Abstract
Intermediate filaments (IFs) in cardiomyocytes consist primarily of desmin, surround myofibrils at Z disks, and transmit forces from the contracting myofilaments to the cell surface through costameres at the sarcolemma and desmosomes at intercalated disks. Synemin is a type IV IF protein that forms filaments with desmin and also binds α-actinin and vinculin. Here we examine the roles and expression of the α and β forms of synemin in developing rat cardiomyocytes. Quantitative PCR showed low levels of expression for both synemin mRNAs, which peaked at postnatal day 7. Synemin was concentrated at sites of cell–cell adhesion and at Z disks in neonatal cardiomyocytes. Overexpression of the individual isoforms showed that α-synemin preferentially localized to cell-cell junctions, whereas β-synemin was primarily at the level of Z disks. An siRNA targeted to both synemin isoforms reduced protein expression in cardiomyocytes by 70% and resulted in a failure of desmin to align with Z disks and disrupted cell–cell junctions, with no effect on sarcomeric organization. Solubility assays showed that β-synemin was soluble and interacted with sarcomeric α-actinin by coimmunoprecipitation, while α-synemin and desmin were insoluble. We conclude that β-synemin mediates the association of desmin IFs with Z disks, whereas α-synemin stabilizes junctional complexes between cardiomyocytes.—Lund, L. M., Kerr, J. P., Lupinetti, J., Zhang, Y., Russell, M. A., Bloch, R. J., Bond, M. Synemin isoforms differentially organize cell junctions and desmin filaments in neonatal cardiomyocytes.
Keywords: Z disk, sarcolemma, intermediate filaments, rat
The intermediate filament (IF) network is a major cytoskeletal component of skeletal, smooth, and cardiac muscle and is important in the regulation of mechanical stress and force transduction (1). Vimentin and desmin are the major IF proteins in muscle. Vimentin predominates in early development, after which its expression decreases, while desmin gradually increases to become the predominant IF in adult muscle (2). As this transition from vimentin to desmin occurs, the IF network organizes around the Z disks of the contractile apparatus of striated muscle (1) and links these structures to the sarcolemma at costameres and, in the heart, at intercalated disks (3–5). The role of the desmin IF network in muscle has been characterized in mice that lack desmin due to homologous recombination. The striated muscles of these mice fail to align the Z disks of adjacent myofibrils, lose most of the costameres at their sarcolemmal membranes, and are weaker than controls (6–9). With age, desmin-null mice develop cardiomyocyte hypertrophy and cardiac dilation (6, 7, 10). Hence, the desmin IF network plays important roles in skeletal and cardiac muscle, but the mechanisms that relate organization of the IF network to heart pathology have not been elucidated.
Striated muscles also express other IF proteins, including lamins at their nuclear membranes (11), keratins (8, 9, 12) and synemin (13–15). Synemin is a type IV IF with a canonical N-terminal IF rod domain and an extended C-terminal tail domain (16). In rats and humans, synemin has at least 2 isoforms, α and β. The α isoform is the result of alternative mRNA splicing that inserts an additional 936 bp, encoding 312 aa, between the two terminal exons of the mRNA (17). Highly expressed in adult skeletal and cardiac muscle (13, 14), synemin is also found in smooth muscle, neurons, glial cells, and hepatic stellate cells (15, 18, 19). In myocytes, synemin integrates into filaments containing desmin or vimentin via its rod domain (14, 20, 21). The rod domain of synemin can also interact with keratins 5 and 6 (22) (although these have not been identified in striated muscles) and 3 components of the dystroglycan complex: dystrophin, utrophin (23), and α-dystrobrevin (24). Furthermore, the C-terminal tail domain of synemin binds α-actinin and vinculin (21, 25). Recent evidence also suggests that the α-specific insert mediates binding of synemin to vinculin and talin (26, 27), which suggests that the two synemin isoforms may have divergent roles. We localized synemin in adult human hearts to the Z disks and intercalated disks and to the sarcolemma and developing Z disks in neonatal rat myocytes (28). These results are consistent with the ability of synemin to associate not only with desmin but also with Z-disk proteins, such as α-actinin, as well as sarcolemmal proteins, including vinculin, talin, and dystrophin. These findings further suggest that synemin links the desmin filament network to both the sarcolemma and Z disks. As it also acts as an A-kinase anchoring protein (AKAP), synemin may be involved in regulating the phosphorylation of proteins at the sarcolemma and Z disks via protein kinase A (28).
Synemin's role in the development of cardiomyocytes remains largely unexplored. Here, we use TaqMan assays to investigate its expression during embryonic and postnatal life, and compare it to several of its binding partners, including desmin, vimentin, vinculin, and α-actinin. We also reduced the expression of synemin to determine its role on the desmin filament network. Our results show that synemin is expressed early in the development of cardiomyocytes; that its α and β isoforms show a preference in distributing to cell-cell junctions and Z disks, respectively; and that its loss results in defective organization of desmin IFs and unstable cell-cell junctions. Our results suggest that α- and β-synemin are found in predominantly different cellular locations, and thus participate in distinctive functions in the developing heart, and that together they are essential for the structure and function of the IF network in cardiac myocytes.
MATERIALS AND METHODS
Cell cultures
Primary cultures of cardiac myocytes were prepared as described previously (29). Hearts were removed from rat pups at postnatal day 1 (P1). Atria were discarded, and ventricular tissues were digested (51 U/ml collagenase, 0.29 mg/ml pancreatin, 116 mM NaCl, 20 mM HEPES, 1 mM NaH2PO4, 5.5 mM glucose, 5.4 mM KCl, and 0.8 mM MgSO4, pH 7.4). Cells were counted with a hemocytometer and plated onto laminin-coated 6-well plates at a density of 2 × 106 cells/well. Cultures were incubated in DMEM/F12 medium (American Type Culture Collection, Manassas, VA, USA) supplemented with 5% horse serum, 1 μg/ml insulin, 10 ng/ml sodium selenite, 1 μg/ml transferrin (Tri-Mix, Invitrogen, Carlsbad, CA, USA), and 0.2% bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA).
Transfection and quantitative RT-PCR
Small inhibitory RNA (siRNA) sequences were selected that are unique to the 3′-UTR region of synemin, as determined by BLAST analysis. Four 22mer sequences (Qiagen, Valencia, CA, USA) were tested for their ability to reduce the levels of mRNA encoding synemin. The siRNA sequence selected for use in further experiments was the most effective (5′-CCGGTACAGATTGCCTTGGTA-3′, synemin siRNA) with >95% of cultured cardiomyocytes transfected, leading to ∼90% decrease in mRNA expression. Transfections of cardiomyocytes with either siRNAs or plasmids were performed by electroporation in a Lonza Nucleofector with the kit for rat neonatal cardiac myocytes (Lonza Inc., Basel, Switzerland). Transfections were performed on 2.5 × 106 cells in a volume of 100 μl of Nucleofector solution (Lonza) immediately after isolation and prior to plating. To test the efficiency of transfection, an Alexa-633 fluoroprobe was conjugated to synemin siRNA. Most (>95%) cardiomyocytes had Alexa-633 fluorescence at 2 d post-transfection. Control cultures were transfected with a scrambled siRNA sequence (Ambion, Austin, TX, USA). Neonatal cardiac myocytes were also transfected with full-length α- or β-synemin as described previously (28).
We used quantitative RT-PCR (qRT-PCR) to assay the reduction in the levels of synemin mRNA after transfection with synemin siRNA. A standard curve for synemin amplification by PCR was generated with serial dilutions of a plasmid containing 800 bp of nucleic acid sequence that is common to both α- and β-synemin. SYBR Green (Bio-Rad, Hercules, CA, USA) fluorescence was measured using an Opticon Thermocycler (Bio-Rad). Copy numbers of synemin mRNA in siRNA-treated and negative control cultures were determined by extrapolation based on the standard curve.
