Abstract
The distribution of postsynaptic glutamate receptors has been shown to be regulated by proimmunocytokine tumor necrosis factor α (TNF-α) signaling. The role of TNF-α receptor subtypes in mediating glutamate receptor expression, trafficking, and function still remains unclear. Here, we report that TNF receptor subtypes (TNFR1 and TNFR2) differentially modulate α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) clustering and function in cultured cortical neurons. We find that genetic deletion of TNFR1 decreases surface expression and synaptic localization of the AMPAR GluA1 subunit, reduces the frequency of miniature excitatory postsynaptic current (mEPSC), and reduces AMPA-induced maximal whole-cell current. In addition, these results are not observed in TNFR2-deleted neurons. The decreased AMPAR expression and function in TNFR1-deleted cells are not significantly restored by short (2 h) or long (24 h) term exposure to TNF-α. In TNFR2-deleted cells, TNF-α promotes AMPAR trafficking to the synapse and increases mEPSC frequency. In the present study, we find no significant change in the GluN1 subunit of NMDAR clusters, location, and mEPSC. This includes applying or withholding the TNF-α treatment in both TNFR1- and TNFR2-deleted neurons. Our results indicate that TNF receptor subtype 1 but not 2 plays a critical role in modulating AMPAR clustering, suggesting that targeting TNFR1 gene might be a novel approach to preventing neuronal AMPAR-mediated excitotoxicity.—He, P., Liu, Q., Wu, J., Shen, Y. Genetic deletion of TNF receptor suppresses excitatory synaptic transmission via reducing AMPA receptor synaptic localization in cortical neurons.
Keywords: clustering, inflammation, neurodysfunction, neurodegeneration, excitotoxicity
α-Amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) and N-methyl-d-aspartic acid receptor (NMDAR) are the main excitatory glutamate receptors in the mammalian brain where they extensively localize and cluster on postsynaptic neural membranes (1–3). They play important roles in mediating excitatory synaptic transmission, and overactivation of these receptors induces excitotoxic damage to neurons (4–6). Therefore, manipulation of the AMPAR/NMDAR distribution and/or activity could be an attractive approach to reduce or prevent neuronal excitotoxicity.
Two subtypes of TNF receptors, TNFR1 and TNFR2, are expressed in both neurons and glia (7–9). They couple to distinct but overlapping intracellular signaling pathways (10, 11) and are associated with the survival or death of cortical neurons (8, 12). TNFR1 can bind to either soluble TNF-α or transmembrane TNF-α, with a preference for soluble TNF-α, and activation of this receptor triggers a complex apoptotic pathway (13, 14). In contrast, TNFR2 is preferentially activated by transmembrane TNF-α (15, 16) and protects neurons against excitotoxicity (8, 17, 18).
It has been suggested that TNFR signaling is correlated with glutamatergic transmission, synaptic plasticity, and scaling (19, 20). Activation of the TNFR increases AMPAR assembly and trafficking to the cell surface while decreasing GABAA receptor surface expression (19, 21–23). Physiologically, TNFR-mediated signaling increases the amplitude and mean frequency of miniature excitatory postsynaptic current (mEPSC; refs. 19, 23) and decreases the amplitude of miniature inhibitory postsynaptic current (mIPSC; ref. 22). Pharmacologically, the inactivation of TNFR signaling by neutralizing endogenous TNF-α or sequestering TNFR1 could prevent basal and tetrodotoxin-induced increases in AMPAR surface expression and mEPSC amplitude, as well as reducing the amplitude of mIPSC (19, 23).
In the observations reported previously, evidence indicates the existence of TNFRs on the cellular surface. Due to specific signaling associations and the overlap of TNFR subtypes 1 and 2, the roles of these subtypes have not been fully determined. Here, we culture cortical neurons and observe the brain tissues following induction of cerebral ischemia injury from the brain tissue of mice that have been genetically modified to lack either TNFR subtype 1 (TNFR1−/−) or 2 (TNFR2−/−). We find that the signaling of TNFR1, but not TNFR2, plays a key role in AMPAR surface distribution, synaptic localization, and excitatory neurotransmitter transmission. However, both TNFR1 and 2 are associated with the maintenance of AMPAR affinity. This effect is highly specific, and neither subtype of TNFR is specifically correlated with NMDAR distribution, synaptic location, or affinity.
MATERIALS AND METHODS
Animals
All mice were of C57BL/6 background. Mice of wild-type (WT), TNFR1 homozygote knockout (TNFR1−/−), and TNFR2 homozygote knockout (TNFR2−/−) were purchased from Jackson Laboratory (Bar Harbor, ME, USA). The TNFR1 heterozygote genotype (TNFR1+/−) was produced by WT breeding with TNFR1−/− mice. The internal breeding of TNFR1+/− mice was performed, and the resulting mice were used to prepare cortical neuron cultures. The postnatal mice used for the cell cultures were genotyped, and the littermates of the same genetic background of WT (TNFR1+/+) and TNFR1−/− were analyzed. Similarly, experimental WT and TNFR2−/− mice were produced, and cell cultures were performed.
Focal cerebral ischemia models
All surgical procedures and animal husbandry protocols were in accordance with Sun Health Research Institute (Sun City, AZ, USA) ethics committee guidelines. Briefly, following overnight food withdrawal, 3-mo-old WT and TNFR1−/− mice (n=5 each) were anesthetized by intraperitoneal injection of chloral hydrate. The extracranial segment of the left internal carotid artery was isolated and ligated to interrupt blood supply for 24 h to the middle cerebral artery territory. The incision wound was sutured and cleaned. The animals were removed from the anesthetic and placed in veterinary incubators. Recovery of consciousness occurred within 10 min postischemia.
Histology
For Gallyas silver stain, brain sections were incubated in 5% periodic acid for 3 min. After washing, the sections were transferred in silver iodide solution for 1 min and then in 0.5% acetic acid for 5 min. Sections were developed to a pale brown/gray for 5–10 min by stopping development with 0.5% acetic acid for 5 min. For hematoxylin stain, the sections were incubated in the hematoxlin solution purchased from Sigma (St. Louis, MO, USA) for 4 min and differentiated with 70% ethanol for 30 s. Following the last histological step, the sections were mounted and dehydrated with ethanol and cleared with xylene.
Immunohistochemistry
The immunostaining was performed as described previously (24). The mice with left focal cerebral ischemia were sacrificed within 24 h of operating manipulation. Mice were perfused with PBS including 10 U/ml of heparin and fixed with 4% paraformaldehyde (PFA). The whole brains were removed, postfixed in 4% PFA for 48 h, and sectioned coronally to 30 μm. Sections were blocked for endogenous peroxidases with −20°C methanol containing 0.33% H2O2 for 10 min. Pretreatment of sections was performed with 0.3% triton X-100 and blocked with 10% goat serum for 30 min. Primary antibodies were applied with polyclonal rabbit anti-GluA1 (former GluR1) subunit of AMPARs (AB1504, 1:100; Millipore, Billerica, MA, USA) and monoclonal mouse anti-synaptophysin (SYP; 5768, 1:500; Sigma). The incubation was performed overnight at 4°C. Secondary antibodies of goat against mouse or rabbit with fluorescence 488 or 568 (1:1000; Invitrogen, Carlsbad, CA, USA) were incubated for 30 min. The images were taken with FluoView FV1000 confocal microscope (Olympus, Tokyo, Japan).
