Abstract
Obesity is a major health concern that contributes to the development of diabetes, hyperlipidemia, coronary artery disease, and cancer. Id proteins are helix-loop-helix transcription factors that regulate the proliferation and differentiation of cells from multiple tissues, including adipocytes. We screened mouse tissues for the expression of Id1 and found that Id1 protein is highly expressed in brown adipose tissue (BAT) and white adipose tissue (WAT), suggesting a role for Id1 in adipogenesis and cell metabolism. Id1−/− mice are viable but show a significant reduction in fat mass (P<0.005) over the life of the animal that was not due to decreased number of adipocytes. Analysis of Id1−/− mice revealed higher energy expenditure, increased lipolysis, and fatty acid oxidation, resulting in reduced triglyceride accumulation in WAT compared to Id1+/+ mice. Serum levels of triglycerides (193.9±32.2 vs. 86.5±33.8, P<0.0005), cholesterol (189.4±33.8 vs. 110.6±8.23, P<0.0005) and leptin (1263±835 vs. 222±260, P<0.005) were significantly lower in aged Id1−/− mice compared to Id1+/+ mice. Id1-deficient mice have higher resting (P<0.005) and total (P<0.05) O2 consumption and lower respiratory exchange ratio (P<0.005), confirming that Id1−/− mice use a higher proportion of lipid as an energy source for the increased energy expenditure. The expression of PGC1α and UCP1 were 2- to 3-fold up-regulated in Id1−/− BAT, suggesting that loss of Id1 increases thermogenesis. As a consequence of higher energy expenditure and reduced fat mass, Id1−/− mice displayed enhanced insulin sensitivity. Id1 deficiency protected mice against age- and high-fat-diet-induced adiposity, insulin resistance, and hepatosteatosis. Our findings suggest that Id1 plays a critical role in the regulation of energy homeostasis and could be a potential target in the treatment of insulin resistance and fatty liver disease.—Satyanarayana, A., Klarmann, K. D., Gavrilova, l O., Keller, J. R. Ablation of the transcriptional regulator Id1 enhances energy expenditure, increases insulin sensitivity, and protects against age and diet-induced insulin resistance and hepatosteatosis.
Keywords: PGC1a, UCP1, adipogenesis, brown adipose tissue, thermogenesis
In mammals, the adipose tissue functions as a major site to control fatty acid (FA) metabolism and energy homeostasis (1). There are two types of functionally distinct adipose tissue in the body: white adipose tissue (WAT) and brown adipose tissue (BAT) (2). The major function of WAT is to store excess energy in the form of triglycerides (TGs) when energy intake exceeds expenditure. Conversely, when energy expenditure exceeds intake, TGs are broken down and release free fatty acids (FFAs) for other tissues to utilize as an energy source. WAT is an active endocrine organ and secretes several cytokines, also known as adipokines, such as leptin, adiponectin, resistin, IL6, and TNF-α, which play important roles in glucose, FA metabolism, and insulin sensitivity (3). In contrast to WAT, BAT is specialized for energy expenditure by dissipating energy as heat, a process termed adaptive thermogenesis (4). This unique metabolic property of production of heat by BAT is attributed to its high mitochondrial density and its exclusive expression of uncoupling protein-1 (UCP1) in the inner mitochondrial membrane (5). During respiration, an electron-motive force established across the inner mitochondrial membrane is dissipated as heat by UCP1, rather than being used to drive the synthesis of ATP (5, 6).
A precise balance between energy intake and expenditure is crucial for maintenance of normal and functional adipose tissue. The adipocyte number, size, and the total amount of WAT are dynamically in proportion to energy levels of the body (1). If the energy intake is persistently higher than expenditure, it leads to abnormally high synthesis of TGs, with a concomitant increase in adipocyte size (hypertrophy) and number (hyperplasia) to accommodate excessive TGs. If this process continues, it ultimately leads to aberrant increase in total body WAT, adipokine disregulation, and associated metabolic complications, such as insulin resistance, impaired glucose tolerance, diabetes, and dyslipidemia (7). In addition, excessive lipids start to accumulate in the non-WAT such as bone marrow, liver (hepatosteatosis), skeletal muscle, and BAT, thereby further impairing the metabolic and thermogenic capacity of the body.
Understanding adipogenesis and the role of adipose tissue in energy balance is absolutely essential for identifying therapeutic targets to treat metabolic disorders. So far, several transcription factors have been identified as critical regulators of adipocyte gene expression and differentiation, such as CCAAT/enhancer binding proteins (C/EBPs), peroxisome proliferator-activated receptor (PPAR) family proteins, adipocyte differentiation determinant-dependent factor 1 (ADD1), retinoid X receptor (RXR) isotypes, GATA, and KLF-4 (1, 8, 9). Deletion of one or more of these genes has direct affect on adipogenesis and therefore alters adipose mass and modulates FA metabolism and energy balance (10–13). Conversely, another set of proteins was identified to have prominent role in cell metabolism and energy balance by regulating thermogenesis in BAT. These genes, termed thermogenic genes, include PGC1α, PPARγ, Cidea, protein kinase IKKε, and transcriptional intermediary factor-2 (TIF2). These proteins respond to certain physiological stimuli and control the expression of uncoupling proteins (UCPs) in BAT, thereby regulating thermogenesis and energy balance (14–18).
Id1 (inhibitor of DNA binding 1) is a helix-loop-helix transcription factor that is implicated in the regulation of multiple cellular processes, such as cell proliferation, differentiation, cell cycle progression, cell fate determination, and angiogenesis (19, 20). Id1 mediates its effects by acting as a negative regulator of basic helix-loop-helix (bHLH) transcription factors, which control cell type-specific gene expression (19). Id genes are expressed in embryonic stem cells, and are known to regulate the earliest cell fate decisions during development. In addition, Id genes are required for embryogenesis since animals that lack two of the Id gene family die during the early stages of development. We have discovered that Id1 is highly expressed in adult adipose tissues, especially brown adipose tissue, suggesting that Id1 might be involved in adipogenesis and metabolism. By utilizing an Id1 knockout mouse model, we demonstrate for the first time that Id1 is a novel regulator of adiposity and energy homeostasis.
MATERIALS AND METHODS
Mice and diet
Mice were housed under standard conditions with a 12-h light-dark cycle, and they were handled in compliance with the U.S. National Institutes of Health guidelines for animal care and use. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the National Cancer Institute at Frederick. For this study, 129Sv/J X C57BL/6J-mixed background mice were used. Mice were fed a standard chow regular diet (RD) containing 5% crude fat (5L79C3N; Purina Mills, St. Louis, MO, USA). For the high-fat-diet (HFD) experiments, 8-wk-old mice were switched from RD to HFD (45 kcal% fat diet; D12451; Research Diets, New Brunswick, NJ, USA) and fed for 4 mo. For cold treatment studies, mice were housed individually in standard cages without bedding, and the cages were placed in the cold room (4°C) for 4 h. During this period, rectal temperatures were recorded at 30-min intervals. After 4 h, mice were killed, and BAT was harvested.
Food consumption, body composition, and metabolic measurements
To measure food consumption, 2-mo-old male mice were housed individually, and each mouse was provided the same amount (200 g) of food. Mouse weight and food weight were measured at weekly intervals for 8 wk, and the food consumption rate was normalized to body weight. Food consumption assays were done with RD and HFD. Whole-body composition of Id1+/+ and Id1−/− mice was measured using NMR analyzer Echo3-in-1 (Eco Medical Systems, Edmonton, AB, Canada). To measure metabolic rate, the mice were acclimated for 24 h, and then they were monitored for 24 h in an indirect open-circuit calorimeter (Oxymax System; Columbus Instruments, Columbus, OH, USA). O2 consumption and CO2 release were measured as volume, and respiratory quotient (RQ) was calculated as the ratio of Vco2/Vo2. The measurements were conducted at room temperature (RT) and normalized to lean mass to account for the disparity in body size between the two groups. Physical activity and ambulatory movements of the mice were measured as described previously (21).
Induction of adipocyte differentiation
Mouse embryonic fibroblasts (MEFs) were cultured in 10-cm dishes and induced to differentiate into adipocytes, as described previously (22). Briefly, E13.5 Id1+/+ and Id1−/− MEFs (passage 2) were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin (15140; Life Technologies, Carlsbad, CA, USA). After the cells became confluent, the medium was replaced with adipocyte differentiation induction medium [DMEM, 10% FBS, 0.5 mM 3-isobutyl-1-methylxanthine (I7018; Sigma, St. Louis, MO, USA), 1 μM dexamethasone (D1756; Sigma), 5 μg/ml insulin (I2643; Sigma), and 1% penicillin/streptomycin]. Medium was replaced with fresh medium after every alternative day for 12 d.
Retrovirus-mediated Id1 overexpression in 3T3-L1 cells
3T3-L1 preadipocyte cell line (CL-173) was purchased from American Type Culture Collection (Manassas, VA, USA). Constitutive overexpression of Id1 in 3T3-L1 cells was achieved by infecting the cells with retrovirus carrying either Id1 (MSCV-Id1-IRES-GFP) or control GFP (MSCV-GFP). These viral vectors were described previously (23). 3T3-L1 cells were transduced using the retronectin method (TAK-T100B; Fisher Scientific, Waltham, MA, USA), according to manufacturer's instructions. After 3 rounds of infection, >95% of the cells were infected, as revealed by the expression of GFP. Then, the cells with highest GFP expression (top 25%) were FACS sorted, cultured, and used for adipogenesis differentiation assays. Adipogenesis was induced as described above. For drug treatment assays, adipogenesis was induced in Id1-overexpressing 3T3-L1 cells, and on d 5 or 8, the cells were treated with either MG132 (20 μM final concentration) or DMSO for 8 h.
Oil-Red-O staining
The differentiation of 3T3-L1 cells and MEFs into adipocytes was detected by Oil-Red-O staining. After the cells were induced to differentiate, at 4, 6, 8, 10, and 12 d, cells were washed with PBS, fixed in 10% neutral buffered formalin solution (NBF; HT501128; Sigma) for 15 min, and stained with 2 ml of 0.5% Oil-Red-O (O0625; Sigma) in isopropanol:distilled water (3:2) for 30 min at RT with gentle shaking (100 rpm) on a rocking platform. Then, the cells were washed with distilled water 3 times, air dried, and photographed (Canon Power Shot G10 Camera; Canon, Tokyo, Japan). High-power (×10) pictures were captured by Leica microscope equipped with Qimaging camera and Metamorph software (Leica Microsystems, Wetzlar, Germany). To quantify staining, 2 ml of isopropanol was added, and the cells were destained by gentle shaking (150 rpm) on a rocking platform for 10 min. The staining intensity was measured by reading the absorbance at 510 nm using Multiskan Spectrum and the software version 1.10 (Thermo Labsystems, Woburn, MA, USA). To stain liver and muscle sections by Oil-Red-O, the sections were rinsed with water for 5 min, followed by brief rinsing in 50% isopropanol. The slides were immersed in Oil-Red-O staining solution (0.2% Oil-Red-O in isopropanol:distilled water, 3:2) for 20 min at RT. Then, the slides were briefly rinsed with 50% isopropanol (10 s) to remove excess stain, followed by a brief rinse in water (10 s), and counterstained in Mayer's hematoxylin solution (MHS1; Sigma) and mounted in aqueous mounting medium.