TaqMan assays
Total RNA was extracted from rat hearts or cultures of neonatal cardiac myocytes with the Quick Prep Total RNA Extraction kit (GE Life Sciences, Piscataway, NJ, USA). RNA was converted into cDNA with the iScript kit (Bio-Rad). cDNA (50 ng) was utilized for TaqMan assays with synemin probes; 20 ng of cDNA was used with all other TaqMan probes. TaqMan assays to detect desmin (Rn 000574732_m1), vimentin (Rn 00667825_m1), vinculin (Rn 01755894_m1), α-actinin (Rn 01470232_m1), and GAPDH (Rn 99999916_s1) were purchased from Applied Biosystems (Foster City, CA, USA). The two custom TaqMan assays for synemin, utilized for quantifying α-synemin or α+β-synemin mRNA, were published previously (19). TaqMan probes, Master Mix (Applied Biosystems), and cDNA for all assays were added to 384-well plates in a 10-μl final volume per reaction (30). Each sample was assayed in triplicate, and the average of the 3 values was plotted for each data point. PCR amplification was performed with a 7900 Thermal Cycler (Applied Biosystems). Results were plotted as either the average comparative cycle time (Ct) for triplicate wells for each gene product at each developmental time, or as 1/Ct (after normalization to an internal reference, GAPDH) (30).
Antibody generation
A monoclonal antibody was produced that recognizes both α- and β-synemin (Syn-MAb) in rats, mice, and humans. Specificity of the Syn-MAb antibody was determined in immunoblots of lysates of CHO cells (Fig. 1A), transfected with either α- or β-synemin cDNAs, subcloned into pcDNA3 (28), and of adult rat hearts (Fig. 1B). A polyclonal antibody (R238) was made in rabbits against the 312 aa specific to human α-synemin (aa 980–1292). Specificity of R238 was determined on Western blots of heart lysates from adult rats (Fig. 1C).
Figure 1.
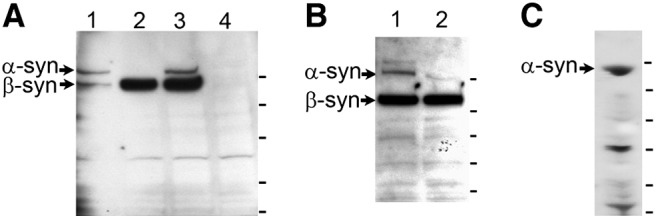
Specificity of new synemin antibodies. We developed a new antibody for use in these studies. A) To test specificity, CHO cells were transiently transfected with pcDNA3-synemin plasmids to express α-synemin (lane 1), β-synemin (lane 2), or both (lane 3). A negative control cell lysate (lane 4) was made from transfected CHO cells that received no DNA. The mouse MAb, Syn-MAb, recognizes both isoforms of synemin on Western blots (210- and 180-kDa SDS-PAGE molecular mass, respectively). α-Synemin and β-synemin are produced from the same mRNA, and in lane 1, some β-synemin is made from splicing of the α-synemin mRNA in CHO cells. B) Western blots of protein extracts from P21 (lane 1) and 6-mo rat hearts (lane 2). Both α- and β-synemin were detected by the Syn-MAb. More α-synemin was detected in P21 lysates than at age 6 mo. Tick marks at right (A, B) represent molecular mass markers: 198, 114, 96, 53, and 36 kDa. C) A rabbit polyclonal antibody, R238, recognizes α-synemin in heart lysates from 6-mo rats. Molecular mass markers (at right): 250, 150, 100, 75, 50, 37 kDa.
Immunofluorescence and microscopy
Cultures of neonatal cardiomyocytes were fixed in 4% paraformaldehyde in PBS and immunostained as described previously (28). Sources and dilutions of the primary antibodies used are presented in Supplemental Table S1. Cultures were blocked, and all antibodies were diluted in Superblock-PBS (Invitrogen). Cultures were washed with 0.1% Triton X-100 in PBS and incubated with secondary antibodies or phalloidin (Supplemental Table S1). Negative controls included cardiomyocytes incubated with individual primary antibodies and both secondary antibodies, or myocytes incubated with secondary antibodies alone. Myocyte-specific markers (desmin and sarcomeric α-actinin) were used to identify cardiomyocytes.
Coverslips were mounted and nuclei were stained using Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA, USA). Immunostaining was visualized with a Nikon TE2000-U inverted fluorescent microscope (Nikon Instruments, Tokyo, Japan) and images were obtained with a Spot digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI., USA) with Spot Advanced 4.0.2 software. Laser scanning confocal microscopy was performed with a Zeiss LSM 410 (Carl Zeiss, Oberkochen, Germany), equipped with a ×63/NA 1.4 objective. Image analysis and fluorescence profiles were performed with ImageJ 1.38 software (Scion Corp., Frederick, MD, USA). To quantify the relative immunofluorescence pixel intensities for desmin and α-actinin at the level of Z disks, we used the plot profile function of ImageJ. The lines used to create the profiles were 10 pixels wide and ran perpendicular to 3 consecutive Z disks. The resulting pixel values were imported into SigmaPlot (Systat Software, Inc., Chicago, IL, USA) and were plotted as line graphs to visualize the relative intensity and overlap of fluorescent signals.
SDS-PAGE and immunoblotting
To produce pellet and supernatant fractions, cultures of rat cardiomyocytes were lysed and resuspended in a minimal volume of a mild detergent solution, Mammalian Protein Extraction Reagent (Pierce, Rockford, IL, USA), supplemented with 300 mM NaCl, 40 mM EDTA, and 10 μg/ml protease inhibitor cocktail (Sigma-Aldrich). Lysates were centrifuged at 4°C and 10,000 g for 10 min. Pellets were resuspended in a reducing and denaturing buffer (4% SDS, 20% glycerol, 10% β-mercaptoethanol, 0.004% bromphenol blue, and 0.125 M Tris-HCl). Aliquots containing 20 μg total protein for pellets, or 50 μg for supernatants or whole-cell lysates, were separated by SDS-PAGE on 4–15% gradient gels (Bio-Rad) (28). Protein concentrations were determined with the BCA assay (Pierce). Fractionated proteins were transferred from gels to PVDF membranes (Pierce), and immunoblotting was performed as described previously (28). Blots were incubated overnight with primary antibody (see Supplemental Table S1). After incubation with secondary antibodies, bands were visualized with a chemiluminescent substrate (SuperSignal West Pico; Pierce). Laser densitometry was performed on digitized images (Kodak Gel Logic; Carestream Health, Rochester, NY, USA) (28). Protein bands were normalized to GAPDH, and differences were compared by 1-tailed t tests. Error bars represent means ± se.
Immunoprecipitation
Protein lysates (800 μg/immunoprecipitation), extracted as described above from cultured neonatal cardiomyocytes, were incubated overnight at 4°C with 2 μg of primary antibody. Antibody-antigen complexes were bound to protein G-Sepharose beads, according to manufacturer's protocol (Pierce). Beads were washed, and specifically bound proteins were eluted in a minimal volume of SDS-PAGE sample buffer. Eluted proteins were divided equally between two lanes on 4–15% gradient SDS-polyacrylamide gels.
RESULTS
Synemin is present in two alternatively spliced forms, α and β. Previous studies revealed synemin at different subcellular locations in cardiac and skeletal muscle, neurons, and glial cells (18, 21, 25, 28, 31) and showed that synemin interacts with several other proteins, including desmin, vimentin, α-actinin, and vinculin (14, 20, 21, 25), which all bind to the β isoform, with additional binding sites for vinculin and talin on α-synemin (26, 27). Here we test the hypothesis that both isoforms of synemin are expressed in the developing heart and that each isoform associates with and differentially stabilizes distinct structures in cardiomyocytes.