Cell culture
Primary neuronal cultures were prepared as described previously (8), with minor modifications. Postnatal (1–3 d) WT, TNFR1−/−, and TNFR2−/− mice were selected, and the cortical tissue was dissected under a stereological microscope. The cortical tissue was cut into 200-μm slices, then digested with DMEM containing 20U of papain/DNase I (Worthington, Lakewood, NJ, USA) in a 37°C incubator with gentle shaking for 30 min. Dissociated cells were seeded on poly-l-lysine substrate-coated 8-well chamber slides (for immunocytochemistry) at a density of 1 × 103 cells/cm2 or 6-well plates (for Western blot) that were precoated with 100 μg/ml poly- l-lysine hydrobromide (PLL, Sigma, catalog: P9155), at a density of 1 × 105 cells/cm2. Cells were grown with the medium based on Neurobasal A supplemented with 2% of B27, 2 mM of L-glutamine, and 1% of N2 supplement (Invitrogen) in a 37°C, 5% CO2 incubator. Half the volume of the medium was changed every 3–4 d. Cells were treated at 13 days in vitro (DIV) for 24 h or 14 DIV for 2 h with vehicle (control) or 100 ng/ml of TNF-α (R&D Systems). Experiments were repeated ≥3 times.
Immunocytochemistry
Cultured cells were immunolabeled as described previously (25). At the end of the culture, cells were washed with PBS 3 times for 5 min each on ice. Cells were fixed with methanol at −20°C for 10 min for immunostaining of SYP and the GluN1 subunit of NMDAR. For other antigen labeling, the cells were then fixed with 4% paraformaldehyde including 4% sucrose at 4°C for 15 min. To stain the antigens located on or around the synaptic structures on the cell surface, the cells were treated without addition of Triton X-100 (for cell permeability). A blockade to prevent nonspecific binding was performed with 3% BSA for 30 min before application of primary antibodies. Immunostaining was performed sequentially with antibodies as follows: polyclonal rabbit antibody against TNFR1 (ab19139, 1:400; Abcam), polyclonal goat anti-TNFR2 (sc-1074, 1:400; Santa Cruz Biotechnology, Santa Cruz, CA, USA), Monoclonal mouse anti-MAP2ab (MAB3418, 1:800; Millipore), polyclonal rabbit anti-GluN1 (former NR1) subunit of NMDAR (AB1516, 1:100; Millipore), monoclonal mouse anti-postsynaptic density protein-95 (PSD-95; MAB1596, 1:100; Millipore), polyclonal rabbit anti-GluA1 subunit of AMPARs (AB1504, 1:100; Millipore), monoclonal mouse anti-SYP (5768, 1:500; Sigma), polyclonal rabbit anti-SYP (sc-9116, 1:400; Santa Cruz Biotechnology). Alexa Fluor 488 (green)- or 568 (red)-conjugated secondary antibodies (donkey or goat anti-rabbit or anti-mouse IgG, 1:1000) were applied (Molecular Probes, Eugene, OR, USA). Control staining was performed using the same procedure with omission of the primary antibody. Chambers were mounted using prolonged antifade mounting solution (Molecular Probes).
Western blot
Cells were treated with cell lysis buffer (10 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1 mM EDTA; 1 mM EGTA; 0.1 M Na3VO4; 10% glycerol; and 0.5% Triton X-100; ref. 25). Protein (25 μg) was separated on an 8% SDS-PAGE gel and transferred to a PVDF membrane. Proteins were probed with the following polyclonal rabbit antibody against the GluA1 subunit of AMPAR (AB1504, 1:100; Chemicon, Temecula, CA, USA) and monoclonal mouse antibody against β-actin (1:20,000; Sigma). Western blot measurements were repeated 3 times independently.
Patch-clamp recording
Whole-cell patch-clamp recordings coupled with a 2-barrel drug application system were used as described previously (26). Glass microelectrodes (GC-1.5; Narishige, Tokyo, Japan) were fashioned using a 2-stage vertical pipette puller (P-830; Narishige), and the resistance of the electrode was consistently 3 to 5 MΩ when filled with internal solution. A tight seal (>2 GΩ) was formed between the electrode tip and the cell surface, which was followed by a transition from on-cell to whole-cell recording by brief suction. Access resistance < 30 MΩ was acceptable during voltage-clamp recordings after formation of the whole-cell configuration. Compensation for the series resistance was not performed in all experiments. Data were acquired using a patch-clamp amplifier (200B; Axon Instruments, Foster City, CA, USA) and filtered at 2 kHz, acquired at 11 kHz, and digitized online (Digidata 1322 series A/D board; Axon Instruments). All experiments were performed at room temperature (22±1°C). The drugs AMPA and NMDA were purchased from Tocris Cookson Inc. (Ballwin, MO, USA). Pipette solution contained the following: 140 mM K-gluconate, 5 mM KCl, 10 mM glucose, 2 mM MgCl2, 10 mM HEPES, 0.2 mM EGTA, 4 mM ATP, 0.3 mM GTP, and 10 mM Na-phosphocreatine, pH 7.2 (with KOH). Cultured cells were superfused with normal extracelluar solution including 135 mM NaCl, 5 mM KCl, 25 mM glucose, 10 mM HEPES, 2 mM CaCl2, and 2 mM MgCl2, pH 7.4 (with Trisbase). When mEPSCs were recorded, the extracelluar solution contained 200 nM tetrodotoxin, 30 μM bicuculin, and 50 μM D-APV. All mEPSCs above a threshold value of 5 pA were included in the data analysis, and each EPSC was verified visually. TNF-α (100 ng/ml) was applied to cultures for 24 h before patch-clamp recording and compared with untreated cultured cells.
Quantification analysis
Identification of glutamate receptor clusters and their colocalization with PSD-95 and synaptic markers was performed with Leica color microscopy (Leica, Wetzlar, Germany). In all experiments, neurons used for analysis were randomly selected. Fluorescent digitized images were obtained with a DEI-470 digital camera (Optronics, Goleta, CA, USA) with the same background and parameters. Neurons (2–3/field) were selected at random and analyzed in each microscopic field (4–5 fields in each condition). All images were analyzed using Image-Pro Plus Image Analysis software (Media Cybernetics, Inc., Silver Spring, MD, USA). Images of the diffuse background fluorescence on the dendritic shaft of neurons were thresholded, and individual clusters were identified as regions with a >2-fold intensity increase in diffuse fluorescence. Due to intense nonspecific binding on the cell body, clusters in well-defined proximal dendrites 5 μm from the soma were counted, and averages were calculated per 20 μm. In the present study, n indicates the segment (20 μm) number of proximal dendritic processes (does not include soma, 3–4 segments/neuron). To quantify synaptic localization, ∼30–40% pixel overlap of GluA1 with PSD-95 clusters or SYP clusters was considered to be a synapse (27). The percentage of colocalization in manipulated and control cultures was calculated as the number of colocalized punctae divided by the number of PSD-95 or SYP clusters. For display in figures, images were processed using Adobe Photoshop software (Adobe Systems, San Jose, CA, USA). Regarding quantitative analysis, investigators were masked to grouping and treatment history of the cells. Raw data presentation in the normalized 8-bit image was analyzed. Each experimental manipulation was performed 3–4 times. Statistics were analyzed using an ANOVA, followed by the Tukey's multiple comparisons test among treatment and each of the WT, TNFR1−/−, and TNFR2−/− groups. All values are shown as means ±se, and differences were considered significant at P ≤ 0.05.
RESULTS
Genetic TNFR1 deletion reduces AMPA receptor clusters
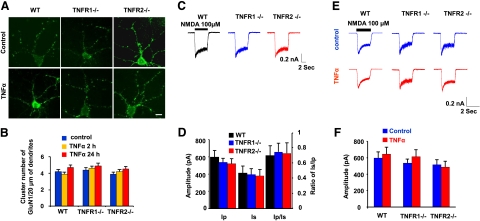
To understand the expression and distribution of TNFR subtypes on cortical neurons (28), cultured neurons were immunostained with antibodies against TNFR1 and TNFR2. Mature neurons were visualized with MAP2ab staining. Immunofluorescence showed that both TNFR1 and TNFR2 are distributed diffusely at the cell surface of the soma, and dendrites in cultured cortical neurons prepared from WT mice (Fig. 1A). We found that all neurons (confirmed by MAP2ab+ staining) express TNFR1. However, only ∼40% of cortical neurons are TNFR2+. TNFR2 expression without TNFR1 is observed only in the cortical neurons prepared from TNFR1−/− mice. Similarly, TNFR1+ staining but not TNFR2 is found on neurons prepared from TNFR2−/− mice (Fig. 1A).