Cell cycle analysis by FACS
3T3-L1 cells that constitutively overexpress Id1 or control GFP were cultured in 10-cm dishes. When the cells reached 70% confluency, they were subjected to cell cycle analysis. The cells were pulse labeled with BrdU (1 h; 51-2354AK, BD Pharmingen FITC-BrdU Flow kit; BD Biosciences, San Jose, CA, USA) and stained according to the manufacturer's protocol. The cells were analyzed by FACS-LSRII (BD Biosciences) and FlowJo software (TreeStar Inc., Ashland, OR, USA).
DNA extraction
Epididymal fat pads were removed from male Id1+/+ and Id1−/− mice fed RD or HFD. Individual fat pads were weighed and then digested overnight at 56°C, and DNA was extracted following the manufacturer's protocol (DNeasy Blood & Tissue Kit, 69504; Qiagen, Venlo, The Netherlands). The DNA concentration was measured by NanoDrop spectrophotometer (ND1000; NanoDrop Technologies, Wilmington, DE, USA) and normalized to 100 mg fat mass.
Hematoxylin and eosin (H&E) staining
Epididymal fat pads and inguinal, retroperitoneal, brown fat, and liver tissues were fixed in 10% NBF solution (HT501128; Sigma) for 24 h, embedded in paraffin, and cut into 5-μm-thick sections. The slides were rinsed with distilled water (2 min), incubated in hematoxylin (7231; Richard-Allan Scientific, Kalamazoo, MI, USA) for 3 min, and then washed with distilled water twice for 2 min. The slides were treated with clarifier (7402; Richard-Allan Scientific) for 2 min, followed by a brief wash with distilled water. The slides were immersed in Bluing reagent (7301; Richard-Allan Scientific) for 1 min and then washed with water (2 min). After incubation in 95% ethanol for 1 min, the sections were incubated with Eosin Y (7111; Richard-Allan Scientific) for 20 s. Later, the slides were incubated in 100% ethanol (3×1 min) followed by xylene (3×1 min). The staining pattern was analyzed and photographed using a Leica microscope equipped with Qimaging camera and Metamorph software.
TG and cholesterol measurement
Mice were starved overnight. To collect whole blood, mice were anesthetized, and blood was drawn from the heart into BD Microtainer serum separator tubes (365956; Becton Dickinson, Franklin Lakes, NJ, USA). Blood serum was obtained by centrifuging the tubes at 4000 rpm for 10 min. Serum TG and cholesterol levels were measured using CHOL and TRIG slides (Vitros chemistry products; Ortho Clinical Diagnostics, Raritan, NJ, USA), and Vitros 250 analyzer (Ortho Clinical Diagnostics), according to manufacturer's instructions.
Extraction of lipids from liver
Livers were harvested from HFD-fed Id1+/+ and Id1−/− mice and weighed; 500 mg of liver tissue was homogenized in 1 ml of ice-cold phosphate-buffered saline (PBS), and lipids were extracted by the method of Bligh and Dyer (24). Triglyceride content was measured as described above.
Glucose, insulin, and adipokine measurements
Blood glucose levels were measured from the tail blood by One Touch Ultra II Glucometer (LifeScan, Milpitas, CA, USA) and One Touch Ultra glucose strips (LifeScan). For insulin and adipokine measurements, mice fed RD or HFD were starved overnight, blood serum was prepared as described above, and the serum samples were portioned into aliquots and stored at −80°C until use. Insulin concentration was determined by insulin (mouse) ultrasensitive EIA kit (80-INSMSU-E01; Alpco, Salem, NH, USA). Leptin and adiponectin levels were measured by leptin (murine/rat) EIA kit (10007609; SPI Bio, Wetherill Park, NSW, Australia) and adiponectin (murine) EIA kit (A05187; SPI Bio). IL6 concentration was measured by mouse IL6-platinum ELISA kit (BMS603TWO; eBioscience, San Diego, CA, USA) kit. IGF-1 levels were measured by IGF-1 (mouse/rat) ELISA (22-IG1MS-E01; Alpco). Nonesterified FAs were measured by serum/plasma FA kit (SFA-5; Zenbio, Research Triangle, NC, USA). For all of the ELISAs, manufacturer's protocols were followed. The ELISA plates were read using Multiskan Spectrum and the software version 1.10 (Thermo Labsystems).
Glucose tolerance tests (GTTs) and insulin tolerance test (ITTs)s
For GTTs, mice were starved overnight then given intraperitoneal injections of 2 mg glucose/g body weight (G7528; Sigma). Blood glucose levels were measured from tail blood using One Touch Ultra II Glucometer (LifeScan) and One Touch Ultra glucose strips (LifeScan) at basal, 15, 30, 60, 90, 120, and 180 min. For ITTs, mice fed RD or HD were starved for 3 h, and 0.75 or I mU insulin/g body weight (I2643; Sigma), respectively, was injected intraperitoneally. Blood glucose levels were measured as in the GTT assay.
Glucose uptake assay
Single-cell suspensions of adipocytes were prepared from fat pads of 12-wk-old male mice, as described previously (25, 26). Glucose uptake assays using the fluorescent glucose analog 2-NBDG (N13191; Invitrogen, Carlsbad, CA, USA) were conducted, as described previously (27). In brief, adipocytes were plated in 6-well plates (1×106 cells/well) containing 2 ml of glucose-free RPMI medium. Then, the plates were placed at 37°C for 20 min to allow the cells to settle and adapt to 37°C. Then, 2-NBDG (10 μM final concentration) and insulin (5 μg/ml) were added to the cells and incubated for 30 min. The cells were then collected, centrifuged, washed 2 times with PBS and 0.2% BSA, and analyzed by FACS-LSRII (BD Biosciences) and FlowJo software.
FA oxidation assays
In vivo FA ([1-14C] oleic acid) oxidation assays were conducted as described previously (28). Mice were injected with [1-14C] oleic acid (1 μCi in 200 μl of saline, intraperitoneal injection; NEC317; Perkin Elmer, Wellesley, MA, USA), and placed into a sealed chamber connected to an air pump (7893B05; Thomas Scientific, Swedesboro, NJ, USA) and a 50-ml tube containing 3 M NaOH solution to trap expired 14CO2. Aliquots (1 ml) were collected at the indicated time points after injection of [1-14C] oleic acid to calculate the oxidation of [1-14C] oleic acid to 14CO2. FFA oxidation rates were measured in isolated soleus muscles as described previously (29).
In vitro lipolysis
Epididymal fat pads were harvested from 2-mo-old male mice. Fat pads were weighed; one fat pad was used for measuring basal lipolysis, and the second fat pad was used for isoproterenol stimulated lipolysis. The fat pads were rinsed with PBS once, minced into ∼0.25-cm pieces, and transferred to 10-cm plates containing 15 ml of KRBH buffer (135 mM NaCl, 2.2 mM CaCl2·2H2O, 1.25 mM MgSO4·7H2O, 0.45 mM KH2PO4, 2.17 mM Na2HPO4, 5 mM d-glucose, 2% BSA, and 10 mM HEPES). Basal and stimulated lipolysis was determined by assaying FFAs released. Lipolysis was induced by adding isoproterenol (10 μM final concentration; I6504; Sigma) to the plates containing WAT explants and KRBH buffer. The plates were incubated at 37°C, and 250-μl samples were collected at 10 and 30 min and 1, 2, 4, 6, and 12 h. FFA levels were measured by serum/plasma FA kit (SFA-5; Zenbio).
Rectal temperature measurement
The body temperature of the mice was measured by superfast waterproof digital thermometer (RT600c; Thermoworks, Lindon, UT, USA). The tip of the thermometer was inserted into the rectum of the mice (∼1 cm), and the temperature (°C) was recorded.
RNA preparation and PCR superarray
Epididymal fat pads and interscapular BAT were harvested from 2-mo-old Id1+/+ and Id1−/− male mice. The tissues were lysed by AGP TR-S0 (AutoGen, Calne, UK) lysis solution, and RNA was extracted by the phenol-chloroform method. The RNA quantity and purity were determined by a Nanodrop spectrophotometer. The RNA quality was determined by an Agilent 2100 Bioanalyzer using the Agilent RNA 6000 Nano kit (5067-1511; Agilent Technologies, Santa Clara, CA, USA). The RNA was used to perform Mouse Obesity RT2 Profiler array (PAMM-017; SABiosciences, Frederick, MD, USA), which profiles the expression of 84 genes related to appetite control and FA metabolism, and genes related to energy expenditure. The PCR superarray (384 well) was run using Applied Biosystems ABI-7900 SDS, according to the manufacturer's instructions, and the data were analyzed by Web-based free PCR array data analysis software provided by the manufacturer (SABiosciences).
Immunoblotting
Whole-cell lysates were prepared from MEFs and 3T3-L1 cells after inducing adipogenesis, and from different tissues of adult mice by using RIPA lysis buffer (89900; Thermo Fisher Scientific, Waltham, MA, USA), supplemented with complete mini-protease inhibitor tablet (11836153001; Roche, Mannheim, Germany). For Western blot analysis, 50 μg of protein was separated on NuPAGE precast gels (Invitrogen), transferred using XCell II Blot module (090707-098; Invitrogen,) onto Immobilon-P membranes (IPVH07850; Millipore, Billerica, MA), and probed with specific primary antibodies: anti-Id1 (BCH-1, 37-2; Biocheck, Foster City, CA, USA), anti-PPARγ (sc-7196; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-aP2 (sc-18661; Santa Cruz Biotechnology), anti-UCP-1 (sc-6529; Santa Cruz Biotechnology), anti-PGC1α (sc-13067; Santa Cruz Biotechnology), anti-Akt (4691S; Cell Signaling, Beverly, MA, USA), anti-pAkt (4060S; Cell Signaling) and anti-β-actin (A5441; Sigma). All the antibodies were used at a dilution of 1:1000 except anti-Id1 and anti-aP2, used at 1:2500 dilution, anti-Akt and anti-pAkt, used at 1:2000 dilution, and anti-β-actin, used at 1:10000 dilution. The following HRP-conjugated secondary antibodies were used: anti-mouse (NA931V; Amersham Biosciences, Piscataway, NJ, USA) anti-rabbit (W401B; Promega, Madison, WI, USA) and anti-goat (A5420; Sigma). For Akt phosphorylation assays, mice fed RD or HFD were starved for 3 h and then injected with 0.75 mU or 1 mU insulin/g body wt, respectively. After 15 min, animals were killed, and tissues were harvested for whole-cell lysate preparation.