Expression of synemin mRNA with development
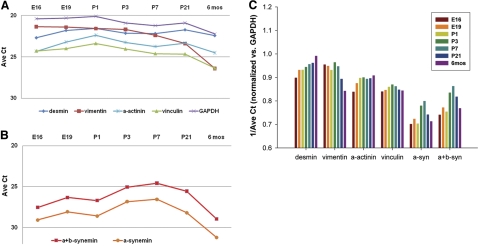
We used TaqMan assays to determine the relative ratio of mRNAs encoding α- and β-synemin vs. GAPDH in the hearts of late embryonic and postnatal rats (Fig. 2). RNA was isolated from rat hearts at embryonic day 16 (E16), E19, P1, P3, P7, P21, and 6 mo (adult). Using a probe that recognized both α- and β-synemin (α+β-synemin), we determined that synemin mRNA showed a small increase after birth between P3 to P7, followed by a return in adult hearts to levels similar to those seen at E16. The decrease in synemin mRNA observed between P21 and adult hearts corresponds to the lesser amounts of protein detected by Western blotting at these two time points (see Fig. 1B). A second TaqMan probe set that specifically amplifies only an α-synemin-specific sequence showed similar, though smaller, changes in expression for this isoform at all time points (Fig. 2B). This trend was also evident after normalization vs. GAPDH (Fig. 2C). This postnatal increase in expression of synemin mRNA corresponds in time to the dramatic increase in vascular resistance and cardiac output occurring after birth that results in tissue remodeling (32).
Figure 2.
Expression of synemin mRNA in cardiac myocytes. A, B) TaqMan assays that detect α-synemin or α+β-synemin cDNAs were used to determine relative ratios of synemin mRNA expression vs. GAPDH in rat hearts as a function of developmental time. Hearts were isolated at E16, E19, P1, P3, P7, P21, and 6 mo. DNA (20 μg) from each was assayed in triplicate to detect desmin, vimentin, α-actinin, vinculin and GAPDH (A), or 50 μg DNA was used to detect α-synemin or α+β-synemin (B). Highest values for α-synemin and α+β-synemin cDNAs were detected at P7. C) After normalization to GAPDH, expression of desmin cDNA was highest in adult rat hearts (6 mo), as opposed to vimentin, which was highest at E16 and decreased into adulthood. Two other binding partners of synemin, α-actinin and vinculin, were also assayed; those mRNAs remained similar across the selected time points.
We also determined cardiac developmental expression of mRNAs for several of synemin's reported binding partners, including α-actinin, vinculin, desmin, and vimentin (Fig. 2C). Consistent with previous reports, the expression of vimentin and desmin showed opposing trends over the course of cardiac development (33). Vimentin was highest during embryonic stages, with a sharp decrease in expression by adulthood, while desmin mRNA expression was lowest in embryonic tissues and showed a steady increase through the postnatal time points and into adulthood. By contrast, the mRNA levels for vinculin and α-actinin did not change appreciably during development.
Localization of synemin in cardiac myocytes
In our prior studies, we immunostained cardiomyocytes isolated from P1 rat pups with a polyclonal antibody that does not distinguish between α- and β-synemin (α/β-synemin; ref. 28). In cardiomyocytes cultured for 2 d, synemin was concentrated at the sarcolemma, but after 4 d in culture, synemin was also present in the cytoplasm in periodic striations with spacing similar to Z disks (28). These experiments suggested that synemin was associated with both the sarcolemma and the developing contractile apparatus. We next asked whether α-synemin and β-synemin preferentially localized to one or the other of these structures.
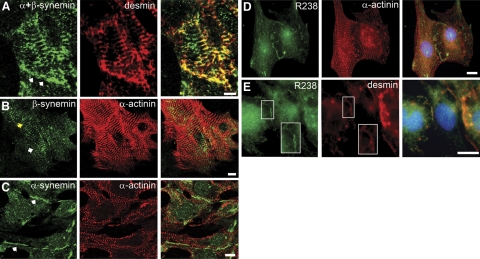
Using cardiomyocytes that were cultured 4 d and then immunostained with anti-synemin (Fig. 3A), we found regions of the sarcolemma at sites of cell-cell contact were stained positively for both desmin and synemin. We then transfected myocytes with plasmids containing synemin cDNAs encoding either α- or β-synemin to learn whether these isoforms concentrate selectively at these different sites. When β-synemin was overexpressed and immunolabeled with a polyclonal antibody to synemin, a striated pattern of immunostaining was observed (Fig. 3B). The β-synemin was present in striations and colocalized with α-actinin, the major Z-disk protein, but not at cell-cell contacts (Fig. 3B). By contrast, regions of cell-cell contact were immunostained following overexpression of α-synemin, but striations were not detected (Fig. 3C). In particular, myocytes that overexpressed β-synemin and were in contact with neighboring cells did not concentrate β-synemin at contact sites (Fig. 3B, yellow arrow), while these contact sites were enriched in α-synemin (Fig. 3C).
Figure 3.
Subcellular localization of synemin in neonatal cardiomyocytes. A) Endogenous synemin in neonatal cardiac myocytes were cultured for 4 d, then immunostained with rabbit polyclonal anti-synemin antibody and an anti-desmin MAb. Synemin immunostaining is at the sarcolemma at sites of cell-cell contact (arrows) and in the cytoplasm in a pattern of periodic striations. B) Cardiomyocytes transfected with pcDNA3-β-synemin show synemin immunostaining at Z disks (arrow) but not at the sarcolemma (yellow arrow) where the myocyte contacts an adjacent myocyte. C) Cardiomyocytes transfected with pcDNA3-α-synemin show positive immunostaining along the sarcolemma (arrows) in regions of cell-cell contact. Myocytes were counterstained with a monoclonal antibody to sarcomeric α-actinin. D, E) A rabbit polyclonal antibody (R238) was used for immunodetection of α-synemin in cardiomyocytes cultured for 4 d. R238 immunostaining shows that α-synemin is concentrated along the plasma membrane. Desmin immunostaining colocalizes with R238 at the plasma membrane (E, inset box) but not α-actinin, which is striated in appearance (D). Scale bars = 10 μm.
We developed a new rabbit polyclonal antibody (R238) that specifically recognized α-synemin (see Fig. 1C). When cardiomyocytes were cultured for 4 d and then immunostained with R238, endogenous α-synemin was concentrated along the plasma membrane (Fig. 3D, E), as was desmin (Fig. 3E) but not α-actinin (Fig. 3D), which was in striations. While our data indicate that α- and β-synemin tend to segregate to different sites within the myoplasm, they do not exclude the presence of α-synemin at Z disks or β-synemin at the sarcolemma.
Reduced expression of synemin
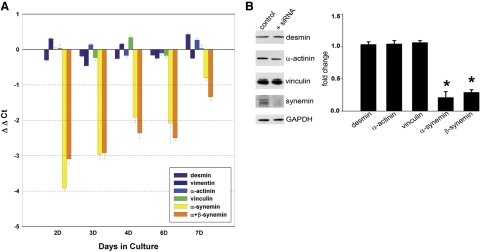
To explore the role of synemin in neonatal cardiomyocytes, we reduced its expression with siRNA. Quantitative PCR revealed a decrease in synemin mRNA of 90 ± 3% compared to controls (n=3; P<0.001, 1-tailed t-test) at 2 d post-transfection. TaqMan assays utilizing RNA extracted from cultured neonatal cardiac myocytes at 2, 3, 4, 6, and 7 d post-transfection confirmed that expression of desmin, vinculin, and α-actinin mRNAs did not change in myocytes treated with synemin siRNA (Fig. 4A). The TaqMan assays of α-synemin and α+β-synemin showed the greatest decrease in mRNA levels at d 2, with a subsequent gradual increase in expression through d 7 (Fig. 4A).
Figure 4.
Effect of synemin knockdown. A) Following transfection with the synemin siRNA, cardiomyocytes were cultured for 2, 3, 4, 6, and 7 d, at which times RNA was extracted. Values were first normalized to GAPDH and then graphed as a ratio of control vs. siRNA-treated cultures (ΔΔCt). The zero point on the graph represents no difference in message level between siRNA-treated and negative control cultures. TaqMan assays for α-synemin or α+β-synemin showed the biggest reduction in mRNA at 2 d post-transfection, with a gradual increase toward control levels by 7 d. TaqMan assays also showed that message levels for desmin, vimentin, α-actinin, and vinculin did not vary appreciably between cultures treated with the synemin siRNA and controls. Error bars = se. B) Total cell proteins from cardiomyocyte cultures were extracted in SDS-PAGE sample buffer at 4 d after transfection with synemin siRNA. Western blots show a 71% decrease in β-synemin (180 kDa) and 79% decrease in α-synemin (210 kDa). Lysates were also probed with antibodies against α-actinin, vinculin, or desmin. After performing densitometry scanning with normalization to an internal reference (GAPDH), all three showed unaltered expression between myocytes treated with synemin siRNA vs. controls. *P < 0.05 vs. control.