Figure 1.
TNFR1 deletion decreases the number of AMPAR clusters. A) Cortical neurons were labeled at 7 DIV by immunofluorescence with antibodies against TNFR1 and TNFR2. TNFR1 (green) and TNFR2 (red) expression is diffuse, little clustering on the dendrite and somatic membrane. B) Immunostaining directed GluA1 subunit of AMPAR is visualized in WT, TNFR1−/−, and TNFR2−/− cortical neurons with and without TNF-α treatment. C) Statistical analysis shows that, in comparison with WT cells, the number of GluA1 clusters significantly decreases in TNFR1−/− neurons, but not in TNFR2−/− cells (P>0.05). With TNF-α treatment for 2 and 24 h, the number of GluA1 clusters significantly increases in WT and TNFR2−/− neurons. However, no significant change in GluA1 clusters is observed in the cells with TNFR1 deletion when exposed to TNF-α. *P < 0.05; **P < 0.01. D) Western blot shows no obvious changes of GluA1 expression levels among WT, TNFR1−/−, and TNFR2−/− cortical neurons without (control) or with TNF-α treatment for 24 h. Scale bars = 5 μm.
We used an immunoglobulin antibody against the GluA1 subunit of AMPARs to demonstrate the role of TNFR1 or TNFR2 signaling in the surface distribution of AMPARs of cortical neurons. Immunostaining showed positive staining for GluA1 on the cellular surface. An immunofluorescent intensity >2-fold the background staining intensity of the dendrite was set as the threshold definition of a cluster (Fig. 1B, top panel). We counted the GluA1 accumulation per 20 μm of dendrite and found that the number of GluA1 clusters was decreased significantly in TNFR1−/− (3.1±0.4, n=49, P<0.01), but not TNFR2−/− neurons (7.2±0.5, n=52, P>0.05), when compared to WT controls (7.0±0.4, n=53) (Fig. 1C). It has been reported that after using inactivating antibodies against TNFR1 or 2, application of TNF-α enhanced surface AMPA receptor clustering in hippocampal neurons via TNFR1 (22). Even though the receptors are functionally inactivated, TNFRs are still present on the cell surface, having not been internalized. To test whether the decreased AMPAR clustering in TNFR1−/− neurons could be restored as a result of TNF-α application, cultured cells were exposed to TNF-α (100 ng/ml) for 2 h. We found an increase in the number of GluA1 clusters in WT and TNFR2−/− neurons but not in TNFR1−/− neurons (Fig. 1B, bottom panel). Statistical analysis showed a significant increase in the GluA1 cluster number in WT (11.3±1.6, n=38, P<0.05) and in TNFR2−/− cells (11.8±1.2, n=36, P<0.05) but not in TNFR1−/− neurons (2.9±0.3, n=47, P>0.05) with TNF-α treatment vs. the corresponding controls (Fig. 1C). Our previous reports demonstrated that TNF-α has a low-affinity binding profile to TNFR2 in vitro (8). Whether a longer-term exposure of TNF-α might promote AMPA receptor clusters has not been concluded, so we extended TNF-α treatment to 24 h. The results showed that, similar to the 2-h treatment, the 24-h application increases GluA1 clusters on the surface of neurons in both WT (12.1±1.7, n=46, P<0.05) and TNFR2−/− (13.0±1.8, n=49, P<0.05) but not in TNFR1−/− neurons (3.4±0.3, n=43, P>0.05) compared to the corresponding controls (Fig. 1C). This finding suggests that even though TNFR2 is present after the TNFR1 gene knockout, the binding and activation of TNFR2 to soluble TNF-α are at very low levels. This finding indicates that TNFR2 signaling may possibly play a minor role in AMPAR clustering, but it is not incredibly potent.
To determine whether changes in AMPAR immunoreactivity reflected a change in overall protein expression level or just a change in the distribution of membrane bound AMPAR, we collected the total cultured cells and performed Western blot analysis. We did not find obvious differences in total GluA1 expression levels among WT, TNFR1−/−, and TNFR2−/− cultured cortical cells (Fig. 1D). We then tested the amount of GluA1 expression after TNF-α application for 24 h. Similarly, no obvious changes are observed in comparison to corresponding vehicle groups, which is consistent with previous reports (ref. 29; Fig. 1D). These results show that TNFR1 signaling is correlated with the surface distribution of GluA1-containing AMPAR and that TNF-α-induced TNFR1 stimulation enhances AMPAR clustering, but is not associated with total AMPAR levels.
TNFR1 deletion decreases synaptic localization of AMPA receptor clusters
Experimental evidence suggests that activation of TNFR1 signaling participates in trafficking AMPAR toward the cell surface (19, 22). However, the role of TNFRs in the stability of AMPAR clustering at synapses is still unknown. A postsynaptic scaffolding protein, PSD-95, is abundant at excitatory postsynaptic sites and tethers AMPAR clusters to postsynaptic membranes (30–38). To understand whether TNFRs could mediate the association of AMPAR with PDS95, double immunostaining was performed with antibodies directed toward GluA1 and PSD-95. Results showed that some of the GluA1 clusters colocalize with PSD-95, but some GluA1 proteins were not associated with PSD-95+ structures (Fig. 2A). The percentage of the colocalization for both GluA1 and PSD-95 to total PSD-95 was calculated. We found a significant decrease in double-staining per 20-μm dendritic segment in TNFR1−/− (40.8±4.6%, n=55, P<0.05) but not in TNFR2−/− (64.4±7.5%, n=51, P>0.05), compared to WT counterparts (60.3±6.1%, n=47, Fig. 2B). After TNF-α treatment for 2 h, we did not find a significant increase in the number of GluA1 colocalizing with PSD-95 from double labeling in TNFR1−/− neurons (43.3±5.2%, n=49, P>0.05) compared to corresponding control groups. However, a significant increase in the percentage of double-labeled structures vs. PSD-95 punctuates is shown in WT (82.3±12.1%, n=45, P<0.05,) and TNFR2−/− (86.8±13.5%, n=51, P<0.05) compared to corresponding vehicle groups (Fig. 2B). To determine whether an extension of TNF-α exposure can increase AMPAR trafficking to synapses in TNFR1−/− neurons, we treated the cells for 24 h. Similar to the 2-h TNF-α treatment group, no significant increase in the percentage of colabeled structures in the TNFR1−/− (44.7±8.6%, n=47, P>0.05) group was observed. A significant increase in the percentage of double-stained elements was observed in WT (92.3±12.6%, n=43, P<0.05,) and TNFR2−/− cells (86.5±11.8%, n=48, P < 0.05) when compared to corresponding control cells (Fig. 2B).
Figure 2.
TNFR1 deletion reduces the synaptic location of AMPA receptors. A) Representative photograph shows the colocalization (yellow) of PSD-95 (red), a postsynaptic protein, and GluA1 clusters (green) with and without TNF-α application. B) A significant decrease in the percentage of the colocalization of GluA1 clusters to PSD-95 is observed in TNFR1−/− neurons. With the treatment of TNF-α for 2 h or 24 h, the percentage of synaptic GluA1 clustering significantly is increased in WT and TNFR2−/− neurons, whereas no significant changes appeared in TNFR1−/− neurons (P>0.05). C) Representative photograph shows the colocalization of SYP (red), a presynaptic protein, and GluA1 (green) with and without TNF-α application. D) A significant increase in the percentage of the colocalization of GluA1 clusters and SYP staining is observed in WT and TNFR2−/− neurons, but no significant changes appeared in TNFR1−/− neurons with the treatment of TNF-α for 2 or 24 h. *P < 0.05.
