Abstract
Under homeostatic conditions, a proportion of senescent CXCR4hi neutrophils home from the circulation back to the bone marrow, where they are phagocytosed by bone marrow macrophages. In this study, we have identified an unexpected role for the anti-inflammatory molecule annexin A1 (AnxA1) as a critical regulator of this process. We first observed that AnxA1−/− mice have significantly increased neutrophil numbers in their bone marrow while having normal levels of GM and G colony-forming units, monocytes, and macrophages. Although AnxA1−/− mice have more neutrophils in the bone marrow, a greater proportion of these cells are senescent, as determined by their higher levels of CXCR4 expression and annexin V binding. Consequently, bone marrow neutrophils from AnxA1−/− mice exhibit a reduced migratory capacity in vitro. Studies conducted in vitro also show that expression of AnxA1 is required for bone marrow macrophages, but not peritoneal macrophages, to phagocytose apoptotic neutrophils. Moreover, in vivo experiments indicate a defect in clearance of wild-type neutrophils in the bone marrow of AnxA1−/− mice. Thus, we conclude that expression of AnxA1 by resident macrophages is a critical determinant for neutrophil clearance in the bone marrow.— Dalli, J., Jones, C. P., Cavalcanti, D. M., Farsky, S. H., Perretti, M., Rankin, S. M. Annexin A1 regulates neutrophil clearance by macrophages in the mouse bone marrow.
Keywords: CXCR4, neutrophil homeostasis
Neutrophils mature in the bone marrow (BM), where their production and release is driven by the cytokine G-CSF. Once in the blood, these cells have a relatively short half-life of ∼6.5 h in humans (1, 2). While it was originally thought that neutrophils are cleared primarily in the spleen (3) and liver (1, 4), more recent analyses of neutrophil clearance in human and in animal models indicate that neutrophils are also cleared in the BM compartment (1, 5–9). Indeed, we have shown that in mice the BM clears ∼30% of circulating neutrophils under homeostatic conditions (4).
Mature neutrophils express high levels of CXCR2, and under inflammatory conditions, both their mobilization from the BM and recruitment into tissues is driven by ELR+ CXC chemokines that act via this receptor (10). It has been reported that as neutrophils age, within a matter of hours, they down-regulate CXCR2 expression and exhibit a reduced capacity to migrate to ELR+ CXC chemokines. Concomitant with CXCR2 down-regulation is the up-regulation of cell surface CXCR4 expression. Thus, senescent CXCR4high neutrophils exhibit reduced chemotaxis to the ELR+ CXC chemokines but acquire the ability to migrate to the chemokine CXCL12 (11, 12). It is important to note, however, that when aged further in vitro neutrophils undergo apoptosis; these apoptotic neutrophils despite expressing the appropriate receptors cannot migrate to chemokines, or any other chemotactic stimuli, as their migratory capacity is impaired due to aging (12).
CXCL12 is expressed constitutively at high levels in the BM, and we have previously shown that in vivo senescent CXCR4high neutrophils preferentially home to the BM in a CXCR4-dependent manner (12). Thus, in vivo, one pathway for neutrophil clearance is dependent on chemokine-driven trafficking back to the BM, where the resident population of macrophages phagocytoses these apoptotic neutrophils. Previous studies have shown that BM macrophages are distinct from other tissue macrophages and play an important role in clearing cellular debris within the BM environment, including, as an example, extruded nuclei from developing reticulocytes (13, 14). Tissue macrophages present in the spleen and liver play a similar function in clearing apoptotic neutrophils from the circulation. Furthermore, the uptake of apoptotic neutrophils by inflammatory macrophages at sites of inflammation is critical to drive the resolution of inflammation (4, 15, 16).
The molecular mechanisms regulating recognition and uptake of apoptotic neutrophils by macrophages have been studied extensively in vitro, with several lines of evidence suggesting that they differ depending on the specific tissue macrophage used (17–19). On the one hand, for example, in vitro studies have shown that uptake of apoptotic cells by peritoneal macrophages is dependent on recognition of phosphatidylserine (20–22). On the other hand, the integrin αV/β3 (23–25) and the anti-inflammatory molecule annexin A1 (AnxA1) (26, 27) have been shown to play a role in the uptake of apoptotic cells by BM-derived macrophages. This finding suggests that the molecular mechanisms regulating neutrophil clearance in vivo may also be tissue dependent, reflecting the specific populations of macrophages present in different tissues. However, this hypothesis has not, as yet, been proven.
Macrophages and neutrophils contain high levels of AnxA1 in their cytoplasm. In human neutrophils, ∼40–60% of AnxA1 protein is stored within the gelatinase granules and can be translocated rapidly to the cell surface on activation, such as in response to an inflammatory stimulus (28). With respect to neutrophil biology, it has been shown that under inflammatory conditions, AnxA1 dampens the vascular response of these cells, reducing their adhesion to and emigration across the postcapillary venules (29, 30). Consistent with these findings, the inflammatory response in AnxA1−/− mice is exacerbated, as reported in a number of experimental models of inflammation (see ref. 31). As such, AnxA1 is an important endogenous anti-inflammatory molecule that limits the extent of the inflammatory response.
Several lines of evidence suggest that AnxA1 is also endowed with proresolving actions that promote neutrophil apoptosis (32) and subsequent phagocytosis by macrophages (33, 34). Thus, exogenous administration of AnxA1 accelerates neutrophil apoptosis (32, 35), while endogenous AnxA1 released from apoptotic neutrophils stimulates macrophages and acts as an “eat-me” signal, inducing them to phagocytose apoptotic neutrophils (34). Furthermore, AnxA1 secreted by macrophages themselves (in response to gluocorticoid treatment) has also been shown to enhance the rate of phagocytosis of apoptotic neutrophils (33). Interestingly, in AnxA1-deficient mice, no defect was found in the clearance of apoptotic neutrophils and the resolution of inflammation under acute inflammatory conditions. It has been concluded, therefore, that this protein is not critical for the recognition or phagocytosis of apoptotic neutrophils by inflammatory macrophages (30). In contrast, others have reported that BM-derived macrophages isolated from AnxA1−/− mice displayed a reduced capacity to phagocytose apoptotic neutrophils in vitro (36). To date, no in vivo information is available assessing the role played by AnxA1 in the clearance of apoptotic neutrophils in the BM. In this study, we addressed this question, reporting abnormally high levels of neutrophils in the BM of AnxA1−/− mice; we demonstrate that this finding is due to a defect in neutrophil clearance by resident BM macrophages, resulting in the accumulation of senescent neutrophils in this tissue compartment.