To measure changes in protein following treatment with synemin siRNA, we prepared Western blots of whole-cell lysates of cardiomyocytes that had been cultured for 4 d post-transfection. We observed a 71% decrease in β-synemin by Western blotting (0.29±0.04; n=4; P<0.002, 1-tailed t test), and a 79% decrease in α-synemin (0.21±0.09; n=4; P≤0.05, 1-tailed t test) (Fig. 4B), in good agreement with our TaqMan assays. When compared to controls, the amounts of α-actinin (1.04±0.05; n=4), vinculin (1.04+0.03; n=4), and desmin (1.03+0.04; n=4) did not change significantly after loss of synemin.
Role for synemin at sites of cell-cell adhesion
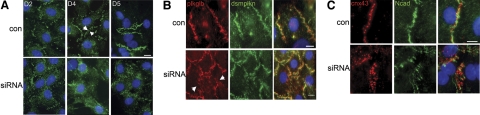
Having localized α-synemin to regions of cell-cell contact (see Fig. 3C–E), we considered potential roles for synemin in cell-cell adhesion. We first examined adherens junctions in cardiomyocytes using an antibody that recognizes the cytosolic tail of N-cadherin (Fig. 5A). Myocytes were cultured for 2 to 5 d following transfection with synemin siRNA or a scrambled control. After 2 d in culture, diffuse cytosolic immunostaining for N-cadherin was evident in both control and synemin siRNA-transfected myocytes. However, by 4 d post-transfection, myocytes treated with scrambled siRNA showed green fluorescent staining of the sarcolemma where adjacent myocytes were in contact, while N-cadherin staining in cultures of myocytes treated with the synemin siRNA remained diffuse and cytosolic with limited sarcolemmal staining on membranes of adjacent myocytes. After 5 d in culture, the presence of nascent intercalated disks was suggested in control cells by bright immunofluorescent N-cadherin staining at the ends of some control cardiomyocytes. In contrast, the siRNA-treated myocytes exhibited N-cadherin staining that was diffuse and disorganized, suggesting that formation of cell-cell adhesion sites was delayed or that the adhesion sites were unstable.
Figure 5.
Reduced expression of synemin disrupts sarcolemmal adhesion sites and gap junctions. A) Cardiomyocytes transfected with synemin siRNA were incubated for 2, 4, or 5 d prior to immunostaining. At 2 d post-transfection, control and siRNA-treated cultures were similar in appearance, exhibiting cytoplasmic and sarcolemmal labeling for N-cadherin on adjacent membranes of neighboring cells (n=3 paired cultures). By d 4, control myocytes showed N-cadherin immunofluorescence on opposing plasma membranes of neighboring cells (arrows), while in myocytes that received the synemin siRNA, labeling for N-cadherin was predominantly cytoplasmic (n=5 paired cultures). At 5 d post-transfection, nascent intercalated disks were identifiable in control cultures but not in siRNA-treated myocytes (n=3 paired cultures). B) Reduced expression of synemin followed by immunostaining for desmosomal proteins also showed less organization of desmoplakin at the sarcolemma, with corresponding increases in the cytoplasm. Control cardiomyocytes showed desmoplakin immunostaining (green) along the sarcolemma at sites of cell-cell contact, which overlapped with plakoglobin (red; arrows, n=5 paired cultures). C) Cardiomyocytes were transfected with synemin or scrambled sequence siRNAs and immunostained to visualize cxn-43 and N-cadherin after 4 d in culture (n=3 paired cultures). In controls, adjacent myocytes (top panel) showed labeling for cnx-43 at the sarcolemma near N-cadherin. Few round structures were seen in the cytoplasm. In myocytes treated with synemin siRNA (bottom panel), cnx-43 fluorescence was disorganized, and labeling for both cnx-43 and N-cadherin was predominantly cytoplasmic, with little organized structure at regions of cell-cell contact. Scale bars = 10 μm.
Desmoplakin binds both plakoglobin and desmin, linking desmin filaments to desmosomes. In control cardiomyocytes, immunolabeling for desmoplakin was evident at the sarcolemma at sites of contact between adjacent myocytes (Fig. 5B). Following treatment of myocytes with synemin siRNA, more desmoplakin was present in the cytosol (Fig. 5B). Double-blinded quantitations by 3 naive observers reliably distinguished between control and siRNA-treated cardiomyocytes (i.e., with correct scores of 80, 80, and 96%; P<0.001, rank sum test).
We next examined connexin-43 (cnx-43) and the gap junctions it forms between adjacent cardiomyocytes following homotypic binding of N-cadherin and formation of cell-cell adhesion complexes (34). This finding suggested that cardiomyocytes with disorganized cell-cell adhesion would also exhibit delayed or disrupted gap junction formation. After 4 d in culture, cnx-43 immunostaining was at the sarcolemma in control cardiomyocytes, with few vesicle-like structures remaining in the cytoplasm (Fig. 5C). However, cardiomyocytes treated with synemin siRNA had many cnx-43-positive cytoplasmic vesicles in addition to disrupted N-cadherin immunostaining at regions of cell-cell contact (Fig. 5C), thus verifying our prediction that synemin influences the stabilization or assembly of the junctional complexes forming between neighboring cardiomyocytes (28).
Effects of reduced synemin on desmin filaments
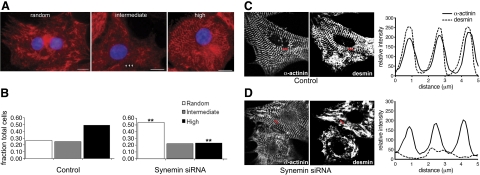
As β-synemin accumulates preferentially at the level of the Z disks, where it colocalizes with desmin, we investigated the effects of reduced synemin expression on the organization of desmin filaments. At 4 d after transfection with either the synemin siRNA or a scrambled siRNA control, desmin immunostaining was striated at the level of Z disks in controls, whereas desmin filaments remained randomly distributed throughout the cytosol of myocytes transfected with synemin siRNA (Fig. 6A). We quantified the relative proportion of cells showing a predominantly striated pattern vs. a random organization (Fig. 6B) in double-blinded studies. Naive observers rated 49% of control myocytes as showing desmin highly organized into striations, and 26% with poor desmin organization (Fig. 6B and Table 1). In cultures transfected with synemin siRNA, this condition was reversed: only 23% of cells showed striated labeling for desmin, while 54% showed random organization (Pearson's χ2 test; P=0.005). Synemin therefore plays an important role in organizing desmin around Z disks. By contrast, the reduced expression of synemin has no significant effect on the organization of α-actinin at Z disks (see Fig. 6D) or vinculin at the sarcolemma (see Fig. 7, top panel). Using ImageJ software, we found that the intensity of labeling for α-actinin was unaffected by transfection with synemin siRNA and showed periodic peaks, consistent with the presence of α-actinin at Z disks. Labeling for desmin, restricted to the Z disk in control myocytes, did not show well-defined peaks in the siRNA-treated cultures (Fig. 6C, D).
Figure 6.
Desmin organization at Z disks. A) Neonatal cardiac myocytes were transfected with synemin siRNA and immunostained with anti-desmin after 4 d in culture. An observer following a blind procedure rated the organization of desmin filaments in treated vs. control cultures as random, moderately organized (intermediate) or highly organized. Random filaments are dispersed in the cytoplasm without a pattern of striations. High organization of filaments shows striations similar to Z disks in 75–100% of the cell body, while intermediate organization shows a limited cytoplasmic region where periodic striations are present (arrows). Scale bars = 10 μm. B) Random and high categories were significantly different when siRNA-treated myocytes and controls were counted for each of the 3 designated categories. Numbers of myocytes in two categories, random and high, differed significantly from one another. **P = 0.005 vs. corresponding control; Pearson's χ2 test. C, D) Control (C) and siRNA-treated cardiomyocytes (D) were coimmunolabeled with antibodies for desmin and α-actinin. Red bars represent lines used for scanning relative intensity with ImageJ. Control myocytes showed periodicity of both α-actinin and desmin. Cardiomyocytes treated with synemin siRNA showed periodicity of α-actinin but not desmin.