To test for changes in the number of synaptic terminals with TNFR deletion, we immunostained presynaptic terminals with an antibody against SYP. We found no significant change in the number of presynaptic SYP immunostains per 20 μm of dendritic segment among WT (n=37), TNFR1−/− (n=40), and TNFR2−/− (n=50) neurons. To further confirm whether TNFR is associated with the localization of the AMPAR to the synapse, SYP was immunostained together with the GluA1 subunit of AMPAR. Results showed that some of the GluA1 clusters colocalized with SYP, but part of GluA1 is not associated with SYP immunolabeling (Fig. 2C). Statistical assays showed a significant decrease in the percentage of double-labeling structures vs. SYP+ terminals in TNFR1−/− (42.5±4.9%, n=43, P<0.05) but not in TNFR2−/− (58.2 ± 6.1%, n=46, P>0.05) compared to WT neurons (60.4±6.2%, n=51) (Fig. 2D). With 100 ng/ml of TNF-α applied for 2 h, similar to the results of double-labeling PSD-95 with GluA1 (Fig. 2B), we found no significant change of a percentage of double-labeling SYP and GluA1 in TNFR1−/− cells (40.3±5.4%, n=43, P>0.05,) but a significant increase in WT (86.2±12.5%, n=48, P<0.05) and in TNFR2−/− neurons (87.1±13.5%, n=49, P<0.05) compared to controls (Fig. 2D). Next, we extended exogenous TNF-α exposure to 24 h to further verify AMPAR clustering at the synapse. Similar to the TNF-α treatment for 2 h, no significant change in the percentage of colabeled structures was observed in TNFR1−/− cells (45.8±7.6%, n=47, P>0.05,). A significant increase was observed in WT (90.3±14.1%, n=50, P<0.05) and in TNFR2−/− neurons (85.5±11.9%, n=49, P<0.05) when compared to corresponding control cells (Fig. 2D). These results suggest that TNFR1, but not TNFR2, could be associated with an apposition and the stability of AMPAR at the synapse.
TNFR1 deletion decreases the localization of AMPAR to synapses in ischemic mouse models
Because TNFR2 preferentially binds to transmembrane TNF-α (15, 16), the soluble TNF-α stimulation in vitro of TNFR1-knockout cells may not be the best activation for TNFR2 function. It also has been suggested that astrocyte-specific transmembrane TNF-α triggers more apparent inflammation and degeneration in the central nervous system than that of neuron-specific (39). Due to low-intensity cell culture, TNFR2 has little contact on neighbor cells producing membrane TNF-α. To further clarify whether TNFR2 plays a minor role in the trafficking of AMAPR to synapses as we observed in vitro (Fig. 2C, D), we used ischemic injury mouse model, which exhibits significant production of proinflammatory cytokines, including both soluble and membrane bound TNF-α. Specifically, we applied ischemic injury on TNFR1-knockout mice and examined whether a different change of AMPAR localization to synapses occurred following induction of focal cerebral ischemia injury. The success of ischemic injury models with a demyelinating cortical region was verified by the Gallyas silver stains (Fig. 3A), and the ipsilateral ischemic tissues showed a structural difference from normal contralateral hemisphere by hematoxylin stain (Fig. 3B). The double immunoreactive staining was performed with antibodies against GluA1 and synapse. Results showed a small part of AMPAR trafficking to synapses in normal contralateral cortex and an apparently increased AMPAR clusters to synapses in the ipsilateral regions following the induction of ischemic injury in 3-mo-old WT mice (Fig. 3C, top panel). Little double-staining of GluA1 and SYP was observed in control contralateral brain of TNFR1−/− mice with ischemic injury, and the phenomenon of GluA1 trafficking to synapses was not obviously increased in the ipsilateral of the injury induction in TNFR1-knockout mice (Fig. 3C, bottom panel), consistent with the finding of spinal cord injury following neutralizing TNFR1 by soluble TNFR1 antibody (40). Combining the findings of in vitro TNFR1−/− cells (Fig. 2C, D), it suggests that TNFR2 play little role in AMPAR trafficking to synapse, indicating little action of TNFR2 in excitotoxicity.
Figure 3.
TNFR1 deletion decreases the synaptic localization of AMPAR in focal cerebral ischemic mice. A) Gallyas silver stain shows the left demyelinating infarct region (red arrow) after the ligation of the left internal carotid artery. B) Hematoxylin histology shows an injury tissue structure following the left internal carotid artery ligation. C) Double immunoreactive staining shows an apparently increased synaptic localization of GluA1 (yellow dots indicate the overlaps of the GluA1 and SYP) in the ipsilateral ischemic tissues in comparison with the contralateral normal control of WT mice with ischemic injury. Little localization of GluA1 to synapses is seen in the contralateral brain tissues of ischemia, and there are no obvious increases in the double-staining of GluA1 and SYP in the ipsilateral tissues of the ischemic TNFR1−/− mice. Scale bars = 100 μm (B); 10 μm (C).
TNFR1 deletion reduces excitatory synaptic transmission and prevents TNF-α-induced increase in excitatory synaptic transmission
To further confirm the hypothesis that genetic deletion of TNFR1 decreases trafficking of AMPARs to synapses, we recorded miniature excitatory postsynaptic currents (mEPSCs) using patch-clamp recordings from primary cultured cortical neurons. Compared to WT neurons (20.6±1.4 pA, n=6), a significant decrease in the average peak amplitude of mEPSCs was observed in TNFR1−/− (14.7±1.9 pA, n=6, P<0.01) but not in TNFR2−/− neurons (19.7±1.7 pA, n=6, P>0.05). When comparing the interval of mEPSCs to WT (428.6±47.2 ms, n=6), a significant interevent interval was extended in TNFR1−/− (609.5±56.4 ms, n=6, P<0.01), but not in TNFR2−/− neurons (452.4±37.2 ms, n=6, P>0.05) (Fig. 4A, B). These results suggest that the genetic deletion of TNFR1 but not TNFR2 causes a significant reduction of synaptic AMPAR function based on physiological measurements.
Figure 4.
TNFR1 deletion decreases synaptic excitatory transmission and prevents TNF-α-induced increase of synaptic excitatory transmission. A) Representative imagography shows mEPSC recording from primary cortex neurons of WT, TNFR1−/−, and TNFR2−/− mice in the absence and presence of TNF-α application. B) Average mEPSC peak amplitude before and after TNF-α treatment. C) Cumulative probability of the peak amplitude and frequency of mEPSC before and after TNF-α treatment. *P < 0.01.
To investigate possible effects of TNFR activation on mEPSCs, we treated neurons with 100 ng/m of TNF-α for 24 h. We normalized peak values and compared the groups exposed to TNF-α for 24 h normalized to the corresponding untreated controls. Results showed that after TNF-α treatment, a significant increase was found in peak amplitude of WT (144.8±14.7%, n=6, P<0.05) and TNFR2−/− (128.1±10.5%, n=6, P<0.05) but not TNFR1−/− neurons (123.7±15.9%, n=6, P>0.05) (Fig. 4C). We also normalized the frequency values of mEPSCs and compared the changes along with TNF-α application for 24 h to the corresponding untreated controls. We found that the TNF-α treatment significantly increased mEPSC frequency in both WT (216.8±23.8%, n=6, P<0.01) and TNFR2−/− cells (190.5±15.7%, n=6, P<0.01) but not TNFR1−/− neurons (113.2±10.5%, n=6, P>0.05) (Fig. 4C). These results suggest that the TNF-α-induced increase of mEPSC amplitude and frequency in WT neurons is mediated by TNFR1, but not TNFR2, and these findings are consistent with the results of TNFR1-mediated AMPAR synaptic clustering.