Statistical programs
Student's t test, GraphPad (San Diego, CA, USA), and Excel (Microsoft, Redmond, WA, USA) were used to calculate the statistical significance and standard deviation. The data are presented as means ± sd.
RESULTS
Id1 is highly expressed in adipose tissues, and Id1 deficiency results in reduced fat mass
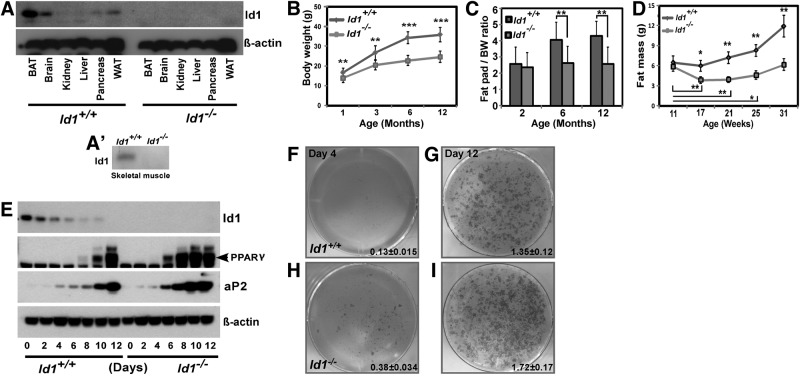
We measured the expression levels of Id1 protein in different adult mouse tissues. Of the tissues tested, we found that Id1 expression was highest in BAT, WAT, and brain, while Id1 expression was low in skeletal muscle and pancreas and undetectable in other tissues (Fig. 1A, A′). Higher expression of Id1 in BAT and WAT suggests that Id1 may function in adipose tissue metabolism. Therefore, we questioned whether Id1 deficiency might have systemic metabolic consequences and analyzed Id1−/− mice. A comparison of Id1−/− and Id1+/+ newborn (1.2±0.05 vs. 1.42±0.09 g, P<0.0001) and P10 pups (5.35±0.53 vs. 6.33±0.64 g, P=0.0011) revealed that Id1−/− mice weighed ∼15% less than controls, suggesting minor growth defects in Id1−/− mice. Similarly, 1-mo-old Id1−/− mice weighed 12–15% less than Id1+/+ mice; however, this disparity in body weight was exacerbated as mice aged, and 3, 6, and 12-mo-old Id1−/− mice weighed ∼30% less than control mice (Fig. 1B and Supplemental Fig. S1A, B). Thus, in addition to early postnatal growth defects, other factors, such as body metabolism, might have contributed to reduced body weight gain in Id1−/− mice during aging.
Figure 1.
Reduced adiposity in Id1−/− mice. A) Expression levels of Id1 protein and loading control β-actin in the indicated mouse tissues. A′) Expression level of Id1 protein in the skeletal muscle of Id1+/+ and Id1−/− mice. B) Body weights of female (n=18–24/group) mice at the indicated age. **P < 0.005; ***P < 0.0005. C) Weight of epididymal fat pads (n=12/group) at the indicated age, normalized to body weight. D) Total fat mass measured by NMR at the indicated age (n=6/group). E) Expression levels of Id1 protein, markers of adipogenesis PPARγ and aP2, and loading control β-actin in Id1+/+ and Id1−/− cells after induction of adipogenesis at the indicated time points. F–I) Representative photos of Oil Red O-stained Id1+/+ (F, G) and Id1−/− (H, I) MEFs undergoing adipocyte differentiation at d 4 (F, H) and d 12 (G, I); n = 6/genotype; 3 independent experiments were conducted. Oil Red O-stained cells were destained with isopropanol; amount of staining was quantified by reading absorbance at 510 nm.
To determine whether Id1 deficiency affects body adiposity, we measured the weight of various tissues, then normalized the values to total body weight. Organs such as heart, kidney (not shown) and liver (Supplemental Fig. S1C) of Id1−/− mice had weights similar to Id1+/+ mice of all ages. Analysis of epididymal fat pads of young (2 mo), adult (6 mo) and aged (12 mo) mice revealed a steady increase in the weight of epididymal fat pads in Id1+/+ mice during aging. In contrast, fat pads in Id1−/− mice did not gain any weight, and compared to Id1+/+ mice, fat pad weight was significantly reduced (Fig. 1C). To confirm the changes in fat mass during aging, we also measured whole-body composition of Id1+/+ and Id1−/− mice of different age groups by NMR. Fat mass steadily increased in Id1+/+ mice, and by 31 wk, fat mass was increased by 90% compared to 11-wk-old Id1+/+ mice. In comparison, Id1−/− mice did not gain any fat mass over the same period of time (Fig. 1D). In contrast, lean mass steadily increased in Id1−/− mice, similar to Id1+/+ mice (Supplemental Fig. S1D). These results suggest that Id1−/− mice failed to gain fat mass during aging.
Id1 is down-regulated during adipogenesis, and lack of Id1 accelerates adipocyte differentiation
To investigate whether defective adipogenesis is responsible for reduced fat mass in Id1−/− mice and whether Id1 is required for adipocyte differentiation, we evaluated the expression pattern of Id1 during in vitro adipogenesis. When MEFs were induced to differentiate into adipocytes, Id1 was strongly expressed before the induction of differentiation, but rapidly disappeared during adipocyte differentiation (Fig. 1E), suggesting that down-regulation of Id1 might be necessary for adipocyte differentiation. Next, we assessed the ability of Id1−/− MEFs to differentiate into adipocytes and found that their differentiation was slightly increased compared to Id1+/+ cells after 4, 8, and 12 d, as revealed by increased number of cells with adipocyte morphology and Oil-Red-O staining (Fig. 1F–I). We also analyzed the expression levels of markers of adipogenesis PPARγ and aP2, and found that their expression was induced earlier in Id1−/− cells compared to Id1+/+ cells (Fig. 1E), suggesting that adipogenesis was accelerated in the absence of Id1.
On the basis of down-regulation of Id1 in wild-type cells during adipogenesis and accelerated adipocyte differentiation in the absence of Id1, we postulated that overexpression of Id1 could delay or block adipogenesis. To test this hypothesis, 3T3-L1 preadipocytes were infected with a retrovirus expressing Id1-GFP or control GFP. After infection and selection of cells with highest GFP expression by flow cytometry, higher levels of Id1 were detected in the MSCV-Id1-transduced 3T3-L1 cells (Supplemental Fig. S1E–N). Surprisingly, Id1-overexpressing 3T3-L1 cells showed the same degree of adipocyte differentiation as control cells, as determined by Oil-Red-O staining (Supplemental Fig. S1L, M) and expression analysis of adipocyte markers PPARγ and aP2 (Supplemental Fig. S1O). Most interestingly, overexpressed Id1 was rapidly decreased during adipogenesis, similar to endogenous Id1 (Supplemental Fig. S1O), which could explain why overexpressed Id1 showed no effect on differentiation. Down-regulation of Id1 during adipogenesis could be due to ubiquitination and degradation, similar to Id1 degradation by the ubiquitin-proteosome pathway during muscle development (30). In this regard, we found that Id1 was heavily ubiquitinated and stabilized in Id1-overexpressing 3T3-L1 cells undergoing adipogenesis that were treated with proteosome inhibitor MG132 (Supplemental Fig. S1P). This suggests that Id1 was subjected to proteosome-mediated degradation during adipogenesis and therefore not able to inhibit adipocyte differentiation as predicted.
Since adipocyte differentiation was accelerated in Id1−/− cells, we asked whether this resulted in an abnormal increase in adipocyte numbers in vivo. Thus, we determined adipocyte numbers by measuring the DNA content in epididymal fat pads, as described previously (18). DNA content per fat pad was not significantly different between 2-mo-old Id1−/− and Id1+/+ mice (73.12±23 vs. 60.16±28.8 μg) and was significantly less in adult Id1−/− mice compared to Id1+/+ mice (57.67±13.52 vs. 95.56±33.75 μg, P<0.005), indicating that Id1 deficiency did not cause abnormal increase in adipocyte number in vivo. These results together suggest that Id1 is targeted for degradation during adipogenesis, and Id1 deficiency merely accelerates adipocyte differentiation, but it does not result in increased adipocyte numbers in vivo. Therefore, defective adipogenesis is not likely to account for the reduced fat mass in Id1−/− mice.
Increased metabolic rate and FA oxidation in Id1-deficient mice
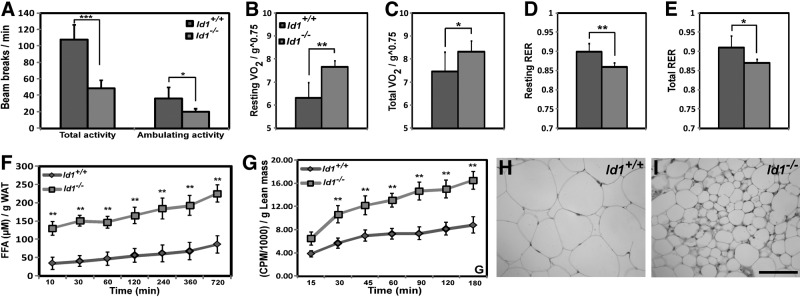
Since adipogenesis is not defective in the absence of Id1, a failure to gain fat mass in Id1−/− mice could be explained by reduced food intake or increased energy expenditure. We found that food intake in the RD groups did not differ significantly between Id1−/− and Id1+/+ mice, after adjusting for body weight (Supplemental Fig. S2A). However, Id1−/− mice showed reduced levels of physical activity, total as well as ambulating activity, compared to Id1+/+ mice (Fig. 2A). Thus, the observed reduction in the WAT mass in Id1−/− mice was not due to differences in food intake or increased physical activity. Therefore, we assessed the effect of Id1 deficiency on whole-body metabolic rates, by measuring O2 consumption and respiratory exchange ratio (RER) with open-circuit indirect calorimetry. Id1−/− mice exhibited higher resting and total O2 consumption (Vo2), and lower RER (RER=Vco2/Vo2; Fig. 2B–E), indicating that energy expenditure is increased in Id1-deficient mice and that they use a relatively higher proportion of lipid as an energy source compared to Id1+/+ mice. Thus, the failure to gain fat mass with age in Id1−/− mice can be explained, in part, by an increase in lipid oxidation and energy expenditure.
