: The leaky competing accumulator model. Psychological Review. 2001;108:550–592. doi: 10.1037/0033-295x.108.3.550. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology and Behavior. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]