MATERIALS AND METHODS
Reagents
Unless otherwise stated, all reagents were purchased from Sigma (Dorset, UK). Rat anti-mouse F4/80-PE (clone CI:A3-I) was from Serotec (Oxford, UK). Rat anti-mouse Ly6G-FITC, Ly6G-PE (clone RB6 8C5), CD115-APC (clone AF598), CXCR4-Alexa 647 (clone 2B11), and F4/80 PercP-Cy5.5 (clone BM8) were from eBioscience (Hatfield, UK). Rat anti-mouse CXCR2-PE (clone 242216) and goat anti-mouse CD33 were from R&D Systems (Abingdon, UK). Rat anti-mouse CD13-PE (clone R3242) and rat anti-mouse Ly-G6-Alexa700 (clone 1A8) were from Becton-Dickinson (Oxford, UK). Rat anti-mouse CD34-PercP (clone MEC14.7) was from St. Cruz (Heidelberg, Germany). Rabbit anti-AnxA1 was from Invitrogen (Paisley, UK). Anti-rabbit Dylight-488 (clone Poly4064) was from BioLegend (Cambridge, UK). Percoll was from GE Healthcare UK Ltd. (Chalfont St. Giles, UK). Annexin V-PE was from Serotec. Human recombinant AnxA1 was generated in-house, as described previously (37). Unless stated, cells were cultured in RPMI containing fetal calf serum (FCS; 10%; Invitrogen), 2 mM l-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin (complete medium) at 37°C in a 5% CO2 humidified incubator.
Mice
Male wild-type (WT) and AnxA1-null (AnxA1−/−) (30, 38) 5- to 6-wk-old BALB/c mice were purchased from B&K Universal (Hull, UK). Chow and water were available ad libitum. UK Home Office guidelines for animal welfare based on the Animals (Scientific Procedures) Act 1986 were strictly observed.
Characterization of the BM and blood cell populations in WT and AnxA1−/− mice
Mice anesthetized using isofluorane were bled via cardiac puncture, and the cells were stained using the following antibody combinations: Ly6G-FITC/CXCR4-Alexa647, Ly6G-FITC/CXCR2-PE, and Ly6G-PE/AnxAV-FITC for 45 min on ice. Cells were then washed, and staining was analyzed using a FACSCalibur (Becton-Dickinson), acquiring ≥20,000 events/sample.
BM from femurs, tibiae, and humeri of WT and AnxA1−/− mice was harvested by flushing with Hanks' balanced salt solution (HBSS; without Ca2+ and Mg2+), 30 mM HEPES, and 15 mM EDTA. A single-cell suspension was created by passing the extracted BM through a 21-gauge needle. Cells were centrifuged (Sorvall Legend RT; Sorvall Ltd., Bishop's Stortford, UK) at 300 g for 10 min and 4°C, followed by differential count. Cells (∼2×105/aliquot) were washed with PBC and incubated with rat serum for 20 min prior to 45 min incubation, at 4°C, with the same combinations of antibodies as above. BM monocyte and macrophage levels were assessed following staining with anti-F4/80 and anti-CD115 antibodies.
Assessment of the phagocytic abilities of the AnxA1−/− BM macrophages was conducted by incubating the isolated BM cells for 20 min with rat serum at 4°C, then washing and incubating with a rat anti-mouse F4/80-PE or an IgG2a-PE control for another 30 min at 4°C. Subsequently, cells were fixed using 4% PFA, permeabilized by incubating with 0.03% Saponin for 10 min at 4°C, and stained using a rat anti-mouse Ly6G-FITC or an IgG2b-FITC for 30 min at 4°C.
Chemotaxis assay
BM-derived neutrophils were isolated from the total BM cell populations using a discontinuous Percoll gradient, as described previously (39). Briefly, BM cells, harvested as above, were layered on a discontinuous (52, 64, and 72%) Percoll gradient (100% Percoll=9:1 Percoll/10× PBS). Neutrophils were isolated from the 64/72% interface and resuspended at 4 × 106 cells/ml in RPMI plus 0.1% BSA. The bottom chambers of a ChemoTx plate (Neuroprobe, Gaithersburg, MD, USA) were loaded with RMPI, CXCL1 (0.5–50 nM; Cambridge BioScience Ltd., Cambridge, UK) or CXCL12 (0.5–50 nM; Peprotech, London, UK). Subsequently, 25 μl of the neutrophil solution, from either the WT or AnxA1−/− animals, was added to the top chambers, and the plate was incubated for 90 min at 37°C in a 5% CO2 humidified incubator. The top part of the chamber was then washed, and the plate was centrifuged at 400 g for 5 min at room temperature. Cells were then transferred to a 96-well plate and incubated for 6 h with 20% AlamarBlue (Serotec) solution. A standard curve with known neutrophil numbers was employed to interpolate the unknown values.
In a distinct set of experiments following neutrophil isolation, the apoptotic cells were removed from both WT and AnxA1−/− mice, using Annexin V+ve microbeads (Miltenyi Biotec Ltd. Surry, UK) per the manufacturer's instructions. The viable cells were then counted, and their chemotactic activity toward CXCL-12 (0.5 nM) was assessed following the protocol outlined above.
Preparation of senescent neutrophils from mouse BM
Neutrophils were isolated as previously outlined and then incubated overnight in complete medium. Following overnight culture, apoptotic cells were removed using Annexin V+ve microbeads (Miltenyi Biotec) per the manufacturer's instructions.
In vivo trafficking of latex (LX)-labeled neutrophils
Senescent neutrophils were loaded with 0.5 μm FITC-conjugated (yellow-gold) Fluoresbrite YG microspheres (2.5% w/v; Polysciences Inc., Warrington, PA, USA), as described previously (4). The cells were then washed to remove any free particles, and 5 × 106 LX+ neutrophils were injected i.v. (labeling efficiency was assessed to be ≥95%). Labeled cells were then injected i.v. into recipient WT and AnxA1−/− mice; 24 h later, the BM was collected from femurs, and single-cell suspension was prepared, centrifuged onto glass slides at 300 g for 5 min using a Cytospin 3 centrifuge (Shandon Scientific Ltd., Runcorn, UK). Slides were then stained using the Kwik-Diff staining kit (ThermoElectron, Pittsburgh, PA, USA) per the manufacturer's instructions, then mounted using Histomount mounting medium (VWR International Ltd., West Chester, PA, USA) and examined at ×100 using a Nikon Eclipse E400 microscope (Nikon, Tokyo, Japan). LX+ve cells were determined by means of fluorescence and subsequently identified by morphological characteristics under light microscopy. A minimum of 200 LX+ve cells/femur were counted. Images were obtained using a Nikon DMX1200 digital camera with Lucia GF software (Laboratory Imaging, Prague, Czech Republic) and processed using Adobe Photoshop CS (Adobe Systems Inc., San Jose, CA, USA).