Figure 2.
Increased energy expenditure in Id1−/− mice. A) Total and ambulatory activity levels over a 24-h period at RT (n=8/group). B, C) Resting (B) and total (C) O2 consumption at RT (n=6/group), normalized to lean mass. D, E) Resting (D) and total (E) respiratory exchange ratio (RER) at RT (n=6/group). F) Amount of FFA released per gram of Id1+/+ and Id1−/− WAT explants in vitro (n=5/group) in the presence of isoproterenol. I) In vivo FA oxidation rates in Id1+/+ and Id1−/− mice (n=5/group) at the indicated time points. H, I) H&E-stained sections of inguinal fat of 6-mo-old Id1+/+ (H) and Id1−/− (I) mice. Scale bar = 100 μm. *P < 0.05; **P < 0.005; ***P < 0.0005.
To elucidate whether lipolysis was enhanced in Id1−/− mice, we determined the amount of FFAs released from WAT explants under basal conditions and after β-adrenergic stimulation by isoproterenol, which stimulates lipolysis by increasing cAMP levels. The amount of FFAs released from Id1−/− WAT was significantly higher compared to Id1+/+ WAT at basal level (26.85±4.47 vs. 14.36±1.97 μM, P<0.01, at 30 min; 39.58±6.88 vs. 22.33±3.85 μM, P<0.01, at 1 h), as well as in the presence of isoproterenol (Fig. 2F), suggesting that lack of Id1 resulted in increased lipolysis in the adipose tissue. Since lipolysis is higher and RER is lower in Id1−/− mice, it appears that Id1−/− mice use relatively higher proportions of FAs than Id1+/+ mice. To evaluate this possibility, we assessed FA oxidation rates in Id1−/− mice by performing in vivo and in vitro FA oxidation assays. Whole-body FA oxidation rates in vivo (Fig. 2G) and FA oxidation rates in the isolated soleus muscles in vitro (Supplemental Fig. 2B), were significantly higher in Id1−/− mice compared to control mice. These data suggest that enhanced lipolysis and FA oxidation contributed to reduced fat mass in Id1−/− mice. Strikingly, histological analysis revealed that in Id1−/− mice, adipocytes from epididymal, retroperitoneal, and inguinal WAT were significantly smaller and heterogeneous in size compared to Id1+/+ WAT (Fig. 2H, I and Supplemental Fig. S2C–F). Analysis of cell diameter of the adipocytes in the WAT confirmed that there was a significant increase in the frequency of smaller adipocytes, and fewer medium-sized and larger adipocytes in Id1−/− WAT compared to Id1+/+ WAT (Supplemental Fig. S2G, H). These data suggest that the failure to gain fat mass in Id1−/− mice during aging resulted from higher energy expenditure and less TG accumulation in adipocytes.
To elucidate the potential physiological consequences of reduced fat mass in Id1−/− mice during aging, we measured serum adipokines, TGs, and cholesterol in different age groups of Id1+/+ and Id1−/− mice (Table 1). Consistent with reduced adiposity in Id1−/− mice, serum leptin levels were markedly decreased in Id1−/− mice compared to Id1+/+ mice of same age groups. TG and cholesterol levels were also significantly reduced in Id1−/− mice in comparison to Id1+/+ mice. Conversely, adiponectin levels were indistinguishable between Id1−/− and Id1+/+ mice of all age groups (Table 1). These results indicate that Id1 deficiency resulted in reduced fat mass, which correlated with lower circulating TGs, cholesterol, and leptin.
Table 1.
Metabolic parameters of Id1+/+ and Id1−− mice
| Parameter |
Id1+/+ age (mo) |
Id1−/− age (mo) |
Id1+/+HFD | Id1−/− HFD | ||||
|---|---|---|---|---|---|---|---|---|
| 2 | 6 | 12 | 2 | 6 | 12 | |||
| Glucose (mg/dl) | 129.4 ± 15.6 | 140.2 ± 17.9 | 142.4 ± 20.8 | 127.9 ± 15.5 | 136.5 ± 18.3 | 136.4 ± 21.3 | 157.2 ± 20.8 | 146 ± 12.2 |
| Insulin (ng/ml) | 0.85 ± 0.53 | 3.62 ± 3.29 | 4.12 ± 2.95 | 0.58 ± 0.1 | 0.48 ± 0.07** | 1.08 ± 0.89** | 4.75 ± 1.82 | 0.45 ± 0.08*** |
| Triglycerides (mg/dl) | 177.3 ± 22.5 | 148.6 ± 30 | 193.9 ± 32.2 | 169.5 ± 25 | 107 ± 26* | 86.5 ± 33.8*** | 124.8 ± 28.3 | 118.4 ± 31.9 |
| FFA (μM) | 112.6 ± 33.2 | 167.9 ± 71 | 117.3 ± 29.2 | 150.1 ± 37.1* | 201.1 ± 54 | 96.4 ± 24.1 | 122.8 ± 28.4 | 169.1 ± 41* |
| Cholesterol (mg/dl) | 173.4 ± 39.5 | 208.5 ± 44.5 | 189.4 ± 33.8 | 125.3 ± 20.9** | 124.6 ± 18.5** | 110.6 ± 8.23*** | 277.4 ± 38.5 | 148.5 ± 17.8*** |
| Leptin (pg/ml) | 133 ± 181 | 1590 ± 1326 | 1263 ± 835 | 25.8 ± 33 | 212 ± 166** | 222 ± 260** | 2406 ± 456 | 475 ± 570*** |
| Adiponectin (ng/ml) | 2.85 ± 0.7 | 2.54 ± 0.8 | 2.77 ± 0.4 | 2.62 ± 0.59 | 2.43 ± 0.51 | 2.71 ± 0.62 | 2.11 ± 0.8 | 2.6 ± 0.7 |
| IL-6 (pg/ml) | 132.6 ± 61 | 272.6 ± 97 | 318.4 ± 281 | 94.8 ± 33 | 169.4 ± 49 | 204 ± 89.2 | 442.6 ± 182 | 292.7 ± 111* |
| IGF-1 (ng/ml) | 6.8 ± 1.3 | 8.2 ± 1.2 | 5.7 ± 1.3 | 7 ± 1.3 | ||||
Comparisons were made between Id1+/+ and Id1−/− mice of same age (n=10–12/genotype at each time point).
P < 0.05;
P < 0.005;
P < 0.0005.
Increased thermogenesis in Id1-deficient mice
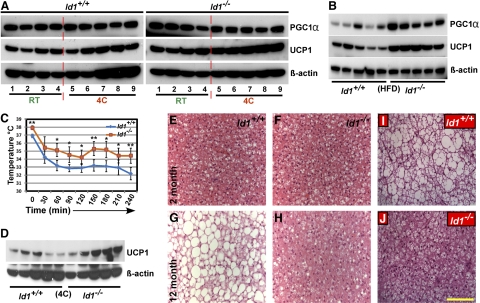
Highest levels of Id1 expression was observed in BAT compared to other tissues (Fig. 1A), suggesting that Id1 might be involved in promoting BAT differentiation or thermogenesis. Thus, loss of Id1 could affect thermogenesis, and therefore further contribute to changes in energy homeostasis. Id1−/− mice had relatively similar amounts of interscapular BAT compared to Id1+/+ mice, when normalized to body weight (not shown). Previous studies determined whether BAT-like cells were present in WAT by examining BAT-specific gene expression, including PGC1α and UCP1 in WAT (31–34). Because Id1 is expressed in BAT as well as WAT, we questioned whether lack of Id1 promotes BAT differentiation, leading to increased BAT abundance, including BAT-like cells within WAT. To this end, we induced adipogenesis in Id1+/+ and Id1−/− MEFs and analyzed the expression of BAT-specific genes PGC1α and UCP1. However, we did not detect expression of either PGC1α or UCP1 in both Id1+/+ and Id1−/− cell lysates (not shown). Similarly, we failed to detect expression of PGC1α or UCP1 in epididymal, retroperitoneal, or inguinal WAT (not shown), suggesting that lack of Id1 does not promote BAT differentiation, and the amount of BAT is not significantly higher in Id1−/− mice.
To investigate the thermogenic response of BAT in Id1−/− mice, we measured the expression levels of PGC1α and UCP1 proteins in the BAT under 3 different conditions: RT; after exposing the mice to cold (4°C) for 4 h; and after feeding the mice with an HFD for 4 mo. We detected enhanced expression of PGC1α and UCP1 in Id1−/− BAT compared to Id1+/+ BAT under all conditions tested (Fig. 3A, B). Most strikingly, UCP1 expression in Id1−/− BAT was 40–50% higher under every condition compared to Id1+/+ BAT, (Fig. 3A, B). Measurement of rectal temperatures at RT revealed that Id1−/− mice were ∼1°C warmer than Id1+/+ mice (37.9±0.3°C vs. 36.9±0.2, P<0.05). Similarly, consistent with increased expression level of UCP1 in Id1−/− mice compared to Id1+/+ mice, measurement of body temperature at 30-min intervals, after exposing the mice to cold, revealed that the body temperature fluctuated between 31 and 36°C in both genotypes, but was higher in Id1−/− mice (Fig. 3C). These data suggest that Id1 deficiency resulted in increased thermogenesis due to induced expression of PGC1α and UCP1. During aging, thermogenic potential declines (35), and since thermogenic response is higher in Id1−/− BAT, we asked whether Id1 deficiency protects against age-associated decline in thermogenesis. Thus, we exposed aged mice to 4°C for 4 h and measured UCP1 levels in BAT. UCP1 expression levels were 70–80% higher in aged Id1−/− mice compared to controls (Fig. 3D), suggesting markedly higher thermogenic response in Id1−/− mice. Furthermore, histological analysis of BAT showed excessive accumulation of lipids and enlarged lipid vacuoles in Id1+/+ BAT in response to HFD or during aging, whereas Id1−/− BAT showed little lipid accumulation (Fig. 3E–J). Thus, increased energy expenditure protected Id1−/− mice from abnormal lipid accumulation in non-WATs, such as BAT, in response to HFD or during aging.
Figure 3.