Table 1.
Desmin organization at Z disks
| Parameter | Random | Intermediate | High | Total |
|---|---|---|---|---|
| Group | ||||
| Control cells | 70 | 66 | 130 | 266 |
| Treated cells | 100** | 42 | 43** | 185 |
| Total | 170 | 108 | 173 | 451 |
| Total cell count | ||||
| Control cells | ||||
| Fields | 30 | |||
| Cells | 266 | |||
| Treated cells | ||||
| Fields | 23 | |||
| Cells | 185 |
Loss of synemin alters desmin organization around Z disks. Neonatal cardiac myocytes were transfected with synemin siRNA and cultured on laminin-coated coverslips for 4 d. After immunostaining with the desmin monoclonal antibody, randomly chosen fields were prepared. An observer rated the organization of desmin filaments as high, intermediate, or random in a masked procedure.
P = 0.005 vs. control; Pearson's χ2 test, df = 2.
Figure 7.
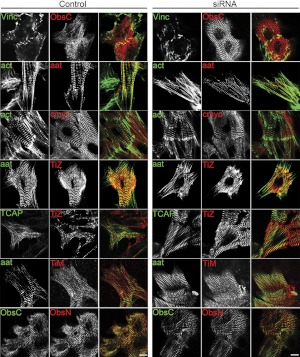
Organization of other sarcomeric proteins. Cardiomyocytes treated with synemin siRNA or scrambled siRNA controls were immunostained after 4 d in culture for other sarcomeric proteins. siRNA-treated cultures showed similar levels of organization of M-line and Z-disk proteins compared to control myocytes. act, actin; aat, α-actinin; cmyo, cardiac myosin heavy chain; TiZ, Z-disk titin; TCAP, TCAP/telethonin; TiM, M-line titin; ObsC, C-terminal obscurin; ObsN, N-terminal obscurin; vinc, vinculin. Scale bars = 10 μm.
Reduced synemin does not disrupt sarcomeric structure
We examined additional sarcomeric proteins to determine whether loss of desmin at Z disks, caused by transfection with synemin siRNA, results in a general disruption of myofibrillar proteins and sarcomeric structure after 4 d in culture. Proteins that assemble at or around the Z disk, including the N-terminal region of titin, obscurin, and telethonin, as well as α-actinin, were striated to similar levels in cardiomyocytes treated with synemin or control siRNAs (Fig. 7). Furthermore, labeling of C-terminal epitopes of titin, as well as obscurin at the M-line, was similar in myocytes transfected with synemin and control siRNAs (Fig. 7). Actin, labeled with a fluorescent phalloidin, and cardiac myosin heavy chain were also not disrupted by the loss of synemin (Fig. 7). Thus, the loss of desmin filaments surrounding the Z disk—and the reduced levels of synemin that cause it—do not result in a general loss or disruption of key sarcomeric structures.
Solubility of synemin and binding partners
In studies primarily utilizing in vitro methods, other investigators have found that synemin forms dimers (14) and tetramers (15). It has also been demonstrated that synemin does not form homopolymeric filaments, but, when another type III IF protein is present, is capable of forming heteropolymers (21–35). Synemin dimers and tetramers are soluble (14, 15), in contrast to filament-associated synemin, which is predominantly insoluble (21). We next asked whether the synemin present in lysates made from cultured neonatal cardiomyocytes is soluble, or, alternatively, whether it is associated with insoluble protein complexes.
Using a mild detergent and nonreducing conditions, we extracted total cellular proteins after cardiomyocytes had been cultured for 4 d. Low-speed centrifugation yielded supernatant and pellet fractions. The proteins in both fractions were analyzed by SDS-PAGE (50 or 20 μg/lane for supernatants and pellets, respectively), followed by Western blotting. In control myocytes, β-synemin was predominantly in supernatants, with faint but detectable amounts also observed in pellets (Fig. 8A). The pellet fractions also contained α-synemin, which was not detectable in supernatants. Consistent with the results summarized earlier, reducing the expression of synemin via transfection with siRNA showed a decrease in the 180-kDa β-synemin by 70% in supernatants (n=3, P<0.002), while α-synemin was undetectable in either pellets or supernatants (Fig. 8A).
Figure 8.
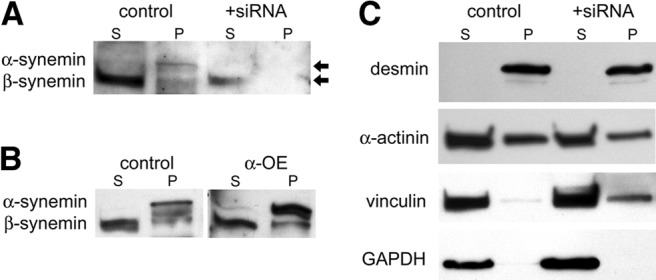
Solubility of synemin and its binding partners. Cardiomyocytes were transfected with synemin siRNA or a scrambled control sequence. At 4 d post-transfection, protein was extracted from cultured myocytes in MPERS buffer. Lysates were centrifuged to produce supernatant (S) and pellet (P) fractions. A) Endogenous β-synemin was predominantly found in supernatants, while α-synemin was observed in pellets. After treatment with synemin siRNA, α-synemin was no longer detected, while some β-synemin remained in the supernatant fraction in reduced amounts. B) Overexpression of α-synemin using pcDNA3-α-synemin confirmed that it concentrated in the pellet fraction, in contrast to endogenous β-synemin, which remained predominantly in supernatants. C) Desmin concentrated in the pellets, while α-actinin and vinculin were enriched in supernatants, with lesser amounts in pellets. GAPDH was found only in supernatants.
Cardiomyocytes express lesser amounts of α-synemin than β-synemin (cf. Figs. 2 and 6B), which made detection of α-synemin difficult on Western blots of pellet and supernatant proteins (Fig. 8A). Therefore, we overexpressed α-synemin in cardiomyocytes using a plasmid encoding α-synemin. We found α-synemin preferentially in pellet fractions, with only negligible amounts detected in supernatants (Fig. 8B). This did not alter the distribution of endogenous β-synemin, which remained largely in the supernatant fraction, with lesser amounts in the pellets (Fig. 8B).
The results of these experiments suggested that α-synemin was among the insoluble proteins in neonatal cardiomyocytes, while β-synemin was predominantly soluble but with a subpopulation that was insoluble. We then asked whether synemin's binding partners are preferentially associated with either pellets or supernatants of cardiomyocyte lysates. Using Western blotting to examine supernatant and pellet fractions for the presence of desmin, α-actinin, and vinculin (Fig. 8C), we found that desmin was present in pellets, whereas α-actinin and vinculin were predominantly in supernatants, although they were detectable in lesser amounts in the pellets as well. Reducing the expression of synemin by transfection of siRNA did not alter these results. Desmin remained in pellets, while vinculin and α-actinin were mainly in supernatants (Fig. 8C).
Association of β-synemin with vinculin and α-actinin
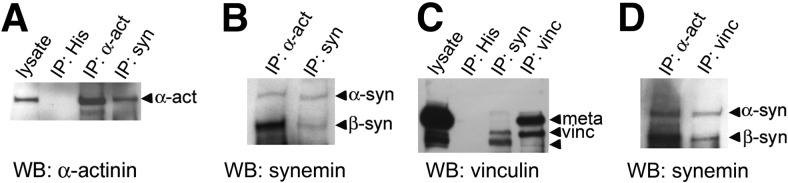
Identifying vinculin and α-actinin as proteins present in myocyte supernatants allowed us to ask whether they interact with β-synemin. We used immunoprecipitation with monoclonal antibodies against vinculin, sarcomeric α-actinin, and synemin with supernatant fractions of cardiomyocytes cultured for 4 d (Fig. 9). Following Western blot analysis, β-synemin (180 kDa) was coimmunoprecipitated with α-actinin (Fig. 9B). This result is consistent with our predictions of an interaction between sarcomeric α-actinin and β-synemin at Z disks. We also observed a preference for interaction of synemin with vinculin (110 kDa) vs. metavinculin (150 kDa) (Fig. 9C). Immunoprecipitation of vinculin from lysates of neonatal cardiomyocytes produced β-synemin as a coprecipitating protein (Fig. 9D). A faintly detectable amount of α-synemin (210 kDa) was also coimmunoprecipitated by both α-actinin and vinculin (Fig. 9B, D). This finding was consistent with our earlier data demonstrating that little α-synemin is present in cardiomyocyte supernatants (Fig. 8B), and suggested that α-synemin, as well as β-synemin, can coimmunoprecipitate with both vinculin and α-actinin.