TNFR1 deletion decreases AMPAR-mediated whole-cell currents, and TNF-α fails to alter AMPAR-mediated whole-cell currents after TNFR1 deletion
To test whether TNFRs affect AMPAR function, 100 μM AMPA was used to induce whole-cell currents in cultured cortical neurons. Results showed that compared to WT neurons (419.3±42.1 pA, n=6), the peak amplitude of whole-cell currents was significantly reduced in TNFR1−/− (186.5±46.7 pA, n=6, P<0.01) but not TNFR2−/− neurons (478.5±78.7 pA, n=6, P>0.05) (Fig. 5A, B). The reduction of AMPAR-mediated whole-cell currents in TNFR1−/− neurons suggests that TNFR1 deletion induces a reduction of total AMPAR numbers on the cell surface.
Figure 5.
TNFR1 deletion decreases AMPAR function in whole-cell recordings. A) Representative whole-cell traces of AMPA (100 μM)-induced inward currents from WT, TNFR1−/−, and TNFR2−/− neurons. B) Calculated peak (Ip), steady state (Is), and kinetics (ratio of Is vs. Ip) values of AMPA-induced inward currents from WT, TNFR1−/−, and TNFR2−/− neurons. C) Representative whole-cell traces of AMPA (100 μM)-induced inward currents from WT, TNFR1−/−, and TNFR2−/− neurons untreated and treated with TNF-α. D) Comparison of calculated Ip values of AMPA-induced inward currents from WT, TNFR1−/−, and TNFR2−/− neurons untreated and treated with TNF-α. **P < 0.01.
To further examine the role of TNF-α and its receptors in modulating AMPAR function, we compared AMPAR-mediated whole-cell currents in neurons before and after exposure of TNF-α. As shown in Fig. 5C, D, after TNF-α exposure for 24 h, no significant change of 100 μM AMPA-induced whole-cell currents was found in all WT, TNFR1−/−, and TNFR2−/− neurons. Statistical analysis showed that the peak amplitudes were 428.67 ± 97.47 pA (n=6) in WT, 160.5 ± 28.5 pA (n=6) in TNFR−/−, and 420.5 ± 45.0 pA (n=6) in TNFR2−/− cells, respectively (Fig. 5C, D). These results suggest that TNF-α, via TNFR1, increases synaptic AMPAR trafficking to the synapse but does not alter total AMPAR expression.
TNFR signaling does not affect NMDA receptor clustering
To determine whether TNFR1 or 2 affected NMDAR clusters, cultured cortical neurons were analyzed by immunolabeling with an antibody against the GluN1 subunit of the NMDAR. We did not find significant changes in the number of GluN1 clusters among WT, TNFR1−/−, and TNFR2−/− neurons (Fig. 6A, top panel). To test whether TNF-α affects NMDAR clustering, cells were exposed to 100 ng/ml of TNF-α for 2 h. We did not find apparent increase in the number of GluN1 clusters in WT, TNFR1−/−, or TNFR2−/− neurons (Fig. 6A, bottom panel). Statistical assays showed no significant changes of GluN1 clustering among WT (n=38, P>0.05), TNFR1−/− (n=47, P>0.05), or TNFR2−/− neurons (n=36, P>0.05) with or without TNF-α treatment (Fig. 6B). These results suggest the stability of NMDAR, at least within the short-term TNF-α treatment. Then we asked whether the stability of NMDAR expression could be changed with TNF-α application over a longer time. TNF-α exposure was extended to 24 h, and no significant changes were observed in the number of GluN1 clusters in WT (n=44), TNFR1−/− (n=43), or TNFR2−/− neurons (n=48) compared with corresponding untreated control cells (P>0.05) (Fig. 6B). In patch-clamp recordings, NMDA-induced inward currents from WT, TNFR1−/−, and TNFR2−/− mice were compared (Fig. 6C). Without TNF-α treatment, no significant differences in the peak amplitude of NMDA-induced current were observed in WT (595.4±77.0 pA, n=6), TNFR1−/− (535.3±50.0 pA, n=6), and TNFR2−/− neurons (515.8±61.1 pA, n=6) (Fig. 6D). After TNF-α treatment (24 h), we did not find significant differences in the peak amplitude of NMDA-induced currents in WT (645.7±81.3 pA, n=6), TNFR1−/− (613.0±86.5 pA, n=6), and TNFR2−/− neurons (485.7±69.9 pA, n=6) (Fig. 6E, F). These results suggest that TNFR signaling has no significant effect on NDMAR trafficking, expression, and function.
Figure 6.
TNF-α treatment does not alter NMDAR whole-cell function. A) Group of representative photographs shows GluN1 subunit clusters of NMDARs in WT, TNFR1−/−, and TNFR2−/− cortical neurons with and without TNF-α treatment. Scale bar = 5 μm. B) Quantitative analysis of GluN1 clusters per 20 μm proximal dendrites shows no significant changes (P>0.05) in all three types of WT, TNFR1−/−, and TNFR2−/− neurons with and without TNF-α treatment for 2 or 24 h. C) Representative whole-cell traces of NMDA (100 μM)-induced inward currents from WT, TNFR1−/−, and TNFR2−/− neurons. D) Ip, Is, and the ratio of Is vs. Ip values of NMDA-induced inward currents from WT, TNFR1−/−, and TNFR2−/− neurons. E) Representative whole-cell traces of NMDA (100 μM)-induced inward currents from WT, TNFR1−/−, and TNFR2−/− neurons untreated and treated with TNF-α. F) Comparison of calculated Ip values of NMDA-induced inward currents from WT, TNFR1−/−, and TNFR2−/− neurons untreated and treated with TNF-α.
DISCUSSION
In this study, we find that the deletion of TNFR1, but not TNFR2, decreases AMPAR cell surface clustering and assembly at the synapse. This cannot be restored by exposure to TNF-α, suggesting a role for TNFR1 signaling in the maintenance of the basal AMPAR membrane stability. Combined with the findings that soluble TNFR1 application, neutralizing TNF-α signaling, lowers AMPAR surface expression (22), it has been suggested that under physiological conditions, TNF-α production by neurons and/or astrocytes (41) is enough to maintain neuronal basal excitatory characteristics. Although significant decreases in AMPAR cell surface clustering were observed, no significant differences were found in AMPAR protein levels, as was demonstrated by Western blot from the cortical neurons of WT, TNFR1−/−, and TNFR2−/− mice. This condition persisted even with the long-term (24 h) TNF-α treatment, suggesting that TNFR signaling might be primarily involved in the trafficking of rapid AMPAR exocytosis but not protein metabolism (19, 22, 42). Leonoudakis et al. (43) showed that at the end of TNF-α application (1 h) the levels of surface GluA2-lacking CP-AMPARs had returned to baseline. Therefore, their data also may implicate more extrasynaptic GluA1 trafficking to the synapse so that the increase is not apparent in the surface expression of GluA1. Our results are supported by the observation of 2-step delivery of AMPARs, first to extrasynaptic and then to synaptic plasma membrane, after LTP induction (44). A report showed that 10 ng/ml of TNF-α treatment on human NT2-N neurons increased GluA1 expression (45), suggesting that TNF-α-NF-κB signaling improved GluA1 synthesis. The difference from our result may suggest that human NT2-N cells are more active than mouse cortical neurons (45). Our results are in agreement with the suggestion that surface AMPARs move laterally to synapses where they are stabilized by synaptic activity (46–49).