Enhanced expression of thermogenic proteins in Id1−/− BAT. A) Whole-cell lysates were prepared from the BAT of 2-mo-old Id1+/+ and Id1−/− mice at RT or after exposing the mice to 4°C for 4 h. Expression levels of PGC1α and UCP1 proteins were measured in Id1+/+ and Id1−/− tissues (n=4–5/genotype) by Western blot analysis under the indicated condition. After running the protein and transferring and probing the membranes with antibodies, the membranes were exposed simultaneously to the same film for same amount of time. B) Expression levels of PGC1α and UCP1 in the BAT of HFD-fed Id1+/+ and Id1−/− mice. C) Rectal temperature of Id1+/+ and Id1−/− mice after exposing them to 4°C for 4 h. Body temperature was recorded at 30-min intervals. D) Expression levels of UCP1 in the BAT of 6-mo-old Id1+/+ and Id1−/− mice after exposing the mice to 4°C for 4 h. β-Actin was used as loading control. E, H) H&E-stained interscapular BAT of 2- and 12-mo-old Id1+/+ (E) and Id1−/− (F) mice. I, J) H&E-stained interscapular BAT of HFD fed Id1+/+ (I) and Id1−/− (J) mice. Scale bar = 100 μm.
Loss of Id1 leads to enhanced expression of genes involved in energy homeostasis
Although mitochondrial thermogenic protein, UCP1 in BAT, is responsible for the generation of heat, several cytokines, peptide hormones, and receptors are involved in the regulation of whole-body energy metabolism (36, 37). The activation of these receptors stimulates specific signaling cascades that ultimately result in the activation of the PGC1α/UCP1 thermogenic pathway (38, 39). To identify whether loss of Id1 alters the expression of genes involved in energy homeostasis, we measured the mRNA levels of 84 genes that are specifically involved in lipid metabolism and energy homeostasis. For this expression analysis, we screened RNA from WAT and BAT of Id1+/+ and Id1−/− mice using the Mouse Obesity PCR superarray. We detected differential expression of 15 genes in BAT and 18 genes in WAT of Id1−/− mice (Tables 2 and 3). Strikingly, >90% of these differentially expressed genes are up-regulated in Id1−/− mice compared to Id1+/+ mice. Most interestingly, in addition to increased expression of cytokines and receptors in Id1−/− mice that enhance energy expenditure, we also observed a 3-fold up-regulation of Ucp1 in BAT, and a 3- to 4-fold up-regulation of pparα, pparγ, and pgc1α in WAT of Id1−/− mice compared to Id1+/+ mice (Tables 2 and 3), suggesting enhanced thermogenesis in Id1−/− mice. However, we cannot exclude the possibility that the differential expression of some of these genes is indirect and might have occurred due to a change in adiposity and/or cytokine levels in Id1−/− mice rather than a direct consequence of loss of Id1.
Table 2.
Differential expression of metabolic genes in the WAT of Id1−/− mice compared to Id1+/+ WAT
| Catalog no. | Gene name | Symbol | Fold regulation | GeneBank no. | Metabolic function |
|---|---|---|---|---|---|
| PPM41799E | Adrenergic receptor, α 2b | Adra2b | 5.4 | NM_009633 | Regulation of lipolysis (49) |
| PPM05338E | Apolipoprotein A-IV | Apoa4 | 157.2 | NM_007468 | Regulation of serum lipid levels (50) |
| PPM04267A | Dopamine receptor D1A | Drd1a | 8.6 | NM_010076 | Regulation of body activity and oral function (51) |
| PPM25148E | Galanin | Gal | 5.5 | NM_010253 | Energy homeostasis (52) |
| PPM04848B | Glucagon receptor | Gcgr | 4 | NM_008101 | Glucose metabolism and energy homeostasis (53) |
| PPM05365F | Growth hormone receptor | Ghr | 3.1 | NM_010284 | Regulation of adiposity, insulin sensitivity and energy homeostasis (54) |
| PPM04851F | Glucagon-like peptide 1 receptor | Glp1r | 10.7 | NM_021332 | Glucose homeostasis and energy metabolism (55) |
| PPM24533A | Islet amyloid polypeptide | Iapp | 3.8 | NM_010491 | Stimulates lipolysis (56) |
| PPM25215A | Insulin II | Ins2 | 6.2 | NM_008387 | Glucose metabolism and energy homeostasis (57) |
| PPM05115E | Insulin receptor | Insr | 3.1 | NM_010568 | Glucose metabolism and energy homeostasis (58) |
| PPM04907E | Neuromedin B receptor | Nmbr | 4.8 | NM_008703 | Activity and behavior (59) |
| PPM29712A | Neurotensin | Nts | 4.1 | NM_024435 | Appetite control (60) |
| PPM03307B | Peroxisome proliferator activated receptor α | Ppara | 4.4 | NM_011144 | Energy homeostasis (61) |
| PPM05108B | Peroxisome proliferator activated receptor γ | Pparg | 3.3 | NM_011146 | Adipogenesis and energy balance (61) |
| PPM03360E | Peroxisome proliferative activated receptor, gamma, coactivator 1 α | Ppargc1a | 4.3 | NM_008904 | Regulation of energy balance (16, 62) |
| PPM24836E | Cholecystokinin | Cck | –32.6 | NM_031161 | Energy intake, glucose homeostasis, lipid metabolism (63) |
| PPM04847A | Galanin receptor 1 | Galr1 | –3.4 | NM_008082 | Energy homeostasis (52) |
| PPM03113F | Tumor necrosis factor | Tnf | –4 | NM_013693 | Insulin resistance, adipose inflammation (3) |
Out of 84 genes in the mouse obesity PCR superarray that are specifically involved in lipid metabolism and energy homeostasis, only 18 genes were differentially regulated in Id1−− WAT.
Table 3.
Differential expression of metabolic genes in the BAT of Id1−/− mice compared to Id1+/+ BAT
| Catalog no. | Gene name | Symbol | Fold regulation | GeneBank no. | Metabolic function |
|---|---|---|---|---|---|
| PPM24968A | Adenylate cyclase activating polypeptide 1 | Adcyap1 | 3.03 | NM_009625 | Energy metabolism (64) |
| PPM05338E | Apolipoprotein A-IV | Apoa4 | 6.3 | NM_007468 | Regulation of serum lipid levels (50) |
| PPM26139A | Colipase | Clps | 4.8 | NM_025469 | Appetite control and fat digestion (65) |
| PPM04603A | Cannabinoid receptor 1 | Cnr1 | 11.5 | NM_007726 | Mitochondrial biogenesis and energy homeostasis (66) |
| PPM04851F | Glucagon-like peptide 1 receptor | Glp1r | 3.4 | NM_021332 | Glucose homeostasis and energy metabolism (55) |
| PPM40421A | Melanin-concentrating hormone receptor 1 | Mchr1 | 4.6 | NM_145132 | Regulation of energy balance (67) |
| PPM25155E | Gastrin-releasing peptide receptor | Grpr | 4.4 | NM_008177 | Regulation of energy balance (68) |
| PPM24533A | Islet amyloid polypeptide | Iapp | 3.4 | NM_010491 | Control of energy expenditure (69) |
| PPM29595A | Melanocortin 3 receptor | Mc3r | 3.1 | NM_008561 | Regulation of energy expenditure (37) |
| PPM05003E | Neuromedin U | Nmu | 3.2 | NM_019515 | Appetite regulation and energy homeostasis (70) |
| PPM37114B | Proopiomelanocortin-α | Pomc | 3.3 | NM_008895 | Regulation of thermogenesis (71) |
| PPM05021A | Somatostatin | Sst | 3 | NM_009215 | Modulation of energy balance (72) |
| PPM41867E | Thyroid hormone receptor β | Thrb | 3.1 | NM_009380 | Regulation of thermogenesis and energy balance (73) |
| PPM05164A | Uncoupling protein 1 (mitochondrial, proton carrier) | Ucp1 | 3.2 | NM_009463 | Regulation of thermogenesis and energy homeostasis (74) |
| PPM03504A | Leptin | Lep | –3.5 | NM_008493 | Insulin sensitivity and appetite regulation (75) |
Of 84 genes in the mouse obesity PCR superarray that are specifically involved in lipid metabolism and energy homeostasis, only 15 genes were differentially regulated in Id1−/− BAT.
Increased insulin sensitivity in Id1−/− mice
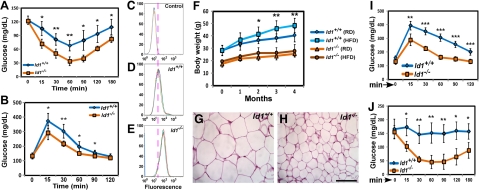
Enhanced energy expenditure reduces body adiposity, and changes in adiposity are often associated with alterations in glucose and insulin homeostasis (40). Because energy expenditure was increased and fat mass was reduced in Id1−/− mice, we asked whether insulin sensitivity was altered in Id1−/− mice. Id1−/− mice of all age groups were normoglycemic (Table 1). However, serum insulin levels were significantly lower in Id1−/− mice compared to control mice (Table 1), suggesting that insulin sensitivity might be higher in Id1−/− mice. To evaluate glucose homeostasis in Id1−/− mice, we performed ITTs and GTTs. Id1−/− mice were more sensitive to insulin, and the rate of glucose clearance on insulin administration was markedly higher in Id1−/− mice of all age groups compared to Id1+/+ controls during ITT (Fig. 4A and Supplemental Fig. S2I, J). Similarly, during GTT, young and adult Id1−/− mice displayed significantly increased glucose clearance and had lower levels of blood glucose compared to control mice (Fig. 4B and Supplemental Fig. S2K), indicating that pancreatic β-cell function was more than sufficient to cope with an exogenous load of glucose. To examine whether the observed enhanced insulin sensitivity in Id1−/− mice was due, in part, to increased glucose uptake by adipose tissue, we assessed glucose uptake by adipocytes. Incubation of adipocytes with the fluorescent glucose analog 2-NBDG, followed by insulin stimulation, led to increased uptake in Id1−/− adipocytes relative to Id1+/+ adipocytes (Fig. 4C–E), suggesting that insulin-mediated glucose uptake was enhanced in Id1−/− adipocytes. To determine whether higher insulin sensitivity in Id1−/− mice could be attributed to increased signaling, we examined insulin signaling in BAT, liver, muscle, and WAT by measuring the activation and phosphorylation of the major marker of insulin signaling, Akt (on Ser473 after insulin administration. Insulin-stimulated Akt phosphorylation was increased by more than 40% in skeletal muscle, ∼25% in liver, and ∼10% in WAT, but significantly decreased in BAT of Id1−/− mice compared to Id1+/+ mice, whereas total Akt levels were unchanged in both genotypes (Supplemental Fig. S2L). These results suggest that whole-body insulin sensitivity and insulin signaling were enhanced in Id1−/− mice.