Human neutrophil isolation
Human neutrophils were isolated from peripheral venous blood drawn from healthy volunteers according to institutional ethical guidelines (06/Q605/40; P/00/029 East London and the City Health Authority, London, UK). Briefly, cells were separated by gradient centrifugation over Ficoll-Paque and plated at 4 × 106 cells/ml. Spontaneous apoptosis (>60%) was achieved by culturing the neutrophils in complete RPMI 1640 for 20 h at 37°C in 5% CO2 atmosphere.
Culture of BM-derived and peritoneal macrophages
BM cells were obtained from WT and AnxA1−/− mice, and BM macrophages were prepared as described previously (33). In brief, BM cells were resuspended in RPMI 1640 supplemented with 25 mM HEPES, 10% FCS, and 20% L929 conditioned medium, and incubated on plastic at 37°C. Fresh culture medium was added on d 3 and 6. On d 7, the cells were incubated with PBS supplemented with 10 mM EDTA and 4 mg/ml lidocaine-HCl for 10 min before removal for cell harvesting, and then seeded on 12-well plates at 1.5 × 106 cells/well. The peritoneum of WT and AnxA1−/− mice was lavaged using a PBS solution containing 25 U/ml of heparin and 3 mM EDTA. Following lavage, the cells were washed, resuspended in complete RPMI, plated in 12-well plates at a concentration of 1.5 × 106 cells/well, and cultured for 2 h at 37°C in 5% CO2 atmosphere. The nonadherent cells were then removed by washing the cells 2× with PBS, and the adherent cells were cultured overnight in complete RPMI at 37°C in 5% CO2 atmosphere.
In vitro neutrophil phagocytosis assay
BM and peritoneal macrophages were washed with RPMI 1640 before coincubation with human apoptotic neutrophils (4×106 cells/well) at 37°C for 30 min. Noningested cells were removed by 3 washes with cold PBS, and phagocytosis was assayed by myeloperoxidase staining of cells fixed with 2.5% glutaraldehyde, as reported previously (40). For each experiment, the number of macrophages containing 1 or more neutrophils in ≥5 fields (minimum of 400 cells analyzed) was expressed as a percentage of the total number of macrophages.
In a separate set of experiments, BM-derived macrophages were incubated with CFDA-labeled apoptotic BM purified neutrophils, isolated and aged as described above, prepared from either WT or AnxA1−/− mice. In one case, AnxA1−/− BM-derived macrophages were preincubated for 5 min with 10 nM hrAnxA, prior to the addition of apoptotic neutrophils.
Evaluation of cell surface and total AnxA1 expression on BM and peritoneal macrophages
Peritoneal macrophages and BM cells were obtained as outlined above. For total AnxA1 expression, cells were first fixed using 2% PFA and then permeabilized using BD Phosflow Perm Buffer III (BD, Oxford, UK), following the manufacturer's instructions. Both permeabilized and intact cells, employed for total and surface AnxA1 expression, respectively, were blocked with 20% rabbit serum at 4°C for 20 min. Subsequently, cells were incubated with a rabbit anti-AnxA1 antibody (1:1000 dilution; Invitrogen) for 30 min at 4°C or with isotype control, prior to washing and incubation with an anti-rabbit Alexa-488 conjugated antibody (clone Poly4064; BioLegend). In a third step, cells were double-stained with an anti-mouse- PerCP-Cy5.5-conjugated anti-F4/80 antibody (clone BM8; eBioscience).
Evaluation of BM PMN senescence
Lipofucsin levels in BM neutrophils were evaluated by staining isolated BM cells with the anti-LyG6-Alexa 700 antibody (clone: 1A8). Since lipofucsin possesses auto fluorescence properties when excited at a wavelength between 320–430 nm with emission λmax at around 570 nm (41), we measured the level of auto fluorescence for the Ly6G positive cells in the FL2 channel.
GM-colony-forming unit (GM-CFU) assay
We used a protocol already described (42). Briefly, 5 × 104 BM cells were added to Methocult medium (StemCell Technologies, Inc., Vancouver, BC, Canada) and incubated for 12 d before quantification of GEMM, GM, M, and G colonies under an inverted microscope.
Hematopoietic progenitor analysis
As described previously (42), 2 × 105 BM cells were added to Methocult medium (StemCell Technologies) and incubated for 12 d before quantification of GEMM, GM, M, and G colonies under an inverted microscope. Numbers of myelomonocytic stem cells and promyelocytes were determined in BM cells by flow cytometry as CD13+ve CD33+ve CD34+ve CD115+ve and CD13+ve CD33+ve CD115−ve CD34−ve, respectively. Since the CD33 antibody was not conjugated to a fluorophore, a second labeling step was employed using a donkey anti-goat FITC-conjugated antibody (R&D Systems).
Data handing and statistical analysis
For the in vitro experiments, each treatment was conducted in quadruplicate and repeated ≥2 times. For the in vivo protocols, ≥5 mice/group were used. In all cases, data are reported as means ± se. Statistical differences between groups were determined either using the Student's t test or by 2-way ANOVA and, if significant, by Bonferroni posttest, taking a probability value of P < 0.05 as significant.
RESULTS
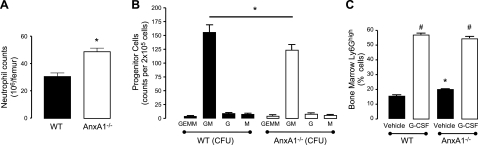
Analysis of neutrophil levels in the BM of WT and AnxA1−/− mice revealed a significant elevation in numbers in the latter genotype (Fig. 1A). To investigate whether this increase in BM granulocyte numbers was the result of enhanced granulopoiesis, we assessed the levels of hematopoietic CFUs in BM of WT and AnxA1−/− mice. Interestingly, G-CFU levels were not significantly different between the mouse genotypes, while a small, but significant decrease was found in the number of GM CFUs in the AnxA1−/− mice (Fig. 1B). Moreover, when we assessed immature granulocytes numbers by flow cytometry, we did not find any significant differences between the two genotypes (Supplemental Fig. S1). We further investigated the effect of G-CSF treatment on neutrophil production by treating mice 1×/d for 4 d with G-CSF. Here, the BM reserve expansion of mature neutrophils was comparable between the WT and AnxA1−/− mice (Fig. 1C). Taken together, the results shown in Fig. 1 indicate that the rate of granulopoiesis is not enhanced in the AnxA1−/− mice, which suggests that the increase neutrophil numbers is not due to an enhanced rate of neutrophil production.