PSD scaffolding proteins are involved in the structure and function of excitatory synapses (30, 50–53). It has been suggested that PSD-95 levels influence AMPA receptor retention and activity at the synapse (35, 37). Overexpression of PSD-95 enhances AMPAR recruitment and excitatory synaptic responses without affecting NMDAR clustering in developing hippocampal neurons (30). A lack of PSD-95 results in reduced AMPAR function (54, 55). Further, the effects of PSD-95 on AMPAR clustering are modulated through association with stargazin and related family members (32, 56, 57). Here we find that a TNFR1 knockout decreases the colocalization of PSD-95 with AMPAR GluA1 subunit. Whether TNFR1 deletion lowers the capacity of PSD-95 binding to AMPAR still needs to be further investigated.
In TNFR1 deletion neurons, the amplitude and frequency of mEPSCs are reduced, which cannot be restored with long-term (24 h) TNF-α treatment, whereas the mEPSCs in TNFR2-knockout cells are not significantly elevated with short-term TNF-α treatment. This evidence suggests that TNFR1 stabilizes glutamate receptor transmission. It has been reported that the activation of presynaptic TNFR1 signaling enhances presynaptic transmission (58) and is likely mediated by activating the cAMP pathway (59). In addition, TNFR signaling likely modulates glutamatergic transmission through both pre- and postsynaptic mechanisms (19, 60). Our electrophysiological results further provide evidence that TNFR1 activation by TNF-α increases AMPAR trafficking to the synapse but not the total AMPAR expression level and function on a whole-cell level. The redistribution of surface-expression might involve a lateral diffusion mechanism of AMPA receptors from extrasynaptic to synaptic loci.
Our results have clearly shown that TNFR signaling does not significantly change the expression, clustering, or synaptic transmission of NMDAR as well as its affinity to NMDA among the neurons of WT, TNFR1−/−, and TNFR2−/−, prior to and after long-term (24 h) TNF-α treatment. This finding suggests that synaptic NMDARs are less mobile than AMPARs, which is consistent with previous reports (22, 61). It has been shown that overactivation of TNFR signaling by chronic TNF-α treatment (24–48 h) increases 30% of calcium current but has no effect in the short-term (<24 h) (62). Whether the TNF-α-induced increase in calcium current is associated with NMDAR needs to be clarified.
Many lines of evidence have shown that TNF-α/TNFR signaling is elevated in a large number of neurological disorders including ischemia (63, 64), traumatic brain injury (65), multiple sclerosis (66–69), Alzheimer's disease (71–73), and Parkinson's disease (7, 74–78). In a previous report, we found that TNFR1 expression and activity are elevated in Alzheimer's disease (9). The elevated TNF-α/TNFR1 signaling can potentiate excitotoxicity to neurons directly, by combining with subthreshold levels of glutamate, through activation of glutamate-NMDAR (79), or indirectly, by inhibiting glial glutamate transporters on astrocytes (80) or by mediating TNFR1 signaling to localize AMPAR to synapses (19, 81, 82). Furthermore, it has been verified that rapid TNF induces Ca2+-permeable GluA2-lacking AMPARs to the neuronal membrane, resulting in excitotoxicity (40, 43). Thereby, from all of these perspectives, it has been indicated that genetically targeting TNFR1 and TNFR2 could be an attractive approach to reduce or prevent excitotoxic damage (40, 43).
Acknowledgments
This work is supported by the U.S. National Institutes of Health, grant NIH NIAAG025888 (Y.S.); an Alzheimer's Association Zenith Award (Y.S.); and a grant from the Arizona Biomedical Research Commission (Y.S.). The authors thank Harrison Stratton for his editorial assistance.
REFERENCES
- 1. Carroll R. C., Beattie E. C., Xia H., Luscher C., Altschuler Y., Nicoll R. A., Malenka R. C., von Zastrow M. (1999) Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc. Natl. Acad. Sci. U. S. A. 96, 14112–14117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lissin D. V., Carroll R. C., Nicoll R. A., Malenka R. C., von Zastrow M. (1999) Rapid, activation-induced redistribution of ionotropic glutamate receptors in cultured hippocampal neurons. J. Neurosci. 19, 1263–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Brien R. J., Lau L. F., Huganir R. L. (1998) Molecular mechanisms of glutamate receptor clustering at excitatory synapses. Curr. Opin. Neurobiol. 8, 364–369 [DOI] [PubMed] [Google Scholar]
- 4. Kobayashi T., Mori Y. (1998) Ca2+ channel antagonists and neuroprotection from cerebral ischemia. Eur. J. Pharmacol. 363, 1–15 [DOI] [PubMed] [Google Scholar]
- 5. Martin L. J., Al-Abdulla N. A., Brambrink A. M., Kirsch J. R., Sieber F. E., Portera-Cailliau C. (1998) Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: A perspective on the contributions of apoptosis and necrosis. Brain Res. Bull. 46, 281–309 [DOI] [PubMed] [Google Scholar]
- 6. Rothman S. M., Olney J. W. (1986) Glutamate and the pathophysiology of hypoxic–ischemic brain damage. Ann. Neurol. 19, 105–111 [DOI] [PubMed] [Google Scholar]
- 7. Boka G., Anglade P., Wallach D., Javoy-Agid F., Agid Y., Hirsch E. C. (1994) Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neurosci. Lett. 172, 151–154 [DOI] [PubMed] [Google Scholar]
- 8. Yang L., Lindholm K., Konishi Y., Li R., Shen Y. (2002) Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways. J. Neurosci. 22, 3025–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheng X., Yang L., He P., Li R., Shen Y. (2010) Differential activation of tumor necrosis factor receptors distinguishes between brains from Alzheimer's disease and non-demented patients. J. Alzheimers Dis. 19, 621–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tartaglia L. A., Weber R. F., Figari I. S., Reynolds C., Palladino M. A., Jr., Goeddel D. V. (1991) The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc. Natl. Acad. Sci. U. S. A. 88, 9292–9296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rath P. C., Aggarwal B. B. (1999) TNF-induced signaling in apoptosis. J. Clin. Immunol. 19, 350–364 [DOI] [PubMed] [Google Scholar]
- 12. Neumann H., Schweigreiter R., Yamashita T., Rosenkranz K., Wekerle H., Barde Y. A. (2002) Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism. J. Neurosci. 22, 854–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boldin M. P., Mett I. L., Varfolomeev E. E., Chumakov I., Shemer-Avni Y., Camonis J. H., Wallach D. (1995) Self-association of the “death domains” of the p55 tumor necrosis factor (TNF) receptor and Fas/APO1 prompts signaling for TNF and Fas/APO1 effects. J. Biol. Chem. 270, 387–391 [DOI] [PubMed] [Google Scholar]
- 14. Shen Y., He P., Zhong Z., McAllister C., Lindholm K. (2006) Distinct destructive signal pathways of neuronal death in Alzheimer's disease. Trends Mol. Med. 12, 574–579 [DOI] [PubMed] [Google Scholar]
- 15. Grell M. (1995) Tumor necrosis factor (TNF) receptors in cellular signaling of soluble and membrane-expressed TNF. J. Inflamm. 47, 8–17 [PubMed] [Google Scholar]
- 16. Grell M., Wajant H., Zimmermann G., Scheurich P. (1998) The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc. Natl. Acad. Sci. U. S. A. 95, 570–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marchetti L., Klein M., Schlett K., Pfizenmaier K., Eisel U. L. (2004) Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J. Biol. Chem. 279, 32869–32881 [DOI] [PubMed] [Google Scholar]