Figure 4.
Id1 deletion protects against HFD-induced insulin resistance. A, B) ITTs (A) and GTTs (B) in 2-mo-old mice (n=6/genotype), showing the changes in blood glucose levels at the indicated time points. C–E) Glucose uptake in adipocytes, measured by FACS following 30 min exposure to 2-NBDG in the presence of insulin; negative control (C), Id1+/+ adipocytes (D), and Id1−/− adipocytes (E) treated with 2-NBDG (n=4/group). F) Body weights of RD- and HFD-fed male mice (n=18/group) at the indicated time points. G, H) H&E-stained sections of inguinal fat from HFD fed Id1+/+ (G) and Id1−/− (H) mice. Scale bar = 100 μm. I, J) GTTs (I) and ITTs (J) in HFD-fed Id1+/+ and Id1−/− male mice (n=5/group), showing the changes in blood glucose levels at the indicated time points. *P < 0.05; **P < 0.005; ***P < 0.0005.
Id1 deficiency protects against HFD-induced adipose hypertrophy and insulin resistance
In wild-type mice, HFD induces obesity, adipocyte hypertrophy, insulin resistance, and diabetes (41). Since Id1-deficient mice exhibited several advantageous metabolic phenotypes, such as higher energy expenditure and increased insulin sensitivity, we questioned whether loss of Id1 protects against deleterious metabolic effects induced by HFD. In response to 4 mo of HFD feeding, Id1+/+ mice gained more weight compared to RD-fed Id1+/+ mice. Strikingly, Id1−/− mice showed minimal weight gain when fed HFD and were not significantly different compared to Id1−/− mice fed RD (Fig. 4F), although food intake was ∼20% higher than in Id1+/+ mice when normalized to body weight (Supplemental Fig. S3A). Histological analysis of WAT disclosed adipocyte hypertrophy and massive lipid accumulation in the WAT of Id1+/+ mice, whereas adipocytes in the WAT of HFD-fed Id1−/− mice were smaller and more heterogeneous in size (Fig. 4G, H and Supplemental Fig. S3B–E), suggesting that Id1−/− mice were resistant to HFD-induced weight gain. Serum leptin levels were significantly lower in HFD-fed Id1−/− mice compared to controls (Table 1), further confirming that most of the adipocytes in the Id1−/− WAT were not hypertrophic and overloaded with TG, which causes increased leptin secretion. Collectively, these data suggest that HFD feeding did not result in abnormal weight gain or adipocyte hypertrophy in Id1−/− mice.
Despite 4 mo of HFD feeding, Id1+/+ and Id1−/− mice did not become diabetic, but the blood glucose levels were slightly higher in Id1+/+ mice (Table 1). However, serum insulin levels were extremely low in HFD-fed Id1−/− mice, compared to Id1+/+ mice (Table 1). On the basis of lower insulin levels, we predicted higher insulin sensitivity in these mice, similar to RD-fed Id1−/− mice, and conducted GTTs and ITTs. HFD-fed Id1+/+ mice displayed glucose intolerance, whereas Id1−/− mice showed normal glucose clearance during GTTs (Fig. 4I). Furthermore, ITTs revealed that Id1+/+ mice developed insulin resistance, as administration of insulin did not result in a significant reduction in blood glucose levels compared to basal levels. In contrast, Id1−/− mice remained sensitive to insulin, and reduction in blood glucose levels was almost comparable to RD-fed Id1−/− mice subjected to ITTs (Fig. 4J and Supplemental Fig. S2I). In a reflection of their normal insulin sensitivity, Id1−/− mice fed HFD exhibited ∼50% higher Akt phosphorylation in insulin-responsive organs (liver, muscle, and WAT) compared to insulin-resistant Id1+/+ mice, without alterations in total Akt protein (Supplemental Fig. S3F). These data suggest that Id1−/− mice were protected from HFD-induced insulin resistance.
Id1 deficiency protects against age and HFD-induced hepatosteatosis
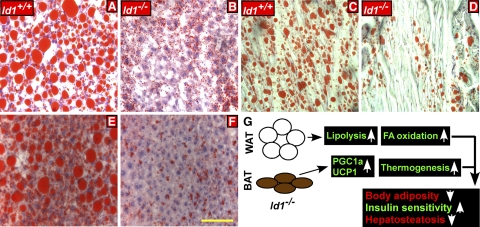
Chronic exposure of mice to HFD leads to saturated lipid storage in WAT, followed by abnormal lipid accumulation in other tissues, such as liver (hepatosteatosis) and muscle, leading to impaired metabolic function of the body (42). Since Id1 deficiency resulted in increased FA oxidation and energy expenditure, we asked whether mice lacking Id1 are protected against HFD-mediated affects. After 4 mo of HFD feeding, liver/body weight ratio was markedly higher in Id1+/+ mice compared to RD fed Id1+/+ mice (5.36±0.64 vs. 4.05±0.82 g, P<0.001) or HFD-fed Id1−/− mice (5.36±0.64 vs. 3.87±0.35 g, P<0.0001). Histological examination revealed a marked difference in the severity of fatty infiltration, with excessive steatosis in Id1+/+ livers, whereas lipid droplets were significantly smaller in Id1−/− livers, as visualized by H&E staining (Supplemental Fig. 3G, H), which was also confirmed by Oil-Red-O staining (Fig. 5A, B). Consistent with staining pattern, the TG content in the livers of Id1−/− mice was significantly lower than those of wild-type mice fed HFD (52±21 vs. 132±29 mg/dl, P<0.0001). Excessive lipid accumulation was also detected in the muscle of Id1+/+ mice but was markedly lower in Id1−/− tissues (Fig. 5C, D). Furthermore, histological analysis of 1-yr-old mice fed RD also revealed that Id1−/− mice were resistant to the onset of age-associated hepatosteatosis, which was observed in Id1+/+ mice, as evident from H&E (Supplemental Fig. 3I, J) and Oil-Red-O staining (Fig. 5E, F) and liver weights (Supplemental Fig. S1C). Thus, Id1 deficiency protected mice against aberrant accumulation of lipids in non-WAT in response to HFD and during aging.
Figure 5.
Id1 deletion prevents HFD-induced hepatic steatosis. A–D) Oil Red O-stained liver sections (A, B) and sections of skeletal muscles (C, D) of HFD fed Id1+/+ (A, C) and Id1−/− (B, D) mice. E, F) Oil Red O-stained liver sections of 1-yr-old Id1+/+ (E) and Id1−/− (F) mice fed RD. Scale bar = 100 μm. G) Schematic showing the consequence of loss of Id1 on adipose tissue metabolism.
DISCUSSION
In the current study, we demonstrated that Id1 is a novel regulator of adiposity and energy balance. Id1 is strongly expressed in adipose tissues (BAT and WAT), and deletion of Id1 resulted in reduced fat mass over the life of Id1−/− mice due to higher energy expenditure, increased lipolysis, and FA oxidation, which resulted in reduced TG accumulation in WAT. Increased thermogenesis also contributed to higher energy expenditure due to up-regulation of PGC1α and UCP1 in the BAT of Id1−/− mice. As a consequence of higher energy expenditure and reduced fat mass, Id1−/− mice displayed enhanced insulin sensitivity and were protected against age and HFD-induced adiposity, insulin resistance, and hepatosteatosis.
Although Id1 has an important role in adipocyte differentiation, defective adipogenesis did not contribute to the reduced fat mass observed in Id1−/− mice. In this regard, the expression of Id1 is down-regulated and PPARγ is up-regulated during adipocyte differentiation in vitro, indicating that down-regulation of Id1 might be necessary for the differentiation of cells into adipocytes. This was supported by the observation that adipocyte differentiation was accelerated with earlier expression of PPARγ in Id1−/− cells. Thus, we speculated that constitutive overexpression of Id1 could either delay or block adipogenesis. However, we were unable to demonstrate that overexpression of Id1 could inhibit adipocyte differentiation, since endogenous and overexpressed Id1 were rapidly down-regulated in cells induced to undergo and progress through adipocyte differentiation. Thus, it appears that Id1 levels need to be low for cells to differentiate into adipocytes, and that levels of Id1 are regulated by ubiquitin-mediated degradation, rather than by transcriptional repression. This was confirmed by the observation that cells treated with a proteosome inhibitor showed high levels of native and ubiquitinated Id1. Although adipocyte differentiation was accelerated in the absence of Id1 in vitro, we did not detect any abnormal increase in adipocyte number in Id1−/− mice. Our results suggest that the role of Id1 in adipogenesis is exactly opposite to the function of other members of this family, including Id2 and Id4, which are induced during adipogenesis, and loss of any one of them results in defective adipogenesis, and that their overexpression enhances adipocyte differentiation. Therefore, Id1 has a distinct function in adipogenesis compared to Id2 or Id4 (43, 44). In this regard, it would be interesting to investigate how Id proteins coordinate with one another during adipogenesis, and our future studies are directed at generating and analyzing compound Id-null mice.
Analysis of metabolic activity of mice revealed increased O2 consumption and reduced RER in Id1−/− mice, indicating higher energy expenditure, and that the Id1−/− mice use a higher proportion of fat as an energy source compared to Id1+/+ mice. This observation was confirmed by the data that Id1 deficiency results in increased lipolysis in the WAT and that whole-body FA oxidation rates were significantly higher in Id1−/− mice. Taken together, lack of Id1 significantly increased energy expenditure and FA oxidation, which resulted in reduced accumulation of TGs in adipocytes and lowered circulating TGs in Id1−/− mice. In addition to FA oxidation, another physiological mechanism that could significantly contribute to increased energy expenditure is BAT-mediated thermogenesis. BAT is specialized for energy expenditure, which is controlled by the transcriptional coactivator PGC1α (15, 45). PGC1α regulates the expression of mitochondrial UCP1, which creates a proton leak in mitochondria that dissipates energy produced by oxidative metabolism (15). We found that Id1 expression was high in BAT compared to other tissues, suggesting a possible role for Id1 in BAT-mediated thermogenesis. PCR superarrays provided evidence that thermogenesis might be increased in Id1−/− mice, as RNA levels of Ucp1, Pgc1α, Pparα, and Pparγ were significantly up-regulated. We found that the expression levels of PGC1α and UCP1 proteins were also higher in Id1−/− BAT under normal conditions (RT and RD), and in response to cold exposure or HFD compared to Id1+/+ BAT, which confirmed that thermogenesis is increased in Id1-deficient mice and thus plays a significant role in the increased energy expenditure observed in Id1−/− mice. Since Id1 acts as a dominant negative regulator of other transcription factors, we speculate that Id1 functions to regulate or suppress the expression of genes involved in the thermogenic pathway, such as PGC1α and its downstream targets, which agrees with the observation that their expression was increased in Id1−/− mice. Because Id1 is one of the major regulators of E, Ets, Rb, and PAX proteins, our future studies are directed at understanding how Id1/E protein signaling regulates the expression and activities of PGC1α network of proteins involved in thermogenesis.