Figure 1.
Elevated neutrophil counts in the AnxA1−/− BM. WT and AnxA1−/− mice were sacrificed, and BM cells were extracted. Differential cell counts were conducted using Turks solution. A) BM neutrophil counts in the BM of WT and AnxA1−/− mice. Results are means ± se of 5 animals/group. *P < 0.05 vs. WT group. B) Granulopoiesis from WT and AnxA1−/− mice was assessed ex vivo. BM cells were cultured in semisolid medium, and GEMM, GM, G, and M CFUs were scored at d 12. C) WT and AnxA1−/− mice were injected with vehicle or G-CSF (100 μg/kg) i.p. daily for 4 d. BM was harvested 24 h after the last injection, and neutrophil levels were quantified. Neutrophil counts in the BM were assessed by flow cytometry (Ly6Ghigh cells). Data represent means ± se of 4–11 animals/group. *P < 0.05 vs. WT group; #P < 0.05 vs. respective vehicle group.
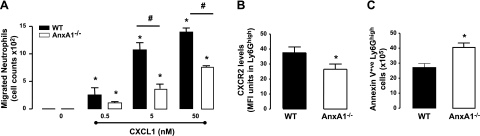
To investigate the functionality of neutrophils, we first assessed their capacity for chemotaxis toward a characterized chemoatttractant, CXCL1, in vitro. Here neutrophils harvested from the BM of AnxA1−/− mice displayed a reduced chemotactic response to this chemokine when compared to their WT counterparts (Fig. 2A). Furthermore, we observed reduced CXCR2 expression in the BM neutrophils obtained from AnxA1−/− animals when compared to WT controls (Fig. 2B). It has previously been reported that as neutrophils age, they express less CXCR2 on their cell surface, which suggests that BM AnxA1−/− neutrophils might represent a senescent population (11, 12). Indeed, as shown in Fig. 2C, the absolute numbers of Annexin V+ve (early apoptotic) neutrophils was significantly higher in the AnxA1−/− mice. Since aged neutrophils lose their capacity to migrate (43, 44), we reasoned the reduced chemotactic activity of BM neutrophils from AnxA1−/− mice might be due to their senescent phenotype.
Figure 2.
Elevated levels of senescent neutrophils in the AnxA1−/− BM. BM cells from WT and AnxA1−/− cells were isolated. A) Chemotatic response to various concentrations of CXCL1 was assessed for purified BM neutrophil populations, derived from both WT and AnxA1−/− animals. B) Expression level of CXCR2 was determined on Ly6Ghigh cells in the BM of WT and AnxA1−/− mice by flow cytometry. C) Total number of apoptotic neutrophils was evaluated as the total number of cells staining positive for anxnexin V+ve/Ly6Ghigh in the cell suspensions isolated from BM of WT and AnxA1−/− mice. Results are means ± se of 5 animals per group. *P < 0.05 vs. WT vehicle group; #P < 0.05.
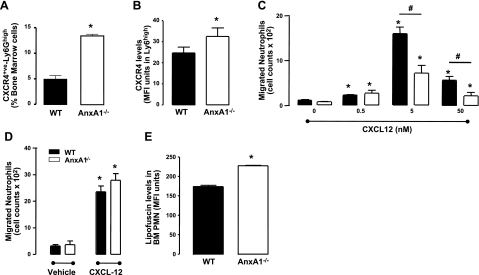
As neutrophils age, cell surface up-regulation of CXCR4 occurs (12). Analysis of CXCR4 expression on BM neutrophils revealed that in WT mice, ∼5% of neutrophils were CXCR4+ve-Ly6Ghigh, this population increasing to ∼13% in AnxA1−/− animals (Fig. 3A). Further, we observed that CXCR4 absolute levels were higher on AnxA1−/− neutrophils (Fig. 3B). Interestingly, while neutrophils isolated from BM of AnxA1−/− mice exhibited increased CXCR4 expression, their ability to migrate in response to CXCL12 was reduced compared to WT (Fig. 3C). However, removal of Annexin V+ve cells from the population of purified neutrophils led to an equivalent chemotactic response for neutrophils obtained from WT and ANXA1−/− mice (Fig. 3D). Finally, we assessed lipofuscin levels in BM neutrophils as another method to determine senescence (41). As assessed by flow cytometry, lipofuscin levels were significantly increased in neutrophils from the BM of AnxA1−/− mice (Fig. 3E). Taken together, the results presented in Fig. 3 indicate that neutrophils in the BM of AnxA1−/− mice exhibit a more senescent phenotype as compared to neutrophils from WT mice.
Figure 3.
Elevated expression of CXCR4 on BM neutrophils from AnxA1−/− mice. BM cells from WT and AnxA1−/− cells were isolated. A, B) Proportion of CXCR4 expressing Ly6Ghigh cells (A) and levels of CXCR4 in Ly6Ghigh cells (B) in these isolated cells were determined following immunostaining. C) Chemotatic response to various concentrations of CXCL12 was assessed for purified BM neutrophil populations from both WT and AnxA1−/− animals. D) Chemotatic response to various concentrations of CXCL12 was assessed for purified BM neutrophil populations from both WT and AnxA1−/− animals that had been depleted of annexin V+ve cells. E) Increased lipofuscin levels in AnxA1−/− BM neutrophils as assessed flow cytometry. Results are means ± se of 5 animals/group. *P < 0.05 vs. WT vehicle group; #P < 0.05.