- 18. Shen Y., Li R., Shiosaki K. (1997) Inhibition of p75 tumor necrosis factor receptor by antisense oligonucleotides increases hypoxic injury and beta-amyloid toxicity in human neuronal cell line. J. Biol. Chem. 272, 3550–3553 [PubMed] [Google Scholar]
- 19. Beattie E. C., Stellwagen D., Morishita W., Bresnahan J. C., Ha B. K., Von Zastrow M., Beattie M. S., Malenka R. C. (2002) Control of synaptic strength by glial TNFalpha. Science 295, 2282–2285 [DOI] [PubMed] [Google Scholar]
- 20. Pickering M., Cumiskey D., O'Connor J. J. (2005) Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp. Physiol. 90, 663–670 [DOI] [PubMed] [Google Scholar]
- 21. Ogoshi F., Yin H. Z., Kuppumbatti Y., Song B., Amindari S., Weiss J. H. (2005) Tumor necrosis-factor-alpha (TNF-alpha) induces rapid insertion of Ca2+-permeable alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA)/kainate (Ca-A/K) channels in a subset of hippocampal pyramidal neurons. Exp. Neurol. 193, 384–393 [DOI] [PubMed] [Google Scholar]
- 22. Stellwagen D., Beattie E. C., Seo J. Y., Malenka R. C. (2005) Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J. Neurosci. 25, 3219–3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stellwagen D., Malenka R. C. (2006) Synaptic scaling mediated by glial TNF-alpha. Nature 440, 1054–1059 [DOI] [PubMed] [Google Scholar]
- 24. He P., Zhong Z., Lindholm K., Berning L., Lee W., Lemere C., Staufenbiel M., Li R., Shen Y. (2007) Deletion of tumor necrosis factor death receptor inhibits amyloid beta generation and prevents learning and memory deficits in Alzheimer's mice. J. Cell. Biol. 178, 829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He P., Shen Y. (2009) Interruption of beta-catenin signaling reduces neurogenesis in Alzheimer's disease. J. Neurosci. 29, 6545–6557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu J., Kuo Y. P., George A. A., Xu L., Hu J., Lukas R. J. (2004) beta-Amyloid directly inhibits human alpha4beta2-nicotinic acetylcholine receptors heterologously expressed in human SH-EP1 cells. J. Biol. Chem. 279, 37842–37851 [DOI] [PubMed] [Google Scholar]
- 27. Elmariah S. B., Crumling M. A., Parsons T. D., Balice-Gordon R. J. (2004) Postsynaptic TrkB-mediated signaling modulates excitatory and inhibitory neurotransmitter receptor clustering at hippocampal synapses. J. Neurosci. 24, 2380–2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patel J. R., Brewer G. J. (2008) Age-related changes to tumor necrosis factor receptors affect neuron survival in the presence of beta-amyloid. J. Neurosci. Res. 86, 2303–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Glazner G. W., Mattson M. P. (2000) Differential effects of BDNF, ADNF9, and TNFalpha on levels of NMDA receptor subunits, calcium homeostasis, and neuronal vulnerability to excitotoxicity. Exp. Neurol. 161, 442–452 [DOI] [PubMed] [Google Scholar]
- 30. El-Husseini A. E., Schnell E., Chetkovich D. M., Nicoll R. A., Bredt D. S. (2000) PSD-95 involvement in maturation of excitatory synapses. Science 290, 1364–1368 [PubMed] [Google Scholar]
- 31. Cho K. O., Hunt C. A., Kennedy M. B. (1992) The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron 9, 929–942 [DOI] [PubMed] [Google Scholar]
- 32. Kistner U., Wenzel B. M., Veh R. W., Cases-Langhoff C., Garner A. M., Appeltauer U., Voss B., Gundelfinger E. D., Garner C. C. (1993) SAP90, a rat presynaptic protein related to the product of the Drosophila tumor suppressor gene dlg-A. J. Biol. Chem. 268, 4580–4583 [PubMed] [Google Scholar]
- 33. Schnell E., Sizemore M., Karimzadegan S., Chen L., Bredt D. S., Nicoll R. A. (2002) Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc. Natl. Acad. Sci. U. S. A. 99, 13902–13907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rumbaugh G., Sia G. M., Garner C. C., Huganir R. L. (2003) Synapse-associated protein-97 isoform-specific regulation of surface AMPA receptors and synaptic function in cultured neurons. J. Neurosci. 23, 4567–4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ehrlich I., Malinow R. (2004) Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J. Neurosci. 24, 916–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beique J. C., Lin D. T., Kang M. G., Aizawa H., Takamiya K., Huganir R. L. (2006) Synapse-specific regulation of AMPA receptor function by PSD-95. Proc. Natl. Acad. Sci. U. S. A. 103, 19535–19540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schluter O. M., Xu W., Malenka R. C. (2006) Alternative N-terminal domains of PSD-95 and SAP97 govern activity-dependent regulation of synaptic AMPA receptor function. Neuron 51, 99–111 [DOI] [PubMed] [Google Scholar]
- 38. Kim M. J., Futai K., Jo J., Hayashi Y., Cho K., Sheng M. (2007) Synaptic accumulation of PSD-95 and synaptic function regulated by phosphorylation of serine-295 of PSD-95. Neuron 56, 488–502 [DOI] [PubMed] [Google Scholar]
- 39. Akassoglou K., Probert L., Kontogeorgos G., Kollias G. (1997) Astrocyte-specific but not neuron-specific transmembrane TNF triggers inflammation and degeneration in the central nervous system of transgenic mice. J. Immunol. 158, 438–445 [PubMed] [Google Scholar]
- 40. Ferguson A. R., Christensen R. N., Gensel J. C., Miller B. A., Sun F., Beattie E. C., Bresnahan J. C., Beattie M. S. (2008) Cell death after spinal cord injury is exacerbated by rapid TNF alpha-induced trafficking of GluR2-lacking AMPARs to the plasma membrane. J. Neurosci. 28, 11391–11400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lebrun-Julien F., Duplan L., Pernet V., Osswald I., Sapieha P., Bourgeois P., Dickson K., Bowie D., Barker P. A., Di Polo A. (2009) Excitotoxic death of retinal neurons in vivo occurs via a non-cell-autonomous mechanism. J. Neurosci. 29, 5536–5545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Diem R., Meyer R., Weishaupt J. H., Bahr M. (2001) Reduction of potassium currents and phosphatidylinositol 3-kinase-dependent AKT phosphorylation by tumor necrosis factor-(alpha) rescues axotomized retinal ganglion cells from retrograde cell death in vivo. J. Neurosci. 21, 2058–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leonoudakis D., Zhao P., Beattie E. C. (2008) Rapid tumor necrosis factor alpha-induced exocytosis of glutamate receptor 2-lacking AMPA receptors to extrasynaptic plasma membrane potentiates excitotoxicity. J. Neurosci. 28, 2119–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oh M. C., Derkach V. A., Guire E. S., Soderling T. R. (2006) Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J. Biol. Chem. 281, 752–758 [DOI] [PubMed] [Google Scholar]
- 45. Yu Z., Cheng G., Wen X., Wu G. D., Lee W. T., Pleasure D. (2002) Tumor necrosis factor alpha increases neuronal vulnerability to excitotoxic necrosis by inducing expression of the AMPA-glutamate receptor subunit GluR1 via an acid sphingomyelinase- and NF-kappaB-dependent mechanism. Neurobiol. Dis. 11, 199–213 [DOI] [PubMed] [Google Scholar]
- 46. Adesnik H., Nicoll R. A., England P. M. (2005) Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron 48, 977–985 [DOI] [PubMed] [Google Scholar]
- 47. Bats C., Groc L., Choquet D. (2007) The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron 53, 719–734 [DOI] [PubMed] [Google Scholar]