Because of higher energy expenditure and reduced fat mass, Id1−/− mice exhibited lower insulin levels and significantly higher insulin response compared to Id1+/+ mice. Accordingly, insulin-stimulated Akt phosphorylation was increased in the liver, skeletal muscle, and WAT of Id1−/− mice compared to Id1+/+ mice, indicating that insulin signaling was enhanced in the absence of Id1. Moreover, HFD-fed Id1+/+ mice developed insulin resistance, whereas Id1−/− mice exhibited normal insulin sensitivity. There are two possible explanations for normal insulin signaling in HFD-fed Id1−/− mice: adipocytes were smaller in Id1−/− mice compared to Id1+/+ mice, and smaller adipocytes are more responsive to insulin than TG-overloaded hypertrophic adipocytes with respect to insulin-stimulated glucose uptake (46, 47); and increased TG accumulation in non-WATs, such as liver and muscle, is a major contributor of impaired insulin sensitivity (48). When fed an HFD, Id1+/+ mice developed fatty liver, and there was excessive accumulation of lipids in the muscle, suggesting saturated lipid storage in the adipose tissue and impaired insulin response in liver and muscle of Id1+/+ mice. However, Id1−/− mice were protected from HFD-mediated hepatosteatosis and skeletal muscle TG accumulation and, therefore, exhibited normal Akt activation and insulin responses. Our finding that Id1 regulates energy homeostasis and body adiposity presents the exciting possibility that Id1 inhibitors could be used to improve insulin sensitivity, treat fatty liver disease, and regulate body weight.
Supplementary Material
Acknowledgments
The authors thank Dr. Robert Benezra (Memorial Sloan-Kettering Cancer Center, New York, NY, USA) for providing the Id1-knockout mice. The authors thank Steven Stull and Juanita Mercado for animal care; Tatyana Chanturiya and William Jou for technical help; and Roberta Smith and the Pathology/Histotechnology Laboratory, NCI–Frederick, for tissue sectioning and staining.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. The authors declare no competing financial interests.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Spiegelman B. M., Flier J. S. (1996) Adipogenesis and obesity: rounding out the big picture. Cell 87, 377–389 [DOI] [PubMed] [Google Scholar]
- 2. Klaus S. (1997) Functional differentiation of white and brown adipocytes. Bioessays 19, 215–223 [DOI] [PubMed] [Google Scholar]
- 3. Galic S., Oakhill J. S., Steinberg G. R. (2010) Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 316, 129–139 [DOI] [PubMed] [Google Scholar]
- 4. Kajimura S., Seale P., Spiegelman B. M. (2010) Transcriptional control of brown fat development. Cell. Metab. 11, 257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ricquier D. (2005) Respiration uncoupling and metabolism in the control of energy expenditure. Proc. Nutr. Soc. 64, 47–52 [DOI] [PubMed] [Google Scholar]
- 6. Argyropoulos G., Harper M. E. (2002) Uncoupling proteins and thermoregulation. J. Appl. Physiol. 92, 2187–2198 [DOI] [PubMed] [Google Scholar]
- 7. De Ferranti S., Mozaffarian D. (2008) The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin. Chem. 54, 945–955 [DOI] [PubMed] [Google Scholar]
- 8. Fajas L., Fruchart J. C., Auwerx J. (1998) Transcriptional control of adipogenesis. Curr. Opin. Cell Biol. 10, 165–173 [DOI] [PubMed] [Google Scholar]
- 9. Kim J. B., Spiegelman B. M. (1996) ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 10, 1096–1107 [DOI] [PubMed] [Google Scholar]
- 10. Rosen E. D., Sarraf P., Troy A. E., Bradwin G., Moore K., Milstone D. S., Spiegelman B. M., Mortensen R. M. (1999) PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4, 611–617 [DOI] [PubMed] [Google Scholar]
- 11. Schroeder-Gloeckler J. M., Rahman S. M., Janssen R. C., Qiao L., Shao J., Roper M., Fischer S. J., Lowe E., Orlicky D. J., McManaman J. L., Palmer C., Gitomer W. L., Huang W., O'Doherty R. M., Becker T. C., Klemm D. J., Jensen D. R., Pulawa L. K., Eckel R. H., Friedman J. E. (2007) CCAAT/enhancer-binding protein beta deletion reduces adiposity, hepatic steatosis, and diabetes in Lepr(db/db) mice. J. Biol. Chem. 282, 15717–15729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shimomura I., Hammer R. E., Richardson J. A., Ikemoto S., Bashmakov Y., Goldstein J. L., Brown M. S. (1998) Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 12, 3182–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang N. D., Finegold M. J., Bradley A., Ou C. N., Abdelsayed S. V., Wilde M. D., Taylor L. R., Wilson D. R., Darlington G. J. (1995) Impaired energy homeostasis in C/EBP alpha knockout mice. Science 269, 1108–1112 [DOI] [PubMed] [Google Scholar]
- 14. Chiang S. H., Bazuine M., Lumeng C. N., Geletka L. M., Mowers J., White N. M., Ma J. T., Zhou J., Qi N., Westcott D., Delproposto J. B., Blackwell T. S., Yull F. E., Saltiel A. R. (2009) The protein kinase IKKepsilon regulates energy balance in obese mice. Cell 138, 961–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feige J. N., Auwerx J. (2007) Transcriptional coregulators in the control of energy homeostasis. Trends Cell Biol. 17, 292–301 [DOI] [PubMed] [Google Scholar]
- 16. Lin J., Wu P. H., Tarr P. T., Lindenberg K. S., St-Pierre J., Zhang C. Y., Mootha V. K., Jager S., Vianna C. R., Reznick R. M., Cui L., Manieri M., Donovan M. X., Wu Z., Cooper M. P., Fan M. C., Rohas L. M., Zavacki A. M., Cinti S., Shulman G. I., Lowell B. B., Krainc D., Spiegelman B. M. (2004) Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 119, 121–135 [DOI] [PubMed] [Google Scholar]
- 17. Picard F., Gehin M., Annicotte J., Rocchi S., Champy M. F., O'Malley B. W., Chambon P., Auwerx J. (2002) SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell 111, 931–941 [DOI] [PubMed] [Google Scholar]
- 18. Zhou Z., Yon Toh S., Chen Z., Guo K., Ng C. P., Ponniah S., Lin S. C., Hong W., Li P. (2003) Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat. Genet. 35, 49–56 [DOI] [PubMed] [Google Scholar]
- 19. Benezra R., Rafii S., Lyden D. (2001) The Id proteins and angiogenesis. Oncogene 20, 8334–8341 [DOI] [PubMed] [Google Scholar]
- 20. Lasorella A., Uo T., Iavarone A. (2001) Id proteins at the cross-road of development and cancer. Oncogene 20, 8326–8333 [DOI] [PubMed] [Google Scholar]
- 21. Chen M., Wang J., Dickerson K. E., Kelleher J., Xie T., Gupta D., Lai E. W., Pacak K., Gavrilova O., Weinstein L. S. (2009) Central nervous system imprinting of the G protein G(s) alpha and its role in metabolic regulation. Cell Metab. 9, 548–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sordella R., Jiang W., Chen G. C., Curto M., Settleman J. (2003) Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell 113, 147–158 [DOI] [PubMed] [Google Scholar]
- 23. Suh H. C., Leeanansaksiri W., Ji M., Klarmann K. D., Renn K., Gooya J., Smith D., McNiece I., Lugthart S., Valk P. J., Delwel R., Keller J. R. (2008) Id1 immortalizes hematopoietic progenitors in vitro and promotes a myeloproliferative disease in vivo. Oncogene 27, 5612–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 25. Chen M., Chen H., Nguyen A., Gupta D., Wang J., Lai E. W., Pacak K., Gavrilova O., Quon M. J., Weinstein L. S. (2010) G(s) alpha deficiency in adipose tissue leads to a lean phenotype with divergent effects on cold tolerance and diet-induced thermogenesis. Cell Metab. 11, 320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kajimura S., Seale P., Tomaru T., Erdjument-Bromage H., Cooper M. P., Ruas J. L., Chin S., Tempst P., Lazar M. A., Spiegelman B. M. (2008) Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev. 22, 1397–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhong L., D'Urso A., Toiber D., Sebastian C., Henry R. E., Vadysirisack D. D., Guimaraes A., Marinelli B., Wikstrom J. D., Nir T., Clish C. B., Vaitheesvaran B., Iliopoulos O., Kurland I., Dor Y., Weissleder R., Shirihai O. S., Ellisen L. W., Espinosa J. M., Mostoslavsky R. (2010) The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 140, 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gautam D., Gavrilova O., Jeon J., Pack S., Jou W., Cui Y., Li J. H., Wess J. (2006) Beneficial metabolic effects of M3 muscarinic acetylcholine receptor deficiency. Cell Metab. 4, 363–375 [DOI] [PubMed] [Google Scholar]
- 29. Heron-Milhavet L., Haluzik M., Yakar S., Gavrilova O., Pack S., Jou W. C., Ibrahimi A., Kim H., Hunt D., Yau D., Asghar Z., Joseph J., Wheeler M. B., Abumrad N. A., LeRoith D. (2004) Muscle-specific overexpression of CD36 reverses the insulin resistance and diabetes of MKR mice. Endocrinology 145, 4667–4676 [DOI] [PubMed] [Google Scholar]
- 30. Sun L., Trausch-Azar J. S., Muglia L. J., Schwartz A. L. (2008) Glucocorticoids differentially regulate degradation of MyoD and Id1 by N-terminal ubiquitination to promote muscle protein catabolism. Proc. Natl. Acad. Sci. U. S. A. 105, 3339–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cederberg A., Gronning L. M., Ahren B., Tasken K., Carlsson P., Enerback S. (2001) FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 106, 563–573 [DOI] [PubMed] [Google Scholar]