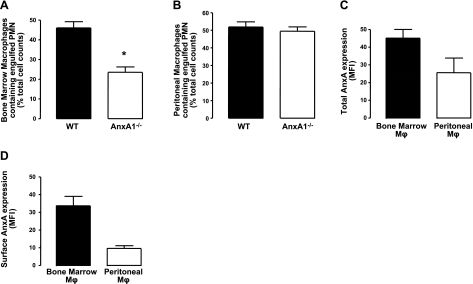
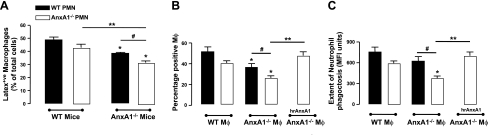
After homing back to the BM, senescent CXCR4high neutrophils are phagocytosed by resident macrophages (4). To assess whether a deregulation in this process was responsible for the accumulation of senescent neutrophils observed in the AnxA1−/− BM, we prepared BM macrophages from both sets of mice and assessed their ability to phagocytose apoptotic neutrophils, using well-established methodologies. This analysis revealed profound defects in the ability of AnxA1−/− BM macrophages to phagocytose apoptotic neutrophils (Fig. 4A), as previously reported (36). Interestingly, we show for the first time that this defect is specific for BM macrophages, since the capacity of peritoneal macrophages to ingest apoptotic neutrophils was not impaired in AnxA1−/− mice (Fig. 4B).
Figure 4.
Altered phagocytosis of human senescent neutrophils by BM, but not peritoneal, AnxA1−/− macrophages in vitro. BM macrophages were obtained following 5 d culture of BM cells obtained from WT and AnxA1−/− mice in GM-CSF-rich medium. Peritoneal macrophages were prepared following peritoneal lavage of WT and AnxA1−/− mice. A, B) Human neutrophils were obtained from healthy volunteers; after an overnight culture they were incubated with BM (A) or peritoneal macrophages (B) for 30 min. C, D) Subsequently, MPO staining was conducted to determine the extent of neutrophil phagocytosis. Total (C) and cell surface-associated (D) AnxA1 levels were determined in both resident BM and peritoneal macrophages; values are normalized to respective isotype control. Results are means ± se of 4 animal cell preparations/group. *P < 0.05 vs. respective control group.
Having observed a difference in the phagocytic ability of these two macrophage populations, we investigated whether this was dictated by different AnxA1 expression levels in these macrophage subsets or by different expression patterns within the cell. Flow cytometric analysis revealed that while a reduction in the total AnxA1 expression was found in the peritoneal macrophages compared to the BM macrophages, this did not reach statistical significance (Fig. 4C). However, BM macrophages were observed to express significantly higher cell surface-associated AnxA1 levels (Fig. 4D).
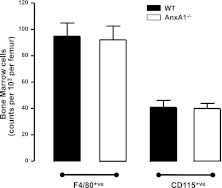
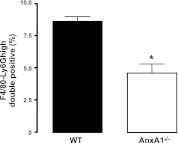
To investigate whether the defect in neutrophil clearance was due to reduced macrophage numbers in the BM of AnxA1−/− mice, we next determined the absolute macrophage and monocyte numbers in both mouse genotypes by flow cytometry. As shown in Fig. 5, no difference was found in the absolute numbers of BM macrophages (F4/80+ve cells) or monocytes (CD115+ve cells) in the BM of these mice. Using two distinct methodologies, we next evaluated whether BM macrophages had a defect in neutrophil clearance in vivo (4), (45). First, we utilized a double-staining protocol to determine the number of macrophages that had engulfed neutrophils in the murine BM under steady state. This technique uses flow cytometry to quantify the number of cells that exhibit extracellular staining for the macrophage-specific marker F4/80 together with high intracellular staining for the neutrophil-specific marker Ly6G. The BM was harvested from untreated WT and AnxA1−/− mice, and flow cytometric analysis revealed a significant reduction in the abundance of Ly6Ghigh-F4/80 double-positive cells in the BM of AnxA1−/− mice as compared to WT mice (Fig. 6). Therefore, despite the higher numbers of Annexin V+ve neutrophils in the BM (Fig. 2C), fewer neutrophils are phagocytosed by BM macrophages.
Figure 5.
Normal numbers of macrophages and monocytes in the BM of AnxA1−/−mice. BM was harvested from WT and AnxA1−/− mice. Numbers of monocyte and macrophages were assessed by flow cytometry (F4/80 and CD115 cells). Data represent means ± se of 4 animals/group.
Figure 6.
Impaired phagocytosis of senescent neutrophils by AnxA1−/− BM macrophages. Extent of neutrophil clearance by BM macrophages was assessed by staining freshly extracted BM cells from WT and AnxA1−/− animals against F4/80 antigen. Subsequently, the cells were fixed, permeabilized, and stained for Ly6G+ve expression. Data represent percentages of F4/80-Ly6G+ve double-positive BM cells from untreated WT and AnxA1−/− mice. Results are means ± se of 4 animal cell preparations/group. *P < 0.05 vs. WT group.
Second, we applied a protocol developed by our group to challenge the system in a dynamic fashion (4). Thus, BM neutrophils isolated from WT or AnxA1−/− mice were labeled with nonactivating fluorescent LX microspheres and then injected into either WT or AnxA1−/− animals. As previously observed (4), analysis of the BM 24 h later revealed macrophages that had phagocytosed neutrophils retained the nondegradable fluorescent beads. The distribution of fluorescent beads was determined between neutrophils and macrophages in BM cytospins. As shown in Fig. 7A, when neutrophils were injected into WT donor mice, ∼50% of the LX+ve cells were found in macrophages, irrespective of whether the injected neutrophils were isolated from WT or AnxA1−/− mice. Thus deletion of AnxA1 in neutrophils does not affect their phagocytosis by BM macrophages in vivo. In contrast, when neutrophils from WT or AnxA1−/− mice were injected into AnxA1−/− mice, there was a significant reduction in percentage of LX+ve BM macrophages, as compared to that observed when the neutrophils were injected into WT mice. Taken together, these results suggest that expression of AnxA1 by BM macrophages is required for their efficient phagocytosis of apoptotic neutrophils in vivo.
Figure 7.
Impaired BM phagocytosis of mouse neutrophils following in vivo administration. A) Senescent neutrophils prepared from the BM of WT and AnxA1−/− mice were loaded with fluorescent LX beads, and 5 × 106 cells were injected i.v. into recipient WT or AnxA1−/− animals. After 24 h, cytospins were prepared from freshly isolated BM. Extent of BM macrophages (Mφ) containing LX beads was determined following counterstaining with hematoxilyn; 200 LX+ve cells/animal were counted. B) Cultured BM-derived macrophages were observed to show the same defect in phagocytosis as in vivo resident BM macrophages. This phenotype could be rescued by addition of 10 nM hrAnxA1 to the cell cultures. C) Number of neutrophils engulfed per macrophage was assessed my measuring mean fluorescence intensity (MFI) per cell. Results are means ± se of 5 animals/group. *P < 0.05 vs. respective WT group; **P < 0.05; #P < 0.05.