Figure 9.
Interactions of synemin with α-actinin and vinculin. A) α-Actinin (110 kDa) was immunoprecipitated from lysates of neonatal cardiomyocytes after 4 d in culture. α-Actinin (arrow) coimmunoprecipitated with synemin when cell lysates were incubated with the Syn-MAb but not with an anti-His antibody (negative control; n=3). B) Both α- and β-synemin (arrows) immunoprecipitated with the Syn-MAb (180 and 210 kDa). Immunoprecipitating with an anti-sarcomeric α-actinin antibody coimmunoprecipitated β-synemin. C) Vinculin (middle arrow) and metavinculin (top arrow), 110 and 150 kDa respectively, immunoprecipitated with the vinculin antibody. However, the Syn-MAb coimmunoprecipitated only vinculin and not metavinculin, along with a second unidentified band (estimated 100 kDa; bottom arrow). D) Immunoprecipitates prepared with antibodies to sarcomeric α-actinin and vinculin showed that β-synemin preferentially coimmunoprecipitated with α-actinin. Detectable amounts of α- and β-synemin were present in vinculin immunoprecipitates.
DISCUSSION
Previous studies of synemin have identified several of its binding partners and suggested that different isoforms of synemin interact preferentially with partners at the plasma membrane or in the contractile apparatus. The isoforms have not been localized to distinct structures, however, nor have their roles at these structures been probed. Here, we show that β-synemin is predominantly soluble and associates with α-actinin in extracts made from cultured cardiomyocytes, and, consistent with this, is preferentially enriched at the level of Z disks. By contrast, α-synemin is more insoluble than the β isoform and preferentially associates with the sarcolemma and intercalated disks. Reducing the expression of synemin with siRNA leads to the disruption of both desmin IFs and cell-cell junctions. Our results are consistent with the hypothesis that α- and β-synemin have distinctive properties and play distinctive roles in the morphogenesis of ventricular cardiomyocytes.
Synemin's relatively low level of expression in developing cardiomyocytes is consistent with previous studies of the assembly of desmin and synemin heterofilaments in skeletal and smooth muscle, which showed that synemin is present in IF fractions in much lower amounts than desmin (13, 22). The increased levels of synemin mRNA that we document in the neonatal heart coincides with the increased workload that occurs following birth and with subsequent remodeling of cardiac tissue (32). Nevertheless, the amounts of synemin mRNA and protein are low compared to some of its binding partners (e.g., desmin, vinculin, α-actinin). In smooth muscle, synemin was estimated to be 1–2% of the amount of desmin synthesized (13). This finding represents a synemin:desmin molar ratio of ∼1:25, which was also determined to be the optimal molar ratio between the two for formation of heterofilaments (22). Those earlier studies did not distinguish between α and β isoforms, however. In our immunoblots of heart tissue, we found more β-synemin in cell lysates than α-synemin (cf. Figs. 1B, 4B, and 8A, B). This finding is consistent with reports demonstrating that β-synemin is the predominant isoform expressed in striated muscle (17). In addition to finding different amounts of the two synemin isoforms in cardiomyocytes, we also find α- and β-synemin preferentially but not exclusively associated with different subcellular locations. Hence, the differences seen in mRNA levels during heart development, as well as the different amounts and localization of each synemin isoform, suggest divergent roles for α- vs. β-synemin within cardiomyocytes.
Our results indicate that α- and β-synemin associate with different structures in neonatal cardiomyocytes: α-synemin associates with the sarcolemma, while β-synemin associates with Z disks. Our localization of β-synemin at Z disks is consistent with our earlier results (28) as well as those of other investigators, who also show β-synemin associated with Z disks in skeletal muscle (36, 37). Expression studies in skeletal myocytes of the 312 amino acid peptide that is unique to α-synemin showed that it associated with focal adhesions at the sarcolemma (26, 27). Our studies support the hypothesis that the preferential association of synemin isoforms with either the sarcolemma or the Z disks depends on the presence of the α-synemin specific sequence. The mechanisms that generate a selective localization of α- and β-synemin have not been identified.
Our results also suggest that the subcellular location and the binding partners that associate with each of the two synemin isoforms are linked. Although several binding partners have been identified for synemin, its ligands in developing cardiomyocytes have not yet been identified. We focused our studies on its interactions with its most prominent binding partners: desmin, vinculin, and α-actinin (13, 21, 23, 25–27, 31, 35, 37, 38). We found that α- and β-synemin each coimmunoprecipitate with both α-actinin and vinculin, consistent with their association in situ (see Fig. 9). Our solubility studies also showed that predominantly β-synemin but little α-synemin was present in the supernatant fraction from whole-cell lysates and that β-synemin coimmunoprecipitates with sarcomeric α-actinin. This association of β-synemin with α-actinin provides an explanation for the selective placement of β-synemin at Z disks, where α-actinin is the major protein. However, other investigators have reported that β-synemin did not coimmunoprecipitate with α-actinin from extracts of adult murine skeletal muscle (35). This discrepancy could be due to developmental or tissue-specific differences.
Our immunoprecipitation experiments using a monoclonal antibody to synemin yielded vinculin, rather than metavinculin, from cardiomyocyte lysates, and the reciprocal immunoprecipitation using an anti-vinculin antibody yielded both synemin isoforms in the immunoprecipitates (see Fig. 9). In prior studies, investigators have shown that the rod domain of synemin (common to both isoforms) mediates binding to vinculin (25). Other studies of the 312-aa peptide unique to α-synemin showed that both vinculin and metavinculin interact with this polypeptide in vitro (27). The question of whether functional differences result when vinculin binds to α-synemin's rod domain, as opposed to its unique peptide sequence, has not been answered. Our studies of the endogenous α and β isoforms show that both associate with vinculin (Fig. 9), presumably via the rod domain in β-synemin, and either of the two binding sites present in α-synemin. As vinculin is predominantly at the sarcolemma of neonatal cardiomyocytes (see Fig. 7, top panel), its presence may influence the nearby accumulation of α-synemin. Other binding partners of synemin, such as talin, dystrophin, and plectin, are also positioned to promote the concentration of α-synemin in developing cardiomyocytes at the sarcolemma and intercalated disks (23, 26, 35).
Our studies demonstrate for the first time that endogenous α-synemin preferentially concentrates at the sarcolemma and intercalated disks of neonatal cardiomyocytes, and that it plays an important role in stabilizing the latter. Proper formation of cell–cell adhesion complexes at intercalated disks is critical for mechanical coupling between cardiomyocytes via adherens junctions and desmosomes, and for electrical and chemical coupling via gap junctions. Several studies have documented the stepwise reformation of cell-cell adhesion sites that occurs when dissociated neonatal heart tissues are subsequently grown as cultures of primary cells (39, 40). An early requirement is the homotypic binding of N-cadherin located on adjacent membranes of neighboring myocytes. Two of synemin's known binding partners, vinculin and α-actinin, are subsequently recruited to the plasma membrane, where they participate in stabilization of nascent adherens junctions (41–44). Vinculin influences the formation of actin filaments and their association with the membrane (44). The newly formed membrane-associated microfilaments are then organized into radial bundles by α-actinin (44). These events contribute to stabilization of the multiprotein adhesion complexes that associate with the cytoplasmic tail of N-cadherin and allow formation of desmosomes to occur, “sealing” adhesion of opposing cell membranes (44). α-Synemin is likely to influence the formation of desmosomes directly, via its interaction with desmin (45), as well as by influencing the overall organization of intercalated disks and associated cytoskeletal structures indirectly, via its AKAP activity (28, 37).