- 32. Chiu C. H., Lin W. D., Huang S. Y., Lee Y. H. (2004) Effect of a C/EBP gene replacement on mitochondrial biogenesis in fat cells. Genes Dev. 18, 1970–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hansen J. B., Kristiansen K. (2006) Regulatory circuits controlling white versus brown adipocyte differentiation. Biochem. J. 398, 153–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scime A., Grenier G., Huh M. S., Gillespie M. A., Bevilacqua L., Harper M. E., Rudnicki M. A. (2005) Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1alpha. Cell. Metab. 2, 283–295 [DOI] [PubMed] [Google Scholar]
- 35. Florez-Duquet M., McDonald R. B. (1998) Cold-induced thermoregulation and biological aging. Physiol. Rev. 78, 339–358 [DOI] [PubMed] [Google Scholar]
- 36. Szekely M., Petervari E., Balasko M. (2010) Thermoregulation, energy balance, regulatory peptides: recent developments. Front. Biosci. 2, 1009–1046 [DOI] [PubMed] [Google Scholar]
- 37. Butler A. A. (2006) The melanocortin system and energy balance. Peptides. 27, 281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Iwen K. A., Senyaman O., Schwartz A., Drenckhan M., Meier B., Hadaschik D., Klein J. (2008) Melanocortin crosstalk with adipose functions: ACTH directly induces insulin resistance, promotes a pro-inflammatory adipokine profile and stimulates UCP-1 in adipocytes. J. Endocrinol. 196, 465–472 [DOI] [PubMed] [Google Scholar]
- 39. Verty A. N., Allen A. M., Oldfield B. J. (2010) The endogenous actions of hypothalamic peptides on brown adipose tissue thermogenesis in the rat. Endocrinology 151, 4236–4246 [DOI] [PubMed] [Google Scholar]
- 40. Hurov J. B., Huang M., White L. S., Lennerz J., Choi C. S., Cho Y. R., Kim H. J., Prior J. L., Piwnica-Worms D., Cantley L. C., Kim J. K., Shulman G. I., Piwnica-Worms H. (2007) Loss of the Par-1b/MARK2 polarity kinase leads to increased metabolic rate, decreased adiposity, and insulin hypersensitivity in vivo. Proc. Natl. Acad. Sci. U. S. A. 104, 5680–5685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lazar M. A. (2005) How obesity causes diabetes: not a tall tale. Science 307, 373–375 [DOI] [PubMed] [Google Scholar]
- 42. Postic C., Girard J. (2008) The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab. 34, 643–648 [DOI] [PubMed] [Google Scholar]
- 43. Murad J. M., Place C. S., Ran C., Hekmatyar S. K., Watson N. P., Kauppinen R. A., Israel M. A. (2010) Inhibitor of DNA binding 4 (ID4) regulation of adipocyte differentiation and adipose tissue formation in mice. J. Biol. Chem. 285, 24164–24173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Park K. W., Waki H., Villanueva C. J., Monticelli L. A., Hong C., Kang S., MacDougald O. A., Goldrath A. W., Tontonoz P. (2008) Inhibitor of DNA binding 2 is a small molecule-inducible modulator of peroxisome proliferator-activated receptor-gamma expression and adipocyte differentiation. Mol. Endocrinol. 22, 2038–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Puigserver P., Spiegelman B. M. (2003) Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr. Rev. 24, 78–90 [DOI] [PubMed] [Google Scholar]
- 46. Bluher M., Michael M. D., Peroni O. D., Ueki K., Carter N., Kahn B. B., Kahn C. R. (2002) Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev. Cell. 3, 25–38 [DOI] [PubMed] [Google Scholar]
- 47. Foley J. E., Laursen A. L., Sonne O., Gliemann J. (1980) Insulin binding and hexose transport in rat adipocytes. Relation to cell size. Diabetologia 19, 234–241 [DOI] [PubMed] [Google Scholar]
- 48. Hulver M. W., Dohm G. L. (2004) The molecular mechanism linking muscle fat accumulation to insulin resistance. Proc. Nutr. Soc. 63, 375–380 [DOI] [PubMed] [Google Scholar]
- 49. Langin D. (2006) Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol. Res. 53, 482–491 [DOI] [PubMed] [Google Scholar]
- 50. Ordovas J. M., Schaefer E. J. (1999) Genes, variation of cholesterol and fat intake and serum lipids. Curr. Opin. Lipidol. 10, 15–22 [DOI] [PubMed] [Google Scholar]
- 51. Gantois I., Fang K., Jiang L., Babovic D., Lawrence A. J., Ferreri V., Teper Y., Jupp B., Ziebell J., Morganti-Kossmann C. M., O'Brien T. J., Nally R., Schutz G., Waddington J., Egan G. F., Drago J. (2007) Ablation of D1 dopamine receptor-expressing cells generates mice with seizures, dystonia, hyperactivity, and impaired oral behavior. Proc. Natl. Acad. Sci. U. S. A. 104, 4182–4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim A., Park T. (2010) Diet-induced obesity regulates the galanin-mediated signaling cascade in the adipose tissue of mice. Mol. Nutr. Food. Res. 54, 1361–1370 [DOI] [PubMed] [Google Scholar]
- 53. Conarello S. L., Jiang G., Mu J., Li Z., Woods J., Zycband E., Ronan J., Liu F., Roy R. S., Zhu L., Charron M. J., Zhang B. B. (2007) Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia 50, 142–150 [DOI] [PubMed] [Google Scholar]
- 54. List E. O., Sackmann-Sala L., Berryman D. E., Funk K., Kelder B., Gosney E. S., Okada S., Ding J., Cruz-Topete D., Kopchick J. J. (2011) Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR-/-) muse. Endocr. Rev. 32, 356–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Baggio L. L., Drucker D. J. (2002) Harnessing the therapeutic potential of glucagon-like peptide-1: a critical review. Treat. Endocrinol. 1, 117–125 [DOI] [PubMed] [Google Scholar]
- 56. Ye J. M., Lim-Fraser M., Cooney G. J., Cooper G. J., Iglesias M. A., Watson D. G., Choong B., Kraegen E. W. (2001) Evidence that amylin stimulates lipolysis in vivo: a possible mediator of induced insulin resistance. Am. J. Physiol. 280, E562–E569 [DOI] [PubMed] [Google Scholar]
- 57. Khan A. H., Pessin J. E. (2002) Insulin regulation of glucose uptake: a complex interplay of intracellular signalling pathways. Diabetologia 45, 1475–1483 [DOI] [PubMed] [Google Scholar]
- 58. Kanzaki M., Pessin J. E. (2001) Signal integration and the specificity of insulin action. Cell. Biochem. Biophys. 35, 191–209 [DOI] [PubMed] [Google Scholar]
- 59. Su P. Y., Ko M. C. (2011) The role of central GRP and NMB receptors in the modulation of scratching behavior in rats. J. Pharmacol. Exp. Ther. 337, 822–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wilding J. P. (2002) Neuropeptides and appetite control. Diabet. Med. 19, 619–627 [DOI] [PubMed] [Google Scholar]
- 61. Evans R. M., Barish G. D., Wang Y. X. (2004) PPARs and the complex journey to obesity. Nat. Med. 10, 355–361 [DOI] [PubMed] [Google Scholar]
- 62. Puigserver P. (2005) Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-alpha. Int. J. Obes. (2005) 29(Suppl. 1), S5–S9 [DOI] [PubMed] [Google Scholar]
- 63. Das U. N. (2010) Obesity: genes, brain, gut, and environment. Nutrition 26, 459–473 [DOI] [PubMed] [Google Scholar]
- 64. Tomimoto S., Ojika T., Shintani N., Hashimoto H., Hamagami K., Ikeda K., Nakata M., Yada T., Sakurai Y., Shimada T., Morita Y., Ishida C., Baba A. (2008) Markedly reduced white adipose tissue and increased insulin sensitivity in adcyap1-deficient mice. J. Pharmacol. Sci. 107, 41–48 [DOI] [PubMed] [Google Scholar]
- 65. D'Agostino D., Cordle R. A., Kullman J., Erlanson-Albertsson C., Muglia L. J., Lowe M. E. (2002) Decreased postnatal survival and altered body weight regulation in procolipase-deficient mice. J. Biol. Chem. 277, 7170–7177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tedesco L., Valerio A., Dossena M., Cardile A., Ragni M., Pagano C., Pagotto U., Carruba M. O., Vettor R., Nisoli E. (2010) Cannabinoid receptor stimulation impairs mitochondrial biogenesis in mouse white adipose tissue, muscle, and liver: the role of eNOS, p38 MAPK, and AMPK pathways. Diabetes 59, 2826–2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Guesdon B., Denis R. G., Richard D. (2010) Additive effects of olanzapine and melanin-concentrating hormone agonism on energy balance. Behav. Brain. Res. 207, 14–20 [DOI] [PubMed] [Google Scholar]
- 68. Ohki-Hamazaki H., Watase K., Yamamoto K., Ogura H., Yamano M., Yamada K., Maeno H., Imaki J., Kikuyama S., Wada E., Wada K. (1997) Mice lacking bombesin receptor subtype-3 develop metabolic defects and obesity. Nature 390, 165–169 [DOI] [PubMed] [Google Scholar]
- 69. Wielinga P. Y., Lowenstein C., Muff S., Munz M., Woods S. C., Lutz T. A. (2010) Central amylin acts as an adiposity signal to control body weight and energy expenditure. Physiol. Behav. 101, 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Budhiraja S., Chugh A. (2009) Neuromedin U: physiology, pharmacology and therapeutic potential. Fundam. Clin. Pharmacol. 23, 149–157 [DOI] [PubMed] [Google Scholar]
- 71. Fan W., Voss-Andreae A., Cao W. H., Morrison S. F. (2005) Regulation of thermogenesis by the central melanocortin system. Peptides 26, 1800–1813 [DOI] [PubMed] [Google Scholar]
- 72. Atrens D. M., Menendez J. A. (1993) Somatostatin and the paraventricular hypothalamus: modulation of energy balance. Brain Res. 630, 238–244 [DOI] [PubMed] [Google Scholar]
- 73. Silva J. E. (1995) Thyroid hormone control of thermogenesis and energy balance. Thyroid 5, 481–492 [DOI] [PubMed] [Google Scholar]
- 74. Enerback S., Jacobsson A., Simpson E. M., Guerra C., Yamashita H., Harper M. E., Kozak L. P. (1997) Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387, 90–94 [DOI] [PubMed] [Google Scholar]
- 75. Jequier E. (2002) Leptin signaling, adiposity, and energy balance. Ann. N. Y. Acad. Sci. 967, 379–388 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.