To underpin the role played by AnxA1 in mediating this phagocytic process, we recapitulated the experimental conditions assessed above in vitro, this time also adding exogenous hrAnxA1 to neutrophils and macrophages prepared from AnxA1−/− mice. By assessing the number of macrophages containing the fluorescent labeled cells, we confirmed our previous observations that expression of AnxA1 by the macrophage is required for efficient phagocytosis of apoptotic neutrophils. Furthermore, the addition of exogenous AnxA1 could rescue the profile of the AnxA1−/− macrophages (Fig. 7B).
Measuring the level of fluorescence contained in each cell, we could also evaluate whether any differences could be found in the phagocytic potential of WT and AnxA1−/− BM-derived macrophages. Here, the only statistically significant reduction in the number of neutrophils engulfed per cell was observed when neutrophils and macrophages derived from AnxA1−/− cells were coincubated. Interestingly, the addition of exogenous hrAnxA1 was once again observed to correct this defect in neutrophil phagocytosis (Fig. 7C).
DISCUSSION
We have shown previously that the BM represents an important site of neutrophil clearance under homeostatic conditions. Senescent neutrophils homing back to the BM in a CXCR4-dependent manner are subsequently phagocytosed by resident tissue macrophages (4, 12). In this study we identify a novel role for the molecule AnxA1, expressed by BM macrophages, in the uptake of apoptotic neutrophils in this tissue.
The observation that AnxA1−/− mice had significantly higher neutrophil numbers in the BM prompted us to investigate a fundamental aspect of neutrophil biology in these mice (46). AnxA1−/− mice, despite having higher numbers of neutrophils in the BM, did not exhibit an increase in numbers of granulocyte progenitor cells, as determined by flow cytometry and CFUs. Further, the extent of granuolopoiesis induced by G-CSF was the same in the WT and AnxA1−/− mice. These results suggest that this increase in BM neutrophil numbers is not due to an enhanced rate of neutrophil production.
Phenotypic analysis of the BM neutrophils showed that in the AnxA1−/− mice, these cells expressed lower CXCR2 and higher CXCR4, lipofuscin, and phosphatidylserine levels, a pattern of expression consistent with a senescent phenotype. Migratory capacity of neutrophils reduces with age, whereby apoptotic neutrophils lose their ability to migrate (43, 44). In particular, it has been reported that aged neutrophils exhibit reduced migration toward CXCL1. Consistent with these observations, neutrophils isolated from the BM of AnxA1−/− mice exhibited a reduced chemotactic activity toward CXCL1 as compared to their WT counterparts. Thus, we propose that this reduced migratory capacity of AnxA1−/− BM neutrophils is due to a higher proportion of senescent and apoptotic cells in the population. Indeed, depletion of annexin-V+ve neutrophils from the BM neutrophil pool restored chemotaxis to levels seen in WT BM neutrophils. These data are consistent with previous studies showing that neutrophil recruitment to sites of inflammation is not impaired in the AnxA1−/− mice (30, 47), thus corroborating the notion that the migratory capacity of neutrophils per se is not impaired by genetic deletion of this protein. We conclude, therefore, that the increased neutrophil numbers observed in the BM of AnxA1−/− mice is not consequent to an enhanced rate of production or expansion of the BM reserve, but rather to the accumulation of senescent neutrophils.
Given that tissue macrophages are responsible for the clearance of apoptotic neutrophils, we next focused our attention on BM macrophages. It has previously been reported that BM-derived macrophages obtained from AnxA1−/− mice have a reduced in vitro capacity to phagocytose human apoptotic neutrophils, as compared to those isolated from WT mice. We show here that this defect in phagocytosis is selective for BM macrophages in that peritoneal macrophages isolated from the AnxA1−/− mice could phagocytose apoptotic neutrophils to the same extent as their WT counterparts. Our data indicate that while AnxA1 is critical for the phagocytosis of apoptotic neutrophils by BM macrophages, its effect may be redundant for peritoneal macrophages. This finding suggests that AnxA1 plays a role in neutrophil clearance in the BM, but not at sites of inflammation: in acute peritonitis, the rate of neutrophil clearance is normal in AnxA1−/− mice (48).
It is now well recognized that phenotypic and functional differences exist between distinct tissue-resident macrophage populations. We show here that peritoneal macrophages express higher levels of MHC class II and CD11b than their BM counterparts, which suggests a more activated phenotype. This finding might provide an insight into why these different sets of tissue macrophages utilize discrete molecular pathways for the uptake of apoptotic neutrophils. Phagocytosis of neutrophils by tissue macrophages in vivo can be reliably assessed by quantifying the number of cells that exhibit extracellular staining for the macrophage-specific marker F4/80 together with high intracellular staining for the neutrophil-specific marker Ly6G (45). Using this technique we could detect that—under homeostatic conditions—the percentage of double-positive cells was significantly reduced in the BM of the AnxA1−/− mice, pointing toward a defect in apoptotic neutrophil clearance in this tissue compartment. As AnxA1 is expressed by both neutrophils and macrophages (28, 49), experiments were designed to assess whether the reduced rate of phagocytosis was due to a lack of AnxA1 in either of these cell types. Our data show that expression of AnxA1 on BM macrophages is required for the recognition and phagocytosis of apoptotic neutrophils. Thus, significantly fewer neutrophils were phagocytosed when WT neutrophils were injected into AnxA1−/− mice, as compared to WT mice. In vitro studies further highlighted this point, showing that maximal phagocytosis of apoptotic neutrophils by BM macrophages required the expression of AnxA1 on macrophages. However, these results do not rule out the role of neutrophil-derived AnxA1 in mediating this process. In any case, addition of exogenous AnxA1 protein restored phagocytosis, suggesting that external expression of this molecule was critical for the clearance of apoptotic neutrophils by BM macrophages. Collectively, the studies presented here and discussed above provide an explanation for the markedly increased numbers of neutrophils in the BM of AnxA1−/− mice.
In summary, we report a novel and unexpected function for endogenous AnxA1 as a molecule expressed by BM macrophages that, under homeostatic conditions, is critical in mediating the clearance of apoptotic neutrophils in the BM.