Conversely, by overexpressing β-synemin in developing cardiomyocytes, we demonstrated that β-synemin is associated preferentially with developing Z disks. Pairing evidence of synemin's association with α-actinin in the soluble fraction of myocyte lysates with our previous finding that synemin organizes at Z disks prior to desmin filaments (28) suggests that β-synemin binds to α-actinin before anchoring desmin to the periphery of Z disks. Our siRNA studies showing reduced levels of synemin inhibit the organization of desmin around Z disks is consistent with these results. Several studies of synemin:desmin heterofilament formation indicate that the rod domain of synemin is incorporated with desmin as filaments form, rather than being bound after the fact to preformed desmin filaments (15, 21, 22, 35). This finding could explain how desmin, after associating with β-synemin that is bound to α-actinin at the Z disk, becomes striated in appearance. It is also consistent with the reorganization of IFs that occurs in relationship to Z disks as myocytes develop, when desmin filaments change from a longitudinal to a transverse orientation (2), and with the ability of synemin to cross-link desmin filaments (13, 21, 25) As the tail domain of β-synemin interacts with α-actinin and its rod domain binds desmin, this model for organizing desmin filaments around the Z disk would require the binding of these two ligands to β-synemin to occur independently.
Our proposed role for β-synemin is similar to the role recently suggested for the large cytolinker protein, plectin (46–48). In muscle cells that lack plectin due to homologous recombination, desmin failed to assemble around Z disks and instead formed large aggregates of protein in the cytoplasm (46). Synemin's association with the Z disk was also disrupted in these studies, which is consistent with reports of a direct interaction between plectin-1 and β-synemin (37, 44). Hence both β-synemin and plectin-1, acting independently or in tandem, may participate in organizing desmin filaments at Z disks.
In summary, our results support the role of synemin as a direct link between the IF network, the myofibrillar contractile apparatus, and adhesion complexes at the sarcolemma and intercalated disks (25). Our data support the idea that the association of synemin with the sarcolemma and the contractile apparatus is regulated by the unique peptide sequence that distinguishes the two isoforms. Studies are in progress regarding synemin's role in developing skeletal muscle and how its associated PKA activity influences morphogenesis and the contractile properties of striated muscle.
Supplementary Material
Acknowledgments
The authors thank Shirley Gaa and Dr. Terry B. Rogers for preparations of cardiomyocytes, Gloria Vives-Rodriguez for technical assistance, and Maureen O'Donnell for help with TaqMan assays and data analysis.
This work was supported by U.S. National Institutes of Health grants NIH RO1-02520711 (to M.B.) and NIH RO1 AR055928RO1 (to R.J.B.) and by The Muscular Dystrophy Association (R.J.B.).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Capetanaki Y., Bloch R. J., Kouloumenta A., Mavroidis M., Psarras S. (2007) Muscle intermediate filaments and their links to membranes and membranous organelles. Exp. Cell Res. 313, 2063–2076 [DOI] [PubMed] [Google Scholar]
- 2. Tokuyasu D., Maher P., Dutton A., Singer S. (1985) Intermediate filaments in skeletal and cardiac muscle tissue in embryonic and adult chicken. Ann. N. Y. Acad. Sci. 455, 200–212 [DOI] [PubMed] [Google Scholar]
- 3. Tokuyasu K., Dutton A., Singer S. (1983) Immunoelectron microscopic studies of desmin (skeleton) localization and intermediate filament organization in chicken cardiac muscle. J. Cell Biol. 96, 1736–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tokuyasu K., Dutton A., Singer S. (1983) Immunoelectron microscopic studies of desmin (skeleton) localization and intermediate filament organization in chicken skeletal muscle. J. Cell Biol. 96, 1727–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lazarides E. (1980) Intermediate filaments as mechanical integrators of cellular space. Nature 283, 249–255 [DOI] [PubMed] [Google Scholar]
- 6. Milner D., Weitzer G., Tran D., Bradley A., Capetanaki Y. (1996) Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J. Cell Biol. 134, 1255–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Z., Colucci-Guyon E., Pincon-Raymond M., Mericshay M., Pourin S., Paulin D., Babinet C. (1996) Cardiovascular lesions and skeletal myopathy in mice lacking desmin. Dev. Biol. 175, 362–366 [DOI] [PubMed] [Google Scholar]
- 8. O'Neill A., Williams M., Resneck W., Milner D., Capetanaki Y., Bloch R. (2002) Sarcolemmal organization in skeletal muscle lacking desmin: Evidence for cytokeratins associated with the membrane skeleton at costameres. Mol. Biol. Cell 13, 2347–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lovering R. M., O'Neill A., Muriel J. M., Prosser B. L., Strong J., Bloch R. J. (2011) Physiology, structure, and susceptibility to injury of skeletal muscle in mice lacking keratin 19-based and desmin-based intermediate filaments. Am. J. Physiol. Cell Physiol. 300, C803–C813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Milner D. J., Taffet G. E., Wang X., Pham T., Tamura T., Hartley C., Gerdes A. M., Capetanaki Y. (1999) The absence of desmin leads to cardiomyocyte hypertrophy and cardiac dilation with compromised systolic function. J. Mol. Cell. Cardiol. 31, 2063–2076 [DOI] [PubMed] [Google Scholar]
- 11. Herrmann H., Foisner R. (2003) Intermediate filaments: novel assembly models and exciting new functions for nuclear lamins. Cell. Mol. Life Sci. 60, 1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ursitti J. A., Lee P. C., Resneck W. G., McNally M. M., Bowman A. L., O'Neill A., Stone M. R., Bloch R. J. (2004) Cloning and characterization of cytokeratins 8 and 19 in adult rat striated muscle. interaction with the dystrophin glycoprotein complex. J. Biol. Chem. 279, 41830–41838 [DOI] [PubMed] [Google Scholar]
- 13. Granger B. L., Lazarides E. (1980) Synemin: A new high molecular weight protein associated with desmin and vimentin filaments in muscle. Cell 22, 727–738 [DOI] [PubMed] [Google Scholar]
- 14. Bilak S. R., Sernett S. W., Bilak M. M., Bellin R. M., Stromer M. H., Huiatt T. W., Robson R. M. (1998) Properties of the novel intermediate filament protein synemin and its identification in mammalian muscle. Arch. Biochem. Biophys. 355, 63–76 [DOI] [PubMed] [Google Scholar]
- 15. Sandoval I. V., Colaco C. A., Lazarides E. (1983) Purification of the intermediate filament-associated protein, synemin, from chicken smooth muscle. Studies on its physicochemical properties, interaction with desmin, and phosphorylation. J. Biol. Chem. 258, 2568–2576 [PubMed] [Google Scholar]
- 16. Becker B., Bellin R. M., Sernett S. W., Huiatt T. W., Robson R. M. (1995) Synemin contains the rod domain of intermediate filaments. Biochem. Biophys. Res. Commun. 213, 796–802 [DOI] [PubMed] [Google Scholar]
- 17. Titeux M., Brocheriou V., Xue Z., Gao J., Pellissier J. F., Guicheney P., Paulin D., Li Z. (2001) Human synemin gene generates splice variants encoding two distinct intermediate filament proteins. European. J. Biochem/FEBS. 268, 6435–6449 [DOI] [PubMed] [Google Scholar]
- 18. Izmiryan A., Franco C. A., Paulin D., Li Z., Xue Z. (2009) Synemin isoforms during mouse development: Multiplicity of partners in vascular and neuronal systems. Exp. Cell Res. 315, 769–783 [DOI] [PubMed] [Google Scholar]