- 48. Ehlers M. D., Heine M., Groc L., Lee M. C., Choquet D. (2007) Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron 54, 447–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Groc L., Heine M., Cognet L., Brickley K., Stephenson F. A., Lounis B., Choquet D. (2004) Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat. Neurosci. 7, 695–696 [DOI] [PubMed] [Google Scholar]
- 50. Kim E., Sheng M. (2004) PDZ domain proteins of synapses. Nat. Rev. Neurosci. 5, 771–781 [DOI] [PubMed] [Google Scholar]
- 51. Prange O., Murphy T. H. (2001) Modular transport of postsynaptic density-95 clusters and association with stable spine precursors during early development of cortical neurons. J. Neurosci. 21, 9325–9333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rao A., Kim E., Sheng M., Craig A. M. (1998) Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture. J. Neurosci. 18, 1217–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Friedman H. V., Bresler T., Garner C. C., Ziv N. E. (2000) Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron 27, 57–69 [DOI] [PubMed] [Google Scholar]
- 54. Ehrlich I., Klein M., Rumpel S., Malinow R. (2007) PSD-95 is required for activity-driven synapse stabilization. Proc. Natl. Acad. Sci. U. S. A. 104, 4176–4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Migaud M., Charlesworth P., Dempster M., Webster L. C., Watabe A. M., Makhinson M., He Y., Ramsay M. F., Morris R. G., Morrison J. H., O'Dell T. J., Grant S. G. (1998) Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 396, 433–439 [DOI] [PubMed] [Google Scholar]
- 56. Chen L., Chetkovich D. M., Petralia R. S., Sweeney N. T., Kawasaki Y., Wenthold R. J., Bredt D. S., Nicoll R. A. (2000) Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 408, 936–943 [DOI] [PubMed] [Google Scholar]
- 57. Dakoji S., Tomita S., Karimzadegan S., Nicoll R. A., Bredt D. S. (2003) Interaction of transmembrane AMPA receptor regulatory proteins with multiple membrane associated guanylate kinases. Neuropharmacology 45, 849–856 [DOI] [PubMed] [Google Scholar]
- 58. Youn D. H., Wang H., Jeong S. J. (2008) Exogenous tumor necrosis factor-alpha rapidly alters synaptic and sensory transmission in the adult rat spinal cord dorsal horn. J. Neurosci. Res. 86, 2867–2875 [DOI] [PubMed] [Google Scholar]
- 59. Wu L. J., Steenland H. W., Kim S. S., Isiegas C., Abel T., Kaang B. K., Zhuo M. (2008) Enhancement of presynaptic glutamate release and persistent inflammatory pain by increasing neuronal cAMP in the anterior cingulate cortex. Mol. Pain 4, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liao D., Scannevin R. H., Huganir R. (2001) Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J. Neurosci. 21, 6008–6017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Allison D. W., Gelfand V. I., Spector I., Craig A. M. (1998) Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J. Neurosci. 18, 2423–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Furukawa K., Mattson M. P. (1998) The transcription factor NF-kappaB mediates increases in calcium currents and decreases in NMDA- and AMPA/kainate-induced currents induced by tumor necrosis factor-alpha in hippocampal neurons. J. Neurochem. 70, 1876–1886 [DOI] [PubMed] [Google Scholar]
- 63. Feuerstein G. Z., Liu T., Barone F. C. (1994) Cytokines, inflammation, and brain injury: role of tumor necrosis factor-alpha. Cerebrovasc. Brain Metab. Rev. 6, 341–360 [PubMed] [Google Scholar]
- 64. Liu T., Clark R. K., McDonnell P. C., Young P. R., White R. F., Barone F. C., Feuerstein G. Z. (1994) Tumor necrosis factor-alpha expression in ischemic neurons. Stroke 25, 1481–1488 [DOI] [PubMed] [Google Scholar]
- 65. Goodman J. C., Robertson C. S., Grossman R. G., Narayan R. K. (1990) Elevation of tumor necrosis factor in head injury. J. Neuroimmunol. 30, 213–217 [DOI] [PubMed] [Google Scholar]
- 66. Hofman F. M., Hinton D. R., Johnson K., Merrill J. E. (1989) Tumor necrosis factor identified in multiple sclerosis brain. J. Exp. Med. 170, 607–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Raine C. S., Bonetti B., Cannella B. (1998) Multiple sclerosis: expression of molecules of the tumor necrosis factor ligand and receptor families in relationship to the demyelinated plaque. Rev. Neurol. (Paris). 154, 577–585 [PubMed] [Google Scholar]
- 68. Rieckmann P., Albrecht M., Kitze B., Weber T., Tumani H., Broocks A., Luer W., Helwig A., Poser S. (1995) Tumor necrosis factor-alpha messenger RNA expression in patients with relapsing-remitting multiple sclerosis is associated with disease activity. Ann. Neurol. 37, 82–88 [DOI] [PubMed] [Google Scholar]
- 69. Selmaj K., Raine C. S., Cannella B., Brosnan C. F. (1991) Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J. Clin. Invest. 87, 949–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sharief M. K., Hentges R. (1991) Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. N. Engl. J. Med. 325, 467–472 [DOI] [PubMed] [Google Scholar]
- 71. Alvarez A., Cacabelos R., Sanpedro C., Garcia-Fantini M., Aleixandre M. (2007) Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disease. Neurobiol. Aging 28, 533–536 [DOI] [PubMed] [Google Scholar]
- 72. Fillit H., Ding W. H., Buee L., Kalman J., Altstiel L., Lawlor B., Wolf-Klein G. (1991) Elevated circulating tumor necrosis factor levels in Alzheimer's disease. Neurosci. Lett. 129, 318–320 [DOI] [PubMed] [Google Scholar]
- 73. Tan Z. S., Beiser A. S., Vasan R. S., Roubenoff R., Dinarello C. A., Harris T. B., Benjamin E. J., Au R., Kiel D. P., Wolf P. A., Seshadri S. (2007) Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology 68, 1902–1908 [DOI] [PubMed] [Google Scholar]
- 74. Bessler H., Djaldetti R., Salman H., Bergman M., Djaldetti M. (1999) IL-1 beta, IL-2, IL-6 and TNF-alpha production by peripheral blood mononuclear cells from patients with Parkinson's disease. Biomed. Pharmacother. 53, 141–145 [DOI] [PubMed] [Google Scholar]
- 75. Hirsch E. C., Hunot S., Damier P., Faucheux B. (1998) Glial cells and inflammation in Parkinson's disease: a role in neurodegeneration? Ann. Neurol. 44, S115–S120 [DOI] [PubMed] [Google Scholar]
- 76. Mogi M., Harada M., Riederer P., Narabayashi H., Fujita K., Nagatsu T. (1994) Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci. Lett. 165, 208–210 [DOI] [PubMed] [Google Scholar]
- 77. Mogi M., Togari A., Kondo T., Mizuno Y., Komure O., Kuno S., Ichinose H., Nagatsu T. (2000) Caspase activities and tumor necrosis factor receptor R1 (p55) level are elevated in the substantia nigra from parkinsonian brain. J. Neural. Transm. 107, 335–341 [DOI] [PubMed] [Google Scholar]
- 78. Nagatsu T., Mogi M., Ichinose H., Togari A. (2000) Changes in cytokines and neurotrophins in Parkinson's disease. J. Neural. Transm. Suppl. 60, 277–290 [DOI] [PubMed] [Google Scholar]
- 79. Zou J. Y., Crews F. T. (2005) TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res. 1034, 11–24 [DOI] [PubMed] [Google Scholar]
- 80. Choi D. W. (1988) Glutamate neurotoxicity and diseases of the nervous system. Neuron 1, 623–634 [DOI] [PubMed] [Google Scholar]
- 81. Hermann G. E., Rogers R. C., Bresnahan J. C., Beattie M. S. (2001) Tumor necrosis factor-alpha induces cFOS and strongly potentiates glutamate-mediated cell death in the rat spinal cord. Neurobiol. Dis. 8, 590–599 [DOI] [PubMed] [Google Scholar]
- 82. Leonoudakis D., Braithwaite S. P., Beattie M. S., Beattie E. C. (2004) TNFalpha-induced AMPA-receptor trafficking in CNS neurons; relevance to excitotoxicity? Neuron Glia Biol. 1, 263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]