Supplementary Material
Acknowledgments
The authors acknowledge the support of the Wellcome Trust (086867/Z/08 and 0851851/Z/08) and the William Harvey Research Foundation. This work forms part of the research themes contributing to the translational research portfolio of Barts and the London Cardiovascular Biomedical Research Unit, which is supported and funded by the National Institute for Health Research (UK). J.D. performed research and collected, analyzed, and interpreted data, and contributed to paper preparation; D.M.C. performed research and collected data; S.F. analyzed and interpreted data; C.J. performed research and collected data; M.P. designed research, analyzed and interpreted data, and contributed to paper preparation; and S.R. designed research, analyzed and interpreted data, and wrote the paper. The authors declare no competing financial interests.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Summers C., Rankin S. M., Condliffe A. M., Singh N., Peters A. M., Chilvers E. R. (2010) Neutrophil kinetics in health and disease. Trends Immunol. 31, 318–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mauer A. M., Athens J. W., Ashenbrucker H., Cartwright G. E., Wintrobe M. M. (1960) Leukokinetic studies. Ii. A method for labeling granulocytes in vitro with radioactive diisopropylfluorophosphate (Dfp). J. Clin. Invest. 39, 1481–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bicknell S., van Eeden S., Hayashi S., Hards J., English D., Hogg J. C. (1994) A non-radioisotopic method for tracing neutrophils in vivo using 5′-bromo-2′-deoxyuridine. Am. J. Respir. Cell Mol. Biol. 10, 16–23 [DOI] [PubMed] [Google Scholar]
- 4. Furze R. C., Rankin S. M. (2008) The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB J. 22, 3111–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lovas K., Knudsen E., Iversen P. O., Benestad H. B. (1996) Sequestration patterns of transfused rat neutrophilic granulocytes under normal and inflammatory conditions. Eur. J. Haematol. 56, 221–229 [DOI] [PubMed] [Google Scholar]
- 6. Rankin S. M. (2010) The bone marrow: a site of neutrophil clearance. J. Leukoc. Biol. 88, 241–251 [DOI] [PubMed] [Google Scholar]
- 7. Saverymuttu S. H., Peters A. M., Keshavarzian A., Reavy H. J., Lavender J. P. (1985) The kinetics of 111indium distribution following injection of 111indium labelled autologous granulocytes in man. Br. J. Haematol. 61, 675–685 [DOI] [PubMed] [Google Scholar]
- 8. Suratt B. T., Young S. K., Lieber J., Nick J. A., Henson P. M., Worthen G. S. (2001) Neutrophil maturation and activation determine anatomic site of clearance from circulation. Am. J. Physiol. Lung Cell. Mol. Physiol. 281, L913–L921 [DOI] [PubMed] [Google Scholar]
- 9. Thakur M. L., Lavender J. P., Arnot R. N., Silvester D. J., Segal A. W. (1977) Indium-111-labeled autologous leukocytes in man. J. Nucl. Med. 18, 1014–1021 [PubMed] [Google Scholar]
- 10. Burdon P. C., Martin C., Rankin S. M. (2008) Migration across the sinusoidal endothelium regulates neutrophil mobilization in response to ELR + CXC chemokines. Br. J. Haematol. 142, 100–108 [DOI] [PubMed] [Google Scholar]
- 11. Nagase H., Miyamasu M., Yamaguchi M., Imanishi M., Tsuno N. H., Matsushima K., Yamamoto K., Morita Y., Hirai K. (2002) Cytokine-mediated regulation of CXCR4 expression in human neutrophils. J. Leukoc. Biol. 71, 711–717 [PubMed] [Google Scholar]
- 12. Martin C., Burdon P. C., Bridger G., Gutierrez-Ramos J. C., Williams T. J., Rankin S. M. (2003) Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 19, 583–593 [DOI] [PubMed] [Google Scholar]
- 13. Sadahira Y., Mori M. (1999) Role of the macrophage in erythropoiesis. Pathol. Int. 49, 841–848 [DOI] [PubMed] [Google Scholar]
- 14. Osmond D. G., Rico-Vargas S., Valenzona H., Fauteux L., Liu L., Janani R., Lu L., Jacobsen K. (1994) Apoptosis and macrophage-mediated cell deletion in the regulation of B lymphopoiesis in mouse bone marrow. Immunol. Rev. 142, 209–230 [DOI] [PubMed] [Google Scholar]
- 15. Fox S., Leitch A. E., Duffin R., Haslett C., Rossi A. G. (2010) Neutrophil apoptosis: relevance to the innate immune response and inflammatory disease. J. Innate Immun. 2, 216–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McColl A., Bournazos S., Franz S., Perretti M., Morgan B. P., Haslett C., Dransfield I. (2009) Glucocorticoids induce protein S-dependent phagocytosis of apoptotic neutrophils by human macrophages. J. Immunol. 183, 2167–2175 [DOI] [PubMed] [Google Scholar]
- 17. Takahashi K., Naito M., Takeya M. (1996) Development and heterogeneity of macrophages and their related cells through their differentiation pathways. Pathol. Int. 46, 473–485 [DOI] [PubMed] [Google Scholar]
- 18. Naito M., Umeda S., Yamamoto T., Moriyama H., Umezu H., Hasegawa G., Usuda H., Shultz L. D., Takahashi K. (1996) Development, differentiation, and phenotypic heterogeneity of murine tissue macrophages. J. Leukoc. Biol. 59, 133–138 [DOI] [PubMed] [Google Scholar]
- 19. Gorgani N. N., Ma Y., Clark H. F. (2008) Gene signatures reflect the marked heterogeneity of tissue-resident macrophages. Immunol. Cell Biol. 86, 246–254 [DOI] [PubMed] [Google Scholar]
- 20. Jitkaew S., Witasp E., Zhang S., Kagan V. E., Fadeel B. (2009) Induction of caspase- and reactive oxygen species-independent phosphatidylserine externalization in primary human neutrophils: role in macrophage recognition and engulfment. J. Leukoc. Biol. 85, 427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kobayashi N., Karisola P., Pena-Cruz V., Dorfman D. M., Jinushi M., Umetsu S. E., Butte M. J., Nagumo H., Chernova I., Zhu B., Sharpe A. H., Ito S., Dranoff G., Kaplan G. G., Casasnovas J. M., Umetsu D. T., Dekruyff R. H., Freeman G. J. (2007) TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity 27, 927–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Greenberg M. E., Sun M., Zhang R., Febbraio M., Silverstein R., Hazen S. L. (2006) Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J. Exp. Med. 203, 2613–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bottcher A., Gaipl U. S., Furnrohr B. G., Herrmann M., Girkontaite I., Kalden J. R., Voll R. E. (2006) Involvement of phosphatidylserine, alphavbeta3, CD14, CD36, and complement C1q in the phagocytosis of primary necrotic lymphocytes by macrophages. Arthritis Rheum. 54, 927–938 [DOI] [PubMed] [Google Scholar]