- 19. Uyama N., Zhao L., Van Rossen E., Hirako Y., Reynaert H., Adams D. H., Xue Z., Li Z., Robson R., Pekny M., Geerts A. (2006) Hepatic stellate cells express synemin, a protein bridging intermediate filaments to focal adhesions. Gut 55, 1276–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khanamiryan L., Li Z., Paulin D., Xue Z. (2008) Self-assembly incompetence of synemin is related to the property of its head and rod domains. Biochemistry 47, 9531–9539 [DOI] [PubMed] [Google Scholar]
- 21. Bellin R. M., Sernett S. W., Becker B., Ip W., Huiatt T. W., Robson R. M. (1999) Molecular characteristics and interactions of the intermediate filament protein synemin. Interactions with alpha-actinin may anchor synemin-containing heterofilaments. J. Biol. Chem. 274, 29493–29499 [DOI] [PubMed] [Google Scholar]
- 22. Hirako Y., Yamakawa H., Tsujimura Y., Nishizawa Y., Okumura M., Usukura J., Matsumoto H., Jackson K. W., Owaribe K., Ohara O. (2003) Characterization of mammalian synemin, an intermediate filament protein present in all four classes of muscle cells and some neuroglial cells: Co-localization and interaction with type III intermediate filament proteins and keratins. Cell Tissue Res. 313, 195–207 [DOI] [PubMed] [Google Scholar]
- 23. Bhosle R. C., Michele D. E., Campbell K. P., Li Z., Robson R. M. (2006) Interactions of intermediate filament protein synemin with dystrophin and utrophin. Biochem. Biophys. Res. Commun. 346, 768–777 [DOI] [PubMed] [Google Scholar]
- 24. Mizuno Y., Puca A. A., O'Brien K. F., Beggs A. H., Kunkel L. M. (2001) Genomic organization and single-nucleotide polymorphism map of desmuslin, a novel intermediate filament protein on chromosome 15q26.3. BMC Genetics. 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bellin R. M., Huiatt T. W., Critchley D. R., Robson R. M. (2001) Synemin may function to directly link muscle cell intermediate filaments to both myofibrillar Z-lines and costameres. J. Biol. Chem. 276, 32330–32337 [DOI] [PubMed] [Google Scholar]
- 26. Sun N., Critchley D. R., Paulin D., Li Z., Robson R. M. (2008) Identification of a repeated domain within mammalian alpha-synemin that interacts directly with talin. Exp. Cell Res. 314, 1839–1849 [DOI] [PubMed] [Google Scholar]
- 27. Sun N., Critchley D. R., Paulin D., Li Z., Robson R. M. (2008) Human alpha-synemin interacts directly with vinculin and metavinculin. Biochem. J. 409, 657–667 [DOI] [PubMed] [Google Scholar]
- 28. Russell M. A., Lund L. M., Haber R., McKeegan K., Cianciola N., Bond M. (2006) The intermediate filament protein, synemin, is an AKAP in the heart. Arch. Biochem. Biophys. 456, 204–215 [DOI] [PubMed] [Google Scholar]
- 29. Wright G., Singh I. S., Hasday J. D., Farrance I. K., Hall G., Cross A. S., Rogers T. B. (2002) Endotoxin stress-response in cardiomyocytes: NF-kappaB activation and tumor necrosis factor-alpha expression. Amer. J. Physiol. Heart Circ. Physiol. 282, H872–H879 [DOI] [PubMed] [Google Scholar]
- 30. Bookout A. L., Mangelsdorf D. J. (2003) Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl. Recep. Signal. 1, e012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jing R., Pizzolato G., Robson R. M., Gabbiani G., Skalli O. (2005) Intermediate filament protein synemin is present in human reactive and malignant astrocytes and associates with ruffled membranes in astrocytoma cells. Glia 50, 107–120 [DOI] [PubMed] [Google Scholar]
- 32. Smolich J. J. (1995) Ultrastructural and functional features of the developing mammalian heart: a brief overview. Reprod. Fertil. Dev. 7, 451–461 [DOI] [PubMed] [Google Scholar]
- 33. Kim H. D. (1996) Expression of intermediate filament desmin and vimentin in the human fetal heart. Anat. Rec. 246, 271–278 [DOI] [PubMed] [Google Scholar]
- 34. Luo Y., Radice G. (2002) Cadherin-mediated adhesion is essential for myofibril continuity across the plasma membrane but not for assembly of the contractile apparatus. J. Cell Sci. 116, 1471–1479 [DOI] [PubMed] [Google Scholar]
- 35. Jing R., Wilhelmsson U., Goodwill W., Li L., Pan Y., Pekny M., Skalli O. (2007) Synemin is expressed in reactive astrocytes in neurotrauma and interacts differentially with vimentin and GFAP intermediate filament networks. J. Cell Sci. 120, 1267–1277 [DOI] [PubMed] [Google Scholar]
- 36. Mizuno Y., Guyon J. R., Watkins S. C., Mizushima K., Sasaoka T., Imamura M., Kunkel L. M., Okamoto K. (2004) Beta-synemin localizes to regions of high stress in human skeletal myofibers. Muscle Nerve 30, 337–346 [DOI] [PubMed] [Google Scholar]
- 37. Hijikata T., Nakamura A., Isokawa K., Imamura M., Yuasa K., Ishikawa R., Kohama K., Takeda S., Yorifuji H. (2008) Plectin 1 links intermediate filaments to costameric sarcolemma through beta-synemin, alpha-dystrobrevin and actin. J. Cell Sci. 121, 2062–2074 [DOI] [PubMed] [Google Scholar]
- 38. Izmiryan A., Peltekian E., Paulin D., Li Z. L., Xue Z. G. (2010) Synemin isoforms in astroglial and neuronal cells from human central nervous system. Neurochem. Res. 35, 881–887 [DOI] [PubMed] [Google Scholar]
- 39. Franke W. W., Schumacher H., Borrmann C. M., Grund C., Winter-Simanowski S., Schlechter T., Pieperhoff S., Hofmann I. (2007) The area composita of adhering junctions connecting heart muscle cells of vertebrates - III: assembly and disintegration of intercalated disks in rat cardiomyocytes growing in culture. Eur. J. Cell Biol. 86, 127–142 [DOI] [PubMed] [Google Scholar]
- 40. Franke W. W., Borrmann C. M., Grund C., Pieperhoff S. (2006) The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins. Eur. J. Cell Biol. 85, 69–82 [DOI] [PubMed] [Google Scholar]
- 41. Shirashi I., Simpson D., Carver W., Price R., Hirozane T., Terracio L., Borg T. (1997) Vinculin is an essential component for normal myofibrillar arrangement in fetal cardiac myocytes. J. Mol. Cell. Cardiol. 29, 2041–2052 [DOI] [PubMed] [Google Scholar]
- 42. Lu M. H., DiLullo C., Schultheiss T., Holtzer S., Murray J. M., Choi J., Fischman D. A., Holtzer H. (1992) The vinculin/sarcomeric-alpha-actinin/alpha-actin nexus in cultured cardiac myocytes. J. Cell Biol. 117, 1007–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goncharova E. J., Kam Z., Geiger B. (1992) The involvement of adherens junction components in myofibrillogenesis in cultured cardiac myocytes. Development (UK) 114, 173–183 [DOI] [PubMed] [Google Scholar]
- 44. Vasioukhin V., Bauer C., Yin M., Fuchs E. (2000) Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 100, 209–219 [DOI] [PubMed] [Google Scholar]
- 45. Pieperhoff S., Borrmann C., Grund C., Barth M., Rizzo S., Franke W. W. (2010) The area composita of adhering junctions connecting heart muscle cells of vertebrates. VII. the different types of lateral junctions between the special cardiomyocytes of the conduction system of ovine and bovine hearts. Eur. J. Cell Biol. 89, 365–378 [DOI] [PubMed] [Google Scholar]
- 46. Konieczny P., Fuchs P., Reipert S., Kunz W. S., Zeold A., Fischer I., Paulin D., Schroder R., Wiche G. (2008) Myofiber integrity depends on desmin network targeting to Z-disks and costameres via distinct plectin isoforms. J. Cell Biol. 181, 667–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fuchs P., Zorer M., Rezniczek G. A., Spazierer D., Oehler S., Castanon M. J., Hauptmann R., Wiche G. (1999) Unusual 5′ transcript complexity of plectin isoforms: Novel tissue-specific exons modulate actin binding activity. Human Mol. Genet. 8, 2461–2472 [DOI] [PubMed] [Google Scholar]
- 48. Rezniczek G. A., Abrahamsberg C., Fuchs P., Spazierer D., Wiche G. (2003) Plectin 5′-transcript diversity: short alternative sequences determine stability of gene products, initiation of translation and subcellular localization of isoforms. Human Mol. Genet. 12, 3181–3194 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.