- 24. Savill J., Hogg N., Ren Y., Haslett C. (1992) Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J. Clin. Invest. 90, 1513–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fadok V. A., Savill J. S., Haslett C., Bratton D. L., Doherty D. E., Campbell P. A., Henson P. M. (1992) Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J. Immunol. 149, 4029–4035 [PubMed] [Google Scholar]
- 26. Fan X., Krahling S., Smith D., Williamson P., Schlegel R. A. (2004) Macrophage surface expression of annexins I and II in the phagocytosis of apoptotic lymphocytes. Mol. Biol. Cell 15, 2863–2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yona S., Buckingham J. C., Perretti M., Flower R. J. (2004) Stimulus-specific defect in the phagocytic pathways of annexin 1 null macrophages. Br. J. Pharmacol. 142, 890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perretti M., D'Acquisto F. (2009) Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 9, 62–70 [DOI] [PubMed] [Google Scholar]
- 29. Lim L. H., Solito E., Russo-Marie F., Flower R. J., Perretti M. (1998) Promoting detachment of neutrophils adherent to murine postcapillary venules to control inflammation: effect of lipocortin 1. Proc. Natl. Acad. Sci. U. S. A. 95, 14535–14539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chatterjee B. E., Yona S., Rosignoli G., Young R. E., Nourshargh S., Flower R. J., Perretti M. (2005) Annexin 1-deficient neutrophils exhibit enhanced transmigration in vivo and increased responsiveness in vitro. J. Leukoc. Biol. 78, 639–646 [DOI] [PubMed] [Google Scholar]
- 31. Damazo A. S., Yona S., D'Acquisto F., Flower R. J., Oliani S. M., Perretti M. (2005) Critical protective role for annexin 1 gene expression in the endotoxemic murine microcirculation. Am. J. Pathol. 166, 1607–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. El Kebir D., Jozsef L., Filep J. G. (2008) Opposing regulation of neutrophil apoptosis through the formyl peptide receptor-like 1/lipoxin A4 receptor: implications for resolution of inflammation. J. Leukoc. Biol. 84, 600–606 [DOI] [PubMed] [Google Scholar]
- 33. Maderna P., Yona S., Perretti M., Godson C. (2005) Modulation of phagocytosis of apoptotic neutrophils by supernatant from dexamethasone-treated macrophages and annexin-derived peptide Ac(2–26). J. Immunol. 174, 3727–3733 [DOI] [PubMed] [Google Scholar]
- 34. Scannell M., Flanagan M. B., deStefani A., Wynne K. J., Cagney G., Godson C., Maderna P. (2007) Annexin-1 and peptide derivatives are released by apoptotic cells and stimulate phagocytosis of apoptotic neutrophils by macrophages. J. Immunol. 178, 4595–4605 [DOI] [PubMed] [Google Scholar]
- 35. Solito E., Kamal A., Russo-Marie F., Buckingham J. C., Marullo S., Perretti M. (2003) A novel calcium-dependent proapoptotic effect of annexin 1 on human neutrophils. FASEB J. 17, 1544–1546 [DOI] [PubMed] [Google Scholar]
- 36. Yona S., Heinsbroek S. E., Peiser L., Gordon S., Perretti M., Flower R. J. (2006) Impaired phagocytic mechanism in annexin 1 null macrophages. Br. J. Pharmacol. 148, 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pederzoli-Ribeil M., Maione F., Cooper D., Al-Kashi A., Dalli J., Perretti M., D'Acquisto F. (2010) Design and characterization of a cleavage-resistant Annexin A1 mutant to control inflammation in the microvasculature. Blood 116, 4288–4296 [DOI] [PubMed] [Google Scholar]
- 38. Hannon R., Croxtall J. D., Getting S. J., Roviezzo F., Yona S., Paul-Clark M. J., Gavins F. N., Perretti M., Morris J. F., Buckingham J. C., Flower R. J. (2003) Aberrant inflammation and resistance to glucocorticoids in annexin 1-/- mouse. FASEB J. 17, 253–255 [DOI] [PubMed] [Google Scholar]
- 39. Dalli J., Norling L. V., Renshaw D., Cooper D., Leung K. Y., Perretti M. (2008) Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood 112, 2512–2519 [DOI] [PubMed] [Google Scholar]
- 40. Godson C., Mitchell S., Harvey K., Petasis N. A., Hogg N., Brady H. R. (2000) Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 164, 1663–1667 [DOI] [PubMed] [Google Scholar]
- 41. Jung T., Hohn A., Grune T. (2010) Lipofuscin: detection and quantification by microscopic techniques. Methods Mol. Biol. 594, 173–193 [DOI] [PubMed] [Google Scholar]
- 42. Pitchford S. C., Furze R. C., Jones C. P., Wengner A. M., Rankin S. M. (2009) Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell 4, 62–72 [DOI] [PubMed] [Google Scholar]
- 43. Wolach B., van der Laan L. J., Maianski N. A., Tool A. T., van Bruggen R., Roos D., Kuijpers T. W. (2007) Growth factors G-CSF and GM-CSF differentially preserve chemotaxis of neutrophils aging in vitro. Exp. Hematol. 35, 541–550 [DOI] [PubMed] [Google Scholar]
- 44. Whyte M. K., Meagher L. C., MacDermot J., Haslett C. (1993) Impairment of function in aging neutrophils is associated with apoptosis. J. Immunol. 150, 5124–5134 [PubMed] [Google Scholar]
- 45. Schwab J. M., Chiang N., Arita M., Serhan C. N. (2007) Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 447, 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Furze R. C., Rankin S. M. (2008) Neutrophil mobilization and clearance in the bone marrow. Immunology 125, 281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gastardelo T. S., Damazo A. S., Dalli J., Flower R. J., Perretti M., Oliani S. M. (2009) Functional and ultrastructural analysis of annexin A1 and its receptor in extravasating neutrophils during acute inflammation. Am. J. Pathol. 174, 177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Damazo A. S., Yona S., Flower R. J., Perretti M., Oliani S. M. (2006) Spatial and temporal profiles for anti-inflammatory gene expression in leukocytes during a resolving model of peritonitis. J. Immunol. 176, 4410–4418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kamal A. M., Flower R. J., Perretti M. (2005) An overview of the effects of annexin 1 on cells involved in the inflammatory process. Mem. Inst. Oswaldo Cruz 100(Suppl. 1), 39–